Abstract
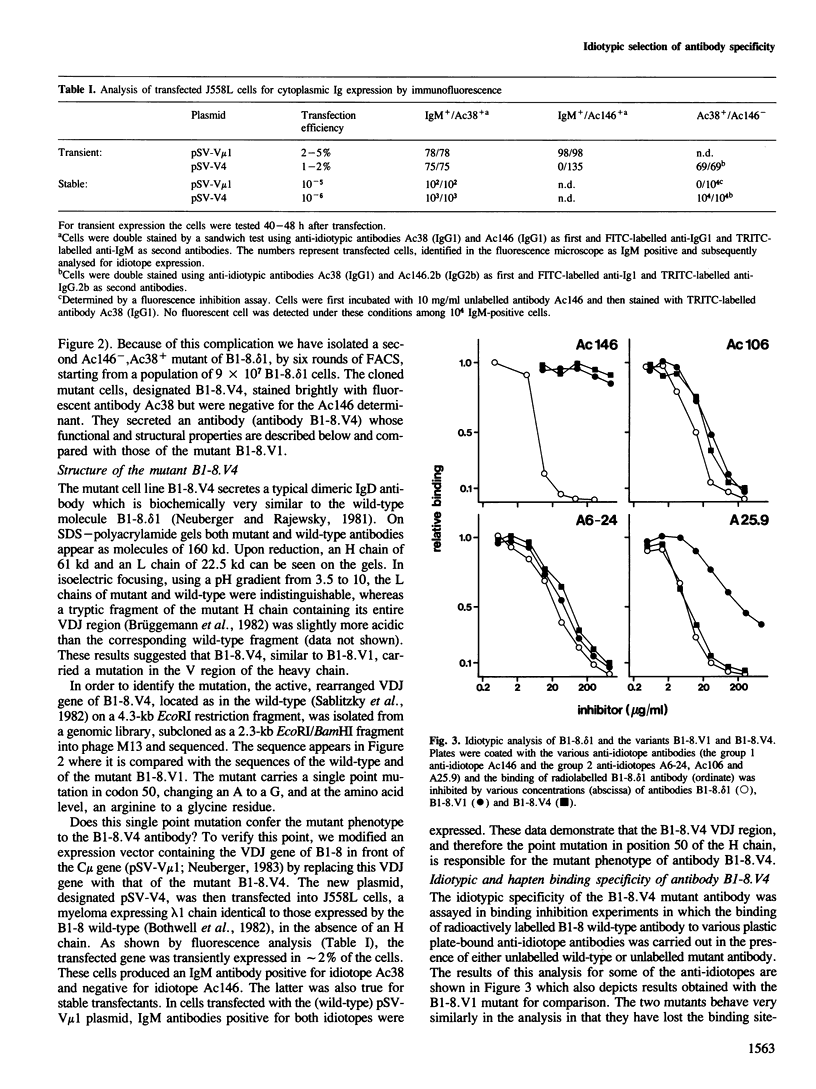
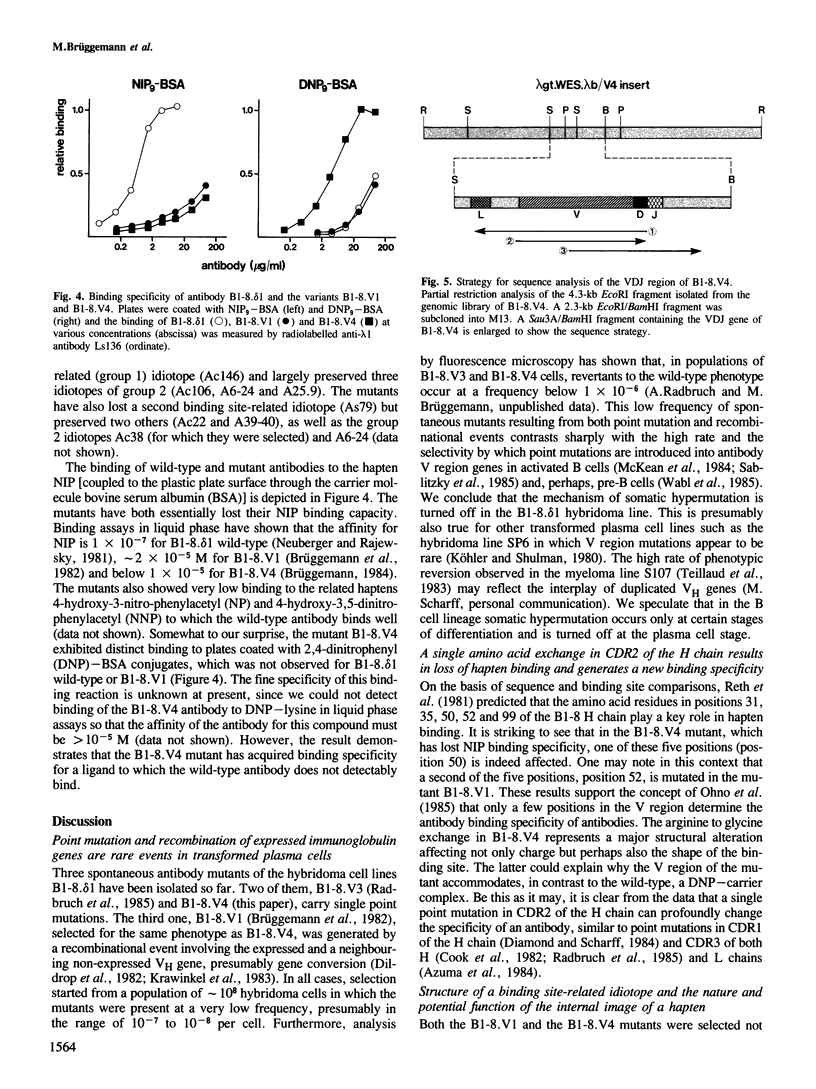
Somatic mutation occurs at a low rate in the rearranged antibody V region genes of the hybridoma line B1-8. delta 1 which expresses an antibody with specificity for the hapten 4-hydroxy-3-nitro-5-iodo-phenylacetyl (NIP). A mutant was selected which had lost a binding site-related idiotope but retained most of its other idiotypic determinants. The mutant had concomitantly lost NIP binding and acquired specificity for dinitrophenylated bovine serum albumin. It carried a single point mutation in position 50 of the heavy chain, resulting in the replacement of an arginine by a glycine.
Full text
PDF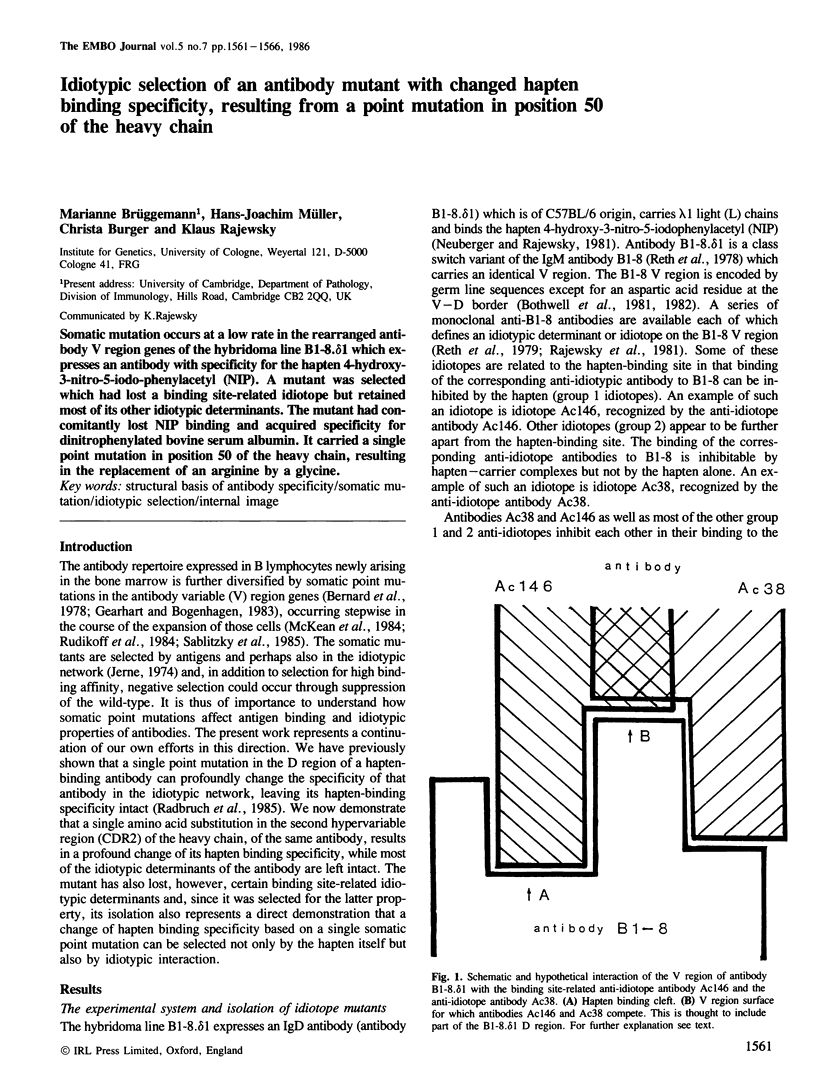



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma T., Igras V., Reilly E. B., Eisen H. N. Diversity at the variable-joining region boundary of lambda light chains has a pronounced effect on immunoglobulin ligand-binding activity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6139–6143. doi: 10.1073/pnas.81.19.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bernard O., Hozumi N., Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978 Dec;15(4):1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersch-Supan M. E., Agarwal S., White-Scharf M. E., Imanishi-Kari T. Heavy chain variable region. Multiple gene segments encode anti-4-(hydroxy-3-nitro-phenyl)acetyl idiotypic antibodies. J Exp Med. 1985 Jun 1;161(6):1272–1292. doi: 10.1084/jem.161.6.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Somatic variants of murine immunoglobulin lambda light chains. Nature. 1982 Jul 22;298(5872):380–382. doi: 10.1038/298380a0. [DOI] [PubMed] [Google Scholar]
- Brüggemann M., Radbruch A., Rajewsky K. Immunoglobulin V region variants in hybridoma cells. I. Isolation of a variant with altered idiotypic and antigen binding specificity. EMBO J. 1982;1(5):629–634. doi: 10.1002/j.1460-2075.1982.tb01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Rudikoff S., Giusti A. M., Scharff M. D. Somatic mutation in a cultured mouse myeloma cell affects antigen binding. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1240–1244. doi: 10.1073/pnas.79.4.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A., Rajewsky K. Structure of primary anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in normal and idiotypically suppressed C57BL/6 mice. Eur J Immunol. 1985 May;15(5):512–520. doi: 10.1002/eji.1830150517. [DOI] [PubMed] [Google Scholar]
- Diamond B., Scharff M. D. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dildrop R., Brüggemann M., Radbruch A., Rajewsky K., Beyreuther K. Immunoglobulin V region variants in hybridoma cells. II. Recombination between V genes. EMBO J. 1982;1(5):635–640. doi: 10.1002/j.1460-2075.1982.tb01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Bogenhagen D. F. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3439–3443. doi: 10.1073/pnas.80.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkamp B., Cramer M., Lemke H., Rajewsky K. Isolation of a cloned cell line expressing variant H-2Kk using fluorescence-activated cell sorting. Nature. 1981 Jan 1;289(5793):66–68. doi: 10.1038/289066a0. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kearney J. F., Lawton A. R. B lymphocyte differentiation induced by lipopolysaccharide. I. Generation of cells synthesizing four major immunoglobulin classes. J Immunol. 1975 Sep;115(3):671–676. [PubMed] [Google Scholar]
- Krawinkel U., Zoebelein G., Brüggemann M., Radbruch A., Rajewsky K. Recombination between antibody heavy chain variable-region genes: evidence for gene conversion. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4997–5001. doi: 10.1073/pnas.80.16.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C. E., Rajewsky K. Isolation of immunoglobulin class switch variants from hybridoma lines secreting anti-idiotope antibodies by sequential sublining. J Immunol. 1983 Aug;131(2):877–881. [PubMed] [Google Scholar]
- Neuberger M. S. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO J. 1983;2(8):1373–1378. doi: 10.1002/j.1460-2075.1983.tb01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Rajewsky K. Switch from hapten-specific immunoglobulin M to immunoglobulin D secretion in a hybrid mouse cell line. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1138–1142. doi: 10.1073/pnas.78.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Mori N., Matsunaga T. Antigen-binding specificities of antibodies are primarily determined by seven residues of VH. Proc Natl Acad Sci U S A. 1985 May;82(9):2945–2949. doi: 10.1073/pnas.82.9.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbruch A., Zaiss S., Kappen C., Brüggemann M., Beyreuther K., Rajewsky K. Drastic change in idiotypic but not antigen-binding specificity of an antibody by a single amino-acid substitution. Nature. 1985 Jun 6;315(6019):506–508. doi: 10.1038/315506a0. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Takemori T. Genetics, expression, and function of idiotypes. Annu Rev Immunol. 1983;1:569–607. doi: 10.1146/annurev.iy.01.040183.003033. [DOI] [PubMed] [Google Scholar]
- Reth M., Hämmerling G. J., Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur J Immunol. 1978 Jun;8(6):393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- Reth M., Imanishi-Kari T., Rajewsky K. Analysis of the repertoire of anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in C 57 BL/6 mice by cell fusion. II. Characterization of idiotopes by monoclonal anti-idiotope antibodies. Eur J Immunol. 1979 Dec;9(12):1004–1013. doi: 10.1002/eji.1830091216. [DOI] [PubMed] [Google Scholar]
- Reth M., Kelsoe G., Rajewsky K. Idiotypic regulation by isologous monoclonal anti-idiotope antibodies. Nature. 1981 Mar 19;290(5803):257–259. doi: 10.1038/290257a0. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Pawlita M., Pumphrey J., Heller M. Somatic diversification of immunoglobulins. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2162–2166. doi: 10.1073/pnas.81.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablitzky F., Radbruch A., Rajewsky K. Spontaneous immunoglobulin class switching in myeloma and hybridoma cell lines differs from physiological class switching. Immunol Rev. 1982;67:59–72. doi: 10.1111/j.1600-065x.1982.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Sablitzky F., Rajewsky K. Molecular basis of an isogeneic anti-idiotypic response. EMBO J. 1984 Dec 1;3(12):3005–3012. doi: 10.1002/j.1460-2075.1984.tb02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablitzky F., Wildner G., Rajewsky K. Somatic mutation and clonal expansion of B cells in an antigen-driven immune response. EMBO J. 1985 Feb;4(2):345–350. doi: 10.1002/j.1460-2075.1985.tb03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori T., Tesch H., Reth M., Rajewsky K. The immune response against anti-idiotope antibodies. I. Induction of idiotope-bearing antibodies and analysis of the idiotope repertoire. Eur J Immunol. 1982 Dec;12(12):1040–1046. doi: 10.1002/eji.1830121210. [DOI] [PubMed] [Google Scholar]
- Teillaud J. L., Desaymard C., Giusti A. M., Haseltine B., Pollock R. R., Yelton D. E., Zack D. J., Scharff M. D. Monoclonal antibodies reveal the structural basis of antibody diversity. Science. 1983 Nov 18;222(4625):721–726. doi: 10.1126/science.6356353. [DOI] [PubMed] [Google Scholar]
- Tesch H., Takemori T., Rajewsky K. The immune response against anti-idiotope antibodies II. The induction of antibodies bearing the target idiotope (Ab3 beta) depends on the frequency of the corresponding B cells. Eur J Immunol. 1983 Sep;13(9):726–732. doi: 10.1002/eji.1830130907. [DOI] [PubMed] [Google Scholar]
- Wabl M., Burrows P. D., von Gabain A., Steinberg C. Hypermutation at the immunoglobulin heavy chain locus in a pre-B-cell line. Proc Natl Acad Sci U S A. 1985 Jan;82(2):479–482. doi: 10.1073/pnas.82.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]



