Abstract
Cryo-electron microscopy (cryo-EM) is a powerful technique capable of investigating samples in a hydrated state, compared to conventional high-vacuum electron microscopy that requires samples to be completely dry. During the drying process, numerous features and details may be lost due to damage caused by dehydration. Cryo-EM circumvents these problems by cryo-fixing the samples, thereby retaining the intact and original features of hydrated samples. This protocol describes a step-by-step cryo-scanning electron microscopy (cryo-SEM) experimental procedure with Chlorella sorokiniana as the subject. By employing filter paper as the sample substrate, we propose a simple and reliable method for cryo-fixation and freeze-fracture of Chlorella sorokiniana in water suspension. The advantage of using filter paper as a substrate lies in its ability to support a thin film of sample, enabling a cold knife to make a cut effortlessly and produce a clean freeze-fractured surface for SEM investigation. By following the approach described in this protocol, both the internal structure and surface morphology of Chlorella sorokiniana can be easily resolved with high quality. This protocol is highly versatile and can be applied to samples dispersed in water or solvents, including cyanobacterial cells, algal cells, and any kind of sample that can be adsorbed onto filter paper.
Key features
• Introducing a reliable way for ideal freeze-fracture of a water-suspended sample using filter paper as substrate.
• Detailed step-by-step descriptions of the entire experiment, covering how to operate the instruments and including some practical experimental tips.
Graphical overview
Keywords: Cryo-SEM, Algae, Chlorella., Freeze fracture, Filter paper
Background
Cryo-SEM is an irreplaceable approach for investigating biological samples in a hydrated state [1–5], and it has been proven to be a powerful tool for studying the microstructure and surface morphology of algae and cells [6–11]. There are four main procedures for sample treatment in cryo-SEM, including cryo-fixation, freeze-fracture, freeze-etching, and sputter coating [12,13]. Cryo-fixation is a technique for rapidly freezing a sample to preserve its structure in a near-native state [14,15]. Typically, a liquid nitrogen bath at atmospheric pressure, liquid nitrogen slush, liquid nitrogen under high pressure (for high-pressure freezing), and liquid ethane and propane are employed as cryogenic substances [14–17]. Freeze-fracture is a technique that fractures the cryo-fixed sample in order to reveal its internal structure, typically by a cold knife or a cryo-ultramicrotome [18]. Freeze-etching is the process of getting the fractured surface etched by sublimation of ice, which reveals fine details of the sample's structure. Sputter coating in cryo-state is similar to the conventional sputter coating, except that the sample is maintained in a cryogenic state.
There are some valuable protocols that have been published regarding plant and animal tissues as well as other biomaterials [1–6,9,13]. Nevertheless, only a limited number of protocols are available for cryo-SEM of algae. Research articles regarding cryo-SEM investigation of algae samples usually include a brief description of the cryo-SEM experiments but do not provide much information about the detailed and visualized experimental procedures. Particularly, information such as how the sample is prepared and how it is freeze-fractured is crucial for obtaining fine details of the internal structures.
The technique of freeze-fracture has been widely used in a broad range of samples, such as fluid samples, hydrated polymers and microgels, and plant tissues [18–20]. In the freeze-fracture procedure, a cold knife in a cryogenic state is commonly employed as the cutting tool: a blade with a sharp edge that is positioned above the sample. It sweeps across the top of the sample in parallel with the sample holder. When it hits the sample, preferably a prominent part or a tip, the sample is then freeze-fractured [1]. During this process, a certain type of sample holder or a specific way to hold the sample is employed. Due to the diverse characteristics of hydrated samples, such as water content, viscosity, strength, shape, or amount, various ways for sample holding are required. Consequently, more and more sample holders or approaches for holding samples are being developed. For example, Mo et al. developed a sample stage (holder) for the freeze-fracture of liquid, semi-liquid, and viscous samples, including chlorella [21]. Nonetheless, reliable protocols with easy and flexible sample-holding approaches that are compatible with various types of samples are still needed.
In this protocol, we propose an easy and reliable approach to hold and support the sample by employing a piece of filter paper as the substrate. The advantage of filter paper lies in its ability to support a thin film of sample, enabling a cold knife to make a cut and produce a clean freeze-fractured surface. This method is versatile and applicable to a broad range of samples, including cyanobacterial cells, algal cells, and any kind of sample that can be adsorbed onto a filter paper. Along with detailed, step-by-step operations for cryo-SEM tests, the authors believe that this protocol will be helpful for researchers to commence the design and operation of their cryo-SEM experiments.
Materials and reagents
Chlorella sorokiniana UTEX 1602 (Institute of Hydrobiology, Chinese Academy of Science, China, FACHB-275). In the following text, Chlorella sorokiniana UTEX 1602 is referred to as Chlorella sorokiniana
Filter paper (Hangzhou Special Paper Industry Co., Ltd, China, NEWSTAR) with a medium filtration speed. The thickness of the filter paper is 0.16 mm, and the pore size is approximately 7 μm. The diameter of a single piece of filter paper is 7 cm
Disposable transfer pipette (1 mL) (Jiangsu KangJian medical apparatus Co., Ltd, China, KANG JIAN, catalog number: KJ619)
Disposable sterile centrifuge tube (50 mL) (Hunan BKMAM Holding Co., Ltd, China, BKMAMLAB, catalog number: 20231206)
Liquid nitrogen (Dalian KeNa Technology Co., Ltd, China)
Equipment
Vacuum cryo transfer system (Leica, model: EM VCT500)
Field emission scanning electron microscopy (JEOL, model: JSM-7610 Plus)
Software and datasets
PC-SEM, v. 4.0.0.8 (Copyright 2006–2018 JEOL Ltd.)
Microsoft Office 2021 (Microsoft, https://www.office.com)
Jiangying Pro (v. 6.0.1, https://www.capcut.cn)
Procedure
Note: Leica EM VCT500 is a system consisting of several independent equipment, including the cryo-module equipped on SEM, which is referred to as VCT500; the Leica EM VCM for cryo-fixation of samples; the Leica EM ACE600 for freeze-fracture, freeze-etching, and sputter coating; and the shuttle to deliver the sample from different equipment in a vacuum and cryogenic state.
-
Preparation of the equipment
-
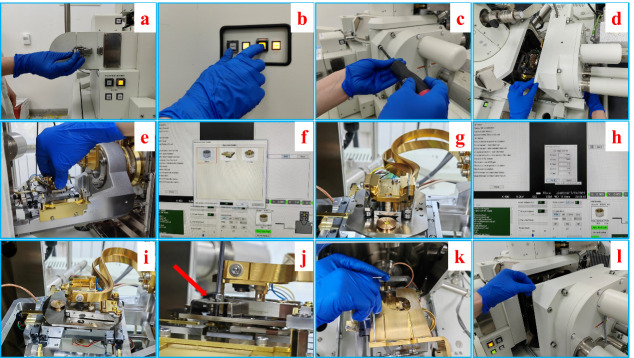
Mount the cryo-stage onto the scanning electron microscope (SEM) (see Figure 1, Video 1).
Loosen the spring snap of the airlock (Figure 1a).
Press the “vent” button on the front panel of the SEM to vent the chamber (Figure 1b).
While venting, unscrew the bolts on the chamber door (Figure 1c).
Pull open the chamber door carefully (Figure 1d).
Slide in the cryo-stage into the dovetail of the SEM stage (Figure 1e).
Set the type and height of the cryo-stage (Figure 1f).
Set the coordinates of the cryo-stage to the right position and angle (Figure 1h).
Mount the stopping piece next to the cryo-stage (red arrow in Figure 1j).
Mount the cold trap on top of the cryo-stage (Figure 1k).
Close the chamber door and evacuate the chamber (Figure 1l).
-
Cool the cryo-stage of the vacuum cryo transfer system (VCT500), ACE600, and the shuttle (see Figure 2, Figure 3).
Fill liquid nitrogen into the dewar of the VCT500, ACE600, and the shuttle, as shown in Figure 2a-2, 2b-2, and 2c-2. Approximately 10 L of liquid nitrogen is required. To help understand the whole procedure, the VCT500, ACE600, and shuttle are shown in Figure 2a-1, b-1, and c-1, with red arrows indicating the main components.
-
Setting temperatures of the cryo-stage of VCT500 and ACE600.
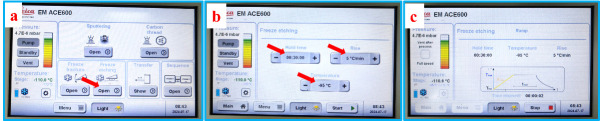
Set the temperature of the cryo-stage of SEM on the control panel of VCT500 and activate the cooling, as shown in Figure 3a. Set the temperature of the cryo-stage of ACE600 and activate the cooling, as indicated in Figure 3b.
Load liquid nitrogen into VCM (see Figure 4b). Fill the reservoir of VCM with liquid nitrogen until 2/3 full.
Set the level of liquid nitrogen on the VCM (see Figure 4c). Select low level on the right of the control panel and press cooling to let liquid nitrogen flow from the reservoir to the liquid nitrogen bath.
-
-
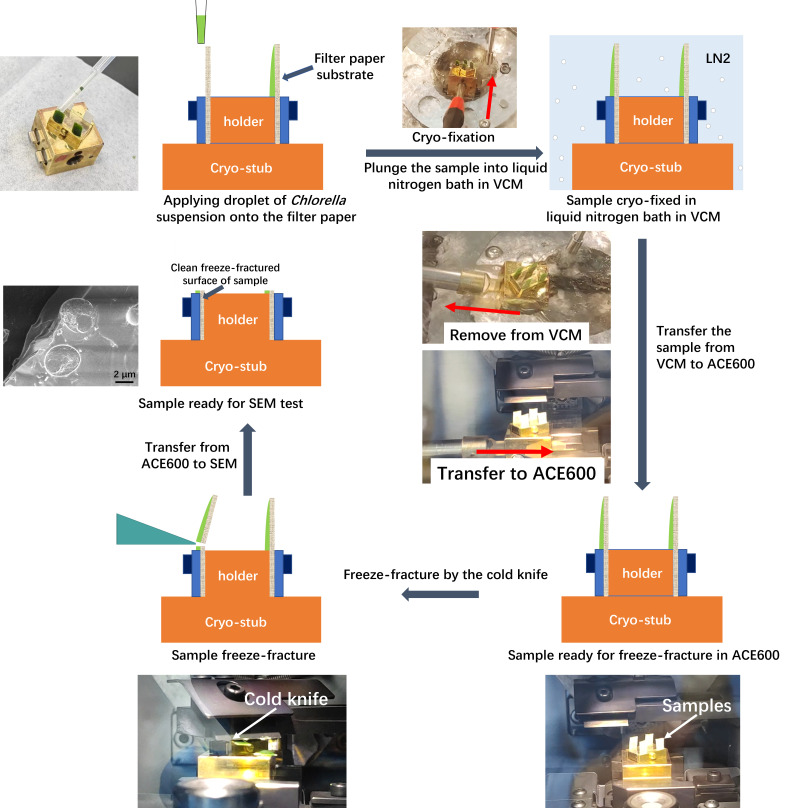
Preparation of the cryo-stub and application of Chlorella sorokiniana onto filter paper substrate
-
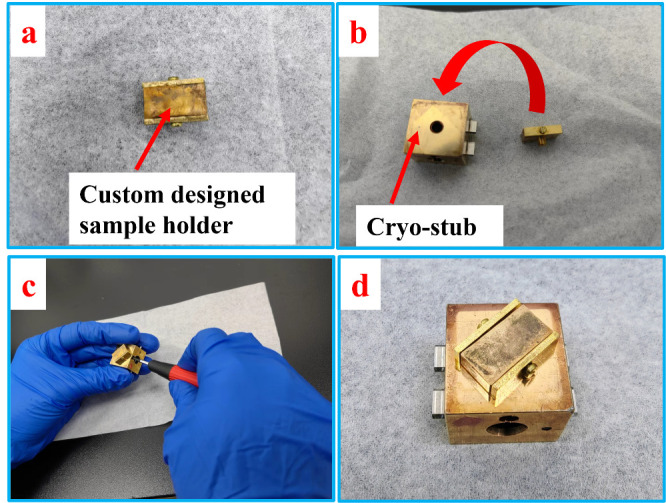
Fix the custom-designed sample holder onto the cryo-stub (Figure 5).
Put the pin of the holder into the hole in the middle of the cryo-stub (Figure 5b).
Tighten the screw in the cryo-stub to hold the pin in place (Figure 5c).
Adjust the holder in the right position on the cryo-stub so that in the consequent freeze-fracture procedure, the filter paper substrate fixed on the holder will be directly facing the cold knife (Figure 5d).
-
Mount the filter paper substrate onto the sample holder.
Cut the filter paper into short strips with 3 mm × 5 mm.
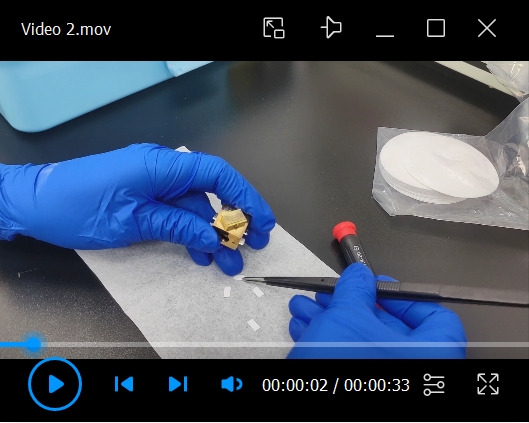
Put the filter paper strips into the slots of the sample holder and tighten the screw to fix the filter paper (see Video 2).
Apply a Chlorella sorokiniana droplet onto the filter paper (see Video 3). Use a 1 mL disposable transfer pipette to draw a small drop of water suspension of Chlorella sorokiniana. Put the tip against the top of the filter paper that is pre-fixed onto the sample holder. Apply the sample slowly onto the filter paper. Wash algal cells in distilled water by centrifugation at a relative centrifugal force of 3,754× g for 5 min.
-
-
Cryo-fixation of the sample in liquid nitrogen bath (see Video 4)
Adjust the position of the cryo-pit inside the liquid nitrogen bath so that the cryo-stub could be slid in.
Use the handling rod to slide the cryo-stub quickly and steadily into the cryo-pit. The cryo-fixation time is approximately 3–5 min.
-
Transfer the cryo-stub from the VCM to ACE600
Hang the shuttle on the dock of VCM and press the attach button to attach the shuttle to VCM.
-
Retrieve the cryo-stub from the VCM to the shuttle (see Video 5).
Adjust the cryo-stub into the right position inside the cryo-pit.
Push the knob at the end of the transfer rod of the shuttle to let the holding-head approach the stub.
Insert the holding head inside the hole of the stub and turn the nob to 180° so the head can hold the stub tightly.
Pull the nob along the transfer rod to retrieve the cryo-stub back to the shuttle.
Press the detach button to pump the shuttle and close the valves, so the stub is in a vacuum and cryogenic state.
-
Remove the shuttle from the VCM and attach it to the ACE600 (see Video 6).
Remove the shuttle from the dock of the VCM.
Hang the shuttle on the dock of the ACE600.
Press the attach button to attach the shuttle to the ACE600.
-
Freeze-fracture of the sample
-
Transfer the cryo-stub from the shuttle to the cryo-stage of the ACE600 (see Video 7).
Push the knob at the end of the transfer rod of the shuttle to slide the cryo-stub onto the stage of ACE600.
Turn the nob to 180° to release the cryo-stub from the holding head.
Pull the nob back to the end of the transfer rod to retrieve the holding head.
-
Freeze-fracture the sample (see Figure 6, Video 8)
Set the height of the cold knife. Select the freeze-fracture module on the control panel of the ACE600, as indicated in Figure 6a. Set the height of the cold knife (Figure 6b).
Sweep the cold knife across the vertically standing samples and make a freeze-fracture (see Video 8). The cold knife is manipulated by a handle on the right side of the ACE600, as indicated by the red arrow in Figure 2b-1. The cold knife moves toward the samples and sweeps across the sample holder by pushing the handle forward. If one of the samples is not completely fractured, make one more sweep of the cold knife to force the upper part of the paper substrate to leave.
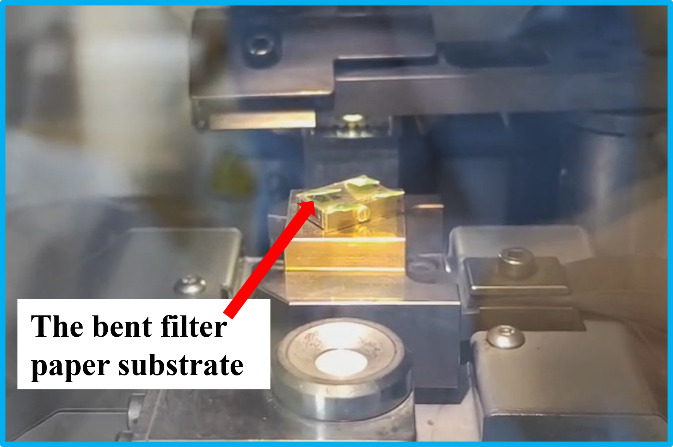
If the position of the cold knife is set too high above the sample holder, the filter paper will be bent instead of completely fractured. This is because the sample suspension film is thicker at the bottom and thinner at the top, where the filter paper might be too soft to be freeze-fractured. If the paper substrate is bent, as shown in Figure 7, the cross-sectional surface of the sample can still be revealed. Therefore, both the surface and cross-section of the sample are exposed at the same time.
Clear the fracture debris on the sample (see Video 9): Pull the cryo-stub from the cryo-stage of ACE600 but keep it inside the chamber, and turn the stub to let the debris fall.
Slide the cryo-stub back to the cryo-stage of ACE600.
-
-
Freeze-etching and sputter coating
Set the freeze-etching parameters. Select the freeze-etching module from the main menu on the touchscreen of ACE600, as shown in Figure 8a. Set the temperature of etching, holding time, and ramp, as shown in Figure 8b.
Bring the cold knife right on top of the fractured sample (see Figure 9) and start the etching process, as indicated in Figure 8c.
Remove the cold knife from the top of the sample holder when the freeze-etching process ends.
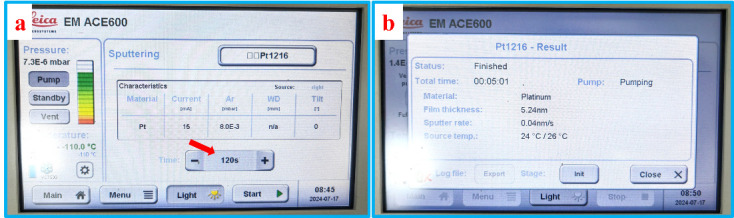
Sputter coating: Select the sputter coating module from the main menu. Set the parameters of sputter coating and press the start button to initiate the sputter coating process, as shown in Figure 10.
-
SEM investigation
-
Retrieve the cryo-stub from ACE600 back to the shuttle and detach the shuttle (see Video 10).
Retrieve the cryo-stub from ACE600 back to the shuttle by manipulating the transfer rod, as described in steps D2b–d.
Detach the shuttle from the ACE600.
-
Attach the shuttle to the VCT500 and transfer the cryo-stub onto the cryo-stage of SEM (see Video 11).
Hang the shuttle on the dock of VCT500 and attach the shuttle.
Before starting to transfer the cryo-stub onto the cryo-stage of SEM, activate the camera on the PC-SEM software and set the cryo-stage in the right position.
When the attaching process is complete, push the knob carefully to slide the cryo-stub onto the cryo-stage of SEM.
-
Operation of SEM (see Video 12).
When the cryo-stub is transferred, adjust the height of it to a working distance of approximately 10 mm.
Turn on the electron beam.
By focusing and adjusting the contrast and brightness, the scattered Chlorella sorokiniana cells in ice can be easily found. The freeze-fractured surface is flat and wide enough to get adequate spots of interest.
-
-
Ending procedures
-
Remove the cryo-stub from the SEM (see Video 13).
Set the cryo-stub into the right position for transfer.
Carefully transfer the cryo-stub from the cryo-stage of the SEM back to the shuttle.
Detach the shuttle from SEM and attach it to the VCM.
Transfer the cryo-stub from the shuttle to the cryo-fixation pit of VCM.
Remove the cryo-stub from the pit and put it on the hot plate.
-
Bake out all the equipment, including VCT500, ACE600, VCM, and the shuttle (see Figure 11).
Press the bake out button on the main menu.
Set the bake out time.
Execute the bake out procedure.
Remove all the lids on the dewar.
-
Figure 1. Step-by-step procedures of the mounting of cryo-stage.
a. Loosening the spring snap of the airlock of the SEM chamber. b. Venting of the SEM chamber by pressing the “vent” button on the front panel. c. Unscrewing the bolts on the chamber door. d. Pulling open the chamber door. e. Sliding in the cryo-stage into the dovetail of the SEM stage. f. Setting the type and height of the cryo-stage. g. Mounted cryo-stage on the stage of SEM. h. Setting the coordinates of the cryo-stage. i. Cryo-stage in ready position for cryo-SEM experiment. j. Mounting the stopping piece next to the cryo-stage. k. Mounting the cold trap on top of the cryo-stage. l. Closing the chamber door.
Video 1. Mounting the cryo-stage into the dovetail of the SEM stage.
Figure 2. Overview of the VCT500, ACE600, and the shuttle, along with the cooling processes.
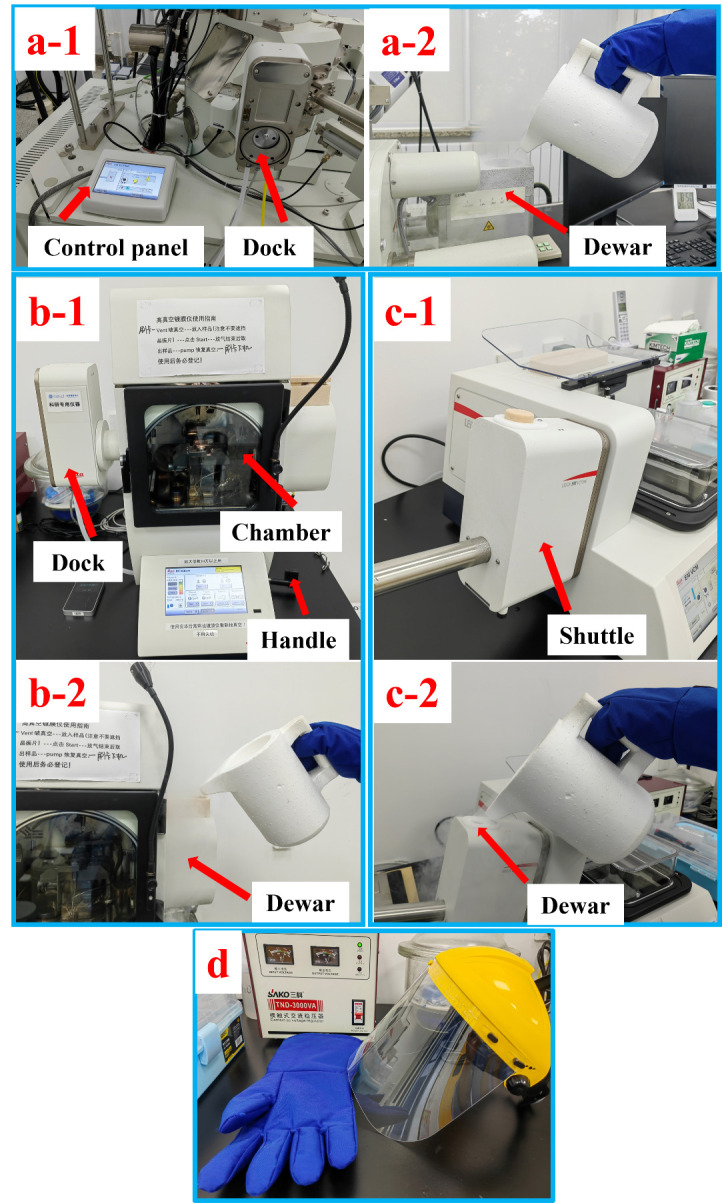
a-1. Control panel and cryo-dock of VCT500. a-2. Filling liquid nitrogen into the dewar of VCT500. b-1. ACE600 with red arrows indicating the dock, chamber, and handle of the cold knife. b-2. Filling liquid nitrogen into the dewar of ACE600. c-1. The shuttle attached to the VCM. c-2. Filling liquid nitrogen into the dewar of the shuttle. d. Mask and cryo-gloves for safety when handling liquid nitrogen.
Figure 3. Setting the temperatures and the activation of cooling on the control panel of VCT500 (a) and ACE600 (b).
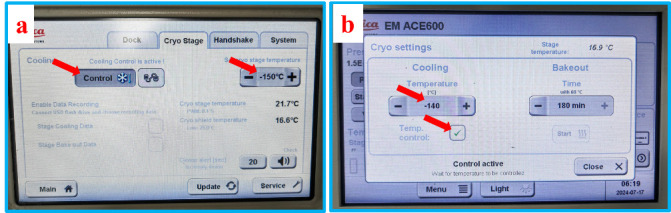
Figure 4. Overview of the VCM and preparations for cryo-fixation.
a. Picture of VCM with the shuttle attached. b. Filling liquid nitrogen into the reservoir of VCM. c. Control panel of VCM. d. Liquid nitrogen bath ready for cryo-fixation.
Figure 5. Setup of the custom-designed sample holder on the cryo-stub.
Video 2. Fixation of the filter paper strips onto the sample holder.
Video 3. Appling a droplet of Chlorella sorokiniana onto the filter paper substrate.
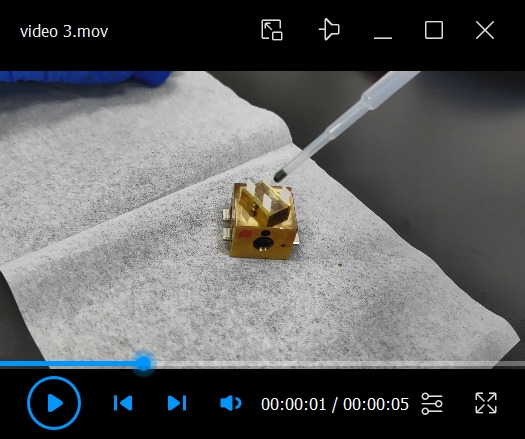
Video 4. Cryo-fixation of the sample in the liquid nitrogen bath.
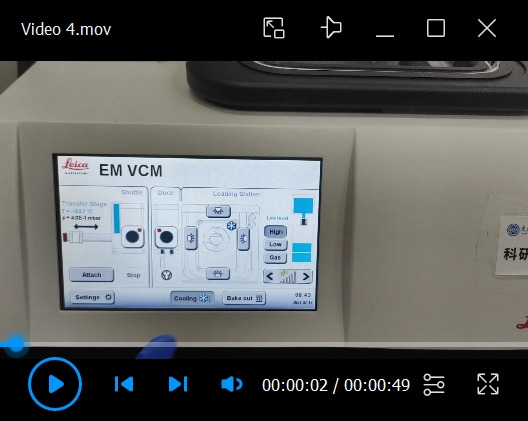
Video 5. Retrieving the cryo-stub from the VCM to the shuttle.
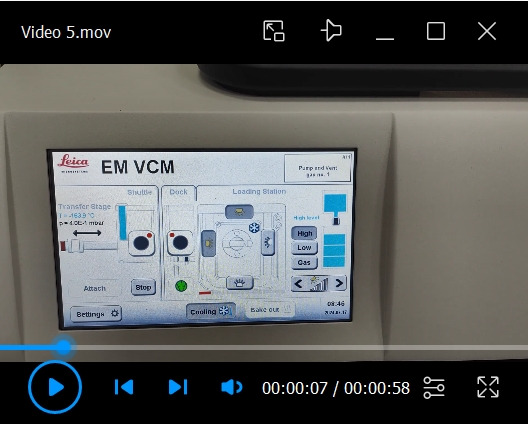
Video 6. Removal of the shuttle from the VCM and its attachment to the ACE600.
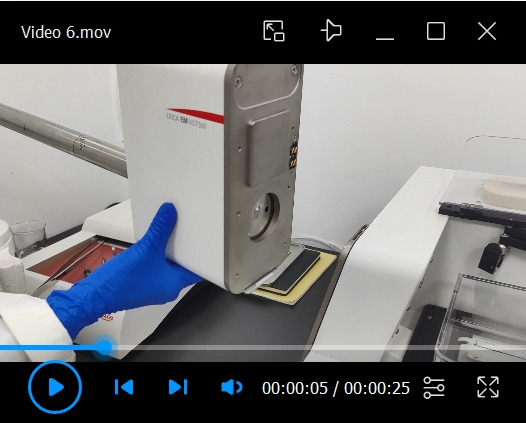
Video 7. Transfer of the cryo-stub onto the cryo-stage of the ACE600.
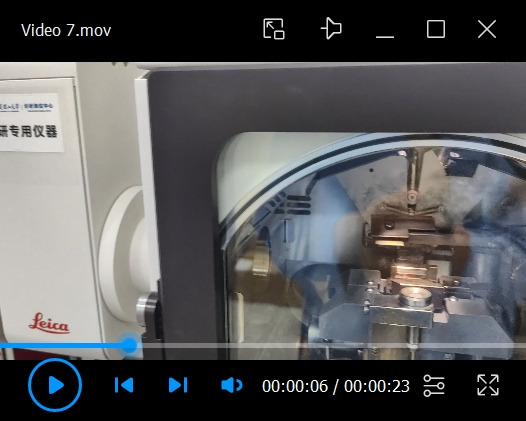
Figure 6. Setting the height of the cold knife.
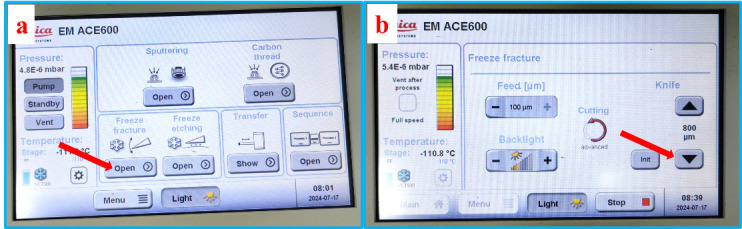
a. Selecting the freeze-fracture module from the main menu. b. Setting the height of the cold knife.
Video 8. Freeze-fracture of the samples with cold knife.
Figure 7. Bent filter paper substrate showing both the cross-section and the surface.
Video 9. Clearing of the debris after freeze-fracture.
Figure 8. Setting up the freeze-etching parameters.
a. Selection of the freeze-etching module from the main menu. b. Setting up the temperature of etching, the holding time, and the ramp. c. Interface of freeze-etching.
Figure 9. Cold knife right on top of the sample during freeze-etching.
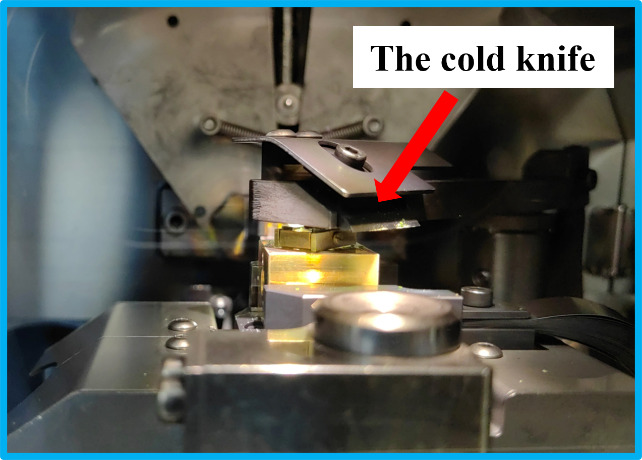
Figure 10. Sputter coating.
a. Setting up the parameters for sputter coating. b. Interface of sputter coating.
Video 10. Retrieving the cryo-stub from ACE600 back to the shuttle.
Video 11. Transferring the cryo-stub from the ACE600 to the cryo-stage of SEM.
Video 12. SEM operation.
Video 13. Retrieving the cryo-stub from the SEM back to the shuttle.
Figure 11. Baking out all the equipment.
a. VCT500. b. ACE600. c. VCM and the shuttle.
Data analysis
The data obtained by this protocol is mainly SEM images, and the data bar is incorporated using Microsoft PowerPoint.
Validation of protocol
The cryo-SEM experiment following this protocol was performed five times, and each replicate showed similar results concerning the freeze-fracture process and the SEM results. SEM images are shown in Figure 12. The images show a clean fractured surface with algae cells embedded in ice (Figure 12a); some cells are cleaved, revealing the internal structure, as shown in Figure 12a-2 and 12a-3. The sample that is etched after freeze-fracture showed scattered algae cells standing on top of the ice, revealing a clearer profile of the cell (Figure 12b). The parameters of freeze-etching are included in general notes.
Figure 12. SEM images of freeze-fractured samples showing the surface and internal structure.
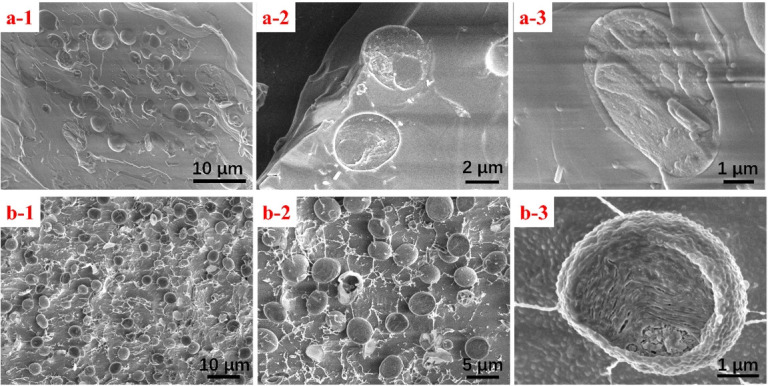
a. Fractured sample of Chlorella sorokiniana without etching. b. After etching.
General notes and troubleshooting
Before venting the SEM chamber, make sure to loosen the spring snap of the airlock; otherwise, the nitrogen flow introduced into the chamber may lead to overpressure and damage the film window of the EDS detector.
When setting the type and height of the cryo-stage, it is recommended to choose the 32 mm holder and set the height as 20 mm to avoid collision with the pole piece.
When mounting the cryo-stage onto the SEM, it is recommended to mount the cryo-stage a day before the cryo-SEM experiments, since the evacuation time could take more than 3 h.
Be cautious when handling liquid nitrogen and make sure to wear a mask and cryo-gloves, as shown in Figure 2d.
As for the temperature of the cryo-stage of SEM, it is recommended to set it to -140 °C or -150 °C. A higher temperature may lead to sample sublimation and contamination of the SEM chamber. As for the temperature of the cryo-stage of ACE600, if a freeze-etching process is performed right after the freeze-fracture, set it to the starting temperature of the etching process. If no freeze-etching is performed, set the temperature to that of the cryo-stage of SEM.
The custom-designed sample holder is made for holding thin film samples vertically so that they can be easily freeze-fractured. The holder is made of copper and both sides are able to hold samples.
The holding head of the transfer shuttle is the component located at the head of the transfer rod. It plays the role of holding the stub during the transfer process. It is made of special resin and is half-transparent.
Before loading the cryo-stub onto the cryo-stage of ACE600, it is important to check the stage temperature prior.
The clearing of the freeze-fractured samples is necessary because it could get rid of debris in the subsequent procedures and avoid the contamination of the SEM chamber.
The freeze-etching procedure plays an important role in getting fine details of the inner structure. Under-etching or over-etching may either cause false surface morphology or damage the Chlorella sorokiniana cells. In this protocol, the freeze-fractured sample is investigated with and without freeze-etching. The freeze-etching parameters are as follows: The temperature of the cryo-stage is -100 °C, the etching temperature is -85 °C, the holding time is 40 min, and the ramp is 5 °C/min. Note that the difference in temperature between the starting temperature and the etching is ideally 15–20 °C.
The cold knife plays as a cold trap during the etching process, so it is recommended to place it as close to the surface of the sample as possible.
Before sputter coating, make sure to remove the cold knife from the top of the samples. The metal used for sputter coating in this experiment is Pt. The ACE600 is equipped with a quartz monitor to record the thickness of Pt. Both filter paper and ice are materials with poor conductivity, so the sputter time is set to 120 s and the thickness of Pt is approximately 5 nm.
The 5-axis coordination of the cryo-stage for sample transfer was set by the supplier upon the installation of the VCT500.
The accelerating voltage of the electron beam in SEM investigation is recommended to be below 3 kV, since an electron beam with higher energy may cause severe surface damage and charging effect.
Frost is one of the primary obstacles to a successful cryo-SEM experiment. It can be a thick layer of nanocrystals covering the sample features, and it can get the valves and cryo-stub stuck during the transfer process. Therefore, operators should minimize the frosting on samples and components of the equipment.
When pulling the cryo-stub from the liquid nitrogen bath of VCM, it will contact with the air for a few seconds. Although VCM has a patent to avoid the formation of frost during this process, it is important that operators perform this transfer as quickly as possible
Be aware that when attached to the VCM, the shuttle is connected to ambient air. A long time of exposure to air will cause severe frosting on the cryo-stage and valves. Make sure to detach the shuttle from VCM whenever the transfer process is done. When transferring the sample from the VCM, only attach the shuttle when the sample is ready for transfer.
Keep in mind that the sample should be immersed in liquid nitrogen throughout the whole cryo-fixation process.
Tools like tweezers and handling rods should be placed on the hot plate to be heated up immediately after being used in liquid nitrogen. They are ready for use again only when the frost is completely gone.
Use liquid nitrogen free of ice crystals, especially the liquid nitrogen used for the LN2 bath for cryo-fixation in the VCM.
The bake out is the process of heating the dewar and the inner components of VCM, ACE600, VCT500, and the shuttle to remove all the frost, water, and contaminants. After the bake-out process, the equipment is dry, clean, and ready for use.
It is recommended to set the bake-out time to maximum. Otherwise, the residual water could turn into ice and get the components stuck in the consequent cryo-SEM experiment.
A sputter-coated sample can technically be freeze-etched to remove excessive ice from the surface, but it is not recommended. A metal film a few nanometers thick could change the freeze-etching process of ice drastically, and the metal film would be obvious on the surface when the ice beneath it is gone.
Carbon tape is not a good option to adhere samples onto stubs and holders in cryo-experiments; use toothpaste or saturated sucrose solution instead.
Acknowledgments
This work was funded by the Foundation of Instrumental Maintenance and Research by Dalian University (No. SYSWX202301).
Competing interests
The authors declare no competing financial interests.
References
- 1. Fujikawa S. and Endoh K.(2020). Cryo-Scanning Electron Microscopy to Study the Freezing Behavior of Plant Tissues. Methods Mol Biol. 2156: 99-117. [DOI] [PubMed] [Google Scholar]
- 2. Gosden R. G., Yin H., Bodine R. J. and Morris G. J.(2010). Character, distribution and biological implications of ice crystallization in cryopreserved rabbit ovarian tissue revealed by cryo-scanning electron microscopy. Hum Reprod. 25(2): 470-478. [DOI] [PubMed] [Google Scholar]
- 3. Nakatomi R., Hayashida T., Fujimoto K., Tohyama K. and Hashikawa T.(2005).Cryo-SEM and subsequent TEM examinations of identical neural tissue specimen. Brain Res Protoc. 14(2): 100-106. [DOI] [PubMed] [Google Scholar]
- 4. Read N. D. and Jeffree C. E.(1991). Low‐temperature scanning electron microscopy in biology. J Microsc. 161(1): 59-72. [DOI] [PubMed] [Google Scholar]
- 5. Yan H., Wang Y., Zhang J., Cui X., Wu J., Zhou J., Chen Y., Lu J., Guo R., Ou M., et al.(2021). Rice Root Hair Phenotypes Imaged by Cryo-SEM. Bio Protoc. 11(11): e4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Y., Centonze V. E., Verkhovsky A. B. and Borisy G. G.(1994). Ultra-High Resolution Cryo-SEM and Specimen Preparation for Cytoskeleton. Acta Histochem Cytochem. 27(5): 507-509. [Google Scholar]
- 7. Fredericq S., Krayesky-Self S., Sauvage T., Richards J., Kittle R., Arakaki N., Hickerson E. and Schmidt W. E.(2019). The Critical Importance of Rhodoliths in the Life Cycle Completion of Both Macro- and Microalgae, and as Holobionts for the Establishment and Maintenance of Marine Biodiversity. Front Mar Sci. 5: e00502. [Google Scholar]
- 8. Jantschke A., Pinkas I., Hirsch A., Elad N., Schertel A., Addadi L. and Weiner S.(2019). Anhydrous β-guanine crystals in a marine dinoflagellate: Structure and suggested function. J Struct Biol. 207(1): 12-20. [DOI] [PubMed] [Google Scholar]
- 9. Mor Khalifa G., Kahil K., Erez J., Kaplan Ashiri I., Shimoni E., Pinkas I., Addadi L. and Weiner S.(2018). Characterization of unusual MgCa particles involved in the formation of foraminifera shells using a novel quantitative cryo SEM/EDS protocol. Acta Biomater. 77: 342-351. [DOI] [PubMed] [Google Scholar]
- 10. Shaked N., Addadi S., Goliand I., Fox S., Barinova S., Lia Addadi. and Weiner S.(2023). Intra- to extracellular crystallization of calcite in the freshwater green algae Phacotus lenticularis. Acta Biomater. 167: 583-592. [DOI] [PubMed] [Google Scholar]
- 11. Yin X., Ziegler A., Kelm K., Hoffmann R., Watermeyer P., Alexa P., Villinger C., Rupp U., Schlüter L., Reusch T. B. H., et al.(2018). Formation and mosaicity of coccolith segment calcite of the marine algae Emiliania huxleyi . J Phycol. 54(1): 85-104. [DOI] [PubMed] [Google Scholar]
- 12. Efthymiou C., Williams M. A. and McGrath K. M.(2017). Revealing the structure of high-water content biopolymer networks: Diminishing freezing artefacts in cryo-SEM images. Food Hydrocolloids. 73: 203-212. [Google Scholar]
- 13. Liang J., Koo B., Wu Y., Manna S., Noble J. M., Patel M., Park J. H., Kozak D., Wang Y., Zheng J., et al.(2022). Characterization of Complex Drug Formulations Using Cryogenic Scanning Electron Microscopy(Cryo‐SEM). Curr Protocol. 2(4): e406. [DOI] [PubMed] [Google Scholar]
- 14. Aston R., Sewell K., Klein T., Lawrie G. and Grøndahl L.(2016). Evaluation of the impact of freezing preparation techniques on the characterisation of alginate hydrogels by cryo-SEM. Eur Polym J. 82: 1-15. [Google Scholar]
- 15. Wu Y., Liang J., Rensing K., Chou T. M. and Libera M.(2014). Extracellular Matrix Reorganization during Cryo Preparation for Scanning Electron Microscope Imaging of Staphylococcus aureus Biofilms. Microsc Microanal. 20(5): 1348-1355. [DOI] [PubMed] [Google Scholar]
- 16. Kaberova Z., Karpushkin E., Nevoralová M., Vetrík M., Šlouf M. and Dušková-Smrčková M.(2020). Microscopic Structure of Swollen Hydrogels by Scanning Electron and Light Microscopies: Artifacts and Reality. Polymers. 12(3): 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishino Y., Miyazaki K., Kaise M. and Miyazawa A.(2022). Fine cryo-SEM observation of the microstructure of emulsions frozen via high-pressure freezing. Microscopy. 71(1): 60-65. [DOI] [PubMed] [Google Scholar]
- 18. Wightman R.(2022). An Overview of Cryo-Scanning Electron Microscopy Techniques for Plant Imaging. Plants. 11(9): 1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Isa L.(2013). Freeze-fracture Shadow-casting(FreSCa) Cryo-SEM as a Tool to Investigate the Wetting of Micro- and Nanoparticles at Liquid–Liquid Interfaces. Chimia. 67(4): 231. [DOI] [PubMed] [Google Scholar]
- 20. Liang J., Xiao X., Chou T. M. and Libera M.(2021). Analytical Cryo-Scanning Electron Microscopy of Hydrated Polymers and Microgels. Acc Chem Res. 54(10): 2386-2396. [DOI] [PubMed] [Google Scholar]
- 21. Mo J., Zhang S., Su Q. and Liu F.(2021). A new cryo-scanning electron microscopy sample stage and its application in morphology characterization of chlorella. 1. J Chin Electron Microsc Soc. 40(3): 294 300 300 .(In Chinese) [Google Scholar]