Abstract
Backgrounds
Many pregnant women suffer from more than one pregnancy complication. However, whether those women experienced a higher risk of adverse birth outcomes is unclear. This study aims to assess the association between the comorbidity of gestational diabetes mellitus (GDM) and hypertension disorders of pregnancy (HDP) and adverse birth outcomes.
Methods
The data was from the Zhoushan Maternal and Child Health Hospital electronic medical recorder system (EMRS) between 2015 and 2022. Multivariate linear regression model was used to analyze the association of GDM, HDP, and comorbidity with birth weight and gestational age, respectively. Multiple logistic regression model was used to analyze the association of GDM, HDP, and comorbidity with adverse birth outcomes.
Results
13645 pregnant women were included. GDM+HDP was significantly associated with a higher risk of composite adverse neonatal outcomes (OR=1.82, 95%CI: 1.02-3.04), including preterm birth, placenta previa, and/or neonatal jaundice, a higher risk of small for gestational age (SGA) (OR=2.2, 95% CI: 1.24 3.92) and large for gestational age (LGA) (OR=2.33, 95% CI: 1.64 3.31) compared with the normal group. Further analysis showed that HDP diagnosed in the 21-27th week comorbid with GDM had the lowest gestational age at delivery (β= -1.57, P=0.0002) and birth weight (β= -189.57, P=0.0138). Moreover, combined hyperglycemia (CH) comorbid with HDP had the strongest association with reduced gestational age (β= -0.83, P=0.0021).
Conclusion
Pregnant women suffering from both GDM and HDP had a higher risk of adverse neonatal outcomes; hence, the prevent and treatment of GDM and HDP, especially their comorbidity, are very important for pregnant women.
Keywords: gestational diabetes mellitus, hypertension disorders, pregnancy, adverse neonatal outcomes, comorbidity
1. Introduction
Gestational diabetes mellitus (GDM) is defined as a new onset or first recognition of glucose intolerance during pregnancy. According to a report, 12.8% of pregnant women suffered from GDM worldwide and the incidence of GDM has reached 14.8% in China, with an increasing growth trend (1). Hypertensive disorders in pregnancy (HDP) are a group of maternal disorders characterized by elevated blood pressure during pregnancy, including gestational hypertension, preeclampsia, and eclampsia. The global prevalence of HDP has been reported to range from 4.6% to 13.1% (2), and among Chinese pregnant women, it is approximately 5% to 10% (3). Both GDM and HDP are associated with a risk of adverse birth outcomes, including newborn birth weight, preterm birth (PTB), placenta praevia, premature rupture of membranes, and placental abruption. Long-term complications of GDM include obesity, diabetes, and cardiovascular disease in the mother and offspring. HDP increases the risk of future coronary artery disease and chronic kidney disease.
Both GDM and HDP are among the most common complications of pregnancy. Recently, the prevalence of GDM and HDP has increased rapidly. Pregnant women with both diseases pose a great challenge for clinical management. Previous studies have shown that GDM and HDP were closely related, and women with GDM were at a significantly increased risk of hypertension and preeclampsia (4). The co-morbidity of GDM and HDP may further increase the risk of adverse birth outcomes. However previous studies have mostly investigated the effect of having only one of these diseases on adverse outcomes. Few studies have been conducted on the co-morbidity of GDM and HDP, whose interaction is unclear.
The relationship between a single condition of GDM or HDP and adverse outcomes has been well established. GDM was associated with adverse outcomes such as macrosomia, pre-eclampsia, low birth weight, birth trauma (shoulder dystocia), respiratory distress, cesarean delivery, neonatal intensive care unit (NICU), and fetal death (5, 6). HDP increased the risk of preterm birth, stillbirth, small for gestational age (SGA), and low birth weight (3, 7). PE significantly increased the risk of placental abruption (8). Studies have shown that in pregnant women with GDM combined with PE, excess gestational weight gain (GWG) led to a more pronounced increase in the risk of preterm delivery and the occurrence of older than gestational age (LGA) infants (9), and the severity of their PE was positively associated with SGA (10), suggesting that co-morbidity of GDM HDP may have a significant impact on adverse birth outcomes. Another study showed that diabetes mellitus combined with hypertension significantly increased the incidence of PTB, but in this study, it was chronic diabetes mellitus rather than GDM (11). A UK study showed that GDM combined with gestational hypertension significantly increased the incidence of LGA and cesarean delivery (6). Another Taiwanese data showed that women with HDP had a higher risk of neonatal PTB and SGA and women with both HDP and GDM had a further increased risk of PTB, SGA, and LGA, but this study did not compare the co-morbidity of GDM and HDP with GDM alone (12).
In summary, only a few studies have explored the effect of GDM and HDP co-morbidity on some adverse birth outcomes, but the relationship between GDM combined with HDP and multiple adverse birth outcomes has not been elucidated. And they didn’t consider that diagnosis of HDP at different periods and GDM subtypes may have different effects on outcomes. Besides, few relevant studies have been conducted in mainland China. Therefore, studies on the co-morbidity of GDM and HDP are inherently innovative. Secondly, the impact of other adverse birth outcomes, such as premature rupture of membranes, placental abruption, and placenta praevia, has been barely studied in the limited studies on the co-morbidity of GDM and HDP, and the joint multiple outcome comparison study of these outcome indicators is also innovative. Thus, the current study aimed at further clarifying the effects of GDM and HDP co-morbidity on multiple adverse birth outcomes using data from the maternal and child health care system and the clinical examination information system of Zhoushan Maternal and Child Care Hospital in Zhoushan, Zhejiang province, China.
2. Materials and methods
2.1. Participants and study design
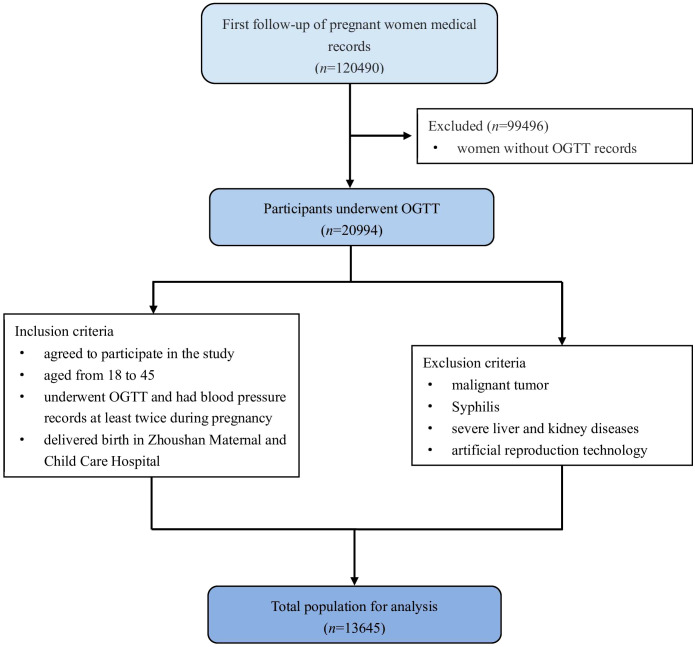
This was a retrospective cohort study using data from the comprehensive electronic medical recorder system (EMRS) of Zhoushan Maternal and Child Care Hospital in Zhoushan, Zhejiang province, China, between 2015 and 2022. The study protocol was approved by the institutional review board of the School of Medicine in Zhejiang University. First follow-up information, oral glucose tolerance test (OGTT), prenatal follow-up blood pressure data, and delivery information were matched with unique IDs. The inclusion criteria were: (1) aged from 18 to 45; (2) underwent OGTT and had blood pressure records at least twice during pregnancy; (3) delivered birth in this hospital. The exclusion criteria were: (1) malignant tumor; (2) Syphilis; (3) severe liver and kidney diseases; (4) artificial reproduction technology. Informed consent was obtained from all individual participants included in the study. The flowchart of participants in the study was showed in Figure 1 .
Figure 1.
Flowchart of participants in the study.
2.2. Measurement of OGTT and definition of GDM and GDM subtypes
GDM screening has become a routine examination among pregnant women in China. OGTT was conducted between the 24th and 28th week of gestation. After an overnight fast (at least 8 h), 75 g glucose resolved in 300 ml water was given and drunk within 5 min the next morning. Venous blood samples were taken at 0 h, 1 h, and 2 h during OGTT for measuring plasma glucose levels. Plasma glucose levels were immediately measured by the hexokinase method with commercially available kits (Beckman AU5800, Beckman Coulter Inc., Brea, CA, USA). The result of OGTT was extracted from EMRS, and GDM was diagnosed based on the criteria set by the IADPSG (13), those whose plasma glucose met at least one of the following criteria were diagnosed as GDM: fasting plasma glucose (FBG) ≥5.1 mmol/l; 1h-postprandial glucose (PG1H) ≥10 mmol/l; 2h-postprandial glucose (PG2H) ≥8.5 mmol/l. Thereby, three subgroups of GDM can be separated: isolated fasting hyperglycemia (IFH), isolated post-load hyperglycemia (IPH), and combined hyperglycemia (CH) according to OGTT results (14).
2.3. Measurement of blood pressure and definition of HDP
The measurements of SBP and DBP were taken as a part of routine perinatal care by physicians, the data of which were extracted from EMRS. BP measurements were performed in a seated position, from the right hand with a standard mercury sphygmomanometer. The gestational age was calculated by the last menstruation date and confirmed by the B ultrasound. HDP encompasses chronic hypertension, gestational hypertension, preeclampsia/eclampsia, and preeclampsia superimposed on chronic hypertension (15). Chronic hypertension was defined as having hypertension before pregnancy or diagnosed before 20 weeks gestation in at least two consecutive examinations (16). The gestational week of HDP onset was defined as the first gestational week of two consecutive examinations of diagnosing gestational hypertension. All participants were classified into three stages based on their gestational week of HDP onset, including ≤20th week, 21-27th week, and ≥28th week.
2.4. Definition of delivery outcomes and covariables
There are two types of birth weight abnormalities according to the gestational age and sex-specific curves in the Chinese Journal of Pediatrics 2020: small for gestational age (SGA, birth weight<10th percentile) and large for gestational age (LGA, birth weight>90th percentile) (17). Preterm birth is defined as births before 37 completed weeks of gestation. Placenta previa is the complete or partial covering of the internal os of the cervix with the placenta (18). Pregnancy BMI was divided into four categories based on the Working Group on Obesity in China (19): underweight, BMI < 18.5 kg/m2; normal, BMI 18.5-23.9 kg/m2; overweight, BMI 24.0-27.9 kg/m2; obesity, BMI ≥ 28 kg/m2. GWG was calculated as the pre-natal weight minus pre-pregnancy weight.
2.5. Statistical analysis
The participants were divided into four groups: normal, GDM only, HDP only, and GDM+HDP (women with both GDM and HDP). The data were missing completely at random and deleted in rows. Continuous and categorical variables were respectively presented as mean ± SD and frequency (percentage). Analysis of variance (ANOVA) and Chi-square test were used for continuous variables and categorical variables, respectively, to compare the characteristics between groups. Multivariable linear regression model was used to analyze the associations of gestational age and birth weight with GDM and HDP. Multiple multivariable or multivariable logistic regression was used to estimate the adjusted odds ratio (aOR) with 95% confidence interval (CI) of each birth outcome measured for each group, relative to the comparison group. To control the confounding bias, the following variables were adjusted based on the distribution of characteristics and important demographic information: age, gravidity, history of 3 or more abortions, gestational age at OGTT, BMI at first visit, pregnancy season, and education. When the outcome variable was birth weight, delivery gestational age was also adjusted. To investigate whether the effects of GDM or HDP were independent of GWG, model 2 was further adjusted for GWG on model 1. All results were considered statistically significant at a value of P < 0.05. Statistical analyses were performed using R software (version 4.2.1) (http://www.R-project.org).
3. Results
There were 13645 pregnant women included in the analyses.10655 (78.1%) women were normal, 2341 (17.2%) women were diagnosed with GDM, 461 (3.4%) women were diagnosed with HDP, and 188 (1.3%) women were diagnosed with GDM and HDP. The maternal age, pre-pregnancy BMI, and weight gain during pregnancy were higher in the GDM+HDP group than in the other three groups, and the proportion of pregnancies with three or more, a history of abortion, and preterm were also higher than in the other three groups ( Table 1 ). The average follow-up time was 27 weeks. The reason for missing follow-up might be pregnant women’s mobility.
Table 1.
Comparison of basic characteristic in normal pregnant women, women with GDM alone, HDP alone, and both GDM and HDP.
| Variable | Normal (N=10655) |
GDM only (N=2341) |
HDP only (N=461) |
GDM+HDP (N=188) |
P |
|---|---|---|---|---|---|
| Mean ± SD | |||||
| Age, years | 28.58 ± 4.21 | 30.25 ± 4.40 | 29.82 ± 4.91 | 31.01 ± 5.39 | <0.001 |
| Gestational age at OGTT, week | 25.83 ± 1.44 | 25.80 ± 1.44 | 25.97 ± 1.42 | 25.95 ± 1.48 | 0.093 |
| BMI at first visit, kg/m2 | 21.06 ± 2.88 | 22.00 ± 3.26 | 23.57 ± 4.11 | 24.90 ± 3.97 | <0.001 |
| Gestational weight gain, kg | 12.73 ± 5.64 | 10.66 ± 4.69 | 12.60 ± 4.80 | 9.52 ± 7.86 | <0.001 |
| N (%) | |||||
| Gravidity | 0.001 | ||||
| 1 | 4371 ± 41.0 | 859 ± 36.7 | 189 ± 41.0 | 68 ± 36.2 | |
| 2 | 2886 ± 27.1 | 634 ± 27.1 | 111 ± 24.1 | 47 ± 25.0 | |
| ≥3 | 3253 ± 30.5 | 820 ± 35.0 | 154 ± 33.4 | 72 ± 38.3 | |
| Missing | 145 ± 1.4 | 28 ± 1.2 | 7 ± 1.5 | 1 ± 0.5 | |
| Education | <0.001 | ||||
| Junior high school and below | 2127 ± 20.0 | 467 ± 19.9 | 112 ± 24.3 | 55 ± 29.3 | |
| High school | 1301 ± 12.2 | 307 ± 13.1 | 56 ± 12.1 | 24 ± 12.8 | |
| College and above | 4817 ± 45.2 | 909 ± 38.8 | 175 ± 38.0 | 55 ± 29.3 | |
| Missing | 2410 ± 22.6 | 658 ± 28.1 | 118 ± 25.6 | 54 ± 28.7 | |
| History of abortion | 0.043 | ||||
| No | 10052 ± 94.3 | 2184 ± 93.3 | 429 ± 93.1 | 171 ± 91.0 | |
| Yes | 603 ± 5.7 | 157 ± 6.7 | 32 ± 6.9 | 17 ± 9.0 | |
| History of preterm | 0.002 | ||||
| No | 10549 ± 99.0 | 2313 ± 98.8 | 451 ± 97.8 | 181 ± 96.3 | |
| Yes | 106 ± 1.0 | 28 ± 1.2 | 10 ± 2.2 | 7 ± 3.7 | |
| Season of last menstruation | 0.001 | ||||
| Spring | 2039 ± 19.1 | 413 ± 17.6 | 115 ± 24.9 | 42 ± 22.3 | |
| Summer | 2507 ± 23.5 | 628 ± 26.8 | 107 ± 23.2 | 47 ± 25.0 | |
| Autumn | 3239 ± 30.4 | 723 ± 30.9 | 131 ± 28.4 | 60 ± 31.9 | |
| Winner | 2870 ± 26.9 | 577 ± 24.6 | 108 ± 23.4 | 39 ± 20.7 | |
HDP, hypertensive disorders complicating pregnancy; GDM, gestational diabetes mellitus; BMI, body mass index; OGTT, oral glucose tolerance test.
3.1. Association of GDM and HDP with gestational week of delivery and preterm birth
As shown in Table 2 , GDM, HDP, and GDM+HDP were all inversely associated with the gestational week of delivery (β=-0.19, P<0.0001 for GDM; β=-0.49, P<0.0001 for HDP; β=-0.43, P=0.0022 for GDM+HDP). After further adjustment for GWG, the association of HDP (β=-0.54, P<0.0001) and GDM+HDP (β=-0.30, P=0.0291) with gestational week of delivery was consistent with that before adjustment, but no significant association was observed between GDM and gestational week of delivery. Thus, the gestational week of delivery was mainly influenced by HDP. However, While GDM and HDP (aOR=1.35, 95%CI: 1.08-1.66 for GDM; aOR=1.74, 95%CI: 1.15-2.53 for HDP) were positively associated with preterm birth, comorbidities were not significantly associated with preterm birth ( Supplementary Table S1 ).
Table 2.
The association of GDM and HDP with gestational week of delivery and fetal birth weight.
| Group | N | Mean ± SD | Model 1 a | Model 2 b | ||
|---|---|---|---|---|---|---|
| β (se) | P | β (se) | P | |||
| Gestational week of delivery | ||||||
| Normal | 10655 | 38.99 ± 1.61 | ref. | – | ref. | – |
| GDM only | 2341 | 38.73 ± 2.42 | -0.19(0.04) | <0.0001 | -0.07(0.04) | 0.2127 |
| HDP only | 461 | 38.42 ± 1.99 | -0.49(0.09) | <0.0001 | -0.54(0.09) | 0.0002 |
| GDM + HDP | 188 | 38.43 ± 1.81 | -0.43(0.14) | 0.0022 | -0.30(0.14) | 0.0291 |
| Birth weight c d | ||||||
| Normal | 10562 | 3314.35 ± 451.64 | ref. | – | ref. | – |
| GDM only | 2317 | 3317.26 ± 503.71 | 3.82(9.83) | 0.6977 | 39.65(9.69) | <0.0001 |
| HDP only | 458 | 3187.61 ± 571.81 | -109.49(20.80) | <0.0001 | -126.52(20.27) | <0.0001 |
| GDM + HDP | 185 | 3265.43 ± 605.96 | -66.01(32.16) | 0.0401 | -23.03(31.37) | 0.4627 |
Model 1 was adjusted for age, gravidity, history of 3 or more abortions, gestational age at OGTT, BMI at first visit, pregnancy season and education.
Model 2 was adjusted variable1s in Model 1 and further adjusted for gestational weight gain.
Model 1 was adjusted for age, delivery gestational age, gravidity, history of 3 or more abortions, gestational age at OGTT, BMI at first visit, pregnancy season and education.
Model 2 was adjusted variable1s in Model 1 and further adjusted for gestational weight gain.
HDP, hypertensive disorders complicating pregnancy; GDM, gestational diabetes mellitus.
3.2. Association of GDM and HDP with composite adverse birth outcomes
As shown in Supplementary Table S2 , GDM was associated with an increased risk of Neonatal Jaundice (aOR=2.21, 95%CI: 1.05-4.40), and HDP was associated with an increased risk of preterm birth (aOR=1.87, 95%CI:1.24-2.74) and premature rupture of membranes (aOR=0.66, 95%CI: 0.46-0.93) in model 2. Considering that GDM and HDP have different effects on outcomes, we further selected preterm birth, placenta praevia, and neonatal jaundice as composite outcomes ( Table 3 ). After adjustment for confounders, GDM, HDP, and GDM+HDP were all positively associated with the risk of composite outcomes (aOR=1.35, 95%CI: 1.10-1.66 for GDM; aOR=1.78, 95%CI: 1.20-2.55 for HDP; aOR=1.82, 95%CI: 1.02-3.04 for GDM+HDP). In Model 2, only HDP was positively associated with the risk of composite outcomes (aOR=1.89, 95%CI: 1.28-2.73), whereas GDM and GDM+HDP were not significantly associated with the risk of composite outcomes ( Table 3 ).
Table 3.
The association of GDM and HDP with adverse birth outcomes.
| Group | Composite pregnancy outcomes* | SGA (< 10th centile) | LGA (> 90th centile) | |||
|---|---|---|---|---|---|---|
| Model 1
a
aOR (95% CI) |
Model 2
b
aOR (95% CI) |
Model 1 a | Model 2 b | Model 1 a | Model 2 b | |
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |||
| Normal | ref | – | ref. | – | ref. | – |
| GDM* only | 1.35 (1.10-1.66) | 1.16 (0.93-1.42) | 1.07 (0.88-1.3) | 0.97 (0.79-1.19) | 1.38 (1.21-1.56) | 1.29 (1.12-1.48) |
| HDP* only | 1.78 (1.20-2.55) | 1.89 (1.28-2.73) | 2.19 (1.57-3.06) | 2.29 (1.64-3.19) | 0.98 (0.74-1.3) | 0.68 (0.5-0.92) |
| GDM + HDP | 1.82 (1.02-3.04) | 1.43 (0.79-2.45) | 2.2 (1.24-3.92) | 1.93 (1.07-3.46) | 2.33 (1.64-3.31) | 1.64 (1.12-2.41) |
* Composite pregnancy outcomes = At least one of Preterm birth, Placenta previa and Neonatal Jaundice.
Model 1 was adjusted for age, gravidity, history of 3 or more abortions, gestational age at OGTT, BMI at first visit, pregnancy season and education.
Model 2 was adjusted variable1s in Model 1 and further adjusted for gestational weight gain.
HDP, hypertensive disorders complicating pregnancy; GDM, gestational diabetes mellitus; aOR, adjusted odds ratio; CI: confidence interval; AGA, appropriate for gestational age; SGA, small for gestational age; LGA, large for gestational age.
3.3. Association of HDP and GDM with birth weight and SGA/LGA
As shown in Table 2 , HDP and GDM+HDP groups were associated with decreased birth weight (β= -109.49, P<0.0001 for HDP; β= -66.01, P=0.0401 for GDM+HDP). In model 2, GDM was associated with increased birth weight (β= 39.65, P<0.0001), but no significant association was observed between GDM+HDP and birth weight. The association between HDP and birth weight was consistent with that before adjustment (β= -126.52, P<0.0001).
As shown in Table 3 , after adjustment for confounders, GDM was associated with an increased risk of delivering an LGA baby (aOR=1.38, 95%CI 1.21-1.56), while HDP was associated with an increased risk of delivering an SGA baby (aOR=2.19, 95%CI 1.57-3.06). Furthermore, GDM+HDP was associated with increased risk of both SGA (aOR=2.2, 95%CI 1.24-3.92) and LGA (aOR=2.33, 95%CI 1.64-3.31), and the associations were stronger than that in only GDM or HDP women. After adjusting for GWG in Model 2, the results were consistent with that before adjustment.
3.4. Association of the comorbidity of GDM and HDP diagnosed at different trimester with gestational week of delivery and birth weight
Considering that diagnosis of HDP at different periods may have different effects on outcomes, we further analyzed the associations of diagnosis of HDP combined with GDM at three periods on gestational weeks and birth weight. As shown in Table 4 , HDP diagnosed at all periods were associated with decreased gestational week of delivery, and HDP diagnosed at 21-27th week have the strongest association (β = -0.96, P<0.001). Furthermore, after being combined with GDM, the association became even stronger (β = -1.57, P<0.001).
Table 4.
The association of GDM and HDP diagnosed at different times with gestational week of delivery and birth weight.
| Group | N | Mean ± SD | Model 1 a | Model 2 b | |||
|---|---|---|---|---|---|---|---|
| β (se) | P | β (se) | P | ||||
| GDM | HDP | Gestational week of delivery | |||||
| No | No | 10562 | 38.98 ± 1.56 | ref | – | ref | – |
| Yes | No | 2317 | 38.75 ± 1.70 | -0.05 (0.04) | 0.1931 | -0.16 (0.04) | <0.0001 |
| No | ≤20th week | 130 | 38.28 ± 2.07 | -0.53 (0.14) | 0.0002 | -0.55 (0.15) | 0.0002 |
| No | 21-27th week | 52 | 38.29 ± 1.85 | -0.96 (0.24) | 0.0001 | -1.05 (0.25) | <0.0001 |
| No | ≥28th week | 43 | 37.88 ± 3.20 | -0.48 (0.10) | <0.0001 | -0.40 (0.10) | 0.0001 |
| Yes | ≤20th week | 18 | 37.22 ± 3.19 | -0.15 (0.22) | 0.5033 | -0.41 (0.23) | 0.0702 |
| Yes | 21-27th week | 285 | 38.53 ± 1.63 | -1.57 (0.43) | 0.0002 | -1.73 (0.43) | 0.0001 |
| Yes | ≥28th week | 115 | 38.63 ± 1.35 | -0.26 (0.16) | 0.1003 | -0.33 (0.16) | 0.0366 |
| GDM | HDP | Birth weight cd | |||||
| No | No | 10562 | 3314.35 ± 451.64 | ref. | – | ref. | – |
| Yes | No | 2317 | 3317.26 ± 503.71 | 4.63 (9.44) | 0.6238 | 36.79 (9.33) | 0.0001 |
| No | ≤20th week | 130 | 3185.54 ± 563.15 | -139.63 (42.05) | 0.0009 | -137.25 (41.08) | 0.0008 |
| No | 21-27th week | 43 | 3053.49 ± 782.53 | -105.85 (45.59) | 0.0203 | -97.99 (44.53) | 0.0278 |
| No | ≥28th week | 285 | 3208.79 ± 536.98 | -66.14 (25.26) | 0.0088 | -96.23 (24.71) | 0.0001 |
| Yes | ≤20th week | 52 | 3200.58 ± 570.57 | -130.45 (64.35) | 0.0426 | -70.51 (62.90) | 0.2624 |
| Yes | 21-27th week | 18 | 3025.88 ± 973.00 | -189.57 (76.97) | 0.0138 | -110.74 (75.26) | 0.1412 |
| Yes | ≥28th week | 115 | 3330.17 ± 544.26 | 19.31 (38.79) | 0.6186 | 39.42 (37.90) | 0.2983 |
Model 1 was adjusted for age, gravidity, history of 3 or more abortions, gestational age at OGTT, BMI at first visit, pregnancy season and education.
Model 2 was adjusted variable1s in Model 1 and further adjusted for gestational weight gain.
Model 1 was adjusted for age, delivery gestational age, gravidity, history of 3 or more abortions, gestational age at OGTT, BMI at first visit, pregnancy season and education.
Model 2 was adjusted variable1s in Model 1 and further adjusted for gestational weight gain.
HDP, hypertensive disorders complicating pregnancy; GDM, gestational diabetes mellitus.
Without GDM, HDP diagnosed before the 20th week was most strongly associated with decreased birth weight (β = -139.63, P<0.001), but after being combined with GDM the association was reduced (β = -130.45, P=0.0426). However, GDM combined with HDP diagnosed at 21-27th week was most strongly associated with decreased birth weight in each group (β = -189.57, P=0.0138) ( Table 4 ). After additional adjustment for GWG in Model 2, the results were consistent with those before the adjustment.
3.5. Association of the comorbidity of HDP and GDM subtypes with gestational week of delivery and birth weight
GDM subtypes might also have different effects on outcomes, we further analyzed the associations of three GDM subtypes combined with HDP with gestational week of delivery and birth weight. As shown in Table 5 , IPH (β = -0.12, P=0.0239) and CoH (β = -0.82, P<0.0001) without HDP were associated with decreased gestational weeks. After being combined with HDP, the association became stronger (β = -0.53, P=0.0100 for HDP+IPH; β= -0.83, P=0.0021 for HDP+CH). In addition, only CH (β = 87.87, P=0.0003) was associated with increased birth weight. However, after being combined with HDP, the association became non-significant, while IPH combined with HDP is associated with decreased birth weight (β= -129.67, P=0.0054) ( Table 4 ). After additional adjustment for GWG in Model 2, CH was associated with increased birth weight (β = 159.42, P=0.0080).
Table 5.
The association of GDM subtypes and HDP with gestational week and birth weight.
| Group | N | Mean ± SD | Model 1 a | Model 2 b | |||
|---|---|---|---|---|---|---|---|
| β (se) | P | β (se) | P | ||||
| HDP | GDM | Gestational week of delivery | |||||
| No | No | 10655 | 38.99 ± 1.61 | ref. | – | ref. | – |
| Yes | IFH | 582 | 39.02 ± 1.46 | -0.03 (0.08) | 0.7180 | 0.01 (0.08) | 0.9277 |
| No | IPH | 1440 | 38.75 ± 1.71 | -0.12 (0.05) | 0.0239 | 0.01 (0.05) | 0.8574 |
| No | CH | 319 | 38.14 ± 5.03 | -0.82 (0.11) | <0.0001 | -0.65 (0.11) | <0.0001 |
| No | No | 461 | 38.42 ± 1.99 | -0.50 (0.09) | <0.0001 | -0.54 (0.09) | <0.0001 |
| Yes | IFH | 50 | 39.02 ± 1.79 | 0.07 (0.26) | 0.8024 | 0.17 (0.26) | 0.5190 |
| Yes | IPH | 88 | 38.32 ± 1.82 | -0.53 (0.20) | 0.0100 | -0.45 (0.20) | 0.0252 |
| Yes | CH | 50 | 38.04 ± 1.68 | -0.83 (0.27) | 0.0021 | -0.55 (0.27) | 0.0388 |
| HDP | GDM | Birth weight cd | |||||
| No | No | 10655 | 3315.09 ± 452.43 | ref. | – | ref. | – |
| No | IFH | 582 | 3384.22 ± 484.15 | 14.41 (18.62) | 0.4389 | 24.79 (18.15) | 0.1719 |
| No | IPH | 1440 | 3281.43 ± 485.99 | -17.32 (11.94) | 0.147 | 25.31 (11.75) | 0.0313 |
| No | CH | 319 | 3361.56 ± 586.49 | 87.87 (24.37) | 0.0003 | 140.43 (23.81) | <0.0001 |
| Yes | No | 461 | 3187.77 ± 570.68 | -107.67 (20.79) | <0.0001 | -124.78 (20.25) | <0.0001 |
| Yes | IFH | 50 | 3321.84 ± 559.93 | -80.04 (60.75) | 0.1877 | -43.40 (59.15) | 0.4631 |
| Yes | IPH | 88 | 3179.77 ± 629.50 | -129.67 (46.63) | 0.0054 | -108.36 (45.40) | 0.0170 |
| Yes | CH | 50 | 3358.78 ± 592.65 | 69.97 (61.60) | 0.2561 | 159.42 (60.06) | 0.0080 |
Model 1 was adjusted for age, gravidity, history of 3 or more abortions, gestational age at OGTT, BMI at first visit, pregnancy season and education.
Model 2 was adjusted variable1s in Model 1 and further adjusted for gestational weight gain.
Model 1 was adjusted for age, delivery gestational age, gravidity, history of 3 or more abortions, gestational age at OGTT, BMI at first visit, pregnancy season and education.
Model 2 was adjusted variable1s in Model 1 and further adjusted for gestational weight gain.
HDP, hypertensive disorders complicating pregnancy; GDM, gestational diabetes mellitus; IFH, isolated fasting hyperglycemia; IPH, isolated post hyperglycemia; CH, Combined fast and post hyperglycemia.
4. Discussion
Our study demonstrated that women with both GDM and HDP had a higher risk of adverse neonatal outcomes than those with one comorbidity. This might be due to the severity, subtype, and time of diagnosis of GDM or HDP. Further analysis showed that women with GDM and HDP diagnosed in the 21-27th week had the lowest gestational age at delivery and birth weight. As for GDM subtypes, GDM-CH+HDP had the greatest association with gestational age at delivery while IPH combined with HDP was associated with decreased birth weight.
It is well established that women with HDP or GDM alone had higher risks of adverse birth outcomes. However, there were only several studies focused on the association with adverse outcomes in women with both comorbidities. Stella et al. (6)reported that, in a Cincinnati cohort based on clinical data of 14,480 women, the rates of LGA were significantly increased in women with combined diagnoses of GDM and gestational hypertension (GH), with an OR of 1.51 (95%CI 1.14-1.98). The study did not include chronic hypertension, preeclampsia, and eclampsia, and did not analyze the risk of both LGA and SGA. Our study found GDM+HDP group had a higher risk in both LGA and SGA, which may due to the severity of HDP or GDM in women with both diseases was not homogenous.
The effect of GDM and HDP in pregnant women varies among populations. A Taiwan cohort study based on 19,442 women (12) showed the women with HDP and GDM had higher risk of adverse neonatal outcomes, compared with those without HDP, with an OR of 4.84 (95%CI 4.34–5.40) for preterm delivery, an OR of 1.90 (95%CI 1.76–2.06) for Jaundice, an OR of 31.7 (95% CI 16.5-60.9) for LGA and an OR of 6.57 (95% CI 5.56-7.75) for SGA. The risk of the outcomes above in our study population might not be higher than in other populations. However, information like BMI was unavailable to adjust for these potential confounders in data analyses and did not establish a group of GDM, therefore the impact associated with GDM alone or with the severity of GDM could not be evaluated in this study. Another recent Chinese cohort of 1398 women with twin pregnancies (20) showed, that no associations were found between HDP alone and adverse neonatal outcomes in monochorionic (MC) twin neonates, whereas MC twins born to women with both GDM and HDP had longer gestational age, heavier birthweight and lower preterm birth risk, which might suggest that neonatal outcomes in twins were more affected by GDM than by HDP. The difference between our results and that might be due to the molecular mechanism and severity of GDM and HDP varied in twin and singleton pregnancy.
The impact of GDM and HDP on neonatal outcomes can be affected by the gestational age of HDP diagnosis and GDM subtypes. A London cross-sectional study reported that earlier onset of hypertension in the mother was positively associated with poorer fetal outcome in the third trimester (28 to 42 weeks of gestation), including gestational age at delivery, birth weight, and Apgar score at 1 min and 5 min (21). However, the study did not access hypertension onset before 28 weeks of gestation We further investigated women with HDP diagnosed at the 21-27th week had the lowest gestational age at delivery, and HDP diagnosed before the 20th week had the lowest birth weight.
The magnitude and significance of adverse pregnancy outcomes differed by various combinations of abnormal OGTT glucose values. A retrospective American report indicated while women with elevated post-load glucose concentrations were at higher risk for hypertension, preterm delivery, or infants with hyperbilirubinemia, women with elevated fasting glucose showed an increased risk of having LGA offspring (22). In addition, Wang et al. (23) observed that the incidence of LGA/macrosomia in women with elevated fasting and post-load glucose concentrations (GDM-CH) was significantly higher than that in women with normal fasting blood glucose and abnormal blood glucose (GDM-IPH); the incidence of GDM and adverse pregnancy outcomes in 4.4 mmol/L pregnant women is very low. The categorization based on abnormal OGTT values seems to provide a practicable basis for clinical risk stratification and future research. Our further investigation found that GDM-CH had the lowest birth weight and decreased gestational week of delivery, followed by GDM-IPH, whereas GDM-IFH was not significantly associated with these outcomes. However, the sample size of women with GDM-IFH was relatively small.
The mechanism for the association of maternal GDM and HDP with neonatal adverse outcomes has not been fully understood. Insulin resistance may be the first consideration as the reason for this association, because it was putative as the pathogenesis of HDP and GDM in pregnant women (24, 25), and cord plasma insulin correlated positively with birthweight and neonatal fat mass (26). We observed that women with both GDM and HDP have higher levels of BMI before pregnancy than the other groups, and BMI is a parameter that reflects insulin resistance. Therefore, the combination of GDM and HDP may imply more severe insulin resistance. Other systemic changes, such as inflammation, oxidative stress, and maternal vasculopathy also play an important part in the pathogenesis of GDM and HDP. Hyperglycemia-promoted inflammation and accumulation of reactive oxidative species (ROS) impair endothelial cells and eventually damage vascular function (27). When GDM is combined with HDP, accompanied by shallow placental implantation and even acute atherosclerosis of placental blood vessels (28), it results in further reduction of vessel dysfunction and placental perfusion, thereby limiting fetal growth and other adverse outcomes. However, it is reasonable to presume that in GDM, the placenta may overcompensate by increasing nutrient transport, even in cases of mild vascular dysfunction, which may lead to excessive fetal growth. Additionally, some studies found that GDM combined with HDP makes uterine smooth muscle more sensitive to oxytocin and more likely to induce preterm birth (29, 30). In short, the pathogenic changes result from a complex interplay between the placenta, the mother, and the fetus.
There were some strengths in the present study. Firstly, this retrospective cohort study evaluates broader birth outcomes in pregnant women with GDM/HDP alone and in those who have both GDM and HDP. Secondly, data on a variety of confounding variables, such as pregnancy season and education level, were collected and used in the final analysis. Thirdly, to the best of our knowledge, this was the first study to analyze birth outcomes in women with HDP in different pregnancies combined with GDM and women with different GDM subtypes combined with HDP. However, several limitations need to be acknowledged. Firstly, the sample size of women with GDM and HDP was relatively small, and the incidence of individual adverse outcomes was low, therefore the association with each outcome might not be accurately assessed. Secondly, our study was a single-center study, and the findings have reference value finiteness for populations in other regions. Multicenter studies should be conducted to clarify the impact of the co-existence of GDM and HDP on neonatal outcomes. Last, information on clinical interventions after diagnosis of GDM and HDP has not been collected, and interference of therapeutic factors cannot be excluded. Lifestyle is also an important confounding factor, but it is not acquired in our data either.
Pregnant women suffering from the comorbidity of GDM and HDP had a higher risk of adverse neonatal outcomes. To prevent poor perinatal outcomes, we recommend individualized management for pregnant women with comorbidities as following: (1) Early detection and monitoring; (2) Diet and lifestyle adjustments; (3) Preventive or therapeutic medications; (4) Frequent Fetal Monitoring; (5) Timely delivery planning. Future studies are needed to further evaluate the effects of the gestational age of HDP diagnosis, GDM subtypes, and their co-morbidities on birth outcomes.
5. Conclusion
In conclusion, pregnant women suffering from both GDM and HDP had a higher risk of adverse neonatal outcomes than those with one comorbidity. Meanwhile, the women with GDM and HDP diagnosed in the 21-27th week had the lowest gestational age at delivery and birth weight, and HDP+GDM-CH had the greatest association with gestational age. This suggested that high attention and active intervention should be paid to this situation.
Acknowledgments
We deeply appreciate the staff and all participants from Zhoushan Maternal and Child Health Hospital.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by 4+X Clinical Research Project of Women's Hospital, School of Medicine, Zhejiang University, and the National Key R&D Program of China [grant number 2022YFC2703505 and 2021YFC2701901].
Abbreviations
GDM, Gestational diabetes mellitus; HDP, Hypertension disorders of pregnancy; EMRS, Electronic medical recorder system; PTB, Preterm birth; SGA, Small for gestational age; LGA, Large for gestational age; CH, Combined hyperglycemia; PE, preeclampsia; GWG, Gestational weight gain; OGTT, Oral glucose tolerance test; IFH, Isolated fasting hyperglycemia; IPH, Isolated post-load hyperglycemia; CH, Combined hyperglycemia.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of Zhejiang University School of Medicine (No.2011-1-002). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XL: Formal analysis, Project administration, Writing – original draft, Writing – review & editing. LuZ: Formal analysis, Writing – original draft, Writing – review & editing. SS: Data curation, Investigation, Writing – review & editing. HC: Data curation, Writing – review & editing. XA: Data curation, Writing – review & editing. YQ: Investigation, Writing – review & editing. YZ: Supervision, Writing – review & editing. YH: Writing – review & editing. LiZ: Writing – review & editing. DA: Writing – review & editing. HL: Writing – review & editing. YY: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1468820/full#supplementary-material
References
- 1. Lin J, Jin H, Chen L. Associations between insulin resistance and adverse pregnancy outcomes in women with gestational diabetes mellitus: A retrospective study. BMC Pregnancy Childbirth. (2021) 21:526. doi: 10.1186/s12884-021-04006-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhuang C, Gao J, Liu J, Wang X, He J, Sun J, et al. Risk factors and potential protective factors of pregnancy-induced hypertension in China: A cross-sectional study. J Clin Hypertension (Greenwich Conn). (2019) 21:618–23. doi: 10.1111/jch.13541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li F, Qin J, Zhang S, Chen L. Prevalence of hypertensive disorders in pregnancy in China: A systematic review and meta-analysis. Pregnancy Hypertension. (2021) 24:13–21. doi: 10.1016/j.preghy.2021.02.001 [DOI] [PubMed] [Google Scholar]
- 4. Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol. (2003) 158:1148–53. doi: 10.1093/aje/kwg273 [DOI] [PubMed] [Google Scholar]
- 5. Mastrogiannis DS, Spiliopoulos M, Mulla W, Homko CJ. Insulin resistance: the possible link between gestational diabetes mellitus and hypertensive disorders of pregnancy. Curr Diabetes Rep. (2009) 9:296–302. doi: 10.1007/s11892-009-0046-1 [DOI] [PubMed] [Google Scholar]
- 6. Stella CL, O’Brien JM, Forrester KJ, Barton JR, Istwan N, Rhea D, et al. The coexistence of gestational hypertension and diabetes: influence on pregnancy outcome. Am J Perinatol. (2008) 25:325–9. doi: 10.1055/s-2008-1078758 [DOI] [PubMed] [Google Scholar]
- 7. Poudel K, Kobayashi S, Miyashita C, Ikeda-Araki A, Tamura N, Ait Bamai Y, et al. Hypertensive disorders during pregnancy (Hdp), maternal characteristics, and birth outcomes among Japanese women: A hokkaido study. Int J Environ Res Public Health. (2021) 18:3342. doi: 10.3390/ijerph18073342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen M, Smith GN, Rodger M, White RR, Walker MC, Wen SW. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PloS One. (2017) 12:e0175914. doi: 10.1371/journal.pone.0175914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang X, Xiao Y. The association between trimester-specific weight gain and severe preeclampsia/adverse perinatal outcome in gestational diabetes mellitus complicated by preeclampsia: A retrospective case study. Diabetes Ther: Res Treat Educ Diabetes Related Disord. (2019) 10:725–34. doi: 10.1007/s13300-019-0589-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xiao Y, Zhang X. Association between maternal glucose/lipid metabolism parameters and abnormal newborn birth weight in gestational diabetes complicated by preeclampsia: A retrospective analysis of 248 cases. Diabetes Ther: Res Treat Educ Diabetes Related Disord. (2020) 11:905–14. doi: 10.1007/s13300-020-00792-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cundy T, Slee F, Gamble G, Neale L. Hypertensive disorders of pregnancy in women with type 1 and type 2 diabetes. Diabetic Med: J Br Diabetic Assoc. (2002) 19:482–9. doi: 10.1046/j.1464-5491.2002.00729.x [DOI] [PubMed] [Google Scholar]
- 12. Lin YW, Lin MH, Pai LW, Fang JW, Mou CH, Sung FC, et al. Population-based study on birth outcomes among women with hypertensive disorders of pregnancy and gestational diabetes mellitus. Sci Rep. (2021) 11:17391. doi: 10.1038/s41598-021-96345-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. (2010) 33:676–82. doi: 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. (2006) 29:1130–9. doi: 10.2337/diacare.2951130 [DOI] [PubMed] [Google Scholar]
- 15. Garovic VD, Dechend R, Easterling T, Karumanchi SA, McMurtry Baird S, Magee LA, et al. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: A scientific statement from the american heart association. Hypertension (Dallas Tex: 1979). (2022) 79:e21–41. doi: 10.1161/hyp.0000000000000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: isshp classification, diagnosis, and management recommendations for international practice. Hypertension (Dallas Tex: 1979). (2018) 72:24–43. doi: 10.1161/hypertensionaha.117.10803 [DOI] [PubMed] [Google Scholar]
- 17. Hui L. Growth standard curves of birth weight,length and head circumference of Chinese newborns of different gestation. Chin J Pediatr. (2020) 58(9):738–46. doi: 10.3760/cma.j.cn112140-20200316-00242 [DOI] [PubMed] [Google Scholar]
- 18. Jauniaux E, Alfirevic Z, Bhide AG, Belfort MA, Burton GJ, Collins SL, et al. Placenta praevia and placenta accreta: diagnosis and management: green-top guideline no. 27a. BJOG: Int J Obstetrics Gynaecol. (2019) 126:e1–e48. doi: 10.1111/1471-0528.15306 [DOI] [PubMed] [Google Scholar]
- 19. Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in chinese adults–study on optimal cut-off points of body mass index and waist circumference in chinese adults. Biomed Environ Sci: BES. (2002) 15:83–96. [PubMed] [Google Scholar]
- 20. Liu Y, Li D, Wang Y, Qi H, Wen L. Impact of gestational diabetes and hypertension disorders of pregnancy on neonatal outcomes in twin pregnancies based on chorionicity. J Clin Med. (2023) 12:1096. doi: 10.3390/jcm12031096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abraham AJM, Bobby Z, Chaturvedula L, Vinayagam V, Syed H, Jacob SE. Utility of time of onset of hypertension, adma and tas in predicting adverse neonatal outcome in hypertensive disorders of pregnancy. Fetal Pediatr Pathol. (2019) 38:460–76. doi: 10.1080/15513815.2019.1619205 [DOI] [PubMed] [Google Scholar]
- 22. Black MH, Sacks DA, Xiang AH, Lawrence JM. Clinical outcomes of pregnancies complicated by mild gestational diabetes mellitus differ by combinations of abnormal oral glucose tolerance test values. Diabetes Care. (2010) 33:2524–30. doi: 10.2337/dc10-1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang CS, Wei YM, Yang HX. Analysis of the effects of gestational diabetes mellitus based on abnormal blood glucose on pregnancy outcomes. Zhonghua Fu Chan Ke Za Zhi. (2013) 48:899–902. [PubMed] [Google Scholar]
- 24. Kalafat E, Sukur YE, Abdi A, Thilaganathan B, Khalil A. Metformin for prevention of hypertensive disorders of pregnancy in women with gestational diabetes or obesity: systematic review and meta-analysis of randomized trials. Ultrasound Obstetrics Gynecol: Off J Int Soc Ultrasound Obstetrics Gynecol. (2018) 52:706–14. doi: 10.1002/uog.19084 [DOI] [PubMed] [Google Scholar]
- 25. Jeon EJ, Hong SY, Lee JH. Adipokines and insulin resistance according to characteristics of pregnant women with gestational diabetes mellitus. Diabetes Metab J. (2017) 41:457–65. doi: 10.4093/dmj.2017.41.6.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brunner S, Schmid D, Hüttinger K, Much D, Heimberg E, Sedlmeier EM, et al. Maternal insulin resistance, triglycerides and cord blood insulin in relation to post-natal weight trajectories and body composition in the offspring up to 2 years. Diabetic Med: J Br Diabetic Assoc. (2013) 30:1500–7. doi: 10.1111/dme.12298 [DOI] [PubMed] [Google Scholar]
- 27. Wan Y, Liu Z, Wu A, Khan AH, Zhu Y, Ding S, et al. Hyperglycemia promotes endothelial cell senescence through aqr/plau signaling axis. Int J Mol Sci. (2022) 23:2879. doi: 10.3390/ijms23052879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carter EB, Conner SN, Cahill AG, Rampersad R, Macones GA, Tuuli MG. Impact of fetal growth on pregnancy outcomes in women with severe preeclampsia. Pregnancy Hypertension. (2017) 8:21–5. doi: 10.1016/j.preghy.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nataly F, Hadas GH, Ohad G, Letizia S, Michal K. Is there a difference in placental pathology in pregnancies complicated with gestational diabetes A2 versus gestational diabetes A1, versus one abnormal value, on 100 gr glucose tolerance test? Placenta. (2022) 120:60–4. doi: 10.1016/j.placenta.2022.02.009 [DOI] [PubMed] [Google Scholar]
- 30. Tong M. Influence of gestational diabetes mellitus complicated with hypertension on the perinatal outcomes and analysis on the related risk factors. Clin Med Eng. (2021) 28:693–4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.