Summary
Background
Prior studies have reported lower effectiveness of XBB.1.5-adapted vaccines against hospitalization related to the Omicron JN.1 variant than the XBB variant. This study evaluated the effectiveness and durability of the BNT162b2 XBB.1.5-adapted vaccine against JN.1-related hospitalization during the 2023–2024 season in Europe.
Methods
A test-negative case–control study was carried out in adults (≥18 y) hospitalized between 2 October 2023 and 2 April 2024 with severe acute respiratory infection (SARI) within the id.DRIVE partnership. This study included nine sites across Belgium, Germany, Italy, and Spain. Cases had a laboratory-confirmed JN.1 infection or a positive SARS-CoV-2 test with symptom onset during JN.1 predominance; controls had a negative SARS-CoV-2 test and symptom onset during JN.1 predominance. The primary objective was to estimate BNT162b2 XBB.1.5-adapted vaccine effectiveness (VE) against COVID-19 hospitalization. One case was matched with up to four controls, according to symptom onset date and site. Multivariable analyses were adjusted for symptom onset date, age, sex, and number of chronic conditions.
Findings
Among 308 test-positive cases and 1117 test-negative controls, BNT162b2 XBB.1.5-adapted VE against hospitalization compared to no vaccination this season was 53.8% (95% CI 38.4–65.4) after a median of 63 days following vaccination. Protection was sustained through five months; VE was 52.2% (95% CI 41.3–61.1) 2 to <4 weeks after vaccination, 48.9% (95% CI 17.9–68.2) at 4 to <8 weeks, and ranged from 54.6% to 59.5% at 4-week intervals from 8 to <22 weeks.
Interpretation
BNT162b2 XBB.1.5-adapted vaccine provided protection against JN.1-related hospitalization, regardless of prior vaccination history, with no evidence of waning through five months. These data support yearly vaccination against COVID-19 to prevent severe illness during the respiratory virus season.
Funding
Pfizer.
Keywords: COVID-19, SARS-CoV-2, COVID-19 vaccination, Vaccine effectiveness, BNT162b2, XBB adapted vaccine, JN.1
Research in context.
Evidence before this study
We searched Medline, Embase, and grey literature for pre-prints and publications related to COVID-19 vaccination, vaccine effectiveness (VE), and XBB.1.5-adapted vaccines available through 11 August 2024 using the following search terms: (XBB∗.ab,ti,kw.) AND (exp SARS-CoV-2 vaccine/exp mRNA Vaccines/exp elasomeran/exp tozinameran/Vaccin$.ti.(Elasomeran or mRNA-1273 or mRNA1273 or RNA-1273 or RNA1273 or spikevax, or moderna).ti,ab.(“Novavax” or “Nuvaxovid” or “Covovax” or “NVX-CoV2373” or “NVX-CoV2601” or “TAK-019” or “nvx cov 2373” or “nvx cov2373” or nvx-cov-2373 or nvx-cov2373 or nvxcov2373 or “sars-cov-2 rs” or “tak 019” or tak019, or NVX-CoV2601).ti,kw,ab.(Tozinameran or ((biontech or Pfizer) adj3 vaccine) or bnt 162b2 or bnt162b2, or comirnaty).ti,ab.) AND (exp Vaccine Efficacy/exp comparative effectiveness/(effectiveness or efficacy).ti,ab,kw.(effective∗ or protect∗ or prevent∗ or efficac$ or “real world” or real-world or rwd or rwe, or durab$).ti,ab.). The literature search was supplemented with articles already known to the authors to be relevant evidence before this study. We also reviewed government reports from countries included in our study (Belgium, Germany, Italy, and Spain) for data on timing of JN.1 variant predominance. JN.1 became predominant the earliest in Spain in early December 2023, followed by Belgium and Germany in late December 2023, and Italy in early January 2024. JN.1 is a descendant of BA.2.86 containing >30 mutations in the spike protein compared to the XBB.1.5 strain. We identified eight studies that reported VE for XBB.1.5-adapted vaccines against JN.1-related hospitalization during the 2023–2024 autumn/winter season, of which only two estimated effectiveness beyond three months post-vaccination. Reported VE against JN.1-related hospitalization ranged from ∼30 to 60% and 23–47% at 1–3 and 3–6 months after XBB.1.5-adapted vaccine receipt, respectively.
Added value of this study
Our study shows that the BNT162b2 XBB.1.5-adapted vaccine was effective against hospitalization related to the JN.1 variant in Europe during the 2023–2024 season and is one of the first studies to evaluate longer-term durability of XBB.1.5-adapted vaccine protection against JN.1. We found an overall VE of 54% compared to no vaccination during the 2023–2024 season, with no evidence of waning protection through five months. Finally, BNT162b2 XBB.1.5-adapted VE against hospitalization was similar regardless of prior vaccination history, including prior receipt of mRNA bivalent BA.4/5 vaccine, ≥2 wildtype mRNA doses, and no prior vaccination.
Implications of all the available evidence
BNT162b2 XBB.1.5-adapted vaccine provided effective protection against hospitalization during the 2023–2024 season against two highly distinct variants, Omicron XBB and JN.1, although protection was lower for JN.1. Further, vaccine protection against hospitalization is likely sustained for at least six months, indicating that vaccination provides protection against severe outcomes throughout the autumn/winter respiratory virus season.
Introduction
On 31 August 2023, the European Medicines Agency (EMA) authorized BNT162b2 XBB.1.5-adapted mRNA vaccine (Pfizer/BioNTech Manufacturing GmbH 2023–2024 formulation; hereafter referred to as BNT162b2 XBB vaccine) for the prevention of coronavirus disease 2019 (COVID-19) in individuals 6 months of age and older for the 2023–2024 autumn/winter season.1 At the time of authorization and launch of the 2023–2024 national vaccine campaigns, most adults in the European Union/European Economic Area (EU/EEA) had already received a first booster of COVID-19 vaccine (median cumulative uptake = 65.5% for age ≥18 years and 84.9% for age ≥60 years),2 and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron XBB-related lineages were predominant. Of the approximately 22.7 million COVID-19 vaccine doses administered in the EU/EEA until January 2024, 97% were BNT162b2 XBB vaccine.3
Early estimates from real-world studies conducted during periods of XBB predominance showed XBB.1.5-adapted vaccine effectiveness (VE) against hospitalization due to SARS-CoV-2 ranging from 66% to 76% effectiveness among older adult populations in the EU/EEA,4, 5, 6, 7 and 63% effectiveness among adults in the United States.8 By December 2023, however, the Omicron JN.1 variant – a highly transmissible descendant of BA.2.86 with over 30 spike protein mutations relative to the XBB.1.5 strain9 – had become predominant across Europe.10 Although XBB.1.5-adapted vaccines have been shown to elicit neutralizing antibodies against BA.2.86 and JN.1,11,12 the early data from studies that evaluated lineage-specific effectiveness – 2 from the United States and 1 from Europe – found that VE was lower for JN.1-related hospitalization (35–57%) than for XBB-related hospitalization (61–74%), suggesting that XBB.1.5-adapted vaccines may have reduced effectiveness against the JN.1 variant.7,13,14 Consistent with these early data, in late April 2024 both EMA and the World Health Organization (WHO) recommended a change to the antigenic composition of authorized COVID-19 vaccines to target JN.1 lineages for the upcoming 2024–2025 vaccination campaign.15,16
This study evaluated the real-world effectiveness and durability of the BNT162b2 XBB vaccine against COVID-19 hospitalization related to the JN.1 variant across four EU countries (Belgium, Germany, Italy, and Spain) during the 2023–2024 autumn/winter respiratory virus season.
Methods
Study design
id.DRIVE is a multi-country, multi-center, test-negative case–control study that was established in Europe in November 2020 to estimate VE against COVID-19 hospitalization, defined as laboratory-confirmed SARS-CoV-2 infection in individuals hospitalized with a severe acute respiratory infection (SARI). The id.DRIVE Master protocol (EUPAS42328) was approved by independent ethics committees (IECs) at all participating hospitals or hospital networks. SARI patients were recruited prospectively at nine study sites including seven individual hospitals and two hospital networks during 2 October 2023 to 2 April 2024 (Supplementary Table S1) in Belgium, Germany, Italy, and Spain. The study start date coincided with 14 days following the date of first availability of XBB-adapted vaccines among the four study countries (Germany: 18 September 2023).
Study objectives
The primary objective of this study was to estimate the VE of at least one dose of BNT162b2 XBB vaccine against hospitalization due to laboratory-confirmed SARS-CoV-2 in SARI patients compared to no receipt of any COVID-19 vaccine (i.e., no receipt of any XBB.1.5-adapted or non-XBB.1.5-adapted vaccine) during the 2023–2024 autumn/winter season regardless of prior COVID-19 vaccine history, including the never vaccinated. Secondary objectives were to estimate VE of at least one dose of BNT162b2 XBB vaccine against confirmed COVID-19 hospitalization in SARI patients compared to various histories of COVID-19 vaccination among those who did not receive any COVID-19 vaccine during the 2023–2024 autumn/winter season: (a) received ≥1 BA.4/5 bivalent dose, defined as receipt of at least one mRNA BA.4/5 bivalent dose as last dose between 12 September 2022 and 31 August 2023, (b) received ≥2 mRNA wildtype (i.e., ancestral) doses only, and (c) have never received any COVID-19 vaccines (‘never vaccinated’). Additionally, as a secondary objective, we estimated VE by age group, number and type of chronic conditions, and time since BNT162b2 XBB vaccination.
Study participants
The study population consisted of individuals admitted to the hospital for at least one overnight stay with SARI, based on the European Centre for Disease Prevention and Control (ECDC) SARI case definition.17 Qualifying SARI symptoms were cough, objective fever ≥38 °C, shortness of breath, anosmia, ageusia, or dysgeusia. Symptom onset must have occurred within 14 days prior to hospital admission. The study included adults aged ≥18 years who were eligible for COVID-19 vaccination according to national or regional immunization recommendations. Patients were excluded if they were hospitalized for COVID-19 in the 3 months prior to the current hospital admission, unable to provide a nasopharyngeal or oropharyngeal specimen, or if their last COVID-19 vaccine dose was a brand not approved by EMA. Patients or their legal representatives were required to provide informed consent except where waivers were authorized by IECs.
SARI patients who were either infected with the JN.1 variant of SARS-CoV-2, confirmed by whole genome sequencing (WGS) or next generation sequencing (NGS), or who experienced symptom onset during a period when JN.1-related lineages comprised ≥70% of samples sequenced as part of national SARS-CoV-2 genomic surveillance, were included. Country-specific JN.1 predominance periods were defined according to national surveillance reports.18, 19, 20, 21 The following dates were used to define the start of the JN.1-predominant period per country: 3 December 2023 for Spain,18 29 December 2023 for Belgium,19 31 December 2023 for Germany,20 and 1 January 2024 for Italy.21 The earliest symptom onset date for a patient infected with JN.1 was 27 November 2023. No patients had a sequencing result of a non-JN.1 lineage during JN.1 predominance periods.
Exposure and outcome definition
The exposed group was defined as patients vaccinated with at least one dose of BNT162b2 XBB vaccine, regardless of prior COVID-19 vaccine history. Patients who received the vaccination within 14 days prior to SARI symptom onset or ≤12 weeks after receipt of any other COVID-19 vaccine were excluded from the analyses. The unexposed group was defined as patients who had not been vaccinated against COVID-19 during the 2023–2024 season. The unexposed group included those never vaccinated and those who have received any COVID-19 vaccine before the 2023–2024 season, which must have occurred >12 weeks prior to SARI symptom onset. This minimum interval of >12 weeks since prior COVID-19 vaccination or SARS-CoV-2 infection was required for both exposed and the unexposed groups, in accordance with national recommendations on eligibility for the 2023–2024 seasonal vaccine.22 The unexposed group was further stratified by prior COVID-19 vaccination categories: 1) patients who have received at least one BA.4/5 bivalent dose, 2) patients who have only received at least two mRNA wild type doses, and 3) patients who have never been vaccinated with any COVID-19 vaccine.
‘Test-positive cases’ were defined as patients who met the SARI case definition and tested positive on at least one of the following: SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR), multiplex PCR, or transcription mediated amplification (TMA) assay. Cases were infected with laboratory-confirmed JN.1 or had a positive SARS-CoV-2 test with symptom onset during a period of JN.1-predominance. ‘Test-negative controls᾿ were defined as patients who met the SARI case definition and tested negative on all SARS-CoV-2 tests conducted, with symptom onset during JN.1 predominance. Specimens for both test-positive cases and test-negative controls must have been collected between 14 days prior to and up to 72 h after hospital admission.
Data collection and management
Data on COVID-19 vaccination were gathered from vaccination registries, medical records, or vaccination cards. Genomic sequencing was performed on SARS-CoV-2-positive samples. Data were collected on age, sex, chronic conditions (Supplementary Table S2), body mass index (BMI), pregnancy status, history of influenza and pneumococcal vaccination, prior SARS-CoV-2 infection, and long-term care facility residence. Study staff gathered data from hospital medical records, general practitioner medical records, or patient interview, which were subsequently entered into an electronic Case Report Form (eCRF, Castor®). Only pseudonymized data were transferred to a secured central research server hosted by P95 for analysis.
Statistical methods
Odds ratios and 95% Wald confidence intervals (CI) that compared the odds of vaccination among test-positive cases to the odds of vaccination among test-negative controls were estimated from multivariable generalized estimating equation (GEE) logistic regression models that accounted for potential intra-cluster correlation among subjects from the same study site23 and adjusted for date of symptom onset, age, sex, and number of chronic conditions. An independent working correlation matrix was recommended to mitigate biased estimates if there is cluster based confounding (CBC), informative cluster sizes (ICS), or both present.24,25 Matching of one case with up to four controls by site and 2-week interval from earliest onset date was performed for each study objective separately (i.e., using separate datasets) to account for potential residual time-varying confounding. Calendar time for symptom onset date was modeled using a cubic spline with two knots. Age was modeled using a cubic spline with two knots at age 50 and 65 years, except when the analysis dataset contained <10% patients aged <50 years, in which case a single knot at age 65 was utilized. The selection of knots was based on the cut-off points used to define age group categories. For analyses stratified by age group, the knot was set at the median age of patients in each group. Spline terms for symptom onset date were penalized to overcome multicollinearity while fitting GEE models using the (modified) Penalized Generalized Estimating Equation (PGEE) package by allowing for a ridge penalty term. Sensitivity analyses were performed to investigate the robustness of results obtained from the PGEE approach and the number and location of the knots for both symptom onset date and age. In addition, sensitivity analyses including stratification by prior COVID-19 infection, adjustment for 2023–2024 seasonal influenza vaccination status, adjustment for the presence of hypertension, and use of a stricter case definition in which symptom onset must have occurred within 7 days prior to hospitalization were performed.
All analyses included only patients with complete data on exposure status, test results, and relevant confounders adjusted in the models.26 Since COVID-19 status was included in the primary data collection, test status information was available for nearly all participants. Additionally, data on exposure status and potential confounders (such as age, sex, number of chronic conditions, and symptom onset dates) were sourced from pre-existing medical records, vaccine registries, and other records. Consequently, performing a complete case analysis was deemed unlikely to produce biased estimates and precision loss. Sample size was calculated to achieve a 95% CI width (upper value minus lower value of the 95% CI) of 50% or less, leading to a target of 820 SARI patients (164 test-positive cases and 656 test-negative controls) for the primary objective. Sample size calculations were simulation-based and relied on assumptions regarding overall vaccine coverage (40%), proportion of vaccinated subjects receiving the BNT162b2 XBB vaccine (90%), case–control ratio (1:4), and presumed VE (70%), taking into account the prespecified statistical analysis approach (GEE). More details regarding model fitting, sample size calculation, and the matching process are provided in Supplementary Material sections A.1, A.2, and A.3, respectively.
All analyses were performed in R version 4.1.2.
Role of the funding source
Pfizer is a partner in the id.DRIVE consortium (https://iddrive.eu/) and funded this study. This study was conducted as a clinical research collaboration between Pfizer and P95, co-coordinator of the id.DRIVE consortium and acting as the study sponsor. Pfizer was involved in the study design, data collection, data interpretation, writing of the manuscript, and decision to submit for publication.
Results
Study population
Of 2759 total patients hospitalized with SARI from 2 October 2023 to 2 April 2024, 1425/2759 (51.6%) patients were included in the analysis, of which 308/1425 (21.6%) were test-positive cases. Of the cases, 45/308 (14.6%) were infected with laboratory-confirmed JN.1 and 263/308 (85.4%) had unknown genetic variant but symptom onset during JN.1 predominance (Supplementary Fig. S1). In total, 0.6% (17/2759) of SARI patients were excluded from analysis due to missing data on number of chronic conditions (n = 12), sex (n = 4), and age (n = 1).
Overall, 78.0% (1111/1425) of all SARI hospitalized patients were aged ≥65 y, 50.0% (713/1425) were male and 88.1% (1256/1425) had at least one chronic condition (Table 1). The most prevalent chronic conditions were hypertension (63.9%), cardiovascular disease (44.4%), lung disease (35.7%), and type 2 diabetes (28.4%). Immunodeficiency or cancer was present in 19.5% of patients. Most (88.0%) SARI patients were recruited at hospitals located in Spain. Supplementary Fig. S2 shows the distribution of enrolled SARI patients over time and for each country.
Table 1.
Characteristics of study participants according to receipt of BNT162b2 XBB.1.5-adapted vaccine and SARS-CoV-2 case classification.
| Total, N (col %) | Received BNT162b2 XBB.1.5-adapted vaccine in 2023–2024 season, (N, col %) |
Did not receive any COVID-19 vaccine in 2023–2024 season, (N, col %) |
|||
|---|---|---|---|---|---|
| Test-positive cases | Test-negative controls | Test-positive cases | Test-negative controls | ||
| Total | 1425 | 120 | 601 | 188 | 516 |
| Prior COVID-19 vaccination | |||||
| Never vaccinated | 85 (6.0) | 0 (0.0) | 5 (0.8) | 18 (9.6) | 62 (12.0) |
| ≥2 mRNA wildtype doses only | 438 (30.7) | 17 (14.2) | 83 (13.8) | 89 (47.3) | 249 (48.3) |
| ≥1 mRNA bivalent BA.4/5 booster | 787 (55.2) | 99 (82.5) | 493 (82.0) | 63 (33.5) | 132 (25.6) |
| Othera | 115 (8.1) | 4 (3.3) | 20 (3.3) | 18 (9.6) | 73 (14.1) |
| Sex | |||||
| Female | 712 (50.0) | 52 (43.3) | 295 (49.1) | 102 (54.3) | 263 (51.0) |
| Male | 713 (50.0) | 68 (56.7) | 306 (50.9) | 86 (45.7) | 253 (49.0) |
| Age, years | |||||
| Median (IQR) | 77.0 (66.0, 86.0) | 84.0 (76.0, 88.0) | 80.0 (73.0, 87.0) | 75.0 (61.0, 84.0) | 70.5 (58.0, 80.0) |
| 18–49 | 110 (7.7) | 0 (0.0) | 7 (1.2) | 23 (12.2) | 80 (15.5) |
| 50–64 | 204 (14.3) | 9 (7.5) | 47 (7.8) | 37 (19.7) | 111 (21.5) |
| ≥65 | 1111 (78.0) | 111 (92.5) | 547 (91.0) | 128 (68.1) | 325 (63.0) |
| Symptom onset, month | |||||
| Nov 2023 | 4 (0.3) | 0 (0.0) | 0 (0.0) | 4 (2.1) | 0 (0.0) |
| Dec 2023 | 717 (50.3) | 58 (48.3) | 295 (49.1) | 95 (50.5) | 269 (52.1) |
| Jan 2024 | 582 (40.8) | 50 (41.7) | 262 (43.6) | 74 (39.4) | 196 (38.0) |
| Feb 2024 | 113 (7.9) | 12 (10.0) | 41 (6.8) | 13 (6.9) | 47 (9.1) |
| Mar 2024 | 9 (0.6) | 0 (0.0) | 3 (0.5) | 2 (1.1) | 4 (0.8) |
| Country of hospitalization | |||||
| Belgium | 10 (0.7) | 1 (0.8) | 6 (1.0) | 1 (0.5) | 2 (0.4) |
| Germany | 15 (1.1) | 0 (0.0) | 0 (0.0) | 4 (2.1) | 11 (2.1) |
| Italy | 146 (10.2) | 12 (10.0) | 17 (2.8) | 33 (17.6) | 84 (16.3) |
| Spain | 1254 (88.0) | 107 (89.2) | 578 (96.2) | 150 (79.8) | 419 (81.2) |
| Body mass index (BMI), kg/m2b | |||||
| Median (IQR) | 25.6 (22.9, 29.5) | 25.4 (22.6, 29.2) | 25.9 (23.1, 29.8) | 25.0 (22.8, 28.0) | 25.4 (22.6, 29.9) |
| Underweight (BMI < 18.5) | 38 (3.8) | 7 (8.2) | 14 (3.1) | 6 (5.0) | 11 (3.2) |
| Normal weight (BMI ≥ 18.5 to <25.0) | 395 (39.1) | 33 (38.8) | 177 (38.6) | 50 (41.3) | 135 (39.0) |
| Overweight (BMI ≥25.0 to <30.0) | 316 (31.3) | 25 (29.4) | 144 (31.4) | 42 (34.7) | 105 (30.3) |
| Obese (BMI ≥30.0)c | 261 (25.8) | 20 (23.5) | 123 (26.9) | 23 (19.0) | 95 (27.5) |
| Missing | 415 | 35 | 143 | 67 | 170 |
| Number of chronic conditions | |||||
| 0–1d | 444 (31.2) | 26 (21.7) | 132 (22.0) | 67 (35.6) | 219 (42.4) |
| 2 | 390 (27.4) | 35 (29.2) | 178 (29.6) | 48 (25.5) | 129 (25.0) |
| ≥3 | 591 (41.5) | 59 (49.2) | 291 (48.4) | 73 (38.8) | 168 (32.6) |
| Type of chronic condition | |||||
| Asthma | 138 (9.7) | 8 (6.7) | 61 (10.1) | 12 (6.4) | 57 (11.0) |
| Lung disease | 509 (35.7) | 45 (37.5) | 243 (40.4) | 60 (31.9) | 161 (31.2) |
| Cardiovascular disease | 633 (44.4) | 64 (53.3) | 318 (52.9) | 84 (44.7) | 167 (32.4) |
| Hypertension | 910 (63.9) | 88 (73.3) | 439 (73.0) | 110 (58.5) | 273 (52.9) |
| Renal disease | 92 (6.5) | 7 (5.8) | 32 (5.3) | 15 (8.0) | 38 (7.4) |
| Liver disease | 220 (15.4) | 27 (22.5) | 107 (17.8) | 31 (16.5) | 55 (10.7) |
| Type 2 diabetes | 404 (28.4) | 45 (37.5) | 202 (33.6) | 44 (23.4) | 113 (21.9) |
| Immunodeficiency or cancere | 278 (19.5) | 24 (20.0) | 109 (18.1) | 48 (25.5) | 97 (18.8) |
| Level of SARI severityb | |||||
| Hospital admission without intensive care unit (ICU) admission and without in-hospital death | 1297 (91.5) | 104 (88.1) | 543 (90.5) | 178 (95.7) | 472 (92.0) |
| ICU admission without in-hospital death | 41 (2.9) | 1 (0.8) | 15 (2.5) | 3 (1.6) | 22 (4.3) |
| In-hospital death | 79 (5.6) | 13 (11.0) | 42 (7.0) | 5 (2.7) | 19 (3.7) |
| Missing | 8 | 2 | 1 | 2 | 3 |
| Length of hospital stay, daysb | |||||
| Median (IQR) | 6.0 (4.0, 10.0) | 6.0 (4.0, 9.0) | 6.0 (4.0, 10.0) | 6.0 (4.0, 10.0) | 6.0 (4.0, 9.0) |
| 1–3 | 275 (19.6) | 20 (17.1) | 107 (17.9) | 36 (19.7) | 112 (22.2) |
| 4–6 | 474 (33.8) | 44 (37.6) | 194 (32.4) | 61 (33.3) | 175 (34.7) |
| 7–13 | 457 (32.6) | 40 (34.2) | 213 (35.6) | 59 (32.2) | 145 (28.7) |
| 14–27 | 168 (12.0) | 11 (9.4) | 75 (12.5) | 23 (12.6) | 59 (11.7) |
| 28–41 | 21 (1.5) | 2 (1.7) | 7 (1.2) | 2 (1.1) | 10 (2.0) |
| ≥42 | 8 (0.6) | 0 (0.0) | 2 (0.3) | 2 (1.1) | 4 (0.8) |
| Missing | 22 | 3 | 3 | 5 | 11 |
| Pregnancyb,f | |||||
| Yes | 2 (0.3) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 1 (0.4) |
| No | 704 (99.7) | 52 (100.0) | 294 (99.7) | 101 (100.0) | 257 (99.6) |
| Missing | 6 | 0 | 0 | 1 | 5 |
| Reported prior SARS-CoV-2 infectionb | |||||
| Yes | 300 (25.4) | 22 (21.8) | 140 (26.7) | 34 (23.3) | 104 (25.5) |
| No | 880 (74.6) | 79 (78.2) | 385 (73.3) | 112 (76.7) | 304 (74.5) |
| Missing | 245 | 19 | 76 | 42 | 108 |
| Influenza vaccination within 12 months prior to hospitalizationb | |||||
| Yes | 822 (58.3) | 112 (93.3) | 566 (94.5) | 51 (27.6) | 93 (18.4) |
| No | 588 (41.7) | 8 (6.7) | 33 (5.5) | 134 (72.4) | 413 (81.6) |
| Missing | 15 | 0 | 2 | 3 | 10 |
| Pneumococcal vaccinationb | |||||
| Yes | 576 (41.1) | 66 (55.0) | 312 (52.3) | 63 (34.4) | 135 (26.9) |
| No | 826 (58.9) | 54 (45.0) | 285 (47.7) | 120 (65.6) | 367 (73.1) |
| Missing | 23 | 0 | 4 | 5 | 14 |
| Smoking historyb | |||||
| Never-smoker | 591 (44.4) | 52 (48.6) | 266 (46.3) | 77 (47.2) | 196 (40.2) |
| Ex-smoker | 488 (36.6) | 43 (40.2) | 233 (40.5) | 59 (36.2) | 153 (31.4) |
| Occasional smoker | 37 (2.8) | 2 (1.9) | 10 (1.7) | 4 (2.5) | 21 (4.3) |
| Daily smoker | 216 (16.2) | 10 (9.3) | 66 (11.5) | 23 (14.1) | 117 (24.0) |
| Missing | 93 | 13 | 26 | 25 | 29 |
| Long-term care facility residentb | |||||
| Yes | 57 (4.1) | 7 (6.0) | 29 (4.9) | 4 (2.2) | 17 (3.3) |
| No | 1350 (95.9) | 110 (94.0) | 568 (95.1) | 180 (97.8) | 492 (96.7) |
| Missing | 18 | 3 | 4 | 4 | 7 |
“Other” includes the following: mRNA bivalent BA.1 vaccine, a single mRNA wildtype dose only, a single mRNA wildtype dose following non-mRNA dose(s), ≥2 mRNA wildtype doses following non-mRNA doses, any number of non-mRNA doses.
Percentages exclude subjects with missing values.
Obese classification was based on both BMI ≥30.0 kg/m2 and obesity as a categorical variable (yes/no).
The categories “0” and “1” among the number of chronic conditions are counted as separate categories of number of chronic conditions, based on the list of chronic conditions provided in Supplementary Table S2. Here, the cell counts among participants having “0–1” chronic conditions are provided as a composite.
The categories “Cancer” and “Immunodeficiency” among chronic conditions are identified as separate types of chronic conditions, as presented in Supplementary Table S2. Here, the cell counts among participants having “Immunodeficiency or cancer” are provided as a composite.
Pregnancy status counts and percentages were counted in female patients.
Median age of test-positive cases was 79.5 years (interquartile range [IQR] 66.0–86.2) and half were male (50.0%, n = 154). Of 1117 test-negative controls, 50.0% were male (n = 559) and median age was 77 years (IQR 66.0–85.0). Test-positive cases had a lower prevalence of asthma than test-negative controls (6.5% vs 10.6%) and higher prevalence of cardiovascular disease (48.1% vs 43.4%), chronic liver disease (18.8% vs 14.5%), and immunodeficiency or cancer (23.4% vs 18.4%).
Overall, 50.6% (721/1425) had received BNT162b2 XBB vaccine (120/308 [38.9%] of test-positive cases and 601/1117 [53.8%] of test-negative controls) (Table 1). No patients had received multiple doses of any XBB-adapted vaccine. Among the 704 patients who had not received any COVID-19 vaccine in the 2023–2024 season, 80 (11.4%) had never been vaccinated, 338 (48.0%) had received ≥2 mRNA wildtype doses only, and 195 (27.7%) had received an mRNA bivalent BA.4/5 booster as last dose. Patients who had not received any COVID-19 vaccine in the 2023–2024 season were younger compared to their counterparts who were vaccinated with the BNT162b2 XBB vaccine [median age of 71 years (IQR 59.0–81.0) vs 81 years (74.0–88.0)]. They also had a lower percentage of ≥3 chronic conditions (34.2% vs 48.5%). Overall, they were more frequently not vaccinated for influenza (77.7% vs 5.7%) or pneumococcal vaccine (69.2% vs 47.0%).
COVID-19 vaccine effectiveness
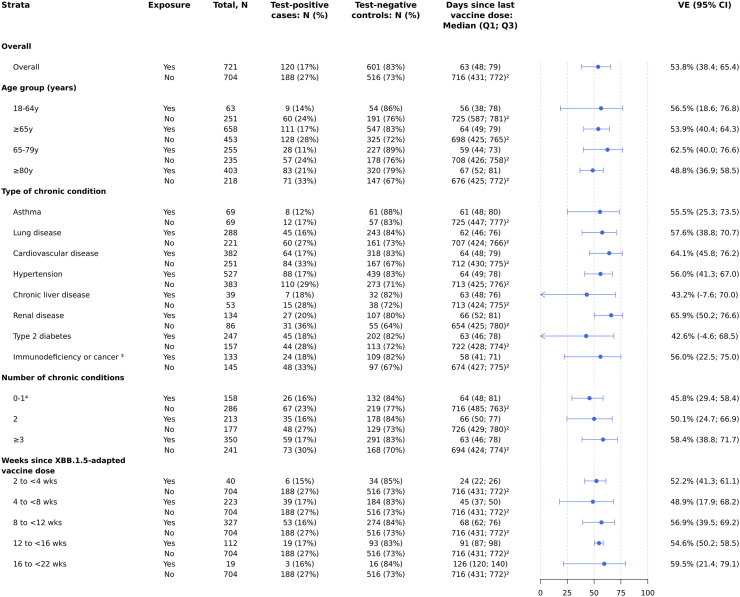
Overall adjusted VE of BNT162b2 XBB vaccine (vs not having received any COVID-19 vaccine during the 2023–2024 season) against hospitalization related to the JN.1 variant was 53.8% (95% CI 38.4–65.4) at a median (IQR) of 63 (48–79) days since vaccination (Fig. 1). In analyses stratified by age group, VE estimates were comparable across age with overlapping CIs, with the oldest age group having the lowest VE; VE was 56.5% (95% CI 18.6–76.8) among those 18–64 y, 62.5% (95% CI 40.0–76.6) among those 65–79 y, and 48.8% (95% CI 36.9–58.5) among those aged ≥80 y. VE was generally similar among patients with 0–1, 2, or ≥3 chronic conditions, and across types of chronic conditions (e.g., lung disease, cardiovascular disease, renal disease). VE estimates for chronic liver disease and type 2 diabetes should be interpreted with caution due to imprecision (wide 95% CIs). In sensitivity analyses stratified by prior COVID-19 infection, VE estimates were comparable across groups; 58.2% (95% CI 34.0–73.5) in patients with documented prior COVID-19 infection and 53.9% (95% CI 27.5–70.7) in patients with no documented prior COVID-19 infection (Supplementary Material section A.4). Adjusted VE by time since BNT162b2 XBB vaccine receipt was 52.2% (95% CI 41.3–61.1) at 2 to <4 weeks, 48.9% (95% CI 17.9–68.2) at 4 to <8 weeks, 56.9% (95% CI 39.5–69.2) at 8 to <12 weeks, 54.6% (95% CI 50.2–58.5) at 12 to <16 weeks, and 59.5% (95% CI 21.4–79.1) at 16 to <22 weeks (maximum follow-up after vaccination was 151 days; Fig. 1). Results by time since last dose were similar when limited to patients aged ≥65 y (Supplementary Fig. S3).
Fig. 1.
Vaccine effectiveness1against COVID-19 hospitalization in SARI patients who received at least one dose of BNT162b2 XBB.1.5-adapted vaccine compared to patients who did not receive any dose of a COVID-19 vaccine in the 2023–2024 autumn/winter season.1Vaccine effectiveness estimates are adjusted for date of symptom onset, age, sex, and number of chronic conditions. 2‘Never vaccinated’ subjects were excluded when calculating the median (IQR) of time since last vaccine dose in the unexposed group (patients who did not receive any dose of a COVID-19 vaccine in the 2023–2024 autumn/winter season). 3The categories “Cancer” and “Immunodeficiency” among chronic conditions are counted as separate types of chronic conditions in all adjusted VE estimates. Here, the VE estimate among participants having “Immunodeficiency or cancer” is provided as a composite. 4The categories “0” and “1” among the number of chronic conditions are counted as separate categories of number of chronic conditions in all adjusted VE estimates. Here, the VE estimate among participants having “0–1”chronic conditions is provided as a composite.
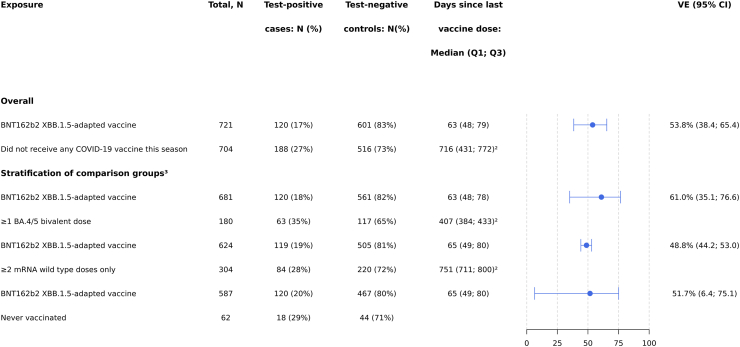
When compared to patients who had not received any COVID-19 vaccine during the 2023–2024 season but had received previous versions of COVID-19 vaccines (i.e., ≥1 BA.4/5 bivalent mRNA dose or ≥2 mRNA wildtype doses only), BNT162b2 XBB VE was similar across vaccination history (VE was 61.0% (95% CI 35.1–76.6) and 48.8% (95% CI 44.2–53.0), respectively; Fig. 2). BNT162b2 XBB VE was also similar when compared to patients who were never vaccinated; overall VE was 52.1% (95% CI 9.8–74.5) (Fig. 2) and protection was sustained through <22 weeks.
Fig. 2.
Vaccine effectiveness1against COVID-19 hospitalization in SARI patients who received at least one dose of BNT162b2 XBB.1.5-adapted vaccine using various prior vaccination categories as reference groups.1Vaccine effectiveness estimates are adjusted for date of symptom onset, age, sex, and number of chronic conditions. 2‘Never vaccinated’ subjects were excluded when calculating the median (IQR) of time since last vaccine dose among patients who did not receive any dose of a COVID-19 vaccine in the 2023–2024 autumn/winter season. 3Stratifications of comparison groups were assessed on matched data per exposure of interest and numbers will therefore not add up to the numbers provided in Table 1. Patient characteristics of these subsets are shown in Supplementary Table S3.
Results from sensitivity analyses that used the PGEE approach to determine the number and location of knots were consistent with the main analysis (Supplementary Material sections A.4.1 and A.4.2). COVID-19 VE estimates were also comparable to results from the main analysis when separately adjusting for hypertension (Supplementary Material section A.4.7) and when applying a stricter SARI case definition of symptom onset within 7 days of hospitalization (Supplementary Material section A.4.8). The overall COVID-19 VE estimate of 53.8% (95% CI: 38.4%-65.4%) was slightly higher (61.8% [95% CI: 56.1%-66.7%]) after adjusting for 2023–2024 seasonal influenza vaccination status (Supplementary Material section A.4.6).
Discussion
This large, multi-center test-negative case–control study conducted in four EU countries shows that the BNT162b2 XBB vaccine was effective against hospitalization related to the JN.1 variant in Europe during the 2023–2024 season and is also one of the first studies to report on the durability of XBB.1.5-adapted vaccine protection against JN.1 beyond 3 months post-vaccination. We observed an overall BNT162b2 XBB VE of 54% (95% CI 38–65) against JN.1 (at a median of 63 days following vaccination) that was sustained for at least 5 months.
Our results are consistent with prior studies reporting XBB.1.5-adapted VE against JN.1-related hospitalization ranging from ∼30 to 60% at 1–3 months post-vaccination7,13,14,27, 28, 29, 30, 31 and no evidence of waning in periods of up to 133–179 days (i.e., 4.5–6 months) since vaccination.13,14,30,31 Two studies have reported VE at 90–179 days (i.e., 3–6 months) after XBB.1.5 vaccine receipt. Nunes and colleagues’ study of adults aged ≥65 years who had completed at least COVID-19 primary series vaccination conducted within the EU/EEA multi-country Vaccine Effectiveness Burden and Impact Studies project Electronic Health Record network (VEBIS-EHR) reported that XBB.1.5-adapted VE against JN.1-related hospitalization was 51% (95% CI 45–56) at 14–89 days, and 47% (95% CI 32–59) at 90–179 days, among adults aged 65–79 years. Among adults aged ≥80 years, vaccine protection was lower; XBB.1.5-adapted VE was 42% (95% CI 36–47) at 14–89 days, and 36% (95% CI 11–54) at 90–179 days.30 Ma et al. utilized the US multi-site inpatient Investigating Respiratory Viruses in the Acutely Ill (IVY) network to evaluate XBB.1.5-adapted VE against XBB- and JN.1-related hospitalization among adults aged ≥18 years. The authors found that VE was lower against JN.1 than against XBB (33% and 54% in the 7–89 days after dose receipt, respectively) and also did not observe evidence of waning vaccine protection against JN.1; VE was 33% (95% CI 2–54) at 7–89 days, and 23% (95% CI −12 to 48) at 90–179 days.31
Notably, we found that the BNT162b2 XBB vaccine showed similar protection against COVID-19 hospitalization related to the JN.1 variant when compared to a variety of prior vaccination histories, including prior receipt of the mRNA bivalent BA.4/5 vaccine, ≥2 wildtype mRNA doses, and no prior vaccination. This is consistent with findings from the United States, in which the BNT162b2 XBB vaccine was similarly effective against COVID-19 hospitalization and ambulatory care visits regardless of comparison group during an XBB predominant period.8 The US study also found that there was little, if any, residual protection retained from non-XBB-adapted COVID-19 vaccine formulations received in prior seasons. Although we did not observe any evidence of waning VE against hospitalization in the first five months following vaccination, altogether the available evidence support yearly immunization with COVID-19 vaccines regardless of previous COVID-19 vaccination history for optimal protection against severe illness, given the eventual waning of vaccine protection to a level akin to never being vaccinated, and the continuous evolution of SARS-CoV-2 that may result in lower effectiveness against new circulating strains.
Our results were robust to a broad range of sensitivity analyses including the number and location of knots for symptom onset date and age, as well as when applying a stricter SARI case definition, and adjusting for hypertension, among other factors. VE was slightly higher when influenza vaccination status was included in the model given that influenza vaccination status is correlated with both COVID-19 vaccination and likelihood of enrollment as a test-negative control, which has been shown to lead to an underestimation of VE.32
One of the key strengths of this multi-center study was the inclusion of sites located across four European countries, which allowed increased coverage for the early detection of SARS-CoV-2 Omicron JN.1 predominance and enabled us to investigate VE against JN.1 up to 22 weeks post-vaccination. We ascertained vaccination status through vaccine registries, vaccination cards, and medical records, which likely limited exposure misclassification. The presence of SARS-CoV-2 was confirmed with RT-PCR or similar molecular assays on nasopharyngeal and/or oropharyngeal swabs, which have high specificity and sensitivity,33 which minimized the risk of case–control misclassification. Finally, use of the test-negative design addresses unmeasured confounding due to health care-seeking behavior,34 as well as due to exposure to infectious diseases, as both cases and controls were required to have respiratory symptoms in order to be eligible for inclusion in the study.35
Several limitations for this study should be noted. First, although VE estimates were adjusted for age, sex, chronic conditions, and date of symptom onset, there may be residual confounding by variables with insufficient or missing data, such as prior infection. Indeed, prior infection is associated with both vaccination status and clinical outcomes, since recent SARS-CoV-2 infection could delay vaccine receipt per national recommendations, and since hybrid immunity may decrease the risk of severe infection.36 Although prior infection was recorded where documented, it frequently could have been undocumented due to asymptomatic/subacute infection or the reduction in case reporting, and this information was missing from at least 17.2% of participants. Nevertheless, sensitivity analyses showed comparable VEs across patients with documented prior COVID-19 and patients with no documented prior COVID-19. However, due to insufficient data for long term care residency (57/1425) and co-infection (8/1425), we could not measure the effect of these factors on the VE estimates. Similar, the effect of potential other unmeasured confounders (such as ethnicity and socio-economic status) could not be assessed. Second, SARS-CoV-2 genetic variant information was unavailable for 85.4% of test-positive cases. We addressed this limitation by using official reports on national JN.1 prevalence to define country-specific JN.1 predominance periods. Even so, some variant misclassification is likely and could influence VE estimates. Third, our findings could differ from future VE reports evaluating JN.1-related outcomes due to expected variant drift, depending on the prevalence of JN.1 descendants (e.g., KP.2, KP.3, LB.1). Fourth, limited sample size for some stratified analyses (e.g., age, chronic conditions, waning) led to a lack of precision in VE estimates. While the point estimates provide valuable insights, they should be interpreted with caution. Fifth, it cannot be excluded that some incidental COVID-19 cases, defined as patients admitted to the hospital for reasons other than COVID-19, may have been included in the study population. Although all patients met the SARI case definition, the primary reason for admission could have nonetheless been unrelated to SARS-CoV-2 infection, potentially leading to an underestimation of VE.37 Finally, the uptake of BNT162b2 XBB vaccine in Europe was highest among older individuals with comorbidities, which may affect the generalizability of our findings.
In conclusion, we found that the BNT162b2 XBB vaccine provided protection against JN.1-related hospitalization in Europe during the 2023–2024 season with no evidence of waning through five months. The level of protection, however, was lower compared to previously reported estimates against XBB.4, 5, 6, 7, 8,13,14 These data support yearly vaccination against COVID-19 to prevent severe illness during the respiratory virus season. Future studies with longer follow-up are needed to assess the long-term durability of XBB.1.5-adapted vaccines, as well as of JN.1- and KP.2-adapted vaccines, against JN.1 sublineages and future variants.
Contributors
This study was designed by members of the id.DRIVE consortium in collaboration with Pfizer. AA, IC, BG, GI, GLtK, C Martin, AM-I, AOS, SO-R, and GR are principal investigators at the study sites and were responsible for data collection and site coordination. Study site principal investigators gathered the data in collaboration with P95 and Pfizer. LD is involved in the coordination of the id.DRIVE study. All statistical analysis was performed by TMPT and EC-S. The data were interpreted by JLN, MMi, HRV, LdM, C Marques, MMu, SV, JY, LJ, JMM and KB. LdM and LD accessed and verified the underlying data in the manuscript. JLN and MMi wrote the first draft of the manuscript. All authors reviewed, provided feedback on the manuscript drafts and approved the manuscript for submission.
Data sharing statement
De-identified data that underlie the results reported in this article (text, tables, figures, and appendices) may be obtained in accordance to id.DRIVE data access policy.
Declaration of interests
All authors have completed the ICMJE disclosure form and declare: Pfizer Inc. funded this study. C Marques, HRV, JLN, JMML, JY, MMu, LJ, and SV are employees of Pfizer Inc. HRV, JLN, JMML, JY, MMu, LJ, and SV hold stocks or stock options in Pfizer Inc. EC-S, KB, LD, LdM, MMi, and TMPT are employees of P95 Epidemiology & Pharmacovigilance, a company providing scientific services in the field of vaccines. KB and TMPT declare stock or stock options in P95. KB has received consulting fees from Pfizer Inc. for conducting this study, royalties for the book ‘Vaccination Programmes: epidemiology, monitoring, evaluation’ by Hahné, Bollaerts, Farrington, and consulting fees from AstraZeneca, Bavarian Nordic, CureVac, Janssen, GSK, Pfizer, Novavax, Valneva, and the World Health Organization. AOS reports that Pfizer partially funded the hospital network for the collection of data via id.DRIVE. IC reports support from Pfizer for congress attendance. AM-I reports being a co-principal investigator from VAHNSI (Fisabio). Fisabio received funding from P95 via id.DRIVE to conduct the study. BG and GI declare payment from the id.DRIVE Consortium to their institutions for conducting the study. BG further declares that data collaboration between P95 and Pfizer is reported. GI has received grants or contracts, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, support for attending meetings and/or travel, and declares participation on a Data Safety Monitoring Board (DSMB) or Advisory Board from GSK, MSD, Sanofi, Pfizer, Seqirus, Moderna, AstraZeneca, Viatris, and Novavax. GR declares payments to his institution by the CAPNETZ foundation, of whose executive board he is the chairman. Furthermore, GR reports personal fees from Astra Zeneca, Atriva, Boehringer Ingelheim, GSK, Insmed, MSD, Sanofi, Novartis, and Pfizer for consultancy during advisory board meetings and personal fees from AstraZeneca, Berlin Chemie, BMS, Boehringer Ingelheim, Chiesi, Essex Pharma, Grifols, GSK, Insmed, MSD, Roche, Sanofi, Solvay, Takeda, Novartis, Pfizer, and Vertex for lectures. SO-R declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from GSK and Sanofi. AÁ, C Martin, EC-S, GLtK, LdM, LD and MMI declare no conflicts of interest.
Acknowledgements
The authors thank all current and former partners of COVIDRIVE and id.DRIVE (AstraZeneca, Bavarian Nordic, CureVac, FISABIO, GSK, Janssen, Novavax, Moderna, Pfizer, Sanofi, THL, Valneva, and P95) for their contribution to the Partnership.
The authors thank Prof. Elizabeth Miller, member of the id.DRIVE Independent Scientific Committee, for her valuable scientific advice to the id.DRIVE studies.
The authors thank Pfizer, Novavax, and Valneva for their funding contribution to the data collection of the study reported in this publication.
The authors thank Hospital Universitario Vall d’Hebrón, Hospital Universitari Germans Trias i Pujol, University Hospital Ulm, Centro Interuniversitario per la Ricerca sull’Influenza e le altre Infezioni Trasmissibili, Universitair Ziekenhuis Antwerpen, Le Centre Hospitalier Universitaire St Pierre, Valencia Hospital Network for the Study of Infectious Diseases (VAHNSI)-Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana (Fisabio – Public Health), and University Hospital Frankfurt for collecting data of the study reported in this publication.
The authors wish to acknowledge the contribution of Griet Rebry (study operations), Juan Hernandez (statistician), Juliette Moyersoen (medical writer) (all of P95), Brenda Marquina Sánchez (coordinator) of FISABIO, and all other members of the id.DRIVE Co-Coordination team.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102995.
Appendix ASupplementary data
References
- 1.European Medicines Agency . 2023. Comirnaty: EMA recommends approval of adapted COVID-19 vaccine targeting Omicron XBB.1.5.https://www.ema.europa.eu/en/news/comirnaty-ema-recommends-approval-adapted-covid-19-vaccine-targeting-omicron-xbb15 [Google Scholar]
- 2.European Centre for Disease Prevention and Control . 2024. COVID-19 vaccine tracker.https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab [Google Scholar]
- 3.European Centre for Disease Prevention and Control . 2024. Interim COVID-19 vaccination coverage in the EU/EEA during the 2023–24 season campaigns.https://www.ecdc.europa.eu/en/publications-data/interim-covid-19-vaccination-coverage-eueea-during-2023-24-season-campaigns [Google Scholar]
- 4.Hansen C.H., Moustsen-Helms I.R., Rasmussen M., Soborg B., Ullum H., Valentiner-Branth P. Short-term effectiveness of the XBB.1.5 updated COVID-19 vaccine against hospitalisation in Denmark: a national cohort study. Lancet Infect Dis. 2024;24(2):e73–e74. doi: 10.1016/S1473-3099(23)00746-6. [DOI] [PubMed] [Google Scholar]
- 5.van Werkhoven C.H., Valk A.W., Smagge B., et al. Early COVID-19 vaccine effectiveness of XBB.1.5 vaccine against hospitalisation and admission to intensive care, The Netherlands, 9 October to 5 December 2023. Euro Surveill. 2024;29(1) doi: 10.2807/1560-7917.ES.2024.29.1.2300703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monge S., Humphreys J., Nicolay N., et al. Effectiveness of XBB.1.5 monovalent COVID-19 vaccines during a period of XBB.1.5 dominance in EU/EEA countries, October to November 2023: a VEBIS-EHR network study. Influenza Other Respir Viruses. 2024;18(4) doi: 10.1111/irv.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson N.W., Thiesson E.M., Pihlström N., et al. Comparative effectiveness of the monovalent XBB.1.5-containing covid-19 mRNA vaccine across three Nordic countries (Preprint) medRxiv. 2024 2024.05.08.24307058. [Google Scholar]
- 8.Tartof S.Y., Slezak J.M., Frankland T.B., et al. Estimated effectiveness of the BNT162b2 XBB vaccine against COVID-19. JAMA Intern Med. 2024;184(8):932–940. doi: 10.1001/jamainternmed.2024.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Planas D., Staropoli I., Michel V., et al. Distinct evolution of SARS-CoV-2 Omicron XBB and BA.2.86/JN.1 lineages combining increased fitness and antibody evasion. Nat Commun. 2024;15(1):2254. doi: 10.1038/s41467-024-46490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . 2024. Executive summary JN.1.https://cdn.who.int/media/docs/default-source/documents/health-topics/sars/jn.1-9-february-2024.pdf?sfvrsn=9a39d825_3 [Google Scholar]
- 11.Modjarrad K., Che Y., Chen W., et al. Preclinical characterization of the omicron XBB.1.5-Adapted BNT162b2 COVID-19 vaccine (preprint) bioRxiv. 2023 doi: 10.1101/2023.11.17.567633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q., Guo Y., Bowen A., et al. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against XBB subvariants and JN.1. Cell Host Microbe. 2024;32(3):315–321. doi: 10.1016/j.chom.2024.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caffrey A.R., Appaneal H.J., Lopes V.V., et al. Effectiveness of BNT162b2 XBB vaccine in the US veterans affairs healthcare system (preprint) medRxiv. 2024 doi: 10.1101/2024.04.05.24305063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tartof S.Y., Slezak J.M., Puzniak L., et al. Effectiveness of BNT162b2 XBB vaccine against XBB and JN.1 sublineages. Open Forum Infect Dis. 2024;11(7) doi: 10.1093/ofid/ofae370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . 2024. Statement on the antigen composition of COVID-19 vaccines.https://www.who.int/news/item/26-04-2024-statement-on-the-antigen-composition-of-covid-19-vaccines [Google Scholar]
- 16.European Medicines Agency EMA recommendation to update the antigenic composition of authorised COVID-19 vaccines for 2024-2025. https://www.ema.europa.eu/en/documents/other/ema-recommendation-update-antigenic-composition-authorised-covid-19-vaccines-2024-2025_en.pdf
- 17.European Centre for Disease Prevention and Control . 2021. ECDC Technical report: core protocol for ECDC studies of COVID-19 vaccine effectiveness against hospitalisation with Severe Acute Respiratory Infection laboratory-confirmed with SARS-CoV-2, version 1.0.https://www.ecdc.europa.eu/sites/default/files/documents/Core-protocol-for-ECDC-studies-of-COVID-19-vaccine-effectiveness-against-hospitalisation-with-SARI.pdf [Google Scholar]
- 18.Instituto de Salud Carlos III . 2024. Vigilancia centinela de Infección Respiratoria Aguda en Atención Primaria (IRAs) y en Hospitales (IRAG): Gripe, COVID-19 y VRS.https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/Temporada_Gripe_23-24.aspx [Google Scholar]
- 19.Sciensano . 2024. Belgium COVID-19 epidemiological situation.https://lookerstudio.google.com/embed/reporting/c14a5cfc-cab7-4812-848c-0369173148ab/page/urrUC [Google Scholar]
- 20.Robert Koch Institut . 2024. SARS-CoV-2 varianten in deutschland.https://public.data.rki.de/t/public/views/IGS_Dashboard/DashboardSublineages?%3Aembed=y&%3AisGuestRedirectFromVizportal=y [Google Scholar]
- 21.Istituto Superiore di Sanita . 2024. Aggiornamento nazionale relativo al periodo 08/01/2024 – 14/01/2024 dei dati della Sorveglianza Integrata COVID-19.https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_17-gennaio-2024.pdf [Google Scholar]
- 22.European Medicines Agency . 2023. EMA and ECDC statement on updating COVID-19 vaccines to target new SARS-CoV-2 virus variants.https://www.ema.europa.eu/en/news/ema-ecdc-statement-updating-covid-19-vaccines-target-new-sars-cov-2-virus-variants [Google Scholar]
- 23.Liang K.-Y., Zeger S.L. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 24.Seaman S., Pavlou M., Copas A. Review of methods for handling confounding by cluster and informative cluster size in clustered data. Stat Med. 2014;33(30):5371–5387. doi: 10.1002/sim.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahan B.C., Li F., Copas A.J., Harhay M.O. Estimands in cluster-randomized trials: choosing analyses that answer the right question. Int J Epidemiol. 2023;52(1):107–118. doi: 10.1093/ije/dyac131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S.-Y. Elsevier; 2011. Handbook of latent variable and related models. [Google Scholar]
- 27.UK Health Security Agency . 2024. COVID-19 vaccine surveillance report: week 29.https://assets.publishing.service.gov.uk/media/669923b20808eaf43b50d1fd/Vaccine_surveillance_report_2024_week_29.pdf [Google Scholar]
- 28.Lin D.Y., Du Y., Xu Y., Paritala S., Donahue M., Maloney P. Durability of XBB.1.5 vaccines against omicron subvariants. N Engl J Med. 2024;390(22):2124–2127. doi: 10.1056/NEJMc2402779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chong C., Wee L.E., Jin X., et al. Risks of severe acute respiratory syndrome coronavirus 2 JN.1 infection and coronavirus disease 2019–associated emergency department visits/hospitalizations following updated boosters and prior infection: a population-based cohort study. Clin Infect Dis. 2024;79(5):1190–1196. doi: 10.1093/cid/ciae339. [DOI] [PubMed] [Google Scholar]
- 30.Nunes B., Humphreys J., Nicolay N., et al. Monovalent XBB.1.5 COVID-19 vaccine effectiveness against hospitalisations and deaths during the Omicron BA.2.86/JN.1 period among older adults in seven European countries: a VEBIS-EHR Network Study (Preprint) medRxiv. 2024 doi: 10.1101/2024.07.04.24309832. [DOI] [PubMed] [Google Scholar]
- 31.Ma K.C., Surie D., Lauring A.S., et al. Effectiveness of updated 2023–2024 (monovalent XBB.1.5) COVID-19 vaccination against SARS-CoV-2 omicron XBB and BA.2.86/JN.1 lineage hospitalization and a comparison of clinical severity—IVY network, 26 hospitals, October 18, 2023–March 9, 2024. Clin Infect Dis. 2024:1–9. doi: 10.1093/cid/ciae405. [DOI] [PubMed] [Google Scholar]
- 32.Doll M.K., Pettigrew S.M., Ma J., Verma A. Effects of confounding bias in coronavirus disease 2019 (COVID-19) and influenza vaccine effectiveness test-negative designs due to correlated influenza and COVID-19 vaccination behaviors. Clin Infect Dis. 2022;75(1):e564–e571. doi: 10.1093/cid/ciac234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filchakova O., Dossym D., Ilyas A., Kuanysheva T., Abdizhamil A., Bukasov R. Review of COVID-19 testing and diagnostic methods. Talanta. 2022;244 doi: 10.1016/j.talanta.2022.123409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dean N.E., Hogan J.W., Schnitzer M.E. Covid-19 vaccine effectiveness and the test-negative design. N Engl J Med. 2021;385(15):1431–1433. doi: 10.1056/NEJMe2113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham S., Tessier E., Stowe J., et al. Bias assessment of a test-negative design study of COVID-19 vaccine effectiveness used in national policymaking. Nat Commun. 2023;14(1):3984. doi: 10.1038/s41467-023-39674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bobrovitz N., Ware H., Ma X., et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23(5):556–567. doi: 10.1016/S1473-3099(22)00801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stowe J., Andrews N., Kirsebom F., Ramsay M., Bernal J.L. Effectiveness of COVID-19 vaccines against Omicron and Delta hospitalisation, a test negative case-control study. Nat Commun. 2022;13(1):5736. doi: 10.1038/s41467-022-33378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.