ABSTRACT
Background
Endovascular procedures are the preferred method for treating peripheral arterial disease. However, limited imaging options during these procedures, such as X‐rays and contrast media, expose patients and healthcare professionals to potentially harmful radiation. This study introduces a robotic ultrasound system (RUSS) for navigating endovascular procedures in order to reduce radiation and provide additional information.
Methods
The RUSS comprises a seven‐degree‐of‐freedom robotic arm that navigates an ultrasound transducer across a specific region of interest. The system is controlled by a self‐programed software designed to navigate the robotic arm in a methodical and reproducible manner using a foot switch.
Results
An endovascular surgeon investigated the guidance and visibility of various guidewires and successfully implanted three stents in a vascular leg phantom using the RUSS without further radiation exposure.
Conclusions
The innovative set‐up has several potential applications, including radiation‐free endovascular procedures as well as health screening and diagnostic support in vascular medicine.
Keywords: 3D ultrasound, 3D vessel model, endovascular procedure without radiation, peripheral arterial disease (PAD), robotic ultrasound guidance, robotic ultrasound system (RUSS)
1. Introduction
Endovascular therapy is widely accepted for treating vascular diseases. Nowadays, this minimally invasive approach offers high technical success rates and lower morbidity and even mortality than open surgery [1]. However, it also poses significant radiation risks to both patients and interventionalists due to the need for X‐ray visualisation and contrast media. In addition, the contrast agent carries a risk of renal failure, especially in patients with comorbidities such as diabetes mellitus and already compromised renal function [2, 3]. Ultrasound guidance is a feasible alternative to fluoroscopy and contrast medium for visualising and navigating endovascular procedures [4, 5, 6]. Ascher et al. [5, 6] have demonstrated repeatedly that vascular reconstruction can be achieved using intraprocedural ultrasound imaging. In addition to avoiding ionising radiation, ultrasound provides the examiner with additional information such as kinetics of the blood flow and haemodynamic relevance of vascular pathologies [7, 8].
Nevertheless, US‐guided endovascular procedures require additional and trained intra‐operative staff; therefore, this technique has not been widely adopted. For this reason, some research groups have already published interesting approaches to robotically guided ultrasound, but none of them have yet proven themselves in everyday clinical practice or are intended for endovascular use [9, 10, 11, 12]. There have been some developments in the field of endovascular robotics, but they remain dependent on X‐rays and only reduce the personnel radiation exposure. However, these have not yet become established in clinical use due to high costs and insufficient benefit [13, 14]. To the best of our knowledge, there is currently no robotic ultrasound system that has been implemented in clinical settings. von Haxthausen et al. [15] demonstrated that a RUSS could scan a leg phantom while maintaining the visible vessel lumen. However, it was not feasible for the interventionalist to direct the robotic arm with the ultrasound probe along the vessel or adjust the ultrasound image in response to the vessel angulation. Consequently, there was a necessity to develop a RUSS with the capability to navigate actively during endovascular procedures and direct the robotic arm accordingly.
This study demonstrates the feasibility of RUSS guidance during endovascular procedures such as visualisation of wires and stent implantation using a specifically designed vascular model.
2. Materials and Methods
The local Ethics Committee of the University of Luebeck, Germany did not raise any concerns regarding the study (IRB: 22‐140) as conducted.
2.1. Ultrasound Capable Vessel Model
An ultrasound‐capable phantom imitating the superficial femoral artery was self‐designed and produced using additive manufacturing, as shown in Figure 1. The Phantom was designed and built in collaboration with the Technische Hochschule (TH) Luebeck. The model consists of a wax gel made of polyethylene and mineral oil with a 00–40 shore hardness rating, resulting in high‐quality sonographic imaging. A plastic femur bone is included to enhance the realism of the model and allows US and fluoroscopic orientation as needed. The vessel system, constructed from silicone tubes with an inner diameter of 6 mm, can be accessed from both sides of the model. It allows for the filling and venting of liquids as well as perfusion simulation [16]. In this study, the vascular system was filled with sterile water.
FIGURE 1.
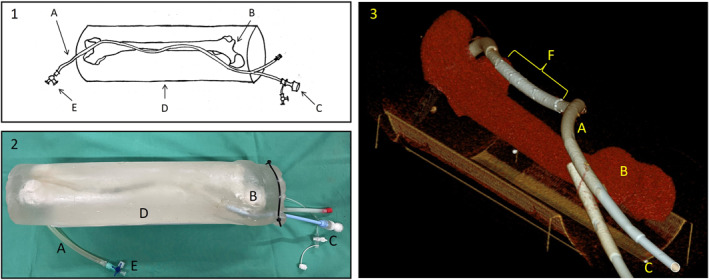
Ultrasound‐capable phantom imitating the superficial femoral artery; sketch of the model (1), image of the model (2) and CT image reconstruction post Stent implantation (3); components of the model: (A) silicone tube inner diameter 6 mm; (B) plastic femur bone; (C) vascular introducer sheath 16F; (D) outer shell made of wax gel; (E) three way stop cock; (F) three endovascular stents in the artificial vessel.
2.2. Robotic Ultrasound System (RUSS)
The RUSS included a lightweight robot (LBR iiwa 14 R820, KUKA, Augsburg, Germany) designed for human‐robot collaboration and a Philips Epiq 7 (Hamburg, Germany) ultrasound station equipped with a 3D ultrasound transducer (XL14‐3 xMATRIX Transducer, Philips, Hamburg, Germany). The transducer was attached to the robot arm using a self‐designed 3D‐printed ultrasound probe holder, as shown in Figure 2. A hybrid force‐motion control was used to maintain a constant force of 5 N on the probe on the phantom during the scan. The robot used has built‐in torque sensors in its joints, which provide an estimate of the force acting on the US probe attached to the end effector. This enables force control without the need for an external sensor. The ultrasound volumes were transmitted in 3D and in real time through the PLUS toolkit [17], which communicated with the US station using a proprietary communications protocol. The 3D ultrasound volumes were streamed continuously at a frequency of 4 Hz. The volume size was 125 × 331 × 96 voxels with a volume spacing of 0.4 × 0.15 × 0.21 mm/voxel. For the experiments, a depth of 5 cm was set at the station. The communication with the robot is enabled by utilising an in‐house middleware. This middleware provides a bidirectional communication between the robot controller and the computer. The software application that controls the robot's movements is written in Python (Python Software Foundation, Beaverton, US) and runs on a standard personal computer. While there are different approaches for automatic vessel detection, the RUSS uses a template matching method, as this has proven to be relatively robust against phantom changes and changes in the settings of the ultrasound station, as shown in Figure 3. The objective was to create a system that produces reproducible results for vessel detection without any black box behaviour, such as that found in neural networks. The system should also be robust to changes in the parameters of the US station without requiring large data sets for training. A similar method was also used and published by von Haxthausen et al. [18].
FIGURE 2.
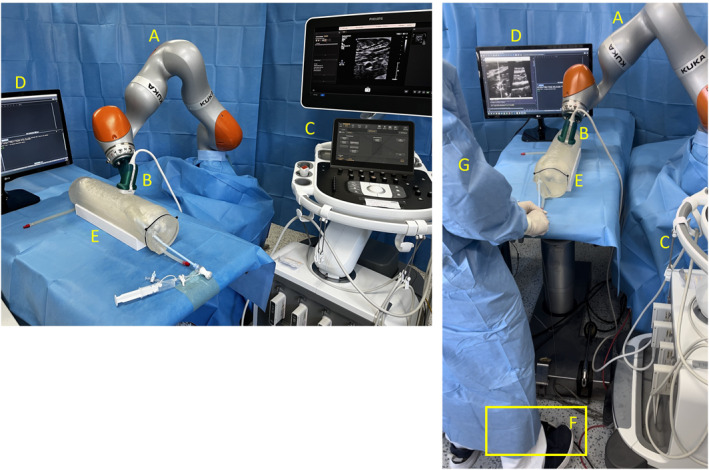
Experimental setup of the robotic‐guided ultrasound system for endovascular interventions including a foot switch for navigation. (A) Robot arm KUKA iiwa 14 LBR 820 with self‐designed 3D printed ultrasound probe holder (B); (C) Ultrasound system Philips Epiq 7; (D) monitor showing ultrasound image in two positions (cross‐section and longitudinal‐section); (E) ultrasound‐capable three‐dimensional vessel model; (F) foot switch Olympus RS31 controlling the robot arm; (G) interventionalist with both hands free for endovascular procedure.
FIGURE 3.
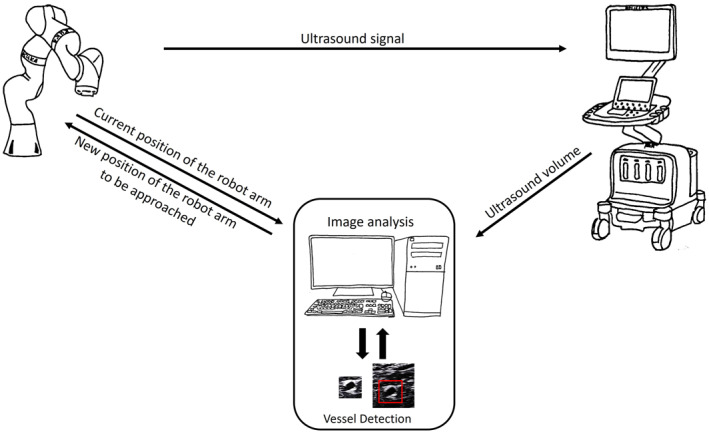
Setup of the robotic ultrasound system (RUSS) for the automatised pilot scan. The ultrasound volume from the ultrasound station is sent to a workstation for visualisation and vessel detection, performed using a template matching method. Once the template matching has been processed, the newly calculated position for the ultrasound probe is sent to the robot arm.
Prior to performing an automatic pilot scan of the target vessel with the robotic ultrasound probe, the probe is manually placed on the selected vessel to allow full visualisation of the vessel cross‐section. At the start of the scan, the physician digitally marks the cross‐section of the vessel to be tracked (refer to Figure 4). This section displays the template T that will be compared to the subsequent US images I. The similarity between the template T and each (x, y)‐position of the image I is determined by calculating the normalised correlation coefficient:
FIGURE 4.
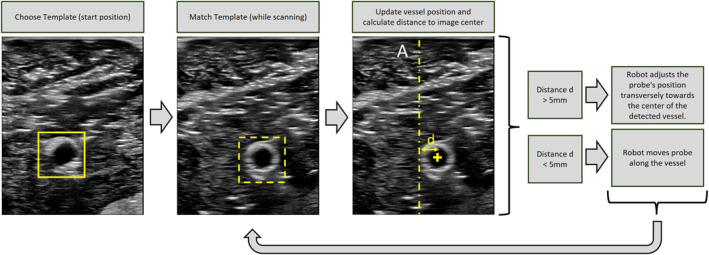
Procedure of template matching vessel tracking. At the beginning of the scan, a vessel template is selected. This template is then searched for in the new ultrasound (US) images during the scan. The position with the highest correlation coefficient is indicated by the thicker dashed yellow box. This position is then updated, and the distance from the vessel's position to the image centre is calculated. If the distance between the detected vessel centre and the centre of the US image displayed is more than 5 mm, the robot will adjust the probe position by moving it towards the centre of the vessel. Otherwise, if the distance is less than 5 mm, the robot moves the probe along the scan.
The resulting matrix C(x, y) contains a similarity value with the template for each (x, y) position in the source image. The best match is then considered the new vessel position. In order to capture the vessel completely and in the centre of the US image, the movement of the probe must be adjusted transversely to the scan direction along the vessel. If the distance between the detected vessel centre and the centre of the US image displayed is more than 5 mm, the robot adjusts the probe position by moving it towards the centre of the vessel. Otherwise, if the distance to the centre of the vessel is less than 5 mm, the robot moves the probe along the scan. Please see Figure 5, detailing all steps involved. The path taken during the pilot scan, which is supervised by the physician and technician, is recorded. The goal of this automatic pilot scan is to obtain a clear visualisation of the vascular path, depicting the cross‐section of the vessel at the centre of the image. In order to guide the US probe on the recorded path along the vessel by the interventionalist, a foot pedal (Olympus RS‐31H foot switch, Hamburg, Deutschland) for controlling the robot arm was integrated into the system. The interventionalist was able to move the RUSS back and forth along the vessel path using buttons 1 and 2 on the foot switch. To ensure reliable visualisation of the vessel in longitudinal section and wire identification, we have successfully developed an option to adapt the longitudinal section of the vessel to the image of the vessel cross‐section using the foot control. The foot control has buttons 3 and 4 that can be used to adjust the vessel section. Please see Figure 5, detailing all steps involved. The experimental setup is illustrated in Figure 2.
FIGURE 5.
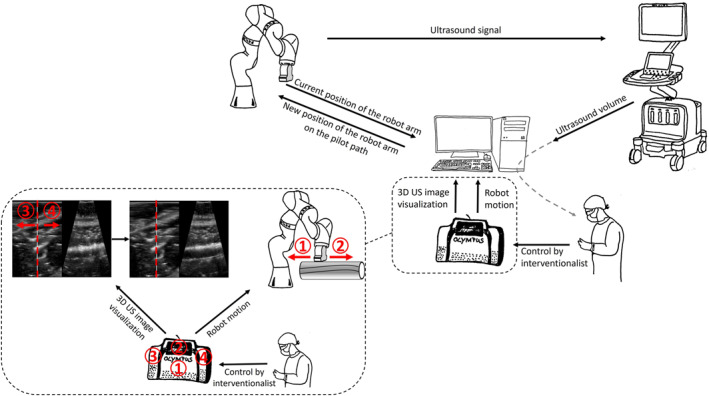
Set‐up of a RUSS equipped with a foot switch for the interventionalist to direct the robotic arm in endovascular procedures. The interventionalist can manipulate the robot's motion on the pilot path, forwards (2) and backwards (1), and adjust the 3D ultrasound image visualisation by correcting the cross section (red dotted line) of the vessel, left (3) and right (4). Technical term abbreviations, including ‘3D’, were explained upon first use.
2.3. Materials for Endovascular Procedure
The visualisation assessment of guide wires during the procedure simulation of the RUSS included several wires: the Back‐Up Meier J‐Tip Steerable Guidewire from Boston Scientific (Massachusetts, US) in a 0.035 in diameter with a length of 185 cm, the Radiofocus Guide Wire M Stiff Type Straight from Terumo (Terumo Europe, Düsseldorf, Germany) with a 0.035 in diameter and a length of 150 cm, the Hi‐Torque Steelcore 18 Guide Wire with Microglide Coating from Abbott (Chicago, US) with a 0.018 in diameter and a length of 300 cm, and the Hi‐Torque Command ES Guide Wire with Hydrophilic Coating from Abbott with a 0.014 in diameter and a length of 300 cm. The stent‐implantation using the RUSS was performed using a self‐expanding stent system over‐the‐wire with a size of 8 mm in diameter, 40 mm in length, and a 75 cm delivery catheter (Boston Scientific Innova).
3. Results
The mapping of the vessel anatomy, visualisation and procedure guidance by the RUSS including subsequent stent implantation was a technical success. The endovascular specialist was able to direct the RUSS along the vessels to the area of interest using an additional foot pedal, enabling a safe endovascular procedure without the need for any radiation in an experimental setting. The path is determined by a previously performed automated pilot scan (refer to Section 2.2). This scan must be performed once for each new case.
3.1. Robot Guided Assessment of Sonographic Guide Wire Visibility
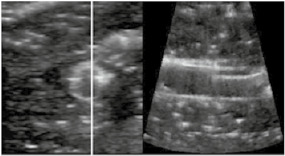
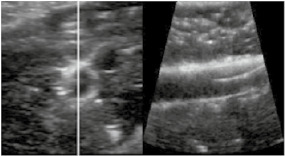
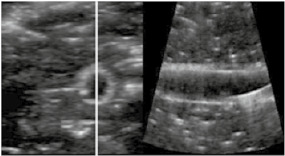
By using RUSS, the endovascular specialist was able to examine a variety of guidewires in terms of their ultrasound visibility. Using the foot switch to adjust the longitudinal and cross‐sectional view, there were no issues in displaying the endovascular guide wires and rating their visibility, as shown in Table 1. The RUSS enables guide wire tip detection and navigation across the phantom using US imaging. Adjustment of the US probe position and the imaging of the area of interest during a procedure was feasible using the foot switch.
TABLE 1.
Endovascular guidewires used during the Intervention and their visibility in the robotic assisted ultrasound volume.
| Guide wire | Length | Diameter | Ultrasound image | Visibility |
|---|---|---|---|---|
| Back‐up Meier J‐Tip Steerable Guidewire from Boston Scientific | 185 cm | 0.05 inch |
|
++ |
| Radiofocus Guide Wire M Stiff Type Straight from Terumo | 150 cm | 0.035 inch |
|
+++ |
| Hi‐Torque Steelcore 18 Guide Wire with Microglide Coating from Abbott | 300 cm | 0.018 inch |
|
+++ |
| Hi‐Torque Command ES Guide Wire with Hydrophilic Coating from Abbott | 300 cm | 0.014 inch |
|
+ |
3.2. Robot Guided Sonographic Stent Implantation
In the same manner as guide wire tracking using RUSS with the foot switch, the interventionalist was able to keep both hands free for performing stent implantation with ease. The sonographic image always offered both longitudinal and cross‐sectional views of the vessel, guaranteeing optimal vessel visualisation before stent implantation (as shown in Figure 6). Three self‐expanding stents were implanted into the vascular model using robotic guided ultrasound guidance alone. The results of the stent implantations were verified via ultrasound imaging by the RUSS.
FIGURE 6.
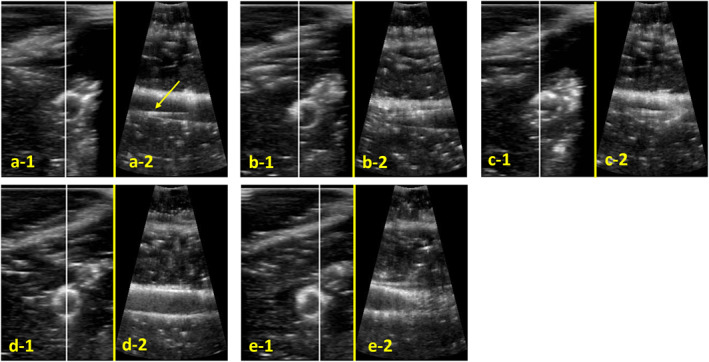
Robot assisted sonographic images during stent implantation, cross section on the left‐hand side (‐1); the white line in the cross section image marks the section of the three‐dimensional image for the longitudinal section on the right‐hand side (‐2): (a) visualisation of guide wire (marked with arrow); (b) visualisation of stent tip; (c) start stent expansion; (d and e) stent completely expanded.
4. Discussion
This study demonstrates the feasibility of performing an endovascular procedure on a vascular model using a robot‐guided ultrasound system. The RUSS was used to visualise the vascular anatomy of the phantom, allowing clear tracking of guidewires and catheters for stent implantation using ultrasound navigation. The ability to guide endovascular procedures using ultrasound imaging instead of radiation has already been demonstrated by Asher et al. [19]. Nevertheless, fluoroscopic guidance is still the most popular imaging modality for endovascular procedures. Considering the health risks associated with X‐rays for both patients and staff, it is imperative to explore alternative imaging methods. The RUSS provides an excellent opportunity for the quick implementation of ultrasound in endovascular procedures, as it does not require the deployment or training of additional specialist staff.
The use of robots in healthcare, particularly surgical robots, has risen notably in recent years [20]. Although initial concerns about patient safety have been addressed by extensive safety measures, some residual risks of technical problems cannot be eliminated entirely [21]. This study did not report any notable malfunction of the robot, such as exerting excessive force on the model or moving in the incorrect direction. The possible risks to individuals regarding robot use must not be ignored. However, adopting a collaborative robot system, like the KUKA LBR iiwa 14 R820, created particularly for human‐robot collaboration, can greatly reduce the occurrence of malfunctions that pose a threat to human safety. Nonetheless, it is crucial to establish relevant safety procedures when handling the systems concerning humans.
The benefits of the RUSS are obvious. The ultrasound probe is manoeuvred calmly and steadily, reducing the risk of human error such as resting tremors. It enables a procedure that typically requires two interventionalists to be carried out by just a single person. In the same way, the use of surgical robots has been shown to have similar benefits, with ergonomic working, tremor stabilisation and 4‐arm control by a single surgeon being just a few of the advantages [22]. In this study, we employed a three‐dimensional ultrasound probe to enabling a vessel view in both longitudinal and cross‐sectional views. The use of a conventional two‐dimensional ultrasound probe is also possible.
Given the shortage of skilled personnel and financial constraints within the healthcare system, the aim of providing additional training and hiring qualified personnel appears distant. To achieve the objective of ultrasound guided peripheral endovascular interventions in the future, a new navigation system needs to be established by combining ultrasound visualisation techniques with robotic guidance of the transducer. The advantages of ultrasound assisted by robots are striking: the robot can perform the same procedure as many times as necessary with the same precision, without tiring [23, 24]. The need for such a system is also seen by Langsch et al., who, however, focus on the endovascular treatment of aneurysms of the abdominal aorta using robotic ultrasound [25]. The RUSS reduces radiation exposure and provides accurate vascular measurement for optimal sizing of devices (balloons, scaffolds). Furthermore, ultrasound enables the assessment of haemodynamic features and quantification of vascular lesions as well as a real time intervention.
There are several reasons to introduce robotic systems into everyday clinical practice in the near future. However, it is essential to collect data from human legs and test the proposed system before using it for endovascular interventions. For future improvements, it is desirable to modify the robot's current translational movements when tracking vessels to be more adaptable. This is particularly important given the knowledge of anatomical differences between the vascular model, which has a continuous cylinder shape with consistent material resistance, and the human leg, which deviates in both shape and resistance. Imaging could potentially be improved by making rotational adjustments to account for the cylindrical shape of the legs. Further study is required to investigate how the RUSS adapts to different resistances.
5. Conclusion
This phantom study indicates that endovascular navigation and intervention as implanting stents using the RUSS are feasible. RUSS offers a new semiautomatic navigation method using ultrasound for peripheral endovascular interventions. To the best of our knowledge, this is the first system to acquire physician‐directed but robot guided ultrasound images of the peripheral arteries. The novel feature of the presented system is that it enables simultaneous navigation of a robot and adjustment of the visualisation of a 3D US volume using a foot pedal. This allows the surgeon to independently control the robot and US view while using both hands to handle endovascular devices such as guidewire catheters and stent systems. In addition, the practical application of RUSS in the context of stent implantation was demonstrated. This phantom study provides promising results and presents the basis for sonographic guided endovascular interventions on peripheral artery disease in humans using a radiation‐free approach.
Author Contributions
The authors confirm contribution to the paper as follows: study conception and design: A. Scheibert, M. Horn, M. Preuss, F. Ernst and M. Kleemann; data collection: J. Osburg, A. Scheibert and M. Horn; analysis and interpretation of results: A. Scheibert, J. Osburg, M. Horn and F. Ernst; draft manuscript preparation: A. Scheibert, M. Preuss, J. Osburg and M. Horn. All authors reviewed the results and approved the final version of the manuscript.
Ethics Statement
The study (IRB: 22‐140) was conducted with the approval of the local Ethics Committee of the University of Luebeck, Germany.
Conflicts of Interest
The authors declare no conflicts of interest.
The University of Luebeck holds a patent for the project under number DE 102020109593 B3, which is titled ‘Method for navigating an ultrasound augmented reality peripheral endovascular intervention and associated assembly for navigating an ultrasoundaugmented reality peripheral endovascular intervention’.
The University of Luebeck holds a patent for the project under number EP2737455 B1 in DE, GB and FR, filed in July 2011, under the title ‘Method for finding the position of a transducer’.
Acknowledgements
This project has received funding from the German Research Foundation (DFG) under project number 445694211 and funding indicator: KL 2457/2‐1 and ER 817/4‐1.
Funding: This project has received funding from the German Research Foundation (DFG) under project number 445694211 and funding indicator: KL 2457/2‐1 and ER 817/4‐1.
Alexandra Scheibert and Mark Preuss contributed equally to this work.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.
References
- 1. Bradbury A. W., Moakes C. A., Popplewell M., et al., “A Vein Bypass First Versus a Best Endovascular Treatment First Revascularisation Strategy for Patients With Chronic Limb Threatening Ischaemia Who Required an Infra‐popliteal, With or Without an Additional More Proximal Infra‐Inguinal Revascularisation Procedure to Restore Limb Perfusion (BASIL‐2): An Open‐Label, Randomised, Multicentre, Phase 3 Trial,” Lancet 401, no. 10390 (2023): 1798–1809, 10.1016/S0140-6736(23)00462-2. [DOI] [PubMed] [Google Scholar]
- 2. Rudnick M. R., Leonberg‐Yoo A. K., Litt H. I., Cohen R. M., Hilton S., and Reese P. P., “The Controversy of Contrast‐Induced Nephropathy With Intravenous Contrast: What Is the Risk?,” American Journal of Kidney Diseases 75, no. 1 (2020): 105–113, 10.1053/J.AJKD.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 3. Rachoin J. S., Wolfe Y., Patel S., and Cerceo E., “Contrast Associated Nephropathy After Intravenous Administration: What Is the Magnitude of the Problem?,” Renal Failure 43, no. 1 (2021): 1311–1321, 10.1080/0886022X.2021.1978490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolt L. J. J., Krasznai A. G., Sigterman T. A., Sikkink C. J. J. M., Schurink G. W. H., and Bouwman L. H., “Duplex‐Guided Versus Conventional Percutaneous Transluminal Angioplasty of Iliac TASC II A and B Lesion: A Randomized Controlled Trial,” Annals of Vascular Surgery 55 (2019): 138–147, 10.1016/J.AVSG.2018.07.047. [DOI] [PubMed] [Google Scholar]
- 5. Ascher E., Marks N. A., Hingorani A. P., Schutzer R. W., and Mutyala M., “Duplex‐Guided Endovascular Treatment for Occlusive and Stenotic Lesions of the Femoral‐Popliteal Arterial Segment: A Comparative Study in the First 253 Cases,” Journal of Vascular Surgery 44, no. 6 (2006): 1230–1237, 10.1016/J.JVS.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 6. Ascher E., Marks N. A., Hingorani A. P., Schutzer R. W., and Nahata S., “Duplex‐Guided Balloon Angioplasty and Subintimal Dissection of Infrapopliteal Arteries: Early Results With a New Approach to Avoid Radiation Exposure and Contrast Material,” Journal of Vascular Surgery 42, no. 6 (2005): 1114–1121, 10.1016/j.jvs.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 7. Parwani D., Ahmed M. A., Mahawar A., and Gorantla V. R., “Peripheral Arterial Disease: A Narrative Review,” Cureus 15, no. 6 (2023): 471–479, 10.7759/CUREUS.40267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harrington A. and Kupinski A. M., “Noninvasive Studies for the Peripheral Artery Disease Patient,” Seminars in Vascular Surgery 35, no. 2 (2022): 132–140, 10.1053/j.semvascsurg.2022.04.009. [DOI] [PubMed] [Google Scholar]
- 9. Mura M., Ciuti G., Ferrari V., Dario P., and Menciassi A., “Ultrasound‐Based Tracking Strategy for Endoluminal Devices in Cardiovascular Surgery,” International Journal of Medical Robotics and Computer Assisted Surgery 11, no. 3 (2015): 319–330, 10.1002/RCS.1603. [DOI] [PubMed] [Google Scholar]
- 10. Janvier M. A., Durand L. G., Cardinal M. H. R., et al., “Performance Evaluation of a Medical Robotic 3D‐Ultrasound Imaging System,” Medical Image Analysis 12, no. 3 (2008): 275–290, 10.1016/J.MEDIA.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 11. Conti F., Park J., and Khatib O., “Interface Design and Control Strategies for a Robot Assisted Ultrasonic Examination System,” in Springer Tracts in Advanced Robotics (Springer Verlag, 2014), Vol. 79, 97–113, 10.1007/978-3-642-28572-1_7. [DOI] [Google Scholar]
- 12. Fang T. Y., Zhang H. K., Finocchi R., Taylor R. H., and Boctor E. M., “Force‐Assisted Ultrasound Imaging System Through Dual Force Sensing and Admittance Robot Control,” International Journal of Computer Assisted Radiology and Surgery 12, no. 6 (2017): 983–991, 10.1007/S11548-017-1566-9/TABLES/3. [DOI] [PubMed] [Google Scholar]
- 13. De Ruiter Q. M. B., Moll F. L., and Van Herwaarden J. A., “Current State in Tracking and Robotic Navigation Systems for Application in Endovascular Aortic Aneurysm Repair,” Journal of Vascular Surgery 61, no. 1 (2015): 256–264, 10.1016/j.jvs.2014.08.069. [DOI] [PubMed] [Google Scholar]
- 14. Cruddas L., Martin G., and Riga C., “Robotic Endovascular Surgery: Current and Future Practice,” Seminars in Vascular Surgery 34, no. 4 (2021): 233–240, 10.1053/J.SEMVASCSURG.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 15. von Haxthausen F., Hagenah J., Kaschwich M., Kleemann M., García‐Vázquez V., and Ernst F., “Robotized Ultrasound Imaging of the Peripheral Arteries – A Phantom Study,” Current Directions in Biomedical Engineering 6, no. 1 (2020): 20200033, 10.1515/cdbme-2020-0033. [DOI] [Google Scholar]
- 16. Kaschwich M., Dell A., Matysiak F., et al., “Development of an Ultrasound‐Capable Phantom With Patient‐Specific 3D‐Printed Vascular Anatomy to Simulate Peripheral Endovascular Interventions,” Annals of Anatomy 232 (2020): 151563, 10.1016/J.AANAT.2020.151563. [DOI] [PubMed] [Google Scholar]
- 17. Lasso A., Heffter T., Rankin A., Pinter C., Ungi T., and Fichtinger G., “PLUS: Open‐Source Toolkit for Ultrasound‐Guided Intervention Systems,” IEEE Transactions on Biomedical Engineering 61, no. 10 (2014): 2527–2537, 10.1109/TBME.2014.2322864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. von Haxthausen F., Aust T., Schwegmann H., et al., “Visual Servoing for Semi‐Automated 2D Ultrasound Scanning of Peripheral Arteries,” Proceedings on Automation in Medical Engineering 1 (2020), 10.18416/AUTOMED.2020. [DOI] [Google Scholar]
- 19. Ascher E., Hingorani A. P., and Marks N., “Duplex‐Guided Balloon Angioplasty of Lower Extremity Arteries,” Perspectives in Vascular Surgery and Endovascular Therapy 19, no. 1 (2007): 23–31, 10.1177/1531003506298139. [DOI] [PubMed] [Google Scholar]
- 20. Scognamiglio P., Stüben B. O., Heumann A., et al., “Advanced Robotic Surgery: Liver, Pancreas, and Esophagus – The State of the Art?,” Visceral Medicine 37, no. 6 (2021): 505–510, 10.1159/000519753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nayyar R. and Gupta N. P., “Critical Appraisal of Technical Problems With Robotic Urological Surgery,” BJU International 105, no. 12 (2010): 1710–1713, 10.1111/J.1464-410X.2009.09039.X. [DOI] [PubMed] [Google Scholar]
- 22. Chuchulo A. and Ali A., “Is Robotic‐Assisted Surgery Better?,” AMA Journal of Ethics 25, no. 8 (2023): E598–E604, 10.1001/AMAJETHICS.2023.598. [DOI] [PubMed] [Google Scholar]
- 23. Swerdlow D. R., Cleary K., Wilson E., Azizi‐Koutenaei B., and Monfaredi R., “Robotic Arm–Assisted Sonography: Review of Technical Developments and Potential Clinical Applications,” American Journal of Roentgenology 208, no. 4 (2017): 733–738, 10.2214/AJR.16.16780. [DOI] [PubMed] [Google Scholar]
- 24. Kojcev R., Khakzar A., Fuerst B., et al., “On the Reproducibility of Expert‐Operated and Robotic Ultrasound Acquisitions,” International Journal of Computer Assisted Radiology and Surgery 12, no. 6 (2017): 1003–1011, 10.1007/S11548-017-1561-1/FIGURES/7. [DOI] [PubMed] [Google Scholar]
- 25. Langsch F., Virga S., Esteban J., Göbl R., and Navab N., “Robotic Ultrasound for Catheter Navigation in Endovascular Procedures,” in 2019 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS) (2019), 5404–5410, 10.1109/IROS40897.2019.8967652. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.


