Abstract
Aim:
This review aims to identify the mechanistic relationships related to periodontal diseases and its possible association with changes in human milk composition and the composition and function of infants’ gut microbiome.
Background:
Maternal health conditions, especially inflammatory, are associated with altered human milk composition. It is not known whether maternal oral inflammatory diseases, including periodontal diseases, deleteriously affect human milk composition.
Methods:
A narrative review was conducted according to SANRA, the Scale for the Assessment of Narrative Review Articles, guidelines. PubMed, Google Scholar, and Cochrane database of systematic reviews were searched from September 2019 up to December 2023 using keywords such as breast/human milk, maternal health/infections, and periodontal diseases. Reference lists of relevant articles were also screened. Our primary outcome of interest was human milk composition (i.e., any changes in macronutrients, immunological components, etc.). Secondary outcomes included changes in human milk microbiome and subsequent changes in the infant gut microbiome. Outcomes were synthesized using a narrative approach where the existing evidence and current literature were summarized. No risk of bias assessment of the studies was performed in this review.
Findings:
The search yielded no studies investigating the relationship between periodontal diseases in nursing mothers and changes in human milk composition. However, a dose–response relationship exists between the severity of periodontal diseases and the risk of adverse pregnancy outcomes such as preterm birth. Mastitis and diabetes affected milk lipids. Immunoglobulin A (sIgA) was increased in mastitis, whereas reduced concentrations were reported in diabetes. Potential biological pathways through which periodontal diseases can negatively affect human milk composition include the systemic dissemination of inflammatory cytokines like IL-6, PGE2, and tumor necrosis factor (TNF)-β that can be up-regulated by bacterial by-products. This biological plausibility needs to be investigated, given the potentially negative impact on the quality of human milk that could be caused by periodontal inflammation.
Keywords: Breast/human milk, breastfeeding, maternal health/infections, periodontal diseases, periodontitis
Introduction
Human milk is critical for optimal newborn growth and development (Andreas et al., 2015). Human milk contains bioactive substances including polyunsaturated fatty acids (PUFAs), immune cells, hormones, growth, and immunological factors like cytokines and immunoglobulins (Andreas et al., 2015). Infants nursed with human milk during their first months of life are less vulnerable to infections, especially those originating in the gastrointestinal and respiratory tracts (Pullan et al., 1980) than those who received formula. However, the mechanisms involved in the transmission of immunity through human milk are still not fully understood. In addition, human milk contains its own microbiome and human milk oligosaccharides that likely shape the infants’ gut microbiome, affecting infant health outcomes both early and later in life (Fitzstevens et al., 2017). There is emerging evidence that supports the role of the human milk microbiome in regulating microbial colonization of the infant gut with health-promoting microbes (Fernández et al., 2013), decreasing the likelihood of allergies (Fernández et al., 2013), irritable bowel syndrome (Fernández et al., 2013), elevated fat mass (Hunt et al., 2011, Sanz, 2011), and other diseases (Sanz, 2011) in the child. However, the specific human milk components that promote and maintain optimal growth and health, and the mechanisms through which these are transmitted to the infant, are incompletely understood.
While nursing, some mothers (referred to as birthing parents thereafter for an inclusive language) may develop infections that could contribute to the overall systemic inflammation throughout the body (Offenbacher et al., 1996, Schieve et al., 1994). Inflammation and infection in the oral cavity and urinary tract in women (referred to as individuals/persons thereafter for an inclusive language) during pregnancy have been associated with adverse pregnancy outcomes such as preeclampsia, preterm birth, and low birth weight (Kass, 1962, Schieve et al., 1994, Baskaradoss et al., 2012, Ide and Papapanou, 2013), suggesting that infections during pregnancy have effects beyond the local niche. This consideration derived studies to further investigate the impact of maternal infections in the postpartum period on the composition of human milk.
Maternal health conditions such as diabetes and mastitis have been shown to be associated with altered human milk composition (Hunt et al., 2013, Groer et al., 2004, Morceli et al., 2011, Smilowitz et al., 2013, Peila et al., 2020), although as regard to the latter this is easier to explain. These alterations in human milk can potentially affect the transmission of immune factors to the nursing infant and have serious implications for the infant’s health (Zárate et al., 2017, Gurven et al., 2016). For example, infants exposed to elevated levels of PUFAs in the first months of their life are at risk of childhood obesity (Zárate et al., 2017). Furthermore, elevated counts of cytokines have been associated in the literature with cardiovascular diseases, metabolic syndromes, and type 2 diabetes (Gurven et al., 2016).
During pregnancy, oral inflammatory conditions such as gingivitis and periodontitis affect 30–100% and 20–50% of pregnant persons, respectively, and both conditions affect 15% of individuals of childbearing aged (Patil et al., 2012, Laine, 2002). Despite that the relationships between periodontal diseases and adverse pregnancy outcomes are well documented (Daalderop et al., 2018, Iheozor-Ejiofor et al., 2017), it is not known whether periodontal diseases affect human milk composition negatively. The extent to which human milk composition is influenced by periodontal diseases needs to be investigated, a key gap in understanding the link between suboptimal oral health and infant outcomes.
Here, we aimed to identify the main immunological components in human milk that are essential for the infants’ growth and development and the impact of maternal systemic diseases on human milk composition. We also aimed to synthesize the evidence on the mechanistic relationships and mechanisms between periodontal diseases and human milk composition. We also aimed to identify and discuss the mechanisms through which periodontal diseases in nursing individuals may influence both the composition and quality of human milk and consequently, the infant health. The fact that periodontal inflammation could negatively impact the composition of human milk (i.e., harming nutritional and immunological contents (Groer et al., 2004, Hunt et al., 2013)) is biologically plausible. Recognizing this gap of knowledge regarding the association between periodontal diseases in birthing parents and their human milk composition, we conducted the following comprehensive review.
Methods
This review of the literature was conducted from September 2019 to December 2023 by searching PubMed, Google Scholar, Cochrane database of systematic reviews, and screening reference lists of relevant articles to investigate the potential relationship between periodontal diseases in birthing parents and human milk composition. This review was prepared considering SANRA, the Scale for the Assessment of Narrative Review Articles, checklist as a guideline (Baethge et al., 2019). In particular, this review aims to answer the following questions:
What are the main immunological components in human milk and how are these elements essential for the infants’ growth and development?
What is the impact of maternal systemic diseases on human milk composition?
What are the mechanistic relationships and mechanisms described in the literature related to periodontal diseases and its possible association with changes in human milk composition?
How maternal health plays a major role in the development of the infants’ intestinal microbiome and the potential impact of periodontal disease in birthing parents on the composition and function of their infants’ gut microbiomes.
What are the implications for future research, practice, and policy development related to the dental and medical field.
The keywords used in the search strategy included breast milk, human milk, breastfeeding, maternal health/infections, periodontal diseases, gingivitis, periodontitis, oral inflammation, and maternal oral health. No study design restrictions were applied; however, the search was limited to studies published only in English language.
Our search strategy was guided by the components of the PECO model (Population, Exposure, Comparison, and Outcome) outlined below:
Population: Pregnant/breastfeeding individuals aged 18 years or older
Exposure: Maternal infections/diseases/periodontal diseases (gingivitis and periodontitis)
Comparison: Healthy individuals/individuals without infections/periodontal diseases
Outcome: 1. Primary outcome included any changes in human milk composition (nutritional or immunological components, etc.) 2. Secondary outcome included changes in human milk microbiome and subsequent changes in infant gut microbiome.
Outcomes were synthesized using a narrative approach where the existing evidence and current literature were summarized. No risk of bias assessment of the studies was performed in this review.
Results
Human breast milk’s main immunological components and infant outcomes
Human milk contains many immunological and nutritional components that are paramount for the infants’ growth and development and immune responses of newborns during the challenging period of immunological adaptation to extra-uterine life. Human milk contains long-chain PUFAs, immunoglobulins, cytokines, immune cells, and other factors that help reduce a newborn’s susceptibility to infections and also facilitate its growth and development (Garofalo, 2010, Gilmore et al., 1994, Andreas et al., 2015) (Table 1). The difference between breast-fed and formula-fed infants in terms of infants’ susceptibility to infections has been discussed in a variety of studies (Feachem and Koblinsky, 1984). A meta-analysis showed that the incidence of diarrhea in breastfed infants is four- to five-fold lower during the first six months of life compared to infants fed with formula (Feachem and Koblinsky, 1984, Morrow and Rangel, 2004). Respiratory infections and death due to infections were also less common in breastfed infants when compared to formula-fed infants (Feachem and Koblinsky, 1984). Accordingly, breastfeeding is conceivably a powerful preventive measure against morbidity and mortality caused by infectious diseases in young children (Morrow and Rangel, 2004, Clavano, 1982).
Table 1.
Summary of breast milk main nutritional and immunological components
| Breast milk components |
Types and concentrations | Suggested role in infants’ growth |
|---|---|---|
| Immune cells (Andreas et al., 2015) | Macrophages (55–60%), neutrophils (30–40%), and lymphocytes T and B (5–10%) are found in human milk | • Help the infant develop a functional immune system through childhood. • The infant’s T-cell activity is stimulated by the help of macrophages which provide adequate protection against pathogens. |
| Cytokines (Murphy et al., 2018, Garofalo, 2010) | Interleukin-1 beta (IL-1b), IL-6, tumor necrosis factor-alpha (TNF-a), interferon-g (IFN-g), IL-10, transforming growth factor-beta (TGF-b) | • IL-1b pro-inflammatory cytokine is involved in the host defense response to injury and recruitment of neutrophils to the mammary gland. • IL-6 pro-inflammatory cytokine has an essential role in the development of the infant’s mucosal immunity. • Tumor necrosis factor-alpha (TNF-a), an important regulator of immune cells, is involved in the chemotactic activity of neutrophils and stimulates human milk’s IL-6 production. • TGF-b has been associated along with IL-6 and IL-10 in the development and differentiation of immunoglobulin A (IgA) producing cells in the mammary gland. It has also been linked with the maturation of the intestinal immune system as well as preventing allergic reactions. • Interferon-g (IFN-g), a cytokine with immuno-stimulatory and immuno-modulatory effects, is also capable of inhibiting virus replication. |
| Colony-stimulating factors (Gilmore et al., 1994) | Granulocyte-colony-stimulating factor (G-CSF), macrophage-colony-stimulating factor (M-CSF), and granulocyte-macrophage-colony-stimulating factor (GM-CSF) | Play a significant role in fetal intestinal development as well as having a beneficial effect on the proliferation, differentiation, and survival of neutrophils. |
| Immunoglobulins (Andreas et al., 2015) | Human breast milk is rich in immunoglobulins (IgA, IgM, and IgG), the most prominent of which is the secretory immunoglobulin A (sIgA) | Protect the infant at the mucosal level by interrupting virus replication, activating the complement system, and promoting phagocytosis. |
| Lactoferrin (Andreas et al., 2015) | A protein with antioxidant, bacteriostatic, and bactericidal properties | • Regulating immune and inflammatory responses. • Efficient in killing many different pathogens without inducing inflammation. |
| Oligosaccharides (Andreas et al., 2015) | The third largest component in breast milk, accounting for 12.9 g/L on average in mature milk and 20.9 g/L at four days postpartum | They act as prebiotics stimulating the proliferation of beneficial bacteria such as Bifidobacterium infantis in the intestines of infants essential for the enhancement of the intestine defense mechanisms against various pathogens. |
| Lipids (Andreas et al., 2015) | One of the major nutritional components of human milk is considered the major source of energy in human milk representing 40–55% of its overall energy. | Essential for central nervous system myelination due to the presence of Sphingomyelins which exist in the membrane of the milk fat globule |
| Polyunsaturated fatty acids (PUFAs) (Andreas et al., 2015) | Human milk is particularly rich in EFAs, GLA, DGLA, AA, EPA, and DHA. | DHA, EPA, and AA are the major LCPUFAs found in the brain and retina. Throughout childhood, neurological and immunological developments rely on the action of these fatty acids. It has been demonstrated that they also play a major role in the brain’s growth spurt occurring from the last trimester of pregnancy to age of 2. |
EFAs, essential fatty acids; GLA, gamma-linolenic acid; DGLA, dihomo-gamma-linolenic acid; AA, arachidonic Acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LCPUFAs, long-chain polyunsaturated fatty acids.
Maternal systemic diseases influence human milk composition and quality
Diabetes
Diabetes is a metabolic disease that induces immunological and biochemical changes in human milk (Morceli et al., 2011). For example, as compared to non-diabetic persons, lower levels of complement component 3 protein (acting as opsonins, which aid in in phagocytosis performed by cells of the immune system) was noted in the colostrum of diabetic persons. In addition, reduced levels of secretory immunoglobulin A (sIgA) and IgG were reported in the human milk of diabetic birthing parents compared to their non-diabetic counterparts (Smilowitz et al., 2013), whereas glucose and lipase enzymes were represented in higher amounts (Morceli et al., 2011). It has been hypothesized that hyperglycemia can affect B-lymphocytes in the serum of diabetic birthing parents, thereby reducing the antibody levels (Smilowitz et al., 2013). Another explanation is that prolactin plays a significant role in humoral immunity by binding to prolactin receptors on B-lymphocytes to stimulate the synthesis of immunoglobulins, and since the concentrations of prolactin are reduced in birthing parents with diabetes, this can impair the secretion of immunoglobulins (Smilowitz et al., 2013, Russell et al., 1985). These findings are consistent with other studies reporting significant 63.6% reduction in the concentration of sIgA in the colostrum of individuals with gestational diabetes mellitus (GDM), compared to those without GDM (Morceli et al., 2011, Smilowitz et al., 2013, Peila et al., 2020).
Given that diabetes is a condition of low-grade systemic inflammation and disruption in the immune system regulation promoting inflammatory responses, changes in the concentrations of cytokines, chemokines, and growth factors in the colostrum of postpartum individuals who had GDM have also been investigated in recent studies (Avellar et al., 2022). Higher concentration of IL-10, interferon-g (IFN-g), IL-15, and IL-6 were observed in the GDM birthing parents’ colostrum, compared to healthy birthing parents (Avellar et al., 2022). With respect to growth factors’ concentration, a statistically significant difference was observed in the level of GM-CSF growth factor between healthy and GDM birthing parents, where lower levels were found in the GDM birthing parents’ colostrum (Avellar et al., 2022). Growth factors in human milk are known to have a significant and pivotal role in the maturation of the infants’ intestinal mucosa (Murphy et al., 2018). Therefore, new studies should focus on how these changes can impact the infant’s health throughout their life. Further, a recent study (Choi et al., 2022) showed alterations in bioactive components in human milk (e.g., insulin, glucose, and C-reactive protein (CRP)) of birthing parents with GDM, where higher levels of CRP and lower levels of insulin and glucose were observed in the GDM birthing parents’ milk at both 1 and 3 months postpartum, compared to birthing parents without GDM.
Macronutrients including fats, proteins, and carbohydrates are also key components of the human milk and are shown to be altered by the metabolic state of the mother (Zhong et al., 2022, Korkut et al., 2022). For example, human milk from diabetic birthing parents has low fat content compared to non-diabetic birthing parents (Bitman et al., 1989). With respect to the fatty acid content, there was a four-fold increase of lipoprotein lipase and increase in the free fatty acids counts (Bitman et al., 1989). Another notable change in the fatty acids composition in the milk of diabetic birthing parents was a decrease in medium-chain fatty acids and an increase in the PUFAs compared to milk from birthing parents without diabetes, which could be the results of improper fatty acid synthesis in the mammary gland and an increase in the hydrocarbon chain elongation, respectively (Bitman et al., 1989). These alterations in lipid metabolism within the mammary glands might be related to changes caused by diabetes, since insulin is known to be involved in fatty acids synthesis through regulating the desaturation and elongation enzymes (Azulay Chertok et al., 2017). Infants exposure to high levels of fatty acids may contribute to development of obesity (Zárate et al., 2017), atherosclerosis, and diabetes later in life and may influence the neonatal neurodevelopment (Keim et al., 2012) in part through the prothrombotic and pro-inflammatory actions of PUFAs and their major role in controlling adipogenesis (Keim et al., 2012, Zárate et al., 2017). Another study (Korkut et al., 2022) that investigated the changes in the macronutrient content in human milk reported an increase in the carbohydrate content in the colostrum of GDM birthing parents, compared to their non-GDM counterparts, whereas no differences were observed in the fat and protein content between the study groups.
Some studies have shown interest in investigating the impact of GDM on the concentrations of metabolic hormones (e.g., adiponectin, leptin, ghrelin, insulin, apelin, etc.) in human milk. A recent systematic review of 12 studies (Suwaydi et al., 2022) showed that although evidence in this area is scarce, the synthesis suggested reduced levels of adiponectin, ghrelin, and irisin in human milk of individuals with GDM, especially during the early stages of lactation. Another recent study (Dou et al., 2023) focused on investigating the difference in human milk oligosaccharides between GDM and non-GDM birthing parents and showed that the total level of the identified 14 oligosaccharides was higher in the colostrum of GDM birthing parents compared to healthy ones. Further, with respect to changes in the level of individual oligosaccharides, lacto-N-neotetraose (LNnT) was significantly higher in GDM birthing parents in the colostrum, transitional, and mature milk.
Mastitis
Mastitis is a common inflammatory disease in lactating persons, affecting between 10 and 27% of individuals (Ingman et al., 2014) and is generally related to infection. Since bacteria plays a role in breast inflammation caused by mastitis, recent studies focused on identifying the bacterial factors of mastitis and evaluate their effects on the physical (i.e., sugar, protein, and fat) and chemical (i.e., pH, density, and freezing temperature) composition of human milk (Shuyang and Qiang, 2021). Birthing parents experiencing mastitis caused by Staphylococcus aureus bacteria exhibited lower sugar levels in their milk, while those with coagulase-negative staphylococci had reduced protein content (Shuyang and Qiang, 2021). This suggests that Staphylococcus aureus might reduce milk sugar through consumption, and coagulase-negative staphylococci may also affect milk protein (Shuyang and Qiang, 2021). In another study, milk produced from symptomatic breasts was shown to have greater lipolysis, generating elevated counts of free fatty acids (Hunt et al., 2011, Say et al., 2016). This was possibly as a result of the action of lipase enzymes produced by infiltrating leukocytes in response to inflammation. Since free fatty acids and monoglycerides of human milk have antibacterial properties, an increase in their numbers might reflect the host’s response to bacteria-induced disease (Hunt et al., 2013). In the presence of mastitis, levels of interleukin (IL)-6, lactoferrin, sIgA, and milk fat globule size were also shown to be higher in comparison to milk from healthy individuals, and the difference in size was larger if accompanied by systemic symptoms like fever (Mizuno et al., 2012). However, it has been proposed that the elevation of certain milk components (e.g., the pro-inflammatory cytokines and immunoglobulins) caused by mastitis might also protect the nursing infant from developing infectious illnesses such as Group B Streptococcus neonatal infection (Buescher and Hair, 2001).
Allergic diseases
Inflammation associated with allergic diseases (e.g., atopic eczema, asthma, and allergic rhinitis) is known to affect the production of inflammatory mediators in the blood (Galli et al., 2008); however, it is unclear whether these diseases also affect their production or levels in human milk. Concentrations of cytokines that are related to allergic reactions and the production of sIgA (e.g., IL-4, -5, -10, and -13) are higher in the human milk of allergic birthing parents compared to their non-allergic counterparts (Böttcher et al., 2000). The data on PUFA concentrations in human milk with maternal atopy are inconsistent. One study observed reduced levels of long-chain n-3 PUFAs in human milk of atopic birthing parents compared to non-atopic birthing parents, despite that birthing parents with atopy in this study consumed high amounts of fish rich in long-chain PUFAs (Johansson et al., 2011). The decrease in human milk PUFAs was suggested to be due to the consumption or exposure to environmental or food allergens, leading to inflammation and overproduction of prostaglandins and leukotrienes that upregulate inflammation, thereby altering the function of mammary gland cells and ultimately the content of human milk (Johansson et al., 2011). However, a second study did not find any differences in human milk PUFA concentrations between birthing parents with and without atopy (Lauritzen et al., 2006).
Obesity and body composition
A number of studies investigated the impact of maternal obesity and body mass index (BMI) on the macronutrients (Daniel et al., 2021, Leghi et al., 2020), fatty acids (de la Garza Puentes et al, 2019), and bioactive components (e.g., insulin, leptin, CRP, and osteopontin) (Sims et al., 2020, Ruan et al., 2022, Zhu et al., 2022) on human milk. In comparison to non-obese/normal weight birthing parents, human milk from obese birthing parents (BMI ≥25 kg/m2) (Ellsworth et al., 2020) was found to have higher fat and lactose concentrations; however, there were no differences in the protein concentrations (Leghi et al., 2020, Daniel et al., 2021, Froń and Orczyk-Pawiłowicz, 2023). With respect to human milk fatty acids concentration, human milk from obese birthing parents had elevated levels of saturated fatty acids and lower levels of PUFAs (α-linolenic acid (ALA) and docosahexaenoic acid (DHA)) and monounsaturated fatty acids compared to human milk from birthing parents with normal weight (de la Garza Puentes et al., 2019, Tekin-Guler et al., 2023). However, studies show conflicting results with respect to the PUFAs concentrations in the milk of obese birthing parents. A recent study (Ellsworth et al., 2020) showed higher levels of dihomo-gamma-linolenic acid (DGLA) and arsenic omega-6 PUFAs in the milk of obese birthing parents compared to birthing parents with normal weight. A review paper (Bardanzellu et al., 2020) found changes in eight specific human milk metabolites, including nucleotide derivatives, 5-methylthioadenosine, sugar alcohols, acylcarnitine and amino acids, polyamines, mono- and oligosaccharides, and lipids, in overweight or obese birthing parents compared to lean birthing parents. Higher levels of insulin (Ramiro-Cortijo et al., 2023, Schneider-Worthington et al., 2021), leptin (Andreas et al., 2014, Schneider-Worthington et al., 2021, De Luca et al., 2016), CRP, and lower levels of oligosaccharides (Saben et al., 2021, Froń and Orczyk-Pawiłowicz, 2023) have also been reported in the human milk of obese birthing parents compared to normal-weight birthing parents (Sims et al., 2020). Further, pre-pregnancy BMI of birthing parents exhibited a positive correlation with osteopontin levels early postpartum (Zhu et al., 2022), and a positive correlation was also found between osteopontin and maternal body composition factors, including body weight, bone mineral content, skeletal muscle mass, body fat, and visceral fat area (Ruan et al., 2022).
Given the significant role of human milk microbiome in shaping the infants’ gut microbiome which can have implications for infants’ health and development, recent studies focused on investigating the links between maternal weight status and the human milk microbiome composition. A recent scoping review of 20 longitudinal and cross-sectional studies showed that some studies reported that overweight or obese individuals displayed higher levels of Staphylococcus genus, reduced abundance of Bifidobacterium, and lower alpha diversity (within-sample diversity) (Daiy et al., 2022). It has been suggested that the above-mentioned changes in the human milk composition of obese birthing parents can put their infants’ at high risk of childhood obesity and complicated health outcomes (Leghi et al., 2020, Lecoutre et al., 2023, Bardanzellu et al., 2020). However, more robust longitudinal studies are needed to further evaluate the impact of maternal obesity on the infants’ health outcomes.
Acute kidney injuries
Although there are limited data, some studies show human milk composition can be negatively affected by acute kidney injuries, which may be a consequence of systemic inflammation, changes in pH, and accumulation of metabolic wastes associated with the disease (Chruscicki et al., 2017). A 2017 case report (Chruscicki et al., 2017) found a decrease in the amino acid concentrations (alanine, glutamine, glutamate, glycine, and valine) in the human milk of a mother with acute kidney injuries and an increase in the levels of creatinine and other catabolites compared to milk from healthy birthing parents, whereas the levels of macronutrients, including lipids and carbohydrates, were similar to their levels in health birthing parents. In early acute kidney injuries, there is increased protein catabolism which may be the reason for decreased amino acid levels in the human milk (Chruscicki et al., 2017). More studies are required to examine the potential relationship between kidney injury and human milk composition.
Celiac disease
Celiac disease (CD) is a serious autoimmune disease where the intestine of affected individuals is highly sensitive to food containing gluten (Olivares et al., 2015, Roca et al., 2018). Studies reported lower levels of immunological factors in human milk including the transforming growth factor (TGF)-β1 and sIgA in the milk of birthing parents with CD, compared to healthy birthing parents (Olivares et al., 2015). It has been proposed that this reduction in the milk’s protective factors can put the infant at risk of developing CD later in life (Olivares et al., 2015). On the other hand, human milk from birthing parents with CD who followed a gluten-free diet showed no difference in the levels of anti-gliadin antibodies (including IgA and IgG) from the milk of healthy birthing parents (Roca et al., 2018). A recent study investigated human milk microbiome composition in birthing parents with CD on a gluten-free diet and found in their human milk elevated levels of three bacterial strains, including one (Rothia mucilaginosa) that has been previously associated with autoimmune conditions, compared to healthy birthing parents (Olshan et al., 2021).
Preeclampsia
Preeclampsia is a systemic inflammatory diseases and a common pregnancy complication affecting 3–5% of pregnancies (Peila et al., 2022). Studies investigating the impact of preeclampsia on human milk composition are scarce; however, a recent review of 15 articles found alterations in the composition of human milk of preeclamptic birthing parents (Peila et al., 2022). Preeclamptic birthing parents had lower concentrations of cytokines (IL-6 and IL-8), elevated levels of adiponectin and DHA, and low vitamin A and E levels in their human milk compared to their healthy counterparts (Peila et al., 2022). However, some studies showed no differences in the macronutrient content (Beser et al., 2022) and activin A levels (a human milk neurobiomarker essential for central nervous system development) (Coscia et al., 2023) of human milk between birthing parents with and without preeclampsia.
Gestational hypertension
Gestational hypertension is another condition that has been recently mentioned in the literature to affect human milk lactogenesis and percentage composition (Sokołowska et al., 2023). Substantial variations were observed in the human milk composition of postpartum birthing parents with gestational hypertension when compared to healthy, normotensive counterparts (Sokołowska et al., 2023). Human milk from birthing parents with gestational hypertension exhibited elevated levels of fat, carbohydrates, and energy compared to healthy ones (Sokołowska et al., 2023). Future studies are needed to further investigate this association and assess the differences in growth rates of the infants.
Inflammatory bowel disease
Few recent studies show that birthing parents with inflammatory bowel disease had low abundance of Bifidobacterium and lower abundance of proteins involved in immune regulation, including thymic stromal lymphopoietin, IL-12 subunit beta, tumor necrosis factor (TNF)-beta, and C-C motif chemokine 20 in their human milk compared to birthing parents without inflammatory bowel disease (Sabino et al., 2023). These changes were shown to have a negative impact on the development of intestinal microbiota of infants.
Vaginal infections
Vaginal yeast infections during pregnancy can affect the immunological and antimicrobial properties of human milk (Nisaa et al., 2023). A recent study investigated the differences in microbiota profiles between birthing parents with and without vaginal yeast infections. Human milk from birthing parents with vaginal infections demonstrated increased alpha diversity at various taxonomic levels and elevated levels of Staphylococcus genus and Streptococcus infantis species, compared to birthing parents without infections (Nisaa et al., 2023).
Pregnancy, postpartum period, and periodontal disease
Pregnancy effects on periodontal disease
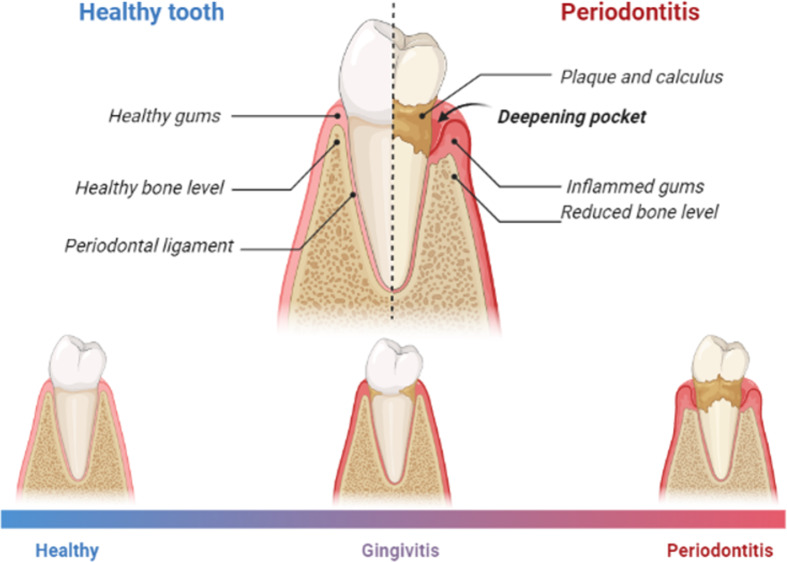
Periodontal diseases (gingivitis and periodontitis) are oral inflammatory conditions that are caused by bacterial plaque, and they result from a disruption in the normal interaction between the host and microflora in the oral cavity (Kinane et al., 2017, Khoury et al., 2020). Bacterial plaque is the oral biofilm that adheres to the tooth surface and gingiva and represents the main local etiologic factor for periodontal diseases (Figure 1) (Kinane et al., 2017, Tatakis and Kumar, 2005, Khoury et al., 2020).
Figure 1.
A diagram showing differences between healthy teeth and teeth with gingivitis and periodontitis. Gingivitis is a reversible inflammation confined to the gingival tissues. The teeth in the oral cavity are supported by a ligament known as the periodontal ligament which attached the teeth to the gingiva and surrounding alveolar bone. The space between the tooth and the gingival tissues is known as the gingival sulcus, and this is where most of the dental plaque accumulates, triggering an inflammatory response. What differentiates gingivitis from periodontitis is that the latter is an irreversible destruction to the periodontal supporting tissues, including the alveolar bone, periodontal ligament, and cementum, which consequently results in tooth loss (Kinane et al., 2017, Tatakis and Kumar, 2005). As sources of inflammation, periodontal diseases contribute to the overall inflammatory burden experienced by individuals (Khoury et al., 2020). Initially, the gingival response to the biofilm is presented in the form of redness, edema, and bleeding. Oral polymorphonuclear neutrophils are then recruited to the sites of inflammation. In a healthy periodontium, oral neutrophils are usually found at the junctional epithelium and base of the sulcus (Khoury et al., 2020) to protect the periodontal tissues by providing a barrier between the junctional epithelium and pathogens within the dental plaque. However, as bacterial counts increase, so does the oral neutrophils count which enhance their activity (Khoury et al., 2020). Any deviation from PMN’s normal production, recruitment, function, or activity can lead to damage in periodontal tissues, and consequently tooth loss. (Prepared using Biorender.com).
Many host-related conditions can affect the severity of the periodontal disease among which are sex hormones that exacerbate the pathogenesis and progression of the disease (Tilling, 2014, Patil et al., 2012). Pregnancy is associated with dynamic changes in the immune, endocrine, and metabolic milieus of the pregnant person (Tilling, 2014), including changes in the levels of estrogen and progesterone, which reach a peak of 6 and 100 ng/ml (10–30 times higher than their levels during the menstrual cycle) by the end of the third trimester, respectively (Patil et al., 2012, Grodstein et al., 1996). Some pregnancy-related changes occur in the oral cavity including gingivitis and periodontitis (Patil et al., 2012, Laine, 2002). The majority of studies reported that the pregnancy-related changes in the oral cavity are confined to gingival tissues (Tilakaratne et al., 2000, Tilling, 2014, Cohen et al., 1971) with studies reporting no changes in the periodontal attachment levels (Cohen et al., 1971). Therefore, pregnancy-related hormonal changes are believed to cause gingivitis during pregnancy; however, they do not likely affect periodontal tissues but may aggravate preexisting periodontal disease (Tilling, 2014). While the relationship between periodontal diseases and common adverse pregnancy outcomes is sufficiently established, this is still a need for more robust randomized clinical trials to confirm the association (Iheozor-Ejiofor et al., 2017).
While many mechanisms have been proposed to explain how pregnancy-related hormonal changes could increase the susceptibility to gingival inflammation, the reason is still unknown (Tilling, 2014). Proposed explanations include: 1. progesterone can increase the vascular permeability and decrease production of IL-6 by fibroblasts (Amar and Chung, 1994), 2. gingival tissue acts as a target for estrogen and progesterone due to the presence of their receptors in the gingival tissue (Offenbacher et al., 2001), 3. progesterone and estrogen have been associated with greater prostaglandin synthesis by macrophages and depressed antibody responses (Amar and Chung, 1994), 4. pregnancy-related hormonal changes are associated with greater polymorphonuclear neutrophils levels in the gingival sulcus and with an alteration of their function, thereby lowering the gingival resistance to bacterial invasion (Barriga et al., 1994), and 5. some bacterial species associated with gingival inflammation, including Bacteroides species and Prevotella intermedia are stimulated by pregnancy hormones (Barak et al., 2003).
Periodontal disease affects pregnancy outcomes
Recent evidence has suggested that there is a bidirectional relationship between periodontal diseases and pregnancy. Not only can pregnancy affect periodontal disease pathogenesis, but the opposite might also occur, and periodontal disease can affect pregnancy outcomes (Baskaradoss et al., 2012, Ide and Papapanou, 2013). As sources of inflammation, periodontal diseases contribute to the systemic inflammatory experienced by the patient (Offenbacher et al., 2001, Moore et al., 2004, Teshome and Yitayeh, 2016), and in the context of pregnancy, this heightened inflammation may have implications for inflammatory-associated processes, such as parturition (Daalderop et al., 2018). Indeed, the first study that reported a positive association between periodontal infection in individuals during pregnancy and preterm low birth weight showed that maternal periodontitis was associated with a seven-fold increased risk for the delivery of a preterm, low birth weight infant (Offenbacher et al., 1996). This finding was consistent with other future studies reporting an association between the severity of periodontal disease and increased odds of low birth weight delivery (Saddki et al., 2008, Khader et al., 2009). These associations, however, are still not entirely settled as evidenced by data shown in other studies reporting no significant association between oral health status and birth weight, except for birthing parents aged over 25 years, where maternal periodontitis was associated with low birth weight (Marin et al., 2005). This can be explained by the fact that age is considered as a predictor of periodontal diseases, so older individuals may have more severe periodontal diseases than younger individuals (Nazir, 2017).
Three main biological pathways might explain the relationship between the severity of periodontal disease and increased risks of adverse pregnancy outcomes. The first is inflammatory product circulation (shown in Figure 2) where the biosynthesis of pro-inflammatory cytokines in the blood serum and gingival fluid (such as IL-1b, IL-6, PGE2, and TNF-a) can be up-regulated by cellular exposure to bacterial products (Gibbs et al., 1992), such as the lipopolysaccharides of the gram-negative anaerobic bacteria of periodontal disease (e.g., Tannerella forsythia, Porphyromonas gingivalis). It is hypothesized that these endotoxins and pro-inflammatory cytokines could enter the bloodstream and reach the maternal–fetal interface (placenta and fetal membranes), thereby stimulating the production of PGE2 and TNF-alpha by the placenta (Gibbs et al., 1992, Romero et al., 1988), resulting in uterine contractility and early rupture of fetal membranes, resulting in preterm delivery (Gibbs et al., 1992).
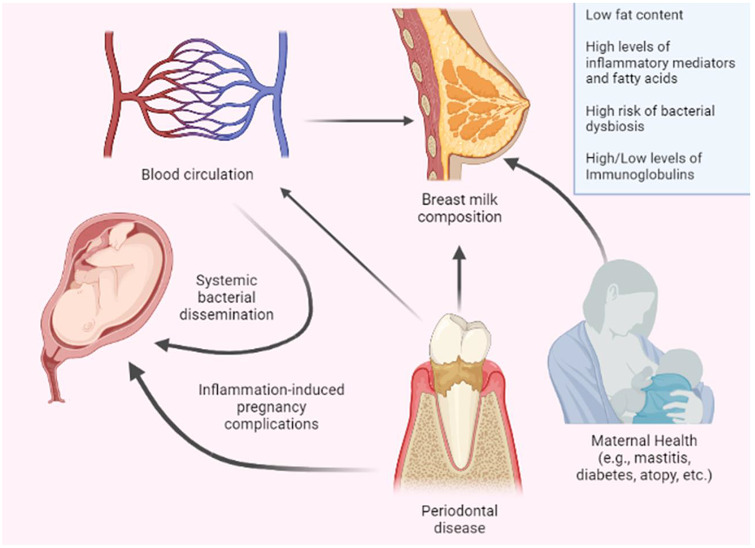
Figure 2.
A simplified diagram showing the potential mechanism explaining the impact of periodontal disease on breast milk composition. Maternal diseases/infections (such as diabetes, mastitis, and atopy) can affect the composition of breast milk. Periodontal diseases have been reported to in the literature to be associated with adverse pregnancy outcomes, including preeclampsia, preterm birth, and low birth weight. Potential biological pathways through which periodontal diseases can negatively affect breast milk composition include the systemic dissemination of inflammatory cytokines like IL-1, IL-6, PGE2, and TNF-β in the blood circulation that can be up-regulated by bacterial by-products. Based on results from this review, it can be hypothesized that milk from mothers with periodontal diseases might have low fat content, high levels of inflammatory mediators and fatty acids, changes in levels of immunoglobulins, and higher risk of bacterial dysbiosis, compared to milk from mothers with good periodontal health. (Prepared using Biorender.com).
This hypothesized mechanism has been supported by studies reporting high levels of PGE2, IL-1b, and IL-6 in the crevicular and amniotic fluid of birthing parents of preterm infants (Offenbacher et al., 1998, Dörtbudak et al., 2005). Another possible pathway that describes the relationship between periodontal diseases and adverse pregnancy outcomes is through the circulation of periodontal pathogens where they enter the bloodstream and invade the placenta (Han, 2011, Han et al., 2009). Intrauterine infections have been associated with placental colonization of Fusobacterium nucleatum and Porphyromonas gingivalis (Fardini et al., 2010, Dörtbudak et al., 2005). Inside the uterus, these pathogens might increase the synthesis of pro-inflammatory cytokines, prostaglandins, and metalloproteases derived from activated neutrophils (Dörtbudak et al., 2005), effects that could potentially stimulate uterine contraction and a preterm birth process (Dörtbudak et al., 2005). The risk of preterm birth could also be related to the immunological response against oral pathogens caused by periodontal disease (Han et al., 2009). Blood samples from the umbilical cords of preterm infants showed elevated levels of specific IgM directed against oral pathogens (Baskaradoss et al., 2012). This fetal immune reaction could be associated with an inflammatory response and/or both mechanisms could act in synergy to elevate the risk (Han et al., 2009).
Periodontal disease can affect human milk composition
A recent prospective cohort study (Badewy et al., 2022) was conducted by our research team to explore the impact of maternal oral inflammation on human milk composition, and how the oral inflammatory load (based on oral polymorphonuclear neutrophil counts) in birthing parents during the first 4 months postpartum impacts the immunological components and nutritional value of human milk including the human milk neutrophil counts and their activation state (based on the expression levels of cluster of differentiation [CD] biomarkers), as well as the content of lipids and fatty acids in human milk. The research was conducted to contribute to filling the knowledge gap in understanding the relationship between periodontal disease and human milk composition. Results from this study showed that birthing parents with moderate to severe oral inflammatory load exhibited a statistically significant decrease in CD64 biomarker expression, an increase in CD14 biomarker expression on human milk neutrophils, and a decrease in eicosapentaenoic acid (C20:5n-3) levels in their human milk at follow-up compared to baseline. This study reveals, for the first time, that maternal oral inflammation can influence human milk composition, emphasizing the need to consider how these alterations may impact long-term infant health outcomes.
Infants’ intestinal microbiome and maternal health
With human milk representing the protective agent against many infantile infections and long-term diseases, the potential impact of maternal oral inflammatory diseases on infant health is worth further investigation. The microbial colonization of the infant’s gut (i.e., the gut microbiome) acts as a barrier against various pathogens and is also essential for modulating the host’s metabolism as well as immune system development and maturation (Tanaka and Nakayama, 2017). Various factors influence the composition and development of the intestinal microbiota of the infant, over which the mother may have the greatest influence on the early acquisition and development of the infant’s microbiome (Richardson et al., 2018). The maternal microbial reservoir at various body sites plays a key role in the development of the infant’s microbiome due to the intimate relation birthing parents have with their infants during birth and while breastfeeding (Funkhouser and Bordenstein, 2013). The maternal microbiome can be transferred to the infants through five sources including fecal matter, vaginal fluids, and skin contact at the time of birth, and through human milk and skin contact after birth, particularly during breastfeeding, and oral bacteria transfer via the bloodstream (Funkhouser and Bordenstein, 2013). The development and composition of the infant’s gut microbiome thus depend on a large extent on maternal health (Richardson et al., 2018). For example, significant reductions in the abundance of Bifidobacteria, considered to be beneficial to health, have been reported in the human milk of allergic birthing parents and birthing parents with CD, compared to their healthy counterparts. (Grönlund et al., 2007) (Olivares et al., 2015).
The composition of an infant’s gut microbiome can have long-term implications on health and the development of its immune and nervous system, relationships which may be modulated by human milk (Figure 3). Various studies show that disturbances in the gut microbiome increase the infant’s susceptibility to autoimmune diseases such as diabetes, inflammatory bowel disease, atopy, and other conditions such as obesity (Arrieta et al., 2015, Turnbaugh et al., 2009, Fujimura et al., 2016). For example, evidence suggests that decreased abundance of Bifidobacteria in the infant gut is associated with obesity later in life (Abrahamsson et al., 2014). Additionally, low gut bacterial diversity (which may indicate a less mature microbiome) in the first month of life has been associated with asthma in children at the age of 7 years (Abrahamsson et al., 2014). Studies have also revealed associations between the gut microbiome and neurobehavioral outcomes in 7-year-old children including autism spectrum disorders and attention deficit hyperactivity disorder, suggesting that gut microbiome development can impact early brain development, resulting in behavioral changes in later life (Curran et al., 2016, Curran et al., 2015). With respect to cognitive development, a recent study (Carlson et al., 2018) investigated the association between gut microbiome in infancy and cognitive development at one and two years of age, where the development of the gut microbiome at one year was shown to predict cognitive communicative behaviors at two years of age. However, more robust longitudinal studies need to be conducted in order to assess the causal association between the studied variables.
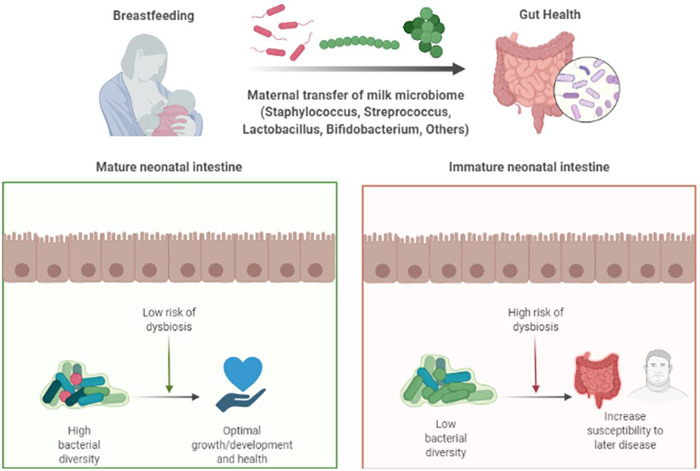
Figure 3.
A simplified diagram showing the modulation of infant gut microbiota via breast milk microbiome. Infants receive various bacterial communities from breast milk which plays a major role in the development and maturation of the infants’ gut microbiome. Breast milk microbiome shapes the neonate’s intestinal maturation. Infants with mature intestine will have high bacterial diversity in their gut (i.e., low risk of bacterial dysbiosis) which protects them from later infections/diseases and is essential for the infants’ growth/development and optimal health. On the other hand, infants with immature intestine, will have low bacterial diversity in their gut (i.e., high risk of bacterial dysbiosis) which increases the infants’ susceptibility to later disease. (Prepared using Biorender.com).
Research, practice, and policy implications
This review summarized existing evidence regarding the mechanisms related to periodontal diseases and its possible association with changes in human milk composition. There is an existing gap of knowledge regarding whether periodontal diseases in birthing parents can impact negatively the composition of their human milk. Future research in this topic could bring exciting outputs that will be significant not just to dentistry, but to obstetrics and gynecology, neonatology, and pediatrics to name a few.
Policy and practice implications
Promoting oral health before and during pregnancy and in the postpartum period is important, but often neglected. Sixty-eight percent of obstetricians (Al-Habashneh et al., 2008) rarely or never encourage pregnant patients to seek dental treatment throughout their pregnancy.
Training primary healthcare professionals in all capacities on the importance of educating birthing parents about the consequences of poor oral health may make the healthcare professionals more confident in addressing this issue with their patients (Heilbrunn-Lang et al., 2015).
Studies showed significant increase in the confidence of midwives who received an oral health education training program, in promoting oral health among pregnant patients (Heilbrunn-Lang et al., 2015).
If it appears that maternal periodontal inflammation also mediates negative effects on human milk, it will be critically important to make these facts known to the birthing parents’ healthcare providers and of course to birthing parents of newborns.
This can be done by advocating for the incorporation of prenatal and postnatal oral health components within the curriculum of pediatric, obstetrics, and gynecology residencies training. With periodontal diseases affecting 20–50% of pregnant persons (Xiong et al., 2007, Madianos et al., 2001), the absence of dental care services can have negative impact on their oral health, quality of life, and health of their pregnancy and baby.
Publicly funded dental programs for birthing parents who are affected by oral inflammatory conditions throughout pregnancy and postpartum do not exist in many countries (Northridge et al., 2020).
Primary healthcare professionals need to be trained to do primary oral screening and referrals to dentists as required.
Research implications
Findings from future studies that aim to investigate the impact of suboptimal oral health of birthing parents and human milk composition might aid in informing the development of a framework for policies and preventive programs to improve the oral health and access to dental care services for individuals of childbearing years.
Future research in this topic could stress the importance of having public dental clinics in primary healthcare settings accessible to all pregnant and nursing individuals, where dentists can deliver basic restorative and preventive measures for birthing parents at-risk of periodontal diseases and suboptimal oral health, in particular individuals with low socio-economic status.
Conclusion
Current evidence suggests that maternal infections and/or the presence of inflammatory disease adversely affect the composition and quality of human milk. Further, disturbances in the composition of an infant’s gut microbiome, in part due to alterations in human milk composition, is associated with adverse health trajectories for that infant and child long term. Given that periodontal diseases affect many individuals during pregnancy and this condition can progress postpartum if left untreated, optimizing and maintaining good oral health are critical for both the health of the mother and her baby. The biological mechanisms that may drive the relationships between periodontal diseases and poor pregnancy and infant outcomes include but are not limited to the systemic dissemination of inflammatory cytokines like IL-1, IL-6, PGE2, and TNF-β that can be up-regulated by bacterial by-products. There is every reason to hypothesize that periodontitis, just as mastitis, diabetes, and maternal infections could affect the composition of human milk, meaning that periodontitis could have similar effects on human milk inflammatory cytokines as well as lipids. Future efforts directed at investigating the potential association between periodontal diseases in individuals of childbearing years and the composition and quality of human milk will help to close the gap in our understanding, prevention, and treatment of oral diseases affecting pregnant and nursing individuals and advocate for integrating oral healthcare within primary healthcare settings.
Author contributions
Below are the specific contributions made by each author:
Rana Badewy: conceptualization, methodology, validation, resources, writing – original draft.
Howard Tenenbaum: review and editing.
Michael Glogauer: review and editing.
Jim Lai: review and editing.
Kristin Connor: review and editing.
Michael Sgro: review and editing.
Richard Bazinet: review and editing.
Amir Azarpazhooh: conceptualization, review and editing, supervision, and project administration.
Funding statement
None.
Competing interests
None.
References
- Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L and Jenmalm MC (2014) Low gut microbiota diversity in early infancy precedes asthma at school age. Clinical and Experimental Allergy 44, 842–850. [DOI] [PubMed] [Google Scholar]
- Al-habashneh R, Aljundi SH and Alwaeli HA (2008) Survey of medical doctors’ attitudes and knowledge of the association between oral health and pregnancy outcomes. International Journal of Dental Hygiene 6, 214–220. [DOI] [PubMed] [Google Scholar]
- Amar S and Chung KM (1994) Influence of hormonal variation on the periodontium in women. Periodontology 2000 6, 79–87. [DOI] [PubMed] [Google Scholar]
- Andreas NJ, Hyde MJ, Gale C, Parkinson JR, Jeffries S, Holmes E and Modi N (2014) Effect of maternal body mass index on hormones in breast milk: a systematic review. Plos One 9, e115043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreas NJ, Kampmann B and Mehring Le-Doare K. (2015) Human breast milk: a review on its composition and bioactivity. Early Human Development 91, 629–635. [DOI] [PubMed] [Google Scholar]
- Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch, S , Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, Subbarao P, Mandhane, P , Becker A, Mcnagny KM, Sears MR, Kollmann T, Mohn,WW , Turvey SE and Finlay BB (2015) Early infancy microbial and metabolic alterations affect risk of childhood asthma. Science Translational Medicine 7, 307ra152. [DOI] [PubMed] [Google Scholar]
- Avellar ACS, Oliveira MN, Caixeta F, Souza R, Teixeira A, Faria AMC, Silveira-Nunes G, Faria ES and Maioli TU (2022) Gestational diabetes mellitus changes human colostrum immune composition. Frontiers in Immunology 13, 910807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azulay chertok IR, Haile ZT, Eventov-Friedman S, Silanikove N and Argov-Argaman N (2017) Influence of gestational diabetes mellitus on fatty acid concentrations in human colostrum. Nutrition 36, 17–21. [DOI] [PubMed] [Google Scholar]
- Badewy R, Azarpazhooh A, Tenenbaum H, Connor KL, Lai JY, Sgro M, Bazinet RP, Fine N, Watson E, Sun C, Saha S and Glogauer M (2022) The association between maternal oral inflammation and neutrophil phenotypes and poly-unsaturated fatty acids composition in human milk: a prospective cohort study. Cells 11, 4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baethge C, Goldbeck-Wood S and Mertens S (2019) Sanra-a scale for the quality assessment of narrative review articles. Research Integrity and Peer Review 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Oettinger-Barak O, Oettinger M, Machtei EE, Peled M and Ohel G (2003) Common oral manifestations during pregnancy: a review. Obstetrical and Gynecological Survey 58, 624–628. [DOI] [PubMed] [Google Scholar]
- Bardanzellu F, Puddu M, Peroni DG and Fanos V (2020) The human breast milk metabolome in overweight and obese mothers. Frontiers in Immunology 11, 1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriga C, Rodriguez AB and Ortega E (1994) Increased phagocytic activity of polymorphonuclear leukocytes during pregnancy. European Journal of Obstetrics and Gynecology and Reproductive Biology 57, 43–46. [DOI] [PubMed] [Google Scholar]
- Baskaradoss JK, Geevarghese A and Al Dosari AA (2012) Causes of adverse pregnancy outcomes and the role of maternal periodontal status - a review of the literature. The Open Dentistry Journal 6, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beser E, Kose Cetinkaya A, Kucukoglu Keser M, Okman E, Sari FN and Alyamac Dizdar E (2022) Evaluation of breast milk macronutrient content in preeclamptic mothers. Breastfeeding Medicine 17, 318–321. [DOI] [PubMed] [Google Scholar]
- Bitman J, Hamosh M, Hamosh P, Lutes V, Neville MC, Seacat J and Wood DL (1989) Milk composition and volume during the onset of lactation in a diabetic mother. The American Journal of Clinical Nutrition 50, 1364–1369. [DOI] [PubMed] [Google Scholar]
- Böttcher MF, Jenmalm MC, Garofalo RP and Björkstén B (2000) Cytokines in breast milk from allergic and nonallergic mothers. Pediatric Research 47, 157–162. [DOI] [PubMed] [Google Scholar]
- Buescher ES and Hair PS (2001) Human milk anti-inflammatory component contents during acute mastitis. Cellular Immunology 210, 87–95. [DOI] [PubMed] [Google Scholar]
- Carlson AL, Xia K, Azcarate-Peril MA, Goldman, BD , Ahn M, Styner MA, Thompson AL, Geng X, Gilmore JH and Knickmeyer RC (2018) Infant gut microbiome associated with cognitive development. Biological Psychiatry 83, 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Nagel EM, Kharoud H, Johnson KE, Gallagher T, Duncan K, Kharbanda EO, Fields DA, Gale C A, Jacobs K, Jacobs DR, jr. and Demerath EW (2022) Gestational diabetes mellitus is associated with differences in human milk hormone and cytokine concentrations in a fully breastfeeding united states cohort. Nutrients 14, 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chruscicki A, Morton AR, Akbari A and White CA (2017) Composition of human breast milk in acute kidney injury. Obstetric Medicine 10, 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavano NR (1982) Mode of feeding and its effect on infant mortality and morbidity. Journal of Tropical Pediatrics 28, 287–293. [DOI] [PubMed] [Google Scholar]
- Cohen DW, Shapiro J, Friedman L, Kyle GC and Franklin S (1971) A longitudinal investigation of the periodontal changes during pregnancy and fifteen months post-partum. Ii. Journal of Periodontology 42, 653–657. [DOI] [PubMed] [Google Scholar]
- Coscia A, Riboldi L, Spada E, Bertino E, Sottemano S, Barbagallo I, Livolti G, Galvano F, Gazzolo D and Peila C (2023) Preeclampsia and its impact on human milk activin a concentration. Nutrients 15, 4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran EA, Cryan JF, Kenny LC, Dinan TG, Kearney PM and Khashan AS (2016) Obstetrical mode of delivery and childhood behavior and psychological development in a british cohort. Journal of Autism and Developmental Disorders 46, 603–614. [DOI] [PubMed] [Google Scholar]
- Curran EA, Dalman C, Kearney PM, Kenny LC, Cryan JF, Dinan TG and Khashan, A. S. (2015) Association between obstetric mode of delivery and autism spectrum disorder: a population-based sibling design study. Jama Psychiatry 72, 935–942. [DOI] [PubMed] [Google Scholar]
- Daalderop LA, Wieland BV, Tomsin K, Reyes L, Kramer BW, Vanterpool SF and Been JV (2018) Periodontal disease and pregnancy outcomes: overview of systematic reviews. JDR Clinical and Translational Research 3, 10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiy K, Harries V, Nyhan K and Marcinkowska UM (2022) Maternal weight status and the composition of the human milk microbiome: a scoping review. Plos One 17, e0274950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel AI, Shama S, Ismail S, Bourdon C, Kiss A, Mwangome M, Bandsma RHJ and O’connor DL (2021) Maternal bmi is positively associated with human milk fat: a systematic review and meta-regression analysis. The American Journal of Clinical Nutrition 113, 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Garza Puentes A, Martí Alemany A, Chisaguano AM, Montes Goyanes R, Castellote AI, Torres-Espínola FJ, García-Valdés L, Escudero-Marín M, Segura M T, Campoy C and López-Sabater MC (2019) The effect of maternal obesity on breast milk fatty acids and its association with infant growth and cognition-the preobe follow-up. Nutrients 11, 2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De luca A, Frasquet-Darrieux M, Gaud MA, Christin P, Boquien CY, Millet C, Herviou M, Darmaun D, Robins RJ, Ingrand P and Hankard R (2016) Higher leptin but not human milk macronutrient concentration distinguishes normal-weight from obese mothers at 1-month postpartum. Plos One 11, e0168568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörtbudak O, Eberhardt R, Ulm M and Persson GR (2005) Periodontitis, a marker of risk in pregnancy for preterm birth. Journal of Clinical Periodontology 32, 45–52. [DOI] [PubMed] [Google Scholar]
- Dou Y, Luo Y, Xing Y, Liu H, Chen B, Zhu L, Ma D and Zhu J (2023) Human milk oligosaccharides variation in gestational diabetes mellitus mothers. Nutrients 15, 1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth L, Perng W, Harman E, Das A, Pennathur S and Gregg B (2020. ) Impact of maternal overweight and obesity on milk composition and infant growth. Maternal and Child Nutrition 16, e12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardini Y, Chung P, Dumm R, Joshi N and Han YW (2010) Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Infection and Immunity 78, 1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feachem RG and Koblinsky MA (1984) Interventions for the control of diarrhoeal diseases among young children: promotion of breast-feeding. Bulletin of the World Health Organization 62, 271–291. [PMC free article] [PubMed] [Google Scholar]
- Fernández L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R and Rodríguez JM (2013) The human milk microbiota: origin and potential roles in health and disease. Pharmacological Research 69, 1–10. [DOI] [PubMed] [Google Scholar]
- Fitzstevens JL, Smith KC, Hagadorn JI, Caimano MJ, Matson AP and Brownell, EA (2017) Systematic review of the human milk microbiota. Nutrition in Clinical Practice 32, 354–364. [DOI] [PubMed] [Google Scholar]
- Froń A and Orczyk-Pawiłowicz M (2023) Understanding the immunological quality of breast milk in maternal overweight and obesity. Nutrients 15, 5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, Lamere B, Rackaityte E, Lukacs NW, Wegienka G, Boushey HA, Ownby DR, Zoratti EM, Levin AM, Johnson CC and Lynch SV (2016) Neonatal gut microbiota associates with childhood multisensitized atopy and t cell differentiation. Nature Medicine 22, 1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkhouser LJ and Bordenstein SR (2013) Mom knows best: the universality of maternal microbial transmission. PLoS Biology 11, e1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Tsai M and Piliponsky AM (2008) The development of allergic inflammation. Nature 454, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo R (2010) Cytokines in human milk. Journal of Pediatrics 156, s36–40. [DOI] [PubMed] [Google Scholar]
- Gibbs RS, Romero R, Hillier SL, Eschenbach DA and Sweet RL (1992) A review of premature birth and subclinical infection. American Journal of Obstetrics and Gynecology 166, 1515–1528. [DOI] [PubMed] [Google Scholar]
- Gilmore WS, Mckelvey-Martin VJ, Rutherford S, Strain JJ, Loane P, Kell M and Millar S (1994) Human milk contains granulocyte colony stimulating factor. European Journal of Clinical Nutrition 48, 222–224. [PubMed] [Google Scholar]
- Grodstein F, Colditz GA and Stampfer MJ (1996) Post-menopausal hormone use and tooth loss: a prospective study. The Journal of American Dental Association 127, 370–377. [DOI] [PubMed] [Google Scholar]
- Groer M, Davis M and Steele K (2004) Associations between human milk siga and maternal immune, infectious, endocrine, and stress variables. Journal of Human Lactation 20, 153–158. [DOI] [PubMed] [Google Scholar]
- Grönlund MM, Gueimonde M, Laitinen K, Kociubinski G, Grönroos T, Salminen S and Isolauri E (2007) Maternal breast-milk and intestinal bifidobacteria guide the compositional development of the bifidobacterium microbiota in infants at risk of allergic disease. Clinical and Experimental Allergy 37, 1764–1772. [DOI] [PubMed] [Google Scholar]
- Gurven MD, Trumble BC, Stieglitz J, Blackwell AD, Michalik DE, Finch CE and Kaplan HS (2016) Cardiovascular disease and type 2 diabetes in evolutionary perspective: a critical role for helminths? Evolution, Medicine, and Public Health 2016, 338–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW (2011) Oral health and adverse pregnancy outcomes - what’s next? Journal of Dental Research 90, 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Shen T, Chung P, Buhimschi,IA and Buhimschi CS (2009) Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. Journal of Clinical Microbiology 47, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbrunn-Lang AY, De Silva AM, Lang G, George A, Ridge A, Johnson M, Bhole S and Gilmour C (2015) Midwives’ perspectives of their ability to promote the oral health of pregnant women in victoria, australia. BMC Pregnancy and Childbirth 15, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KM, Foster JA, Forney LJ, Schütte UM, Beck DL, Abdo Z, Fox LK, Williams JE, Mcguire MK and Mcguire MA (2011) Characterization of the diversity and temporal stability of bacterial communities in human milk. Plos One 6, e21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KM, Williams JE, Shafii B, Hunt MK, Behre R, Ting R, Mcguire MK and Mcguire MA (2013) Mastitis is associated with increased free fatty acids, somatic cell count, and interleukin-8 concentrations in human milk. Breastfeeding Medicine 8, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide M. and Papapanou PN (2013) Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes--systematic review. Journal of Clinical Periodontology 40, s181–94. [DOI] [PubMed] [Google Scholar]
- Iheozor-Ejiofor Z, Middleton P, Esposito M and Glenny AM (2017) Treating periodontal disease for preventing adverse birth outcomes in pregnant women. The Cochrane Database of Systematic Reviews 6, cd005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingman WV, Glynn DJ and Hutchinson MR (2014) Inflammatory mediators in mastitis and lactation insufficiency. Journal of Mammary Gland Biology Neoplasia 19, 161–167. [DOI] [PubMed] [Google Scholar]
- Johansson S, Wold AE and Sandberg AS (2011) Low breast milk levels of long-chain n-3 fatty acids in allergic women, despite frequent fish intake. Clinical and Experimental Allergy 41, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass EH (1962) Pyelonephritis and bacteriuria. A major problem in preventive medicine. Annals of Internal Medicine 56, 46–53. [DOI] [PubMed] [Google Scholar]
- Keim SA, Daniels JL, Siega-Riz AM, Herring AH, Dole N and Scheidt PC (2012) Breastfeeding and long-chain polyunsaturated fatty acid intake in the first 4 post-natal months and infant cognitive development: an observational study. Maternal and Child Nutrition 8, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader Y, Al-Shishani L, Obeidat B, Khassawneh M, Burgan S, Amarin ZO, Alomari M and Alkafajei A (2009) Maternal periodontal status and preterm low birth weight delivery: a case-control study. Archives of Gynecology and Obstetrics 279, 165–169. [DOI] [PubMed] [Google Scholar]
- Khoury W, Glogauer J, Tenenbaum HC and Glogauer M (2020) Oral inflammatory load: neutrophils as oral health biomarkers. Journal of Periodontal Research 55, 594–601. [DOI] [PubMed] [Google Scholar]
- Kinane DF, Stathopoulou PG and Papapanou PN (2017) Periodontal diseases. Nature Reviews Disease Primers 3, 17038. [DOI] [PubMed] [Google Scholar]
- Korkut S, Köse Çetinkaya A, Işik Ş, Özel Ş, Gökay N, Şahin A and Alyamaç Dizdar E (2022) Macronutrient composition of colostrum in mothers with gestational diabetes mellitus. Breastfeeding Medicine 17, 322–325. [DOI] [PubMed] [Google Scholar]
- Laine M A (2002) Effect of pregnancy on periodontal and dental health. Acta Odontologica Scandinavica 60, 257–264. [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Halkjaer LB, Mikkelsen TB, Olsen SF, Michaelsen KF, Loland L and Bisgaard H (2006) Fatty acid composition of human milk in atopic danish mothers. The American Journal of Clinical Nutrition 84, 190–196. [DOI] [PubMed] [Google Scholar]
- Lecoutre S., Maqdasy S, Lambert M and Breton C (2023) The impact of maternal obesity on adipose progenitor cells. Biomedicines 11, 3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leghi GE, Netting MJ, Middleton PF, Wlodek ME, Geddes DT and Muhlhausler A. B. S. (2020) The impact of maternal obesity on human milk macronutrient composition: a systematic review and meta-analysis. Nutrients 12, 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madianos PN, Lieff S, Murtha AP, Boggess, KA , Auten RL, jr., Beck JD and Offenbacher S (2001) Maternal periodontitis and prematurity. Part ii: maternal infection and fetal exposure. Annals of Periodontology 6, 175–182. [DOI] [PubMed] [Google Scholar]
- Marin C, Segura-Egea JJ, Martínez-Sahuquillo A and Bullón P (2005) Correlation between infant birth weight and mother’s periodontal status. Journal of Clinical Periodontology 32, 299–304. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Hatsuno, M , Aikawa K, Takeichi H, Himi T, Kaneko A, Kodaira K, Takahashi H and Itabashi K (2012) Mastitis is associated with il-6 levels and milk fat globule size in breast milk. Journal of Human Lactation 28, 529–534. [DOI] [PubMed] [Google Scholar]
- Moore S, Ide M, Coward P Y, Randhawa M, Borkowska E, Baylis R and Wilson RF (2004) A prospective study to investigate the relationship between periodontal disease and adverse pregnancy outcome. British Dental Journal 197, 251–258. [DOI] [PubMed] [Google Scholar]
- Morceli G, França EL, Magalhães VB, Damasceno DC, Calderon IM and Honorio-França AC (2011) Diabetes induced immunological and biochemical changes in human colostrum. Acta Paediatrica 100, 550–556. [DOI] [PubMed] [Google Scholar]
- Morrow AL and Rangel JM (2004) Human milk protection against infectious diarrhea: implications for prevention and clinical care. Seminars in Pediatric Infectious Diseases 15, 221–228. [DOI] [PubMed] [Google Scholar]
- Murphy J, Pfeiffer RM, Lynn BCD, Caballero AI, Browne EP, Punska EC, Yang HP, Falk RT, Anderton DL, Gierach GL, Arcaro KF and Sherman M E. (2018) Pro-inflammatory cytokines and growth factors in human milk: an exploratory analysis of racial differences to inform breast cancer etiology. Breast Cancer Research and Treatment 172, 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir M. A. (2017) Prevalence of periodontal disease, its association with systemic diseases and prevention. International Journal of Health Sciences 11, 72–80. [PMC free article] [PubMed] [Google Scholar]
- Nisaa AA, Oon CE, Sreenivasan S, Balakrishnan V, Rajendran D, Tan JJ, Roslan FF, Todorov SD, Jeong W., Zhao F, Nasir NSM, Deris ZZ., Zhang H, Park Y H, Liu G and Liong MT (2023) Vaginal infections during pregnancy increase breast milk microbiome alpha diversity and alter taxonomic composition. Preventive Nutrition and Food Science 28, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northridge ME, Kumar A and Kaur R (2020) Disparities in access to oral health care. Annual Review of Public Health 41, 513–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S, Jared HL, O’reilly PG, Wells SR, Salvi GE, Lawrence HP, Socransky SS and Beck JD (1998) Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Annals of Periodontology 3, 233–250. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, Mckaig R and Beck J (1996) Periodontal infection as a possible risk factor for preterm low birth weight. Journal of Periodontology 67, 1103–1113. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Lieff S, Boggess KA, Murtha AP, Madianos PN, Champagne CM, Mckaig RG, Jared HL, Mauriello SM, Auten R. L, jr., Herbert, WN and Beck, J. D. (2001) Maternal periodontitis and prematurity. Part i: obstetric outcome of prematurity and growth restriction. Annals of Periodontology 6, 164–174. [DOI] [PubMed] [Google Scholar]
- Olivares M, Albrecht S, De Palma G, Ferrer MD, Castillejo G, Schols HA and Sanz Y (2015) Human milk composition differs in healthy mothers and mothers with celiac disease. European Journal of Nutrition 54, 119–128. [DOI] [PubMed] [Google Scholar]
- Olshan KL, Zomorrodi AR, Pujolassos M, Troisi J, Khan N, Fanelli B, Kenyon V, Fasano A and Leonard MM (2021) Microbiota and metabolomic patterns in the breast milk of subjects with celiac disease on a gluten-free diet. Nutrients 13, 2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil SN, Kalburgi NB, Koregol AC, Warad SB, Patil S and Ugale MS (2012) Female sex hormones and periodontal health-awareness among gynecologists - a questionnaire survey. The Saudi Dental Journal 24, 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peila C, Bertino E, Cresi F and Coscia A (2022) Interactions between preeclampsia and composition of the human milk: what do we know? The Journal of Maternal-Fetal & Neonatal Medicine 35, 6219–6225. [DOI] [PubMed] [Google Scholar]
- Peila C, Gazzolo D, Bertino E, Cresi F and Coscia A (2020) Influence of diabetes during pregnancy on human milk composition. Nutrients 12, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan CR, Toms GL, Martin AJ, Gardner PS, Webb JK and Appleton DR (1980) Breast-feeding and respiratory syncytial virus infection. British Medical Journal 281, 1034–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro-Cortijo D, Singh P, Herranz Carrillo G, Gila-Díaz A, Martín-Cabrejas MA, Martin CR and Arribas SM (2023) Association of maternal body composition and diet on breast milk hormones and neonatal growth during the first month of lactation. Frontiers in Endocrinology 14, 1090499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Kerna N and Tulp O (2018) Fundamental factors affecting the development and function of the pediatric microbiome. 157.
- Roca M, Vriezinga SL, Crespo-Escobar P, Auricchio R, Hervás D, Castillejo G, Mena MC, Polanco I, Troncone R, Mearin ML and Ribes-Koninckx C (2018) Anti-gliadin antibodies in breast milk from celiac mothers on a gluten-free diet. European Journal of Nutrition 57, 1947–1955. [DOI] [PubMed] [Google Scholar]
- Romero R, Hobbins JC. and Mitchell MD (1988) Endotoxin stimulates prostaglandin e2 production by human amnion. Obstetrics and Gynecology 71, 227–228. [PubMed] [Google Scholar]
- Ruan H, Tang Q, Zhao X, Zhang Y, Zhao X, Xiang Y, Geng W, Feng Y and Cai W (2022) The levels of osteopontin in human milk of chinese mothers and its associations with maternal body composition. Food Science and Human Wellness 11, 1419–1427. [Google Scholar]
- Russell D H, Kibler R, Matrisian L, Larson DF, Poulos B and Magun BE (1985) Prolactin receptors on human t and b lymphocytes: antagonism of prolactin binding by cyclosporine. Journal of Immunology 134, 3027–3031. [PubMed] [Google Scholar]
- Saben JL, Sims CR, Abraham A, Bode L and Andres A (2021) Human milk oligosaccharide concentrations and infant intakes are associated with maternal overweight and obesity and predict infant growth. Nutrients 13, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino J, Tarassishin L, Eisele C, Hawkins K, Barré A, Nair N, Rendon A, Debebe A, Picker M, Agrawal M, Stone J, George J, Legnani P, Maser E, Chen CL, Thjømøe A, Mørk E, Dubinsky M, Hu J, Colombel JF, Peter I and Torres J (2023) Influence of early life factors, including breast milk composition, on the microbiome of infants born to mothers with and without inflammatory bowel disease. Journal of Crohn’s and Colitis 17, 1723–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddki N, Bachok N, Hussain NH, Zainudin SL and Sosroseno W (2008) The association between maternal periodontitis and low birth weight infants among malay women. Community Dentistry Oral Epidemiology 36, 296–304. [DOI] [PubMed] [Google Scholar]
- Sanz Y (2011) Gut microbiota and probiotics in maternal and infant health. The American Journal of Clinical Nutrition 94, 2000s–2005s. [DOI] [PubMed] [Google Scholar]
- Say B, Dizdar EA, Degirmencioglu H, Uras N, Sari FN, Oguz S and Canpolat FE (2016) The effect of lactational mastitis on the macronutrient content of breast milk. Early Human Development 98, 7–9. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Handler A, Hershow R, Persky V and Davis F (1994) Urinary tract infection during pregnancy: its association with maternal morbidity and perinatal outcome. American Journal of Public Health 84, 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Worthington CR, Bahorski JS, Fields DA, Gower BA, Fernández JR and Chandler-Laney PC (2021) Associations among maternal adiposity, insulin, and adipokines in circulation and human milk. Journal of Hum Lactation 37, 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuyang X and Qiang Y (2021) Bacterial factors of mastitis in lactating women and its effect on the physical properties and chemical composition of breast milk. Cell Mol Biol (Noisy-Le-Grand) 67, 172–177. [DOI] [PubMed] [Google Scholar]
- Sims CR, Lipsmeyer ME, Turner DE and Andres A (2020) Human milk composition differs by maternal bmi in the first 9 months postpartum. The American Journal of Clinical Nutrition 112, 548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilowitz JT, Totten SM, Huang, J , Grapov D, Durham HA, Lammi-Keefe CJ, Lebrilla C and German JB (2013) Human milk secretory immunoglobulin a and lactoferrin n-glycans are altered in women with gestational diabetes mellitus. The Journal of Nutrition 143, 1906–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokołowska EM, Jassem-Bobowicz JM, Drążkowska I, Świąder Z and Domżalska-Popadiuk I (2023) Gestational hypertension and human breast milk composition in correlation with the assessment of fetal growth-a pilot study. Nutrients 15, 2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwaydi MA, Zhou X, Perrella SL, Wlodek ME, Lai CT., Gridneva Z and Geddes DT (2022) The impact of gestational diabetes mellitus on human milk metabolic hormones: a systematic review. Nutrients 14, 3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M and Nakayama J (2017) Development of the gut microbiota in infancy and its impact on health in later life. Allergology International 66, 515–522. [DOI] [PubMed] [Google Scholar]
- Tatakis D N and Kumar PS. (2005) Etiology and pathogenesis of periodontal diseases. Dental Clinics of North America 49, 491–516. [DOI] [PubMed] [Google Scholar]
- Tekin-Guler T, Koc N, Kara-Uzun A and Fisunoglu M (2023) The association of pre-pregnancy obesity and breast milk fatty acids composition and the relationship of postpartum maternal diet, breast milk fatty acids and infant growth. Breastfeeding Medicine 18, 475–482. [DOI] [PubMed] [Google Scholar]
- Teshome A and Yitayeh A (2016) Relationship between periodontal disease and preterm low birth weight: systematic review. The Pan African Medical Journal 24, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilakaratne A, Soory M, Ranasinghe AW, Corea SM, Ekanayake SL and De Silva M (2000) Periodontal disease status during pregnancy and 3 months post-partum, in a rural population of sri-lankan women. Journal of Clinical Periodontology 27, 787–792. [DOI] [PubMed] [Google Scholar]
- Tilling E (2014). Effect of pregnancy on periodontal and oral health. 10, 72–75.
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath A C, Knight R and Gordon JI (2009) A core gut microbiome in obese and lean twins. Nature 457, 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Buekens P, Vastardis S and Yu S. M (2007) Periodontal disease and pregnancy outcomes: state-of-the-science. Obstetrical and Gynecological Survey 62, 605–615. [DOI] [PubMed] [Google Scholar]
- Zárate R, El Jaber-Vazdekis N, Tejera N, Pérez JA and Rodríguez C (2017) Significance of long chain polyunsaturated fatty acids in human health. Clinical Translational Medicine 6, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Zhang J, Xia J, Zhu Y, Chen C, Shan C and Cui X (2022) Influence of gestational diabetes mellitus on lipid signatures in breast milk and association with fetal physical development. Frontiers in Nutrition 9, 924301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yu X, Wang Y, Bai S, Lai J, Tong X and Xing Y (2022) Longitudinal changes of lactopontin (milk osteopontin) in term and preterm human milk. Frontiers in Nutrition 9, 962802. [DOI] [PMC free article] [PubMed] [Google Scholar]