Abstract
Background
Our objective was to analyze the clinical and imaging features of Chlamydia psittaci pneumonia to enhance its diagnostic accuracy.
Methods
We systematically reviewed the cases of Chlamydia psittaci diagnosed by next-generation sequencing at the Hunan University of Medicine General Hospital between March 2019 and June 2024, summarizing and analyzing their clinical characteristics and imaging features.
Results
A total of 50 cases that met the inclusion criteria were ultimately included in the study analysis. The median age of the patients was 62.96±11.08 years. Notably, 46 patients (92%) had potential exposure to birds or poultry. Forty-six patients (92%) temperature ≥39.0 °C, 37 patients (74%) had cough, 34 patients (68%) had moist rale, and 39 patients (78%) had a relatively slow pulse. Laboratory tests indicated that over 70% of patients exhibited elevated infection markers, accompanied by abnormalities in liver and renal function, electrolyte levels, and cardiac enzymes. Meanwhile, the patient’s white blood cell count was normal. Chest CT (Computed Tomography) frequently revealed flaky shadows (94%), thoracic effusions (68%), and thickened pleura (54%). Bronchoscopy revealed significant bronchial mucosal hyperemia, swelling (93.478%), and reduced secretion (56.522%). A total of 44 patients (88%) showed a gradual improvement over 12 days. Forty patients (80%) were treated with doxycycline. Fifty patients were classified into two groups according to whether severe pneumonia occurred after admission. WBC (white blood cell), N% (percentage of neutrophils), NLR (Neutrophil-to-lymphocyte ratio), PLR (platelet/lymphocyte ratio), SII (platelet*Neutrophil/lymphocyte ratio), and PCT (procalcitonin) are indicators that suggest severe cases.
Conclusion
The combination of the following indicators is expected to aid in diagnosis of Chlamydia psittaci pneumonia: opportunities to come into contact with birds or poultry, high fever, relatively slow pulse, and elevated infection indicators accompanied by organ injury. Treatment with doxycycline was effective and resulted in favorable prognosis.
Keywords: Chlamydia psittaci, metagenomic next-generation sequencing, clinical features, treatment
Background
Chlamydia psittaci (C. psittaci) pneumonia is an infrequent pulmonary infectious illness that accounts for only 1% of community-acquired pneumonia (CAP) cases.1,2 Despite its low prevalence, the disease is characterized by several severe cases and rapid progression. The diagnosis of C. psittaci pneumonia remains challenging owing to nonspecific clinical symptoms and the limited sensitivity and specificity of conventional diagnostic techniques.3 Therefore, identifying efficient and accurate methods for early detection of C. psittaci pneumonia, particularly in severe cases, is of paramount importance. In recent years, with the metagenomic next-generation sequencing (mNGS) technology widespread application, there has been a notable increase in reports of C. psittaci pneumonia.4,5 Despite this trend, few studies have compared severe and non-severe cases of C. psittaci pneumonia cases. In this study, 50 cases of C. psittaci were diagnosed using the mNGS method at Hunan University of Medicine General Hospital within the past 5 years. We analyzed clinical and imaging characteristics to increase the level of clinical diagnosis and treatment of this disease. The clinical features of 22 severe and 28 non-severe cases were compared, providing a valuable reference for distinguishing between the two groups.
Patients and Methods
Patients
In this retrospective study, we collected data from 50 cases of C. psittaci pneumonia diagnosed using mNGS at Hunan University of Medicine General Hospital from March 2019 to June 2024. All patients were included in the study and were diagnosed according to the following criteria: (I) diagnostic criteria by the guide for community-acquired pneumonia;6 (II) mNGS detection of C. psittaci specific gene fragments in lung tissue, sputum, or bronchoalveolar lavage fluid (BALF) showing that the positive results were consistent with those reported by Miao et al7 and (III) exclusion of other pathogens through a comprehensive analysis of mNGS results and clinical data.
Study Design
Data including age, sex, underlying diseases, symptoms, physical examinations, laboratory outcomes, imaging studies, treatment patterns, and clinical outcomes were extracted from the patients’ electronic health records. This study was approved by the Ethics Committee of First People’s Hospital of Huaihua (KY-2022052606).
Severe Pneumonia
One major criterion or at least three minor criteria.
The main criteria:1) Patient requiring endotracheal intubation for mechanical ventilation; 2) Patients with septic shock still requiring vasoactive drugs after fluid resuscitation.
The secondary criteria:1) respiratory rate ≥30 times/min; 2) PaO2/FiO2 ≤250mmHg;3) multi-lobar infiltrates; 4) confusion/disorientation; 5) blood urea nitrogen ≥ 7.14 mmol/L;6) systolic blood pressure (SBP) < 90 mmHg requiring active fluid resuscitation.
Definition of Returned to Normal
Following treatment, normalization of body temperature, improvement in liver and kidney function, normalization of myocardial enzyme levels and electrolyte balance, gradual reduction in infection markers, and slow resolution of chest CT abnormalities were observed.
mNGS Detection Method
Standard procedures were followed for the collection of lung tissue, sputum, or bronchoalveolar lavage fluid samples from patients.
Single-stranded DNA (ssDNA) libraries were prepared by DNA fragmentation, terminal repair, linker ligation, modification of a single strand, and circulation. Subsequently, DNA nanospheres were produced by rolling circle amplification of ssDNA, which was then loaded into a flow cytometer for sequencing using the Illumina platform.
High-quality sequencing data were obtained by filtering low-quality and short reads (length<35 bp), human reference genome sequences, and low-complexity sequences. The remaining data were compared with microbial genome databases of bacteria, fungi, viruses, and parasites.
The sequencing platform utilized was MGI200.
Analysis software:
1. The quality control software for the raw data of the machine was fast.
2. To remove the host sequence, the alignment software bowtie2 and the software samtools for processing bam files were employed.
3. The pathogen identification software utilized was kraken2.
4. The sequence alignment software was blast.
5. The fasta processing software was seqkit.
Data Analysis
A relatively slow pulse was defined as a pulse increasing <18 bpm for temperature every one degree Celsius, or a pulse was <120 bpm when the body temperature was >38.9°C.8,9
SPSS software (version 26.0) was used for data analysis. Measurement data with normal distribution are expressed as mean± standard deviation, and measurement data with non-normal distribution are expressed as median (interquartile range [IQR]). Categorical data are summarized as ratios or composition ratios. An independent sample t-test was used to compare normally distributed data, the Mann–Whitney U-test was used for the comparison of non-normally distributed data, and the χ2 test or Fisher’s exact test probability method was used for the comparison of count data.
Statistical comparisons between normally distributed, non-normally distributed, and categorical data were conducted using the independent sample t-test, Mann–Whitney U-test, and χ2 test or Fisher exact test, respectively. All reported p values were bilateral, and statistical significance was set at P<0.05.
Results
Patient Characteristics
In this retrospective study, we collected data from 50 hospitalized patients diagnosed with C. psittaci pneumonia using mNGS. Their median age was 62.96±11.08 years, including 17 women and 33 men. Twenty-seven cases had basic disorders including high blood pressure, Glycuresis, Autoimmune diseases, Hepatitis, Coronary heart disease, and auricular fibrillation. Forty-six of the patients resided in the countryside (with neighbors raising poultry). All patients had fever, and 46 (92%) had a temperature of 39.0°C. Cough (74%), expectoration (56%), chills (48%), shortness of breath (38%), coarse breath sounds (60%), low breath sounds (28%), moist rales (68%) and relatively slow pulses (78%).
Fifty patients were classified into two groups according to whether severe pneumonia occurred after admission. Regarding clinical features, marked differences were noted in shortness of breath and moist rale between the two groups (P < 0.05); temperature, age, sex, residence in rural areas, underlying diseases, cough, expectoration, chills, coarse breath sounds, low breath sounds, and relatively slow pulse among the two groups were not statistically significant. The clinical features of the patients are summarized in Table 1.
Table 1.
Analysis of Clinical Features of Patients with Severe Group or Non- Severe Group
| Clinical Features | Severe groups (n=22) | Non-severe groups (n=28) | P value |
|---|---|---|---|
| Demographic data | |||
| Sex (males /female) | 15/7 | 18/10 | 0.773 |
| Residing in rural areas | 22 (100%) | 24 (85.714%) | 0.242 |
| Age (years) | 63.23±12.067 | 62.75±10.458 | 0.882 |
| Underlying diseases | 10 (45.455%) | 10 (35.714%) | 0.283 |
| Symptoms | |||
| Mean temperature (°C) | 39.55 (39–40) | 39.5 (39–40) | 0.700 |
| Cough | 16 (72.727%) | 21 (75%) | 0.856 |
| Expectoration | 9 (40.909%) | 17 (60.714%) | 0.449 |
| Chills | 9 (40.909%) | 15 (53.571%) | 0.374 |
| Shortness of breath | 14 (63.636%) | 5 (17.857%) | 0.001 |
| Characteristics | |||
| Coarse breath sounds | 16 (72.727%) | 14 (50%) | 0.103 |
| Low breath sounds | 7 (31.818%) | 7 (25%) | 0.594 |
| Moist rale | 19 (86.364%) | 15 (53.571%) | 0.014 |
| Relatively slow pulse | 18 (81.818%) | 21 (75%) | 0.734 |
Laboratory Examination
Most patients exhibited significantly elevated N% (percentage of neutrophils), NLR (Neutrophil-to-lymphocyte ratio), CRP (C-reactive protein), PCT (procalcitonin), ESR (erythrocyte sedimentation rate), IL-6 (interleukin-6), ALT (alanine aminotransferase), AST (aspartate aminotransferase), LDH (lactate dehydrogenase), and MYO (myoglobin) levels, with over half displaying normal white blood cell (WBC) counts. Additionally, more than 60% of patients showed decreased levels of ALB, blood calcium, and orthostatic calcium. Detailed results of the initial laboratory examinations upon hospital admission are shown in Table 2. Patients classified in the severe group demonstrated significantly higher increases in WBC, N%, NLR, LWR (lymphocyte/leukocyte ratio), PLR (platelet/lymphocyte ratio), SII (platelet*Neutrophil/lymphocyte ratio), PCT, ALT, AST, LDH, and CK-MB (creatine kinase isoenzyme), as well as a more pronounced decrease in ALB compared to those in the non-severe group, with all differences were statistically significant (P<0.05). Laboratory examination results are presented in Table 3.
Table 2.
Analysis of Laboratory Examination Results in Patients with Chlamydia Psittaci Pneumonia
| Laboratory Test (Unit) | Chlamydia psittaci pneumonia | Abnormal proportion (%) |
|---|---|---|
| Elevated above normal range | ||
| WBC (*109/L) | 8.36±2.763 | 30% (15/50) |
| PLT (*109/L) | 167.98±74.921 | 24% (12/50) |
| N(%) | 88.858±7.525 | 96% (48/50) |
| NLR | 13.45 (7.82,29.49) | 92% (46/50) |
| CRP (mg/L) | 187.388±95.013 | 100% (50/50) |
| PCT (ug/L) | 1.425 (0.575,4.785) | 100% (50/50) |
| ESR (mm/h) | 67.29±34.156 | 97.561% (40/41) |
| IL-6 (pg/mL) | 109.2 (65.55,468.4) | 96.774% (30/31) |
| ALT (IU/L) | 50.5 (29,87.75) | 58% (29/50) |
| AST (IU/L) | 77 (39.75,112.75) | 72% (36/50) |
| TBIL (umol/L) | 12.85 (8.275, 22.425) | 30% (15/50) |
| DBIL (umol/L) | 6.4 (4.075, 15.425) | 48% (24/50) |
| LDH (U/L) | 337 (266.25, 507.75) | 82% (41/50) |
| CK (U/L) | 169 (89, 421) | 34% (17/50) |
| CK-MB (IU/L) | 14 (10, 20) | 10% (5/50) |
| MYO (ug/L) | 149.5 (83.25, 582.25) | 68% (34/50) |
| Below the normal lower limit | ||
| ALB (g/L) | 31.05±6.122 | 94% (47/50) |
| Na (mmol/L) | 134.8 (132.05, 140) | 60% (30/50) |
| Ca (mmol/L) | 1.97 (1.915, 2.04) | 96% (48/50) |
| Corrective Ca (mmol/L) | 2.017 (1.953,2.103) | 96% (48/50) |
Note: *WBC, white blood cell; PLT, platelets; N%, percentage of neutrophils; NLR,Neutrophil to lymphocyte ratio; CRP, C-reactive protein; PCT, procalcitonin; ESR, erythrocyte sedimentation rate; IL-6, interleukin-6; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; LDH, lactate dehydrogenase; CK, creatine kinase; CK-MB, creatine kinase isoenzyme; MYO, myoglobin; ALB, albumin.
Table 3.
Analysis of Laboratory Examination Results of Severe or Non-Severe Group Patients
| Laboratory Test (Unit) | Severe groups (n=22) | Non-severe groups (n=28) | P value |
|---|---|---|---|
| WBC (*109/L) | 9.382±3.236 | 7.557±2.047 | 0.027 |
| PLT (*109/L) | 187.64±94.418 | 134.5 (121.25, 181.75) | 0.197 |
| N(%) | 91.509±5.403 | 88.85 (83.025, 91.8) | 0.028 |
| NLR | 24.27 (11.645,40.16) | 12.37 (6.123, 17.513) | 0.005 |
| MLR | 0.578±0.281 | 0.531 (0.332, 0.703) | 0.777 |
| LMR | 1.75 (1.18, 3.488) | 1.885 (1.424, 3.015) | 0.777 |
| LWR | 0.051±0.036 | 0.068 (0.05, 0.107) | 0.011 |
| PLR | 512.615±356.067 | 316.569 (202.186, 378.309) | 0.028 |
| SII | 4212 (1489.215, 7142.585) | 2071.477±1494.532 | 0.006 |
| CRP (mg/L) | 187.605 (132.583,220.725) | 177.082±105.033 | 0.249 |
| PCT (ug/L) | 3.075 (1.035,7.528) | 0.82 (0.383, 2.618) | 0.004 |
| ESR (mm/h) | 68.71±32.388 | 66.29±36.01 | 0.827 |
| IL-6 (pg/mL) | 150.6 (65.09, 783.7) | 81.275 (66.63, 377.34) | 0.363 |
| ALT (IU/L) | 64 (35.5, 140.75) | 39 (20.75, 75) | 0.03 |
| AST (IU/L) | 100.5 (73.75, 205.25) | 48.5 (28.5, 91.25) | 0.001 |
| ALB (g/L) | 27.259±3.408 | 34.029±6.17) | 0.000 |
| TBIL (umol/L) | 21.673±15.591 | 12.5 (9.125, 15.65) | 0.257 |
| DBIL (umol/L) | 11.08 (3.8, 25.5) | 6.1 (4.825, 7.725) | 0.278 |
| CREA-S (umol/L) | 95.5 (62.25, 138.75) | 92 (77.25, 106) | 0.899 |
| UA (umol/L) | 180 (124.5, 262) | 240.11±83.319 | 0.115 |
| LDH (U/L) | 429.5 (345.5, 579.25) | 282.5 (232.5, 363.75) | 0.000 |
| CK (U/L) | 248 (42, 682) | 139 (91, 279.75) | 0.386 |
| CK-MB (IU/L) | 16 (13, 25) | 13.79±7.32 | 0.041 |
| MYO (ug/L) | 461 (84, 814) | 134 (82.25, 390) | 0.202 |
| Na (mmol/L) | 136.809±8.466 | 134.8 (132.8,140) | 0.872 |
| Ca (mmol/L) | 1.946±0.156 | 1.994±0.153 | 0.291 |
| Corrective Ca (mmol/L) | 2.017±0.152 | 2.026±0.133 | 0.823 |
Note: *WBC, white blood cell; PLT, platelets; N%, percentage of neutrophils; NLR:Neutrophil to lymphocyte ratio; MLR, monocyte/lymphocyte ratio; LMR, lymphocyte/monocyte ratio; LWR, lymphocyte/leukocyte ratio; PLR, platelet/lymphocyte ratio; SII:platelet*Neutrophil/lymphocyte ratio; CRP, C-reactive protein; PCT, procalcitonin; ESR, erythrocyte sedimentation rate; IL-6, interleukin-6; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; CREA-S, serum creatinine; UA, uric acid; LDH, lactate dehydrogenase; CK, creatine kinase; CK-MB, creatine kinase isoenzyme; MYO, myoglobin; ALB, albumin.
Imaging
All patients underwent chest computed tomography (CT) examination. Chest CT showed unilateral lesions in 22 (44%) cases, patchy or patchy shadows in 47 (94%), pleural effusion in 34 (68%), pericardial effusion in 15 (30%), pleural thickening in 27 (54%), mediastinal lymph node enlargement in 22 (44%), nodular changes in the lungs in 8 (16%), and bronchial inflation in 6 (12%). The lesion range was located within the superior lobe of the left lung was present in 31 (62%) cases, the left inferior pulmonary lobe in 28 (56%), the superior lobe of the right lung in 36 (72%), and the inferior lobe of the right lung in 35 (70%).
Bronchoscopy Examination
Forty-six patients Performed bronchoscopic examination. The findings indicated that 43 (93.478%) patients exhibited bronchial mucosa hyperemia and swelling and 26 (56.522%) patients had reduced secretion.
Treatment and Prognosis
Before diagnosis, most patients received empirical treatment with β-lactam antibiotics, which proved to be ineffective. Subsequently, BALF, lung tissue, and sputum samples were collected for mNGS analysis to confirm the diagnosis and guide appropriate treatment adjustments. Following treatment modification, 44 patients (88%) showed clinical and laboratory gradual improvement within 12 days. Specifically, 33 patients were treated solely with doxycycline, 7 patients received quinolone treatment alone, 2 patients received a combination of doxycycline and azithromycin, and 2 patients received a combination of doxycycline and quinolones. The recovery time for patients treated with doxycycline alone was 8(6,7) days, compared to approximately 14 days for those treated with quinolones alone. Patients treated with a combination of doxycycline and azithromycin had a recovery time of approximately 12 days, which was similar to those treated with a combination of doxycycline and quinolones. Among the six unrecovered patients, three received doxycycline treatment and two received quinolones before diagnosis.
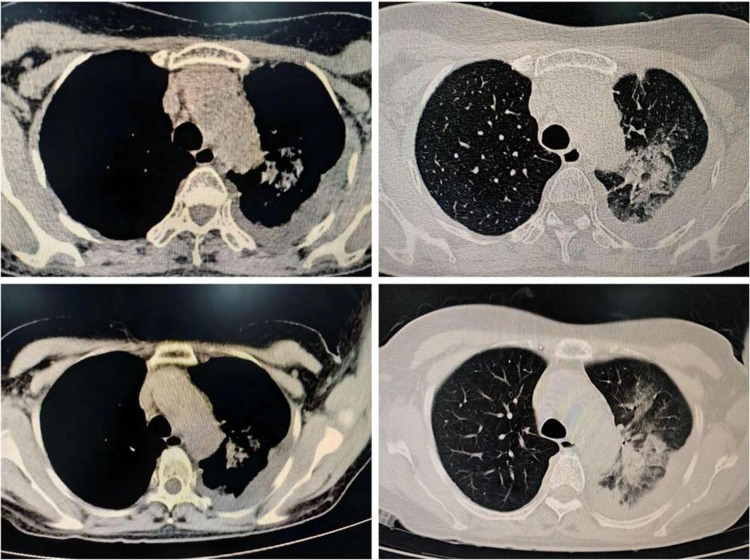
The transfer of 10 patients to the intensive care unit (ICU) took place due to unstable vital signs while receiving treatment in the general ward, necessitating tracheal intubation and mechanical ventilation. Following treatment, four patients were discharged from the hospital after recovery. Among the remaining six patients, four had underlying diseases, four were male, five were over 60 years old, and five were diagnosed with severe pneumonia. One patient, a 67-year-old man, was not initially diagnosed with severe pneumonia upon admission to the hospital because of fever and shortness of breath for one week. Pulmonary physical examination revealed no discernible abnormalities, whereas laboratory tests indicated that WBC, PLT, and NLR were within normal limits. However, N %, CRP, and IL-6 levels increased significantly, with only slight increases in liver and kidney function, PCT levels, and ESR. Chest CT tomography revealed bilateral lesions with patchy shadows along with pleural and pericardial effusions, bronchial inflation signs, pleural thickening, and mediastinal lymph node enlargement. Initially, piperacillin sodium and tazobactam sodium were administered as anti-infective therapies, but the efficacy of these treatments was ineffective. Bronchoscopy was conducted on the ninth day of illness, during which bronchoalveolar lavage fluid was collected for analysis. Subsequently, an alternative anti-infective agent was replaced with doxycycline therapy, based on the results obtained on the eleventh day. The chest CT results of the patients are shown in Figure 1.
Figure 1.
Chest CT scan of non critically ill patients who have not recovered.
Discussion
C. psittaci pneumonia is a form of atypical infectious pneumonia resulting from infection with the bacterium Chlamydia psittaci, which causes epidemics in animals.10 C. psittaci is a gram-negative pathogen that is strictly intracellular and belongs to the Chlamydia genus within the Chlamydiaceae family.11,12 Transmission typically occurs through inhalation of respiratory secretions or dried aerosols containing the bacterium, leading to its replication in mononuclear phagocytes within the liver and spleen before spreading through the bloodstream to the lungs and other organs, resulting in disease.13 Other possible modes of exposure include mouth-to-beak contact and handling of infected birds’ plumage and tissues;14 human-to-human transmission is rare.15 In this study, 46 patients living in rural areas and their nearby neighbors were involved in poultry feeding. We considered the risk factors for contracting C. psittaci infections in poultry.
The clinical presentation of C. psittaci infection is systemic12 and affects various body systems, including the breathing, digestion, circulation, nerves, hepatic, and musculoskeletal systems.
The severity of the infection can vary, with some cases rapidly progressing to respiratory failure necessitating tracheal intubation and mechanical ventilation. In severe cases, extracorporeal membrane oxygenation (ECMO) treatment may be necessary.16 The primary symptoms are elevated body temperature, difficulty in breathing, and a non-productive cough,17 while the predominant signs include dry and wet rales and a relatively slow pulse.18 This study found that the typical symptoms of C. psittaci pneumonia include a high heart rate, cough, and sputum production. Common signs observed were coarse breath sounds, moist crackles, and relatively slow pulses. Compared with non-severe cases, patients diagnosed with severe C. psittaci pneumonia exhibited a higher incidence of shortness of breath and moist rales.
Complications of C. psittaci pneumonia may include carditis, endocarditis, hepatitis, arthritis, conjunctivitis, and meningitis et al.4 The novel inflammatory indicators MLR, LMR, LWR, PLR, and SII have been shown to accurately and sensitively indicate the inflammatory state of the body, allowing for the assessment of various diseases and their prognosis.19,20 Previous research has indicated that patients may suffer from pneumonia caused by C. psittaci with normal or slightly elevated leukocytes and a nuclear-left-shift phenomenon.8 Additionally, studies have shown that patients with C. psittaci pneumonia have significantly elevated levels of CRP, PCT, IL-6, and other infection markers.21 Laboratory examination showed that most patients exhibited symptoms of multisystem dysfunction: (1) most patients exhibited normal or slightly elevated WBC; however, infection indicators, such as N%, CRP, NLR, ESR, IL-6, and PCT, were significantly increased. (2) Most patient had abnormal liver function, myocardial enzymes, and electrolyte results, with a minority experiencing renal dysfunction. Compared with non-severe cases, patients in the severe group demonstrated significantly higher levels of N %, NLR, LWR, PLR, SII, PCT, ALT, AST, LDH, and CK-MB. Higher WBC. (3) A notable decrease in albumin levels was observed, which was likely attributed to the more pronounced inflammatory response and increased consumption in the severe group.
Pulmonary imaging alterations in C. psittaci pneumonia are characterized by different degrees of exudation and consolidation, along with the presence of a bronchial inflation sign and pleural effusion.22 Additionally, certain patients may exhibit ground-glass density shadows, halo signs, and anti-halo sign.23 Research conducted by Su Shanshan et al indicated that C. psittaci pneumonia typically presents unilaterally in the lower lobe during the initial phase, progressing to bilateral lesions in later stages.6 The study also revealed patchy shadows, pleural effusion, pleural thickening, and mediastinal lymph node enlargement on the chest CT. The lesions predominantly exhibited a bilateral distribution. The imaging features of C. psittaci pneumonia are subtle, complicating its differentiation from pneumonia induced by other pathogens. Diagnosis primarily depends on pathogen detection; however, traditional culture methods are challenging to implement in most hospital settings and yield low positivity rates. Serological tests are more appropriate for retrospective diagnosis; however, serum complement fixation tests (CFT) cannot differentiate between the various types of C. psittaci. Numerous prior studies have demonstrated that mNGS is a reliable tool for the rapid and accurate identification of C. psittaci.21,24,25
C. psittaci pneumonia is frequently managed with doxycycline, either as a monotherapy or in combination with moxifloxacin.26 For early mild cases of C. psittaci pneumonia, quinolones alone are recommended.27 Macrolides are also effective and are particularly recommended for patients allergic to tetracycline drugs as well as for children and pregnant women. Generally, C. psittaci pneumonia has a favorable prognosis, with timely and accurate therapy reducing the mortality rate by approximately 1%. In this study, most patients were initially treated empirically with β-lactam antibiotics prior to diagnosis. The primary mechanism of action of β-lactam antibiotics involves the disruption of bacterial cell wall synthesis. However, C. psittaci lacks a cell wall, rendering the β-lactam drugs ineffective. Most patients exhibited clinical improvement following a switch to doxycycline or quinolone monotherapy. A minority of the patients received combination therapy with doxycycline and quinolones. Nonetheless, a subset of patients did not respond to either doxycycline or quinolone treatment, and most non-responders were diagnosed with severe pneumonia. The severity of illness in these cases was considered a contributing factor to the lack of therapeutic response. The overall prognosis for the 50 patients diagnosed with Chlamydia psittaci pneumonia included in this study was favorable, with only 6 patients not achieving recovery.
This study comprehensively analyzed the clinical and radiographic features of patients with C. psittaci pneumonia, thereby contributing to the enhancement of diagnostic and therapeutic strategies for this disease. Additionally, the study compared the clinical characteristics of patients with severe and non-severe patients, offering a valuable reference for distinguishing between these two groups. Notably, C. psittaci pneumonia is often associated with opportunities to contact birds and poultry. The clinical presentation predominantly featured a temperature of ≥39.0 °C, cough, expectoration, chills, coarse breath sounds, moist rale, relatively slow pulse, normal white blood cell count, remarkably elevated infection markers, and abnormal liver function, kidney function, electrolytes, and myocardial enzymes. Chest CT tomography revealed patchy infiltrates, pleural effusion, pleural thickening, and mediastinal lymph node enlargement. Bronchoscopy revealed pronounced bronchial mucosal hyperemia and swelling, with reduced secretion. The overall prognosis for C. psittaci pneumonia is favorable, with doxycycline being the most effective treatment. However, patients in the severe category were more likely to experience shortness of breath, moist rales, markedly abnormal inflammatory indicators, liver function, myocardial enzymes, and electrolytes. The proportion of patients with a poor prognosis was slightly higher in the severe group.
This study had a retrospective design, which precluded the dynamic monitoring and comparison of relevant indicators. Although pathogenic bacteria were identified in the specimens using mNGS, traditional methods proved insufficient for verification.
Conclusions
The majority of patients diagnosed with C. psittaci pneumonia have the opportunity to come into contact with birds or poultry. Clinically, most patients exhibit a body temperature ≥39.0°C, along with symptoms such as cough, expectoration, chills, coarse breath sounds, moist rales, and a relatively slow pulse. Additionally, infection markers were significantly elevated and were frequently associated with damage to other organs. Patients with severe pneumonia exhibited a higher likelihood of experiencing shortness of breath, moist rales, significantly elevated infection markers, and more extensive damage to other organs. Treatment with doxycycline was effective, and the prognosis was favorable.
Acknowledgments
We express our gratitude to the patients who participated in the study.
Funding Statement
This study was sponsored by the Huaihua Science and Technology Plan Project (2020R3331). The sponsor did not participate in study design, collection, analysis, interpretation, or manuscript drafting.
Abbreviations
Mngs, metagenomic next-generation sequencing; PCR, polymerase-chain reaction; MIF, micro immunofluorescence test; CAP, community-acquired pneumonia; NLR, Neutrophil-to-lymphocyte ratio; MLR, monocyte/lymphocyte ratio; LMR, lymphocyte/monocyte ratio; LWR, lymphocyte/leukocyte ratio; PLR, platelet/lymphocyte ratio; SII:platelet*Neutrophil/lymphocyte ratio; PCT, procalcitonin; CRP, C-reactive protein; IL-6, interleukin-6; ESR, erythrocyte sedimentation rate; CT, computed tomography; WBC, white blood cell; PLT, platelets; N%, percentage of neutrophils; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; CREA-S, serum creatinine; UA, uric acid; LDH, lactate dehydrogenase; CK, creatine kinase; CK-MB, creatine kinase isoenzyme; MYO, myoglobin; ALB, albumin; BALF, Bronchoalveolar Lavage Fluid.
Ethics Approval and Informed Consent
The Ethics Committee (LYF-2022052606) of Hunan University of Medicine General Hospital (formerly named Huaihua First People’s Hospital). All patients and legal guardians provided informed consent. This study complied with the principles of the Declaration of Helsinki.
Authors’contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ni YY, Zhong HH, Gu Y, et al. Clinical features, treatment, and outcome of psittacosis pneumonia: a multicenter study. Open Forum Infect Dis. 2023;10:ofac518. doi: 10.1093/ofid/ofac518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teng XQ, Gong WC, Qi TT, et al. Clinical Analysis of Metagenomic Next-Generation Sequencing Confirmed Chlamydia psittaci Pneumonia: a Case Series and Literature Review. Infect Drug Resist. 2021;14:1481–1492. doi: 10.2147/IDR.S305790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo CH, Lin YP, Chen CW, et al. Diagnosis of severe Chlamydia psittaci pneumonia by metagenomic next-generation sequencing: 2 case reports. Respir Med Case Rep. 2022;38:101709. doi: 10.1016/j.rmcr.2022.101709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu JM, Gao Y. Tigecycline in the treatment of severe pneumonia caused by chlamydia psittaci: a case report and literature review. Front Med Lausanne. 2022;9:1040441. doi: 10.3389/fmed.2022.1040441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang FX, Li JJ, Qi B, et al. Clinical symptoms and outcomes of severe pneumonia caused by chlamydia psittaci in southwest China. Front Cell Infect Microbiol. 2021;11:727594. doi: 10.3389/fcimb.2021.727594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su SS, Su XQ, Zhou LP, et al. Severe chlamydia psittaci pneumonia: clinical characteristics and risk factors. Ann Palliat Med. 2021;10:8051–8060. doi: 10.21037/apm-21-1502 [DOI] [PubMed] [Google Scholar]
- 7.Miao Q, Ma YY, Wang QQ, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–S240. doi: 10.1093/cid/ciy693 [DOI] [PubMed] [Google Scholar]
- 8.Jin WZ, Liang RZ, Tian XJ, et al. Clinical features of psittacosis in 46 Chinese patients. Enferm Infecc Microbiol Clin (Engl Ed). 2023;41:545–548. doi: 10.1016/j.eimc.2022.05.012 [DOI] [PubMed] [Google Scholar]
- 9.Zhang YP, Song P, Zhang RH, et al. Clinical Characteristics of chronic lung abscess associated with parvimonas micra diagnosed using metagenomic next-generation sequencing. Infect Drug Resist. 2021;14:1191–1198. doi: 10.2147/IDR.S304569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi YF, Chen JX, Shi XH, et al. A case of chlamydia psittaci caused severe pneumonia and meningitis diagnosed by metagenome next-generation sequencing and clinical analysis: a case report and literature review. BMC Infect Dis. 2021;21:621. doi: 10.1186/s12879-021-06205-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Gier B, Hogerwerf L, Dijkstra F, et al. Disease burden of psittacosis in the Netherlands. Epidemiol Infect. 2018;146:303–305. doi: 10.1017/S0950268817003065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul L, Comstock J, Edes K, et al. Gestational psittacosis resulting in neonatal death Identified by next-generation RNA sequencing of postmortem, formalin-fixed lung tissue. Open Forum Infect Dis. 2018;5:ofy172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogerwerf L, Roof I, de Jong MJK, et al. Animal sources for zoonotic transmission of psittacosis: a systematic review. BMC Infect Dis. 2020;20:192. doi: 10.1186/s12879-020-4918-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw KA, Szablewski CM, Kellner S, et al. Psittacosis outbreak among workers at chicken Slaughter plants, Virginia and Georgia, USA, 2018. Emerging Infect Dis. 2019;25:2143–2145. doi: 10.3201/eid2511.190703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang ZJ, Zhou H, Cao H, et al. Human-to-human transmission of chlamydia psittaci in China, 2020: an epidemiological and aetiological investigation. Lancet Microbe. 2022;3:e512–e520. doi: 10.1016/S2666-5247(22)00064-7 [DOI] [PubMed] [Google Scholar]
- 16.Meijer R, van Biezen P, Prins G, et al. Multi-organ failure with necrotic skin lesions due to infection with chlamydia psittaci. Int Infect Dis. 2021;106:262–264. doi: 10.1016/j.ijid.2021.03.091 [DOI] [PubMed] [Google Scholar]
- 17.Balsamo G, Maxted AM, Midla JW, et al. Compendium of measures to control chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis), 2017. J Avian Med Surg. 2017;31:262–282. doi: 10.1647/217-265 [DOI] [PubMed] [Google Scholar]
- 18.Fukui S, Kawamura W, Uehara Y, et al. A patient with psittacosis from a pigeon: a reminder of the importance of detailed interviews and relative bradycardia. IDCases. 2021;25:e01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yevich S, Gaspar N, Tselikas L, et al. Percutaneous computed tomography-guided thermal ablation of pulmonary osteosarcoma metastases in children. Ann Surg Oncol. 2016;23:1380–1386. doi: 10.1245/s10434-015-4988-z [DOI] [PubMed] [Google Scholar]
- 20.Saumet L, Deschamps F, Marec-Berard P, et al. Radiofrequency ablation of metastases from osteosarcoma in patients under 25 years: the SCFE experience. Pediatr Hematol Oncol. 2015;32:41–49. doi: 10.3109/08880018.2014.926469 [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Cao K, Wei Y, et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by chlamydia psittaci. Infection. 2020;48:535–542. doi: 10.1007/s15010-020-01429-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branley JM, Weston KM, England J, et al. Clinical features of endemic community-acquired psittacosis. New Microbes New Infect. 2014;2:7–12. doi: 10.1002/2052-2975.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Li SJ, Tan WM, et al. Metagenomic next-generation sequencing in the family outbreak of psittacosis: the first reported family outbreak of psittacosis in China under covid-19. Emerg Microbes Infect. 2021;10(1):1418–1428. doi: 10.1080/22221751.2021.1948358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian YY, Wang HY, Zhou Y, et al. Improving Pulmonary Infection diagnosis with metagenomic next generation sequencing. Front Cell Infect Microbiol. 2020;10:567615. doi: 10.3389/fcimb.2020.567615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Zhan DT, Chen DD, et al. Next-generation sequencing diagnosis of severe pneumonia from fulminant psittacosis with multiple organ failure: a case report and literature review. Ann Transl Med. 2020;8:401. doi: 10.21037/atm.2020.03.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucanska M, Hajtman A, Calkovsky V, et al. Upper airway cough syndrome in pathogenesis of chronic cough. Physiol Res. 2020;69(Suppl 1):S35–S42. doi: 10.33549/physiolres.934400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katsura D, Tsuji S, Kimura F, et al. Gestational psittacosis: a case report and literature review. J Obstet Gynaecol Res. 2020;46:673–677. doi: 10.1111/jog.14217 [DOI] [PubMed] [Google Scholar]