Abstract
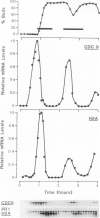
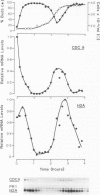
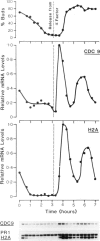
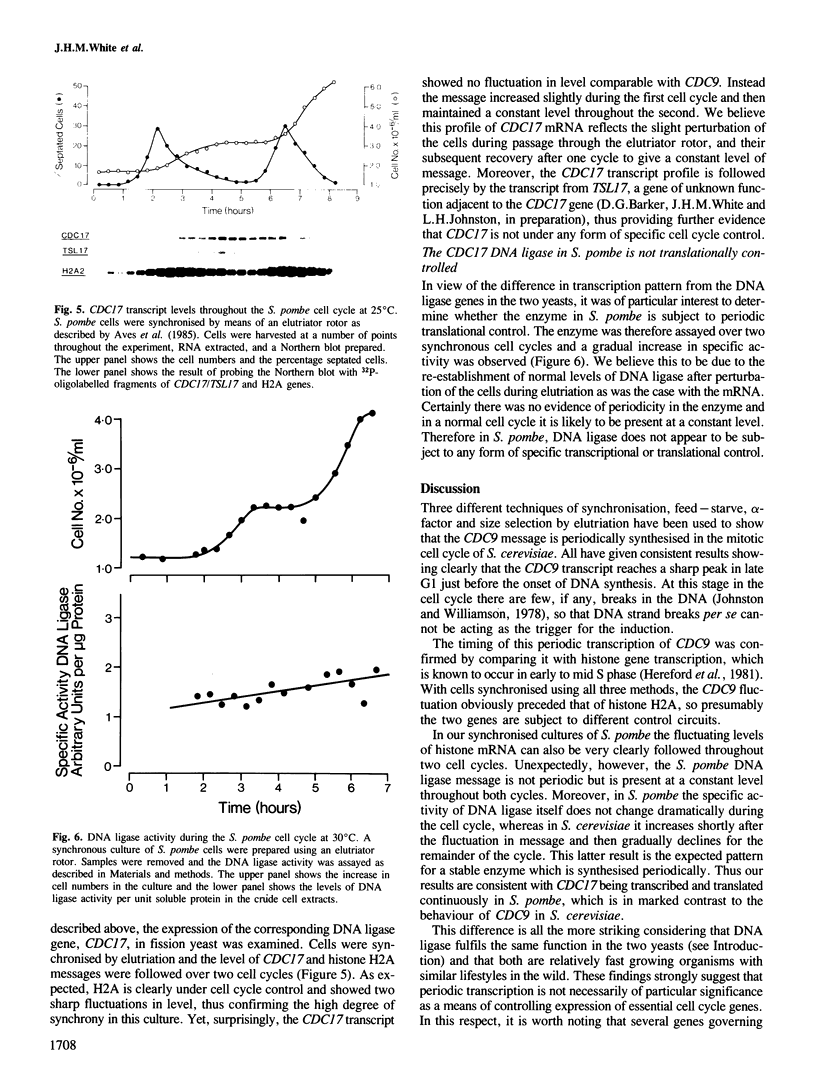
Using cultures synchronised by three independent procedures, we have shown that the CDC9 gene, coding for DNA ligase, is periodically expressed in the Saccharomyces cerevisiae cell cycle. The level of CDC9 transcript increases many fold in late G1 reaching a peak at about the G1/S phase boundary and preceding the peak in histone message by some 20 min. The level of DNA ligase itself also fluctuates, showing the expected pattern for a stable enzyme synthesised periodically. In contrast, the transcript from the DNA ligase gene (CDC17) of Schizosaccharomyces pombe is present at a constant level throughout the cell cycle, and no fluctuation in amount was detected, although the histone H2A showed the expected periodic synthesis. Furthermore, DNA ligase activity remains at a constant level during the S. pombe cell cycle showing that there is unlikely to be any form of translational control. These contrasting modes of expression of the DNA ligase genes in the two organisms suggests that when periodic transcription is observed from an essential cell cycle gene, it may have no particular significance for regulating progress through the cell cycle. Also, regulatory circuits may be less well conserved between organisms than the processes they control and thus different organisms may utilise quite different modes of control to achieve the same ends.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aves S. J., Durkacz B. W., Carr A., Nurse P. Cloning, sequencing and transcriptional control of the Schizosaccharomyces pombe cdc10 'start' gene. EMBO J. 1985 Feb;4(2):457–463. doi: 10.1002/j.1460-2075.1985.tb03651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. G., Johnson A. L., Johnston L. H. An improved assay for DNA ligase reveals temperature-sensitive activity in cdc9 mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1985;200(3):458–462. doi: 10.1007/BF00425731. [DOI] [PubMed] [Google Scholar]
- Barker D. G., Johnston L. H. Saccharomyces cerevisiae cdc9, a structural gene for yeast DNA ligase which complements Schizosaccharomyces pombe cdc17. Eur J Biochem. 1983 Aug 1;134(2):315–319. doi: 10.1111/j.1432-1033.1983.tb07568.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Hereford L., Fahrner K., Woolford J., Jr, Rosbash M., Kaback D. B. Isolation of yeast histone genes H2A and H2B. Cell. 1979 Dec;18(4):1261–1271. doi: 10.1016/0092-8674(79)90237-x. [DOI] [PubMed] [Google Scholar]
- Johnston L. H., Barker D. G., Nurse P. Cloning and characterization of the Schizosaccharomyces pombe DNA ligase gene CDC17. Gene. 1986;41(2-3):321–325. doi: 10.1016/0378-1119(86)90114-9. [DOI] [PubMed] [Google Scholar]
- Johnston L. H., Game J. C. Mutants of yeast with depressed DNA synthesis. Mol Gen Genet. 1978 May 3;161(2):205–214. doi: 10.1007/BF00274189. [DOI] [PubMed] [Google Scholar]
- Johnston L. H., Williamson D. H. An alkaline sucrose gradient analysis of the mechanism of nuclear DNA synthesis in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1978 Aug 17;164(2):217–225. doi: 10.1007/BF00267387. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsumoto S., Yanagida M. Histone gene organization of fission yeast: a common upstream sequence. EMBO J. 1985 Dec 16;4(13A):3531–3538. doi: 10.1002/j.1460-2075.1985.tb04113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975 Aug 14;256(5518):547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- Peterson T. A., Prakash L., Prakash S., Osley M. A., Reed S. I. Regulation of CDC9, the Saccharomyces cerevisiae gene that encodes DNA ligase. Mol Cell Biol. 1985 Jan;5(1):226–235. doi: 10.1128/mcb.5.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H. Replication of the nuclear genome in yeast does not require concomitant protein synthesis. Biochem Biophys Res Commun. 1973 Jun 8;52(3):731–740. doi: 10.1016/0006-291x(73)90998-4. [DOI] [PubMed] [Google Scholar]