Abstract
Pancreatic lobules pulse-labeled with [3H]leucine have been incubated at temperatures between 0 and 37 degrees C in the presence or absence of ongoing oxidative phosphorylation. Subcellular fractionation methods and electron microscopic autoradiography have been used to monitor the progress of intracellular transport of newly synthesized secretory proteins. Over the period studied, exit from the rough endoplasmic reticulum (RER) occurs only at greater than 10 degrees C while traversal of the Golgi complex and entry into condensing vacuoles requires greater than 22 degrees C. Both steps of transport require ongoing ATP production. Incubation at 10 or 20 degrees C does not diminish ATP levels, relative to 37 degrees C controls. Remarkable and unprecedented alterations of the ultrastructure of transitional elements of the RER accompany the arrest of exit from the RER: at 10 degrees C transitional elements are much more numerous and longer than in controls; in the absence of ATP production they are essentially absent. These observations are interpreted in terms of a cyclic model of RER-to-Golgi vesicular traffic. Inhibition of ATP production also causes an increase in the rigid cisternae and coated elements in the distal Golgi area.
Full text
PDF
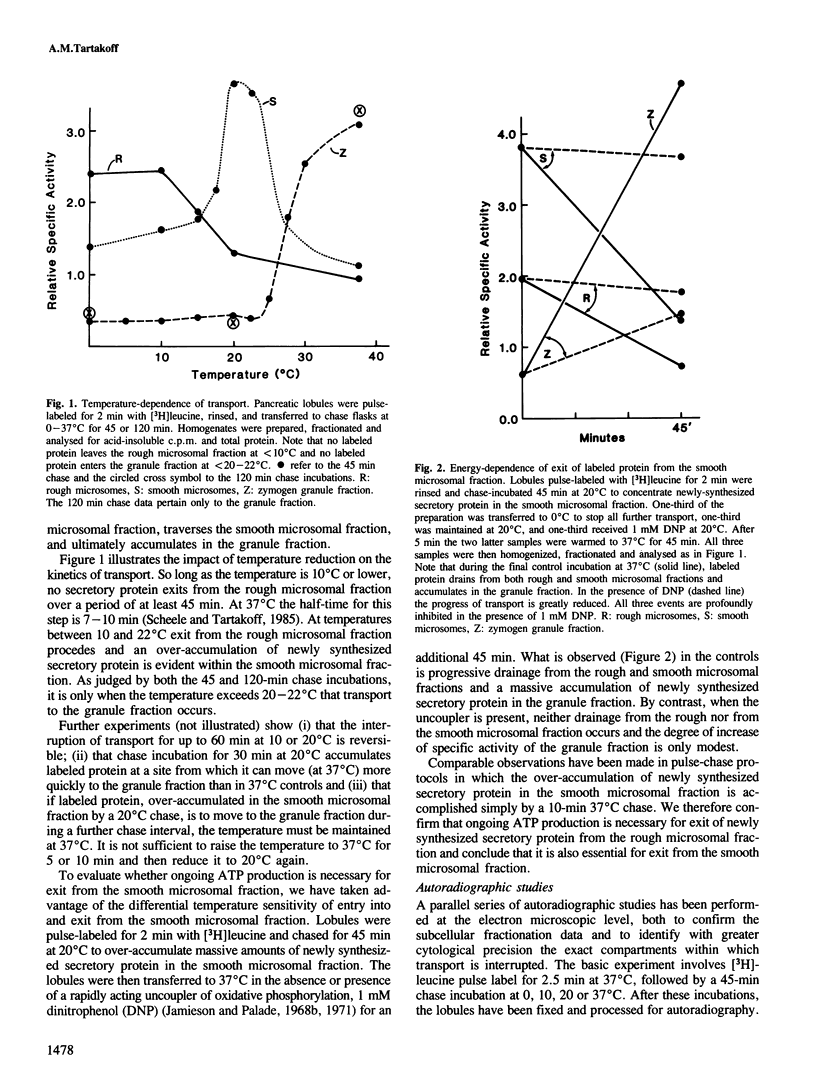


Images in this article
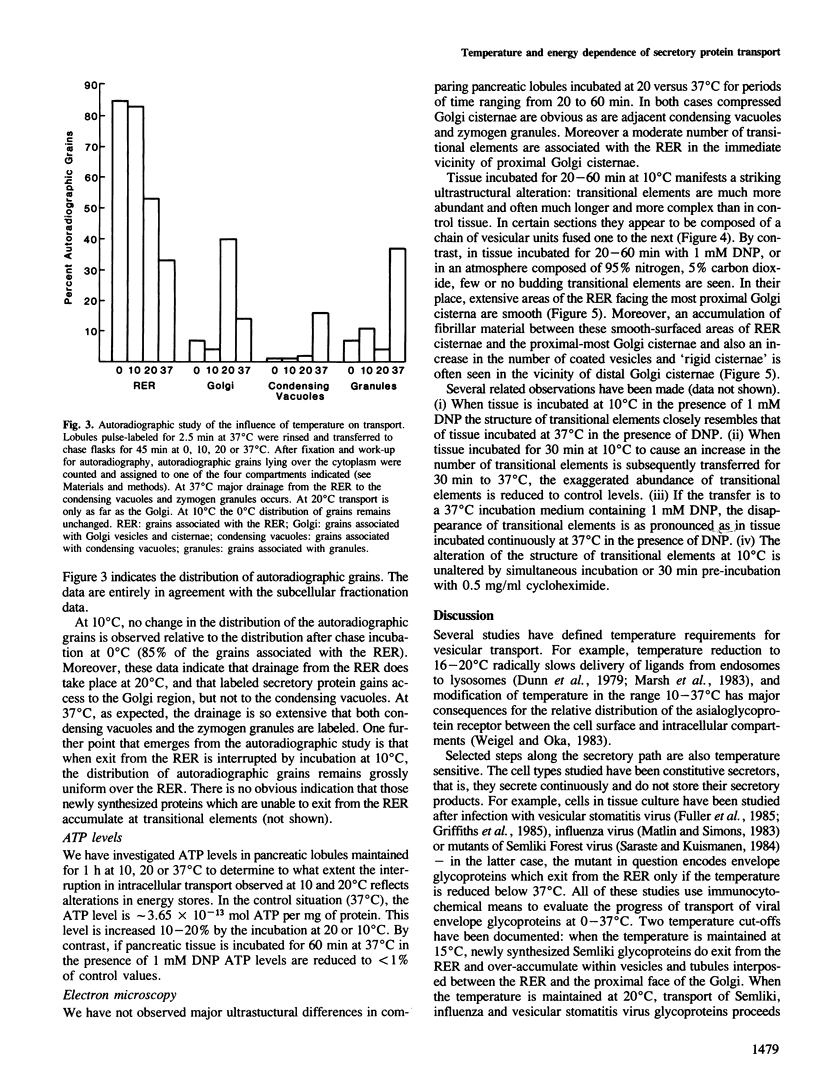
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Glick B. S., Rothman J. E. Sequential intermediates in the pathway of intercompartmental transport in a cell-free system. Cell. 1984 Dec;39(3 Pt 2):525–536. doi: 10.1016/0092-8674(84)90459-8. [DOI] [PubMed] [Google Scholar]
- Braell W. A., Schlossman D. M., Schmid S. L., Rothman J. E. Dissociation of clathrin coats coupled to the hydrolysis of ATP: role of an uncoating ATPase. J Cell Biol. 1984 Aug;99(2):734–741. doi: 10.1083/jcb.99.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands R., Snider M. D., Hino Y., Park S. S., Gelboin H. V., Rothman J. E. Retention of membrane proteins by the endoplasmic reticulum. J Cell Biol. 1985 Nov;101(5 Pt 1):1724–1732. doi: 10.1083/jcb.101.5.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie D. A. Golgi complex beads in vertebrates and their relationship with clathrin coats. Tissue Cell. 1982;14(2):253–262. doi: 10.1016/0040-8166(82)90023-4. [DOI] [PubMed] [Google Scholar]
- Dunn W. A., Hubbard A. L., Aronson N. N., Jr Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980 Jun 25;255(12):5971–5978. [PubMed] [Google Scholar]
- Featherstone C., Griffiths G., Warren G. Newly synthesized G protein of vesicular stomatitis virus is not transported to the Golgi complex in mitotic cells. J Cell Biol. 1985 Dec;101(6):2036–2046. doi: 10.1083/jcb.101.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting T., Kabat D. Evidence for a glycoprotein "signal" involved in transport between subcellular organelles. Two membrane glycoproteins encoded by murine leukemia virus reach the cell surface at different rates. J Biol Chem. 1982 Dec 10;257(23):14011–14017. [PubMed] [Google Scholar]
- Fries E., Gustafsson L., Peterson P. A. Four secretory proteins synthesized by hepatocytes are transported from endoplasmic reticulum to Golgi complex at different rates. EMBO J. 1984 Jan;3(1):147–152. doi: 10.1002/j.1460-2075.1984.tb01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D., Bravo R., Simons K. An enzymatic assay reveals that proteins destined for the apical or basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J. 1985 Feb;4(2):297–307. doi: 10.1002/j.1460-2075.1985.tb03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Pfeiffer S., Simons K., Matlin K. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J Cell Biol. 1985 Sep;101(3):949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. 3. Dissociation of intracellular transport from protein synthesis. J Cell Biol. 1968 Dec;39(3):580–588. doi: 10.1083/jcb.39.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. IV. Metabolic requirements. J Cell Biol. 1968 Dec;39(3):589–603. doi: 10.1083/jcb.39.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Snider M., Strous G. J. Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature. 1983 Jul 7;304(5921):80–83. doi: 10.1038/304080a0. [DOI] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983 Aug;34(1):233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Jamieson J. D., Palade G. E. Composition of cellular membranes in the pancreas of the guinea pig. II. Lipids. J Cell Biol. 1971 Apr;49(1):130–149. doi: 10.1083/jcb.49.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Mori M., Quintana N., Yam A. Studies of the secretory process in the mammalian exocrine pancreas. I. The condensing vacuoles. J Cell Biol. 1977 Oct;75(1):148–165. doi: 10.1083/jcb.75.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Saraste J., Kuismanen E. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell. 1984 Sep;38(2):535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Scheele G. A., Palade G. E., Tartakoff A. M. Cell fractionation studies on the guinea pig pancreas. Redistribution of exocrine proteins during tissue homogenization. J Cell Biol. 1978 Jul;78(1):110–130. doi: 10.1083/jcb.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G. Pancreatic lobules in the in vitro study of pancreatic acinar cell function. Methods Enzymol. 1983;98:17–28. doi: 10.1016/0076-6879(83)98135-1. [DOI] [PubMed] [Google Scholar]
- Scheele G., Tartakoff A. Exit of nonglycosylated secretory proteins from the rough endoplasmic reticulum is asynchronous in the exocrine pancreas. J Biol Chem. 1985 Jan 25;260(2):926–931. [PubMed] [Google Scholar]
- Schlossman D. M., Schmid S. L., Braell W. A., Rothman J. E. An enzyme that removes clathrin coats: purification of an uncoating ATPase. J Cell Biol. 1984 Aug;99(2):723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., Braell W. A., Schlossman D. M., Rothman J. E. A role for clathrin light chains in the recognition of clathrin cages by 'uncoating ATPase'. Nature. 1984 Sep 20;311(5983):228–231. doi: 10.1038/311228a0. [DOI] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Stubbs C. D., Smith A. D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984 Jan 27;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Jamieson J. D. Subcellular fractionation of the pancreas. Methods Enzymol. 1974;31:41–59. doi: 10.1016/0076-6879(74)31006-3. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. The surface content of asialoglycoprotein receptors on isolated hepatocytes is reversibly modulated by changes in temperature. J Biol Chem. 1983 Apr 25;258(8):5089–5094. [PubMed] [Google Scholar]
- Yamamoto A., Masaki R., Tashiro Y. Is cytochrome P-450 transported from the endoplasmic reticulum to the Golgi apparatus in rat hepatocytes? J Cell Biol. 1985 Nov;101(5 Pt 1):1733–1740. doi: 10.1083/jcb.101.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]