Abstract
Purpose
Thoracic surgery is among the most painful surgeries, postoperative pain can lead to a poor prognosis. This study aimed to explore the analgesic effect of ultrasound-guided continuous rhomboid intercostal and sub-serratus (RISS) plane block Comparison of thoracoscopic intercostal nerve block (ICNB) on postoperative pain management and recovery in patients who underwent Video-Assisted Thoracic Surgery (VATS) Lobectomy.
Methods
This prospective randomized controlled study enrolled patients after VATS Lobectomy who received ultrasound-guided continuous RISS plane block (RISS group) or ICNB (Control group) for postoperative pain. The primary outcome was the visual analogue scale (VAS) score. The secondary outcomes included non-invasive blood pressure (NIBP), heart rate (HR), the time to ambulation after surgery, the timing of drain removal, and the duration of postoperative hospitalization.
Results
A total of 98 participants were collected (53.08 ± 13.63; 43 (43.88%) males); each group included 49 patients. The RISS group displayed significantly lower visual analogue scale (VAS) scores during rest and when coughing at postoperative 12, 24, and 48h compared to the Control group (P < 0.001). The total consumption of sufentanil and remifentanil was smaller in the RISS group than in the control group. The NIBP,HR in the RISS group were significantly lower than in the Control group at immediately after skin incision (T1), upon entering the thoracic cavity (T2), 5 min after entering the thoracic cavity (T3) (P < 0.001). The patients in the RISS group were more likely to exhibit a shorter time to the first postoperative ambulation compared to the Control group (8.84 ± 2.87,15.43 ± 4.50, P < 0.001).
Conclusion
Continuous RISS may be a safe and effective strategy for postoperative pain management after thoracoscopic surgery.
Keywords: postoperative pain, thoracoscopic surgery, rhomboid intercostal and sub-serratus plane block, intercostal nerve block
Introduction
Thoracic surgery involves deep incisions, working through a dense network of chest wall nerves, and factors such as lung tissue damage, respiratory movements, irritation from drainage tubes, and peripheral nerve injuries, which collectively contribute to severe postoperative pain.1,2 During the early post-thoracoscopy phase, up to 75% of the patients experience moderate to severe pain.2,3 Acute pain is not only debilitating in the early period after surgery, but it also carries the risk of developing into chronic pain,4,5 and effective pain management has the potential to reduce the incidence of chronic postoperative pain.6,7 In addition, acute pain significantly impedes patient recovery8,9 and postoperative quality of life.10 Severe pain can lead to systemic dysfunctions, including heightened sympathetic nervous system activity and shallow breathing, resulting in lung collapse.11–13 Hence, pain management after thoracic surgery is paramount.
The available methods for managing postoperative pain include systemic pharmacologic therapy, local, intra-articular, or topical techniques, regional anesthetic techniques, neuraxial anesthetic techniques, and non-pharmacologic therapies (eg, cognitive modalities, physical therapy, transcutaneous electrical nerve stimulation).9 Unfortunately, no techniques alone have been deemed completely effective, safe, or applicable to many patients.14 Opioids after thoracic surgery are associated with side effects, risk of secondary hyperalgesia, and persistent postoperative pain.15 In addition, systemic opioids carry a risk of addiction, which is a significant risk in the current opioid crisis.16
Thoracoscopic intercostal nerve block (ICNB) has shown promise for the management of pain after thoracic surgery by decreasing the stimulation of intercostal nerves and has been commonly used after thoracic surgery.17 A major disadvantage of ICNB in thoracic surgery is the necessity of performing several ICNBs if multiple levels or bilateral levels are involved, and ICNBs do not provide complete analgesia after thoracic surgery.18 Patient-controlled intravenous analgesia (PCIA) is another hallmark of postoperative pain control,19 but PCIA alone is insufficient to control pain after thoracic surgery and is an adjunct to other methods and is usually combined to other modalities (eg, thoracoscopic ICNB).20
In 2018, Elsharkawy et al21 found that the RISS block induced anesthesia in the lateral cutaneous branches of the thoracic intercostal nerves, offering versatile application for chest wall and upper abdominal analgesia across diverse clinical settings. The RISS plane block involves the injection of local anesthetics into fascial planes, theoretically allowing for catheter placement to achieve continuous analgesia.21 Successful RISS plane blocks have been reported in various procedures, including lung transplantation,22 radical mastectomy,23 and nephrectomy,24,25 strongly suggesting favorable outcomes in postoperative pain relief. Unfortunately, continuous RISS plane blocks for post-thoracoscopic pain management remain unexplored in China.
Therefore, this study aimed to assess the analgesic effect of ultrasound-guided continuous RISS plane block comparison of ICNB on postoperative pain management and recovery in patients who underwent thoracoscopic surgery.
Methods
Study Design and Patients
This prospective randomized controlled study enrolled patients after VATS Lobectomy, at the Anesthesia and Surgery Center of Chifeng College Affiliated Hospital between June and September 2023. Inclusion criteria: (1) aged 18–78 years old; (2) ASA physical status classification I–III; (3) body mass index (BMI) of 15–35 kg/m2; and 4) preparedness for VATS Lobectomy. Exclusion criteria: (1) known allergies to anesthetics and local anesthetics; (2) abnormal coagulation function or compromised immunity, rendering nerve blocks contraindicated; (3) block failure(The criteria for block failure is considered to be a block where the bolus of local anesthetic completely misses its target, and surgery cannot proceed after allowing an adequate time of approximately 30 minutes); (4) psychiatric conditions affecting cooperation; (5) significant intraoperative hemorrhage; (6) alterations in surgical procedures during the operation; or (7) incomplete follow-up information. This project belonged to the Inner Mongolia Autonomous Region Health Science and Technology Plan Project (project #202202337). Ethical approval for this study was granted by the Chifeng College Affiliated Hospital Ethics Committee (no. fsyy2023037). All participants and their parents provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki.
Procedures
The participants were randomly grouped into the RISS and Control groups according to a computer-generated sequence of numbers in a 1:1 ratio by a researcher who was not involved in the study. Te researcher assigned a random ID to each patient, and a blinded anesthesiologist used this ID while collecting the data.
General Anesthesia
The participants underwent standard preoperative fasting of at least 8 h. Upon entering the operating room, they received oxygen through a face mask at a rate of 5 L/min. Heart rate, blood pressure, blood oxygen saturation, and bispectral index (BIS) were continuously monitored using a Mindray T8 monitor. Blinded anesthesiologists performed rapid intravenous induction based on group allocation, administering midazolam 1mg, sufentanil 0.4–0.5µg/kg, propofol 1.5–2.5mg/kg, or etomidate 0.2–0.3mg/kg, and rocuronium 0.6mg/kg. Endotracheal intubation was performed once patients lost consciousness, followed by assisted ventilation to maintain end-tidal carbon dioxide concentration between 35–40mmHg. During surgery, anesthesia was maintained with 2% sevoflurane and 50% oxygen inhalation, along with continuous intravenous infusion of remifentanil (0.1–1µg/kg/min) and propofol (2–4µg/kg/min) to target a BIS value of 40–60. Blood pressure was controlled within 20% of baseline, with ephedrine and atropine used as needed. Following surgery, neostigmine and atropine were routinely administered to reverse residual neuromuscular blockade, and endotracheal tubes were removed for patients with an Aldrete score of 8 or higher before transfer to the recovery room.
Riss
For the participants in the RISS group, before induction, a continuous RISS plane block under ultrasound guidance was performed. The participants assumed a lateral decubitus position with the surgical side facing upward. Oxygen supplementation and relevant monitoring were started, including blood pressure, pulse, and electrocardiogram. High-frequency linear probes were used for the RISS plane block. The probe was initially placed vertically at the T4-5 intercostal level on the inner aspect of the scapula and subsequently rotated to the oblique sagittal position. The ultrasound image sequentially revealed layers from superficial to deep, encompassing the serratus anterior, rhomboid major, intercostal muscles, pleura, and lung. Following the administration of 1 mL of lidocaine 1% at the puncture site, a 16 G epidural puncture needle was introduced using an in-plane technique from the upper inner to the lower outer direction. Upon reaching the plane between the rhomboid major and intercostal muscles, the two layers of fascia were separated using a hydrodissection technique. Subsequently, 15 mL of ropivacaine 0.4% was injected, and an epidural catheter was placed under ultrasound guidance, securing it at a depth of approximately 3–4 cm. The ultrasound probe was positioned on the lower outer side at the T7-8 level, corresponding to the far posterior axillary line behind the lower angle of the scapula. The ultrasound image depicted layers from superficial to deep, revealing the latissimus dorsi, serratus anterior, intercostal muscles, pleura, and lung. Following the administration of 1 mL of lidocaine 1% at the puncture site, a 22-gauge nerve block puncture needle was inserted using an in-plane technique from the upper inner to lower outer direction. Once the plane between the serratus anterior and intercostal muscles was reached and no blood return was confirmed, 20 mL of ropivacaine 0.4% was injected. Patients were vigilantly observed for 20 min to detect potential pneumothorax, local anesthetic toxicity, and puncture site hematoma. Subsequently, a pinprick test was performed to assess pain perception on the punctured side of the chest and upper abdomen, and the level of anesthesia was documented. Postoperatively, the RISS group received patient-controlled nerve analgesia (PCNA) consisting of ropivacaine hydrochloride 400 mg and 200 mL of normal saline at a background infusion rate of 4 mL/h, a patient-controlled bolus dose of 2 mL per administration and a lockout time of 15 min. All procedures in the RISS group were conducted by the same senior attending physician with over 5 years of experience in ultrasound-guided techniques. An anesthesia nurse with a senior qualification and over 5 years of experience provided assistance.
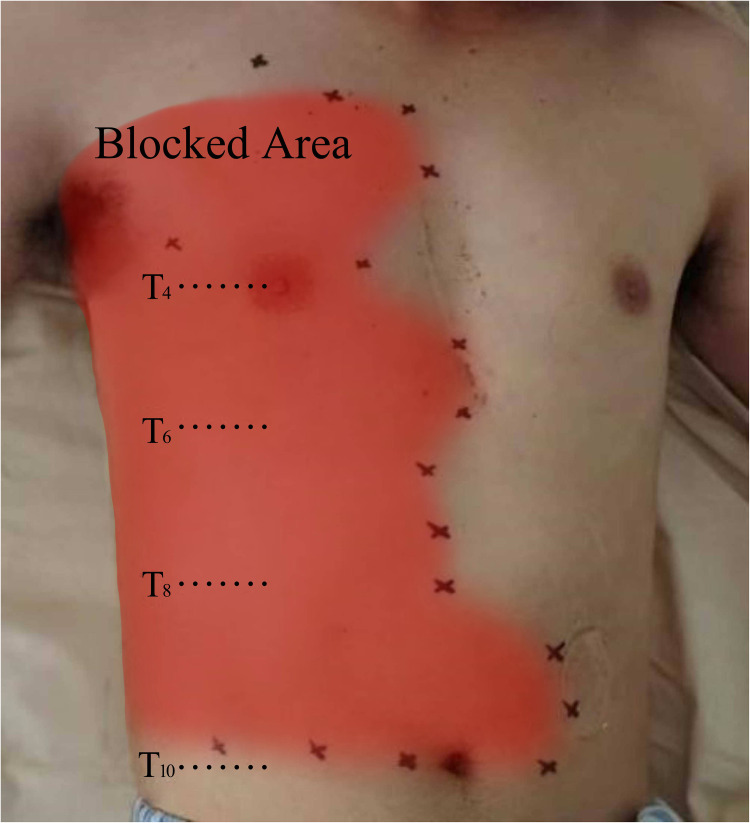
Analgesic effect of RISS block: The participants in the RISS group exhibited a pain level extending primarily from T4 to T8 at 20 min post-block. The upper boundary of the sensory deficit plane could extend as high as T2, while the lower boundary encompassed T10. The anterior extent reached the midclavicular line, and the posterior extent reached the scapular line (Figure 1).
Figure 1.
Schematic representation of the sensory loss plane under ultrasound-guided continuous rhomboid intercostal and sub-serratus (RISS) plane block.
Icnb
In the Control group, the surgical procedures for all patients were performed by the same team of thoracic surgeons as the RISS group. At the end of the operation in the Control group, the surgeon administered a total of 20 mL of ropivacaine 0.4% evenly distributed to the incision and its adjacent upper and lower intercostals spaces under thoracoscopic vision to perform multi-point intercostal nerve block. The participants in the Control group were connected to a PCIA pump comprising a sufentanil injection of 100 µg, dezocine 30 mg, granisetron injection of 9 mg, and 86 mL of normal saline, with a background infusion rate of 2 mL/h, a patient-controlled bolus dose of 2 mL per administration, and a lockout time of 15 min.
Outcomes
The primary outcome was the visual analogue scale (VAS) scores upon admission to the PACU, and at postoperative 6, 12, 24, and 48 h for pain at rest and when coughing. The secondary outcomes included non-invasive blood pressure (NIBP), including systolic blood pressure (SBP) and diastolic blood pressure (DBP), and heart rate (HR) at various time points: before anesthesia induction (T0), immediately after skin incision (T1), upon entering the thoracic cavity (T2), 5 min after entering the thoracic cavity (T3), 15 min after entering the thoracic cavity (T4), and 5 min after extubation (T5), the time to ambulation after surgery, the timing of drain removal, and the duration of postoperative hospitalization. The time to ambulation was defined as the number of steps the patient took, with a criterion of walking at least 10 steps in the postoperative period. The moment for drain removal was determined when the postoperative drainage volume fell below 50 mL within 8 h, in conjunction with complete lung re-expansion, allowing for drain removal. The duration of the postoperative hospital stay started on the day following the surgical procedure and continued until the day of discharge. The members of the data collection team conducted postoperative visits when the participants were admitted to the post-anesthesia care unit (PACU) and at 6, 12, 24, and 48 h. Data on the use of analgesics in the PACU and the ward were documented.
Statistical Analysis
The primary outcome measure was the Visual Analogue Scale (VAS) score, and employing a repeated measures analysis of variance (ANOVA)(α=0.05,1-β=0.8),the G*Power 3.1(F. Faul, Uni Duisburg-Essen, GER) software calculated a required sample size of 82 cases. Considering a dropout rate of 20%, a minimum sample size of 100 cases is necessary. Data analysis was performed using SPSS 25.0 (IBM, Armonk, NY, USA). Continuous data conforming to the normal distribution were expressed as means ± standard deviations (SD) and compared between groups using the t-test for independent samples or ANOVA. Categorical data were expressed as n (%) and analyzed using the chi-squared test. For ordinal data, the rank sum test was used. Two-sided P-values < 0.05 indicated statistical significance.
Results
A total of 100 patients were enrolled in this study; one patient was excluded because of a change in the surgical approach due to excessive intraoperative bleeding, while another patient was excluded due to block failure, and finally, 98 participants completed the study and follow-up (aged 53.08±13.63 years old; 43 (43.88%) males), each group included 49 patients. The general participant characteristics, including patient age, sex, body mass index (BMI), history of hypertension and diabetes, surgical duration, anesthesia duration, ASA classification, surgical incision side ratio, intraoperative fluid volume, and intraoperative blood loss, exhibited no statistically significant differences between RISS and Control groups (all P > 0.05), but there were significant differences in the total intraoperative analgesic amount of sufentanil and remifentanil, total postoperative analgesic amount of sufentanil, and analgesia pump press at postoperative 24 and 48 h (all P < 0.05) (Table 1).
Table 1.
Basic Characteristics
| Variables | RISS (n=49) | Control Group (n=49) | P |
|---|---|---|---|
| Age, mean ± SD | 52.10 ± 14.78 | 54.06 ± 12.44 | 0.654 |
| Sex, male, n (%) | 20 (40.8%) | 23 (46.9%) | 0.541 |
| Body mass index (kg/m2), mean ± SD | 25.05 ± 3.99 | 24.16 ± 3.87 | 0.265 |
| Comorbidities, n (%) | 19 (38.8%) | 20 (40.8%) | 0.836 |
| Hypertension, n (%) | 13 (26.5%) | 15 (30.6%) | 0.655 |
| Diabetes, n (%) | 9 (18.4%) | 6 (12.2%) | 0.400 |
| Surgical duration (min), mean ± SD | 109.18 ± 36.33 | 107.76 ± 40.79 | 0.855 |
| Anesthesia duration (min), mean ± SD | 150.20 ± 43.77 | 142.45 ± 42.56 | 0.376 |
| ASA class I /II, n (%) | 44 (89.8%) | 44 (89.8%) | > 0.999 |
| Surgical incision (left/right chest) | 16/33 | 20/29 | 0.402 |
| Intraoperative fluid volume (mL), mean ± SD | 900.00 ± 328.35 | 861.22 ± 414.99 | 0.420 |
| Intraoperative blood loss (mL), mean ± SD | 33.47 ± 36.19 | 24.08 ± 19.38 | 0.468 |
| Total intraoperative analgesic, mean ± SD | |||
| Sufentanil (µg) | 21.43 ± 3.39 | 23.37 ± 3.87 | 0.002 |
| Remifentanil (µg) | 750.21 ± 287.48 | 1049.41 ± 459.26 | < 0.001 |
| Total postoperative analgesic dose, mean ± SD | |||
| Sufentanil (µg) | 48.06 ± 10.55 | 160.41 ± 32.40 | < 0.001 |
| Postoperative analgesia pump press times, mean ± SD | |||
| 24 h | 0.08 ± 0.28 | 0.80 ± 0.76 | < 0.001 |
| 48 h | 0.10 ± 0.37 | 1.78 ± 0.74 | < 0.001 |
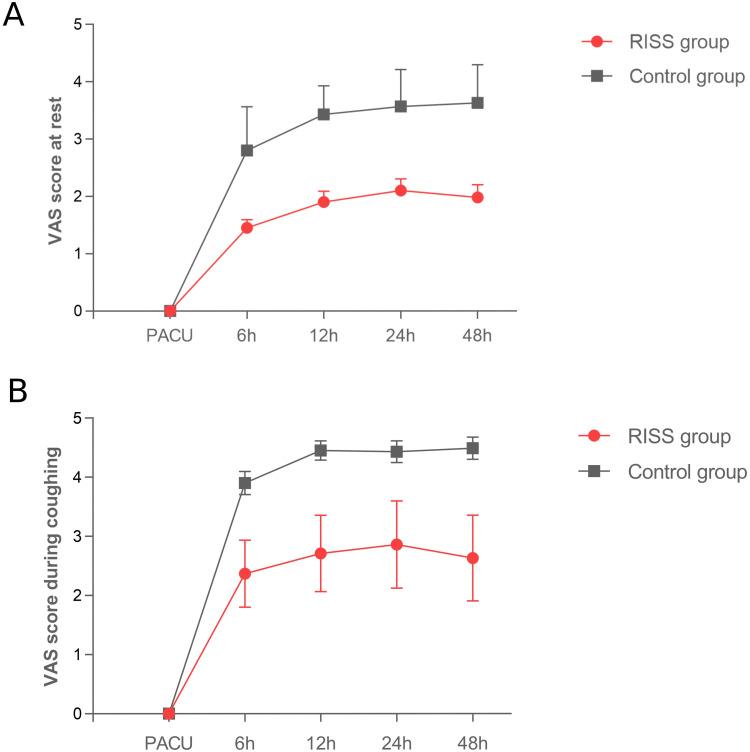
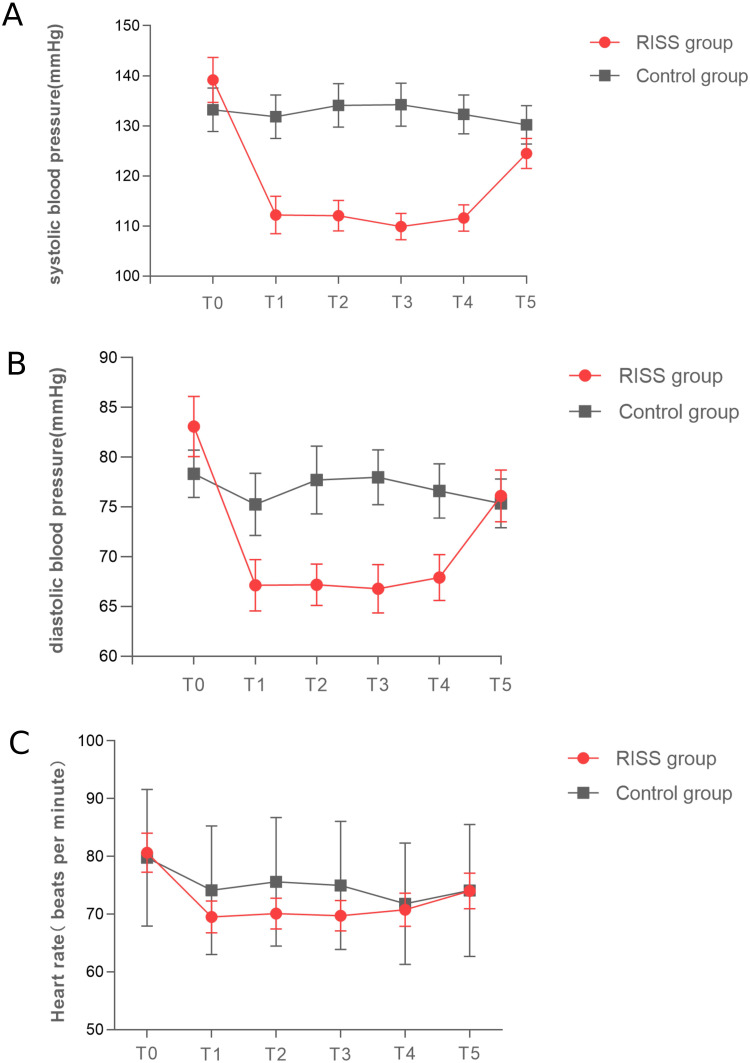
The RISS group displayed significantly lower VAS scores during rest and when coughing at 6, 12, 24, and 48h postoperatively compared to the Control group (P < 0.001) (Table 2 and Figure 2). The SBP, DBP in the RISS group were significantly lower than in the Control group at T1-T4 (P < 0.001), and the heart rates in the RISS group were significantly lower than in the Control group at T1-T3 (P < 0.001). There were significant interaction effects between time and group (P < 0.001) (Table 3 and Figure 3). The RISS group displayed a shorter time for the first postoperative ambulation compared with the Control group (8.84 ± 2.87h, 15.43 ± 4.50h, P < 0.001). There were no statistically significant differences in chest tube removal time and length of postoperative hospital stay between the two groups (all P > 0.05) (Table 4). In addition, there were no significant differences in postoperative nausea and vomiting between the RISS and Control groups (P > 0.05). One case of peripheral nerve-controlled analgesia catheter misplacement occurred in the RISS group postoperatively. Neither group experienced other adverse reactions, such as pneumothorax, local anesthetic toxicity, hypotension, urinary retention, or respiratory depression (Table 4).
Table 2.
Comparison of VAS Scores at Rest and During Coughing Postoperatively
| Measurement | Time | RISS (n=49) | Control Group (n=49) | T-value | P-Value |
|---|---|---|---|---|---|
| VAS score during coughing, mean ± SD | 6 h | 2.37 ± 0.57 | 3.90 ± 0.68 | 7.865 | <0.001 |
| 12 h | 2.71 ± 0.65 | 4.45 ± 0.58 | 8.234 | <0.001 | |
| 24 h | 2.86 ± 0.74 | 4.43 ± 0.65 | 7.814 | <0.001 | |
| 48 h | 2.63 ± 0.73 | 4.49 ± 0.65 | 8.225 | <0.001 | |
| F | 13.269 | 20.650 | |||
| P | P<0.001 | P<0.001 | |||
| Intergroup effect | F=238.678 | P<0.001 | |||
| Intra-group effect | F=26.598 | P<0.001 | |||
| Intergroup * intra-group effect | F=3.943 | P=0.011 | |||
| VAS score at rest, mean ± SD | 6 h | 1.45 ± 0.50 | 2.80 ± 0.76 | 7.276 | <0.001 |
| 12 h | 1.90 ± 0.65 | 3.43 ± 0.50 | 8.085 | <0.001 | |
| 24 h | 2.10 ± 0.71 | 3.57 ± 0.65 | 7.554 | <0.001 | |
| 48h | 1.98 ± 0.78 | 3.63 ± 0.67 | 7.609 | <0.001 | |
| F | 21.727 | 22.371 | |||
| P | P<0.001 | P<0.001 | |||
| Intergroup effect | F=43.725 | P<0.001 | |||
| Intra-group effect | F=1.879 | P<0.001 | |||
| Intergroup * intra-group effect | F=190.703 | P=0.157 |
Figure 2.
Comparison of the visual analogue scale (VAS) scores at rest and during coughing postoperatively in RISS and Control group. (A) VAS score at rest (upon admission to the post-anesthesia care unit (PACU), postoperative 6h, 12h, 24h, 48h. (B) VAS score during coughing (upon admission to the post-anesthesia care unit (PACU), postoperative 6h, 12h, 24h, 48h. The Orange lines represent the RISS group, and the blue lines represent the Control group.
Table 3.
Comparison of Blood Pressure and Heart Rate at Various Time Points During Surgery
| Measurement | Time | RISS (n=49) | Control Group (n=49) | T-Value | P-Value |
|---|---|---|---|---|---|
| Systolic blood pressure (mmHg), mean ± SD | Pre-Induction (T0) | 139.16 ± 15.68 | 133.22 ± 15.19 | −1.904 | <0.060 |
| Incision (T1) | 112.2 ± 13.12 | 131.82 ± 15.14 | 6.853 | <0.001 | |
| Upon chest entry (T2) | 112.08 ± 10.68 | 134.1 ± 15.05 | 8.352 | <0.001 | |
| 5 minutes into chest entry (T3) | 109.92 ± 9.20 | 134.22 ± 14.95 | 9.695 | <0.001 | |
| 15 minutes into chest entry (T4) | 111.61 ± 9.16 | 132.29 ± 13.52 | 8.863 | <0.001 | |
| 5 minutes after extubation (T5) | 124.51 ± 10.43 | 130.20 ± 13.41 | 2.346 | <0.001 | |
| F | 38.430 | 1.115 | |||
| P | <0.001 | 0.366 | |||
| Intergroup effect | F=51.801 | P<0.001 | |||
| Intra-group effect | F=17.781 | P<0.001 | |||
| Intergroup * intra-group effect | F=20.902 | P<0.001 | |||
| Diastolic blood pressure (mmHg), mean ± SD | Pre-induction (T0) | 83.08 ± 10.58 | 78.33 ± 8.26 | −2.480 | 0.015 |
| Incision (T1) | 67.14 ± 8.94 | 75.30 ± 10.92 | 4.028 | <0.001 | |
| Upon chest entry (T2) | 67.18 ± 7.28 | 77.89 ± 9.57 | 5.314 | <0.001 | |
| 5 minutes into chest entry (T3) | 66.78 ± 8.51 | 77.98 ± 9.61 | 6.108 | <0.001 | |
| 15 minutes into chest entry (T4) | 67.90 ± 8.02 | 76.61 ± 9.46 | 4.919 | <0.001 | |
| 5 minutes after extubation (T5) | 76.12 ± 9.05 | 75.37 ± 8.52 | −0.425 | 0.671 | |
| F | 24.354 | 1.762 | |||
| P | < 0.001 | P=0.141 | |||
| Intergroup effect | F=17.065 | P<0.001 | |||
| Intra-group effect | F=16.222 | P<0.001 | |||
| Intergroup * intra-group effect | F=11.750 | P<0.001 | |||
| Heart Rate (beats per minute), mean ± SD | Pre-induction (T0) | 80.63 ± 11.78 | 79.76 ± 11.86 | −0.368 | 0.714 |
| Incision (T1) | 69.51 ± 9.68 | 74.16 ± 11.15 | 2.206 | 0.030 | |
| Upon chest entry (T2) | 70.10 ± 9.27 | 75.59 ± 11.13 | 2.653 | 0.009 | |
| 5 minutes into chest entry (T3) | 69.73 ± 9.14 | 74.98 ± 11.08 | 2.557 | 0.012 | |
| 15 minutes into chest entry (T4) | 70.78 ± 10.09 | 71.8 ± 10.52 | 0.490 | 0.625 | |
| 5 minutes after extubation (T5) | 74.00 ± 10.87 | 74.12 ± 11.45 | 0.054 | 0.957 | |
| F | 12.791 | 6.506 | |||
| P | P< 0.001 | P< 0.001 | |||
| Intergroup effect | F=2.111 | P=0.149 | |||
| Intra-group effect | F=15.448 | P< 0.001 | |||
| Intergroup * intra-group effect | F=4.171 | P=0.002 |
Figure 3.
Comparison of blood pressure and heart rate at various time points during surgery in RISS and Control group. (A) Systolic blood pressure (SBP, mmHg). (B) Diastolic blood pressure (DBP, mmHg). (C) Heart rate (HR, beats per minute). The Orange lines represent the RISS group, and the blue lines represent the Control group.
Table 4.
Comparison of Postoperative Recovery and Adverse Reactions Between the Two Groups
| Indicators | RISS (n=49) | Control Group (n=49) | P |
|---|---|---|---|
| Time to ambulation (h), mean ± SD | 8.84 ± 2.87 | 15.43 ± 4.50 | < 0.001 |
| Duration of drain removal (days), mean ± SD | 3.08 ± 1.32 | 3.20 ± 1.61 | 0.876 |
| Length of postoperative hospital stay (days), mean ± SD | 7.76 ± 3.02 | 8.16 ± 3.35 | 0.706 |
| Complications [cases (n, %)] | |||
| Nausea and vomiting | 2 (4) | 7 (14) | 0.706 |
| Catheter displacement | 1 (2) | 0 | 0.317 |
| Other* | 0 | 0 | – |
Notes: *Other: including pneumothorax, local anesthetic toxicity, hypotension, urinary retention, or respiratory depression.
Discussion
This study found that ultrasound-guided continuous RISS led to lower VAS scores, NIBP, and HR compared to the Control group, which showed that ultrasound-guided continuous RISS may contribute to a better analgesic effect (despite a smaller consumption of sufentanil and remifentanil) and faster postoperative recovery. RISS appears safe and effective for postoperative pain management in thoracoscopic surgery patients.
Elsharkawy et al21 demonstrated that a single RISS plane block offers analgesia for 12–24 h. In the present study, ultrasound-guided needle placement in the RISS plane and the subsequent placement of a patient-controlled peripheral nerve analgesia pump extended postoperative pain relief significantly, as shown by the postoperative VAS scores at rest and when coughing, as previously observed.21,22,24,26 It resulted in fewer button presses on the postoperative analgesia pump and the need for rescue analgesia compared with the Control group. Common regional block techniques employed for post-thoracic surgery pain management include thoracic paravertebral block,27,28 thoracic epidural analgesia (TEA),29 and erector spinae plane block.30 Compared with these three techniques, the RISS plane block offers several advantages. The ultrasound images of the rib and muscle gaps during the RISS plane block are easy to interpret. It carries low risks of pneumothorax, epidural hematoma, or spinal cord injury, has low demands on coagulation function, and exerts a minor impact on circulatory and respiratory functions, as observed in the present study. In addition, it does not lead to respiratory depression, addiction, or drug tolerance. As a result, it is a straightforward, relatively safe technique with easy catheter fixation and prolonged analgesic duration and is conducive to widespread clinical adoption. Furthermore, the postoperative pain scores were lower with RISS than with ICNB, which may provide safe and effective postoperative analgesia and accelerate rapid postoperative recovery. No severe complications were observed in the present study.
The RISS plane extends medially to the erector spinae muscles and laterally to the serratus anterior muscle.21 Early studies have shown that the RISS plane block provides analgesia for the anterior and posterior aspects of the chest wall and upper abdomen.21,22,24 In a cadaveric study involving fresh cadavers, T5-T6 intercostal nerves’ lateral branches were found to be stained with methylene blue, and T7-T8 low serratus anterior muscle block led to methylene blue diffusion spanning from T4 to T10.21 In the present study, the injection sites at T4-5 and T7-8 were used. At 20 min post-block, the pain loss plane primarily covered T4-T8, aligning with previous clinical observations.21,23 Given that thoracoscopic surgery incisions are primarily innervated by the T4-T6 spinal nerves, the local anesthetic diffusion range adequately covers the surgical pain.
This study revealed distinct hemodynamic responses between RISS and Control groups. Pre-induction (T0), RISS patients exhibited elevated SBP, DBP, and HR, suggesting heightened sympathetic activity. However, post-induction (T1 to T5), RISS maintained significantly lower SBP, DBP, and HR compared to Control group. Significant interaction effects underscored temporal hemodynamic differences, suggesting RISS provides a more stable intraoperative cardiovascular environment.31 Intraoperative HR and NIBP serve as vital anesthesia indicators, with RISS demonstrating stable levels. These findings inform anesthesia management and suggest RISS as a potential strategy for stable anesthesia. Further research is needed to explore long-term implications and underlying mechanisms.
In the present study, there were no significant differences in postoperative nausea and vomiting between RISS and Control groups. Since RISS involves leaving a catheter in place after surgery, there is always a risk of catheter displacement, which occurred in one patient. No cases of pneumothorax, local anesthetic toxicity, hypotension, urinary retention, or respiratory depression were observed, suggesting the safety of RISS.26,32 Still, considering the relatively small number of patients, the results should be validated in future studies.
However, this present study still had several limitations. Firstly, the investigation exclusively compared RISS comparison of ICNB without evaluating it against other fascial plane blocks. Secondly, the study solely observed the analgesic effects within the first 48 h postoperatively, leaving a gap in our understanding of the long-term impact of these two block methods on pain management. A time point of 3 months should be included in future studies. Thirdly, postoperative quality of life was not assessed.
Conclusion
In conclusion, ultrasound-guided continuous RISS plane block may be a safe and effective approach for pain control after thoracoscopy surgery as compared with ICNB.
Funding Statement
This project belonged to the Medical and Health Technology Plan Project of Inner Mongolia Autonomous Region Health Commission (project #202202337). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Sharing Statement
All the data will be shared 6 months after publication with the patients’ relevant data de-identification. Other documents will be available as well, including the study protocol, statistical analysis plan and analytical code. Other researchers may request the data set by emailing to the corresponding author. The results of this study will be submitted for publication in peer-reviewed publications.
Ethics Approval
Ethical approval for this study was granted by the Chifeng College Affiliated Hospital Ethics Committee (no. fsyy2023037).All participants and their parents provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author(s) report no conflicts of interest in this work.
References
- 1.Marshall K, McLaughlin K. Pain Management in Thoracic Surgery. Thorac Surg Clin. 2020;30(3):339–346. doi: 10.1016/j.thorsurg.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 2.Ross JDW, Cole CMW, Lo W, Ura M. Postoperative Pain in Thoracic Surgical Patients: an Analysis of Factors Associated With Acute and Chronic Pain. Heart Lung Circ. 2021;30(8):1244–1250. doi: 10.1016/j.hlc.2020.12.001 [DOI] [PubMed] [Google Scholar]
- 3.Hu LH, Xu X, Shen WY, Qi Y, Tian H, He JX. Application of thoracoscopy-guided thoracic paravertebral block for analgesia after single-port video-assisted pulmonary lobectomy. Zhonghua Yi Xue Za Zhi. 2020;100(33):2596–2600. doi: 10.3760/cma.j.cn112137-20200525-01647 [DOI] [PubMed] [Google Scholar]
- 4.Thapa P, Euasobhon P. Chronic postsurgical pain: current evidence for prevention and management. Korean J Pain. 2018;31(3):155–173. doi: 10.3344/kjp.2018.31.3.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537–1546. doi: 10.1016/S0140-6736(19)30352-6 [DOI] [PubMed] [Google Scholar]
- 6.Lopes A, Seligman Menezes M, Antonio Moreira de Barros G. Chronic postoperative pain: ubiquitous and scarcely appraised: narrative review. Braz J Anesthesiol. 2021;71(6):649–655. doi: 10.1016/j.bjane.2020.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Small C, Laycock H. Acute postoperative pain management. Br J Surg. 2020;107(2):e70–e80. doi: 10.1002/bjs.11477 [DOI] [PubMed] [Google Scholar]
- 8.Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–2298. doi: 10.2147/JPR.S144066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horn R, Kramer J. Postoperative Pain Control. Treasure Island (FL): StatPearls; 2023. [Google Scholar]
- 10.Blichfeldt-Eckhardt MR, Andersen C, Ording H, Licht PB, Toft P. From acute to chronic pain after thoracic surgery: the significance of different components of the acute pain response. J Pain Res. 2018;11:1541–1548. doi: 10.2147/JPR.S161303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinese Society of Anesthesiology. Expert Consensus on Adult Postoperative Pain Management. J Clin Anesthesiol. 2010;26(3):911–917. [Google Scholar]
- 12.Sengupta S. Post-operative pulmonary complications after thoracotomy. Indian J Anaesth. 2015;59(9):618–626. doi: 10.4103/0019-5049.165852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan L, Wang H, Zhang X. The impact of postoperative analgesia with dexmedetomidine combined with sufentanil on immune function and pulmonary infection complications in elderly patients with lung cancer. Chin J Geriatr. 2019;38(100):1158–1161. [Google Scholar]
- 14.Pennefather SH, McKevith J. Pain Management After Thoracic Surgery. In: Slinger P, editor. Principles and Practice of Anesthesia for Thoracic Surgery. Second Edition ed. Cham: Springer Nature Switzerland AG; 2019. [Google Scholar]
- 15.Krakowski JC, Hallman MJ, Smeltz AM. Persistent Pain After Cardiac Surgery: prevention and Management. Semin Cardiothorac Vasc Anesth. 2021;25(4):289–300. doi: 10.1177/10892532211041320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lancet Regional Health-Americas T; The Lancet Regional H-A. Opioid crisis: addiction, overprescription, and insufficient primary prevention. Lancet Reg Health Am. 2023;23:100557. doi: 10.1016/j.lana.2023.100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed Z, Samad K, Ullah H. Role of intercostal nerve block in reducing postoperative pain following video-assisted thoracoscopy: a randomized controlled trial. Saudi J Anaesth. 2017;11(1):54–57. doi: 10.4103/1658-354X.197342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gadsden J, Kwofie K, Shastri U. Continuous intercostal versus paravertebral blockade for multiple fractured ribs. J Trauma Acute Care Surg. 2012;73(1):293–294. doi: 10.1097/TA.0b013e31825aaeb5 [DOI] [PubMed] [Google Scholar]
- 19.Pastino A, Lakra A. Patient-Controlled Analgesia. Treasure Island (FL): StatPearls; 2023. [PubMed] [Google Scholar]
- 20.Clairoux A, Issa R, Belanger ME, Urbanowicz R, Richebe P, Brulotte V. Perioperative pain management for thoracic surgery: a narrative review of the literature. Curr Challenges Thor Surg. 2023;5:1. [Google Scholar]
- 21.Elsharkawy H, Maniker R, Bolash R, Kalasbail P, Drake RL, Elkassabany N. Rhomboid Intercostal and Subserratus Plane Block: a Cadaveric and Clinical Evaluation. Reg Anesth Pain Med. 2018;43(7):745–751. doi: 10.1097/AAP.0000000000000824 [DOI] [PubMed] [Google Scholar]
- 22.Elsharkawy H, Ince I, Pawa A. Rhomboid intercostal and sub-serratus (RISS) plane block for analgesia after lung transplant. J Clin Anesth. 2019;56:85–87. doi: 10.1016/j.jclinane.2019.01.042 [DOI] [PubMed] [Google Scholar]
- 23.Kozanhan B, Aksoy N, Yildiz M, Tutar MS, Canitez A, Eryilmaz MA. Rhomboid Intercostal and Subserratus Plane block for modified radical mastectomy and axillary curettage in a patient with severe obstructive sleep apnea and morbid obesity. J Clin Anesth. 2019;57:93–94. doi: 10.1016/j.jclinane.2019.03.026 [DOI] [PubMed] [Google Scholar]
- 24.Elsharkawy H, Hamadnalla H, Altinpulluk EY, Gabriel RA. Rhomboid intercostal and subserratus plane block -a case series. Korean J Anesthesiol. 2020;73(6):550–556. doi: 10.4097/kja.19479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou X, Chai B, Lin W, Li S, Ma B. The effect of ultrasound-guided Rectus-Intercostal Serratus-Subserratus plane block on postoperative analgesia in patients undergoing laparoscopic nephrectomy. J Clin Anesth. 2020;36(4):322–325. [Google Scholar]
- 26.Deng W, Hou XM, Zhou XY, Zhou QH. Rhomboid intercostal block combined with sub-serratus plane block versus rhomboid intercostal block for postoperative analgesia after video-assisted thoracoscopic surgery: a prospective randomized-controlled trial. BMC Pulm Med. 2021;21(1):68. doi: 10.1186/s12890-021-01432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotze A, Scally A, Howell S. Efficacy and safety of different techniques of paravertebral block for analgesia after thoracotomy: a systematic review and metaregression. Br J Anaesth. 2009;103(5):626–636. doi: 10.1093/bja/aep272 [DOI] [PubMed] [Google Scholar]
- 28.Giang NT, Van Nam N, Trung NN, et al. Patient-controlled paravertebral analgesia for video-assisted thoracoscopic surgery lobectomy. Local Reg Anesth. 2018;11:115–121. doi: 10.2147/LRA.S184589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottschalk A, Cohen SP, Yang S, Ochroch EA, Warltier D. Preventing and treating pain after thoracic surgery. Anesthesiology. 2006;104(3):594–600. doi: 10.1097/00000542-200603000-00027 [DOI] [PubMed] [Google Scholar]
- 30.Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The Erector Spinae Plane Block: a Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg Anesth Pain Med. 2016;41(5):621–627. doi: 10.1097/AAP.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 31.Luo F, Zhang J, Cheng P, et al. Clinical Application of Ultrasound-guided RISS Plane Block for Postoperative Analgesia After Minimally Invasive McKeown Esophagectomy: a Prospective Randomized Controlled Study. Medical Journal of Peking Union Medical College Hospital. 2024;15(3):624–631. [Google Scholar]
- 32.Ökmen K, Gürbüz H, Özkan H. Application of unilateral rhomboid intercostal and subserratus plane block for analgesia after laparoscopic cholecystectomy: a quasi-experimental study. Korean J Anesthesiol. 2022;75(1):79–85. doi: 10.4097/kja.21229 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data will be shared 6 months after publication with the patients’ relevant data de-identification. Other documents will be available as well, including the study protocol, statistical analysis plan and analytical code. Other researchers may request the data set by emailing to the corresponding author. The results of this study will be submitted for publication in peer-reviewed publications.