Abstract
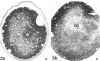
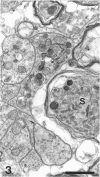
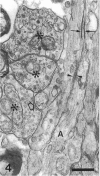
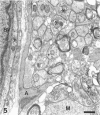
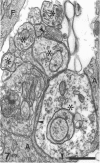
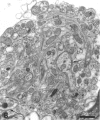
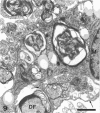
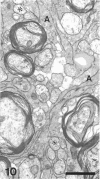
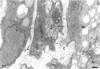
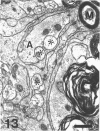
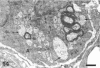
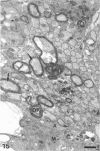
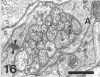
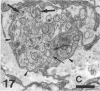
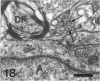
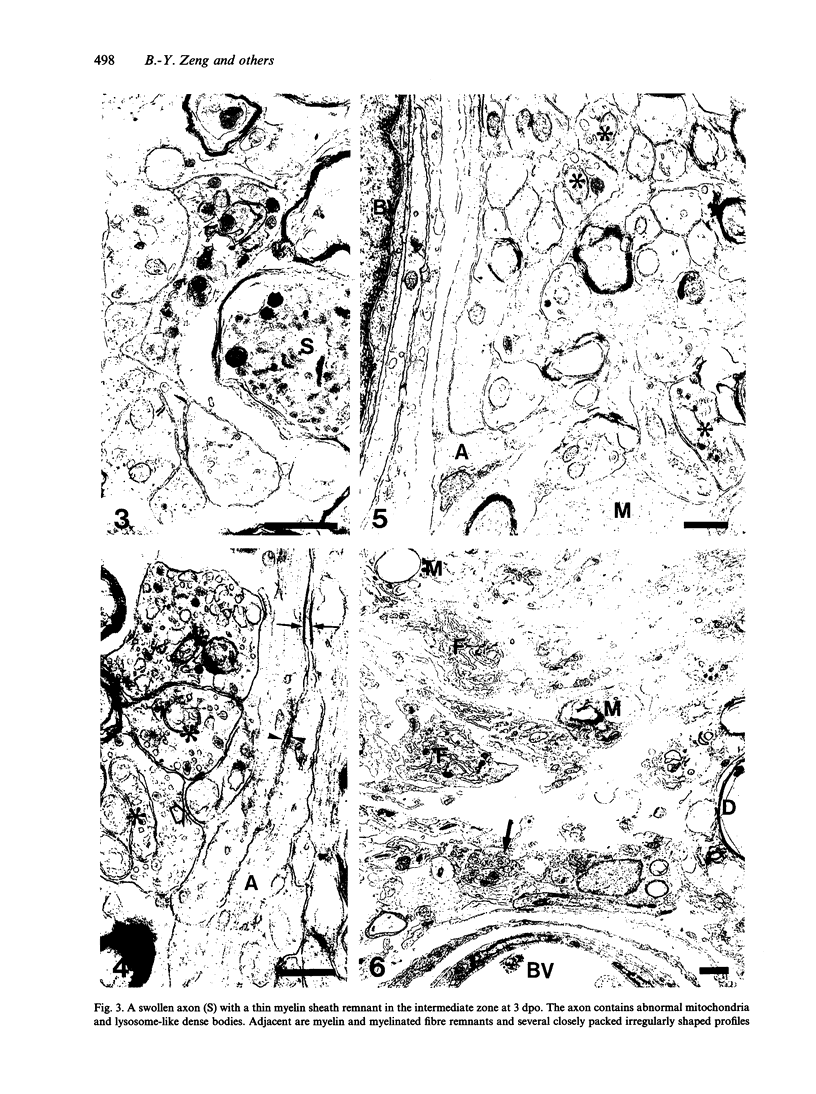
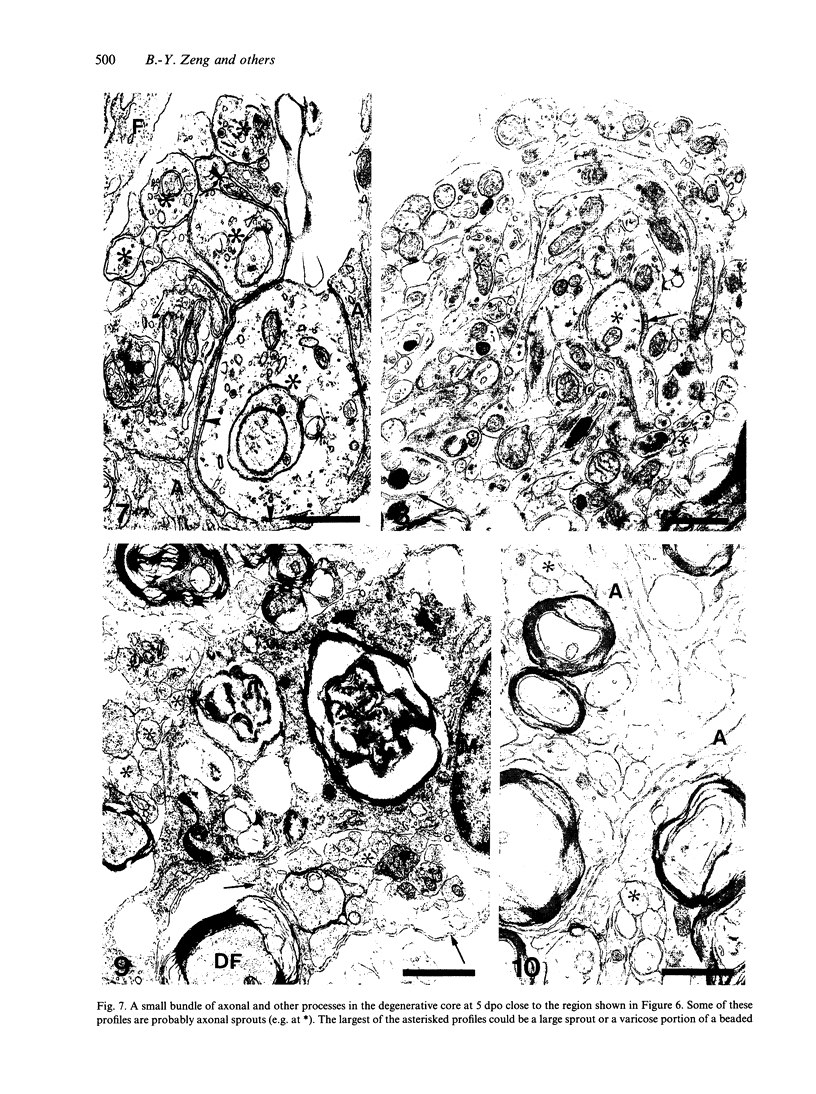
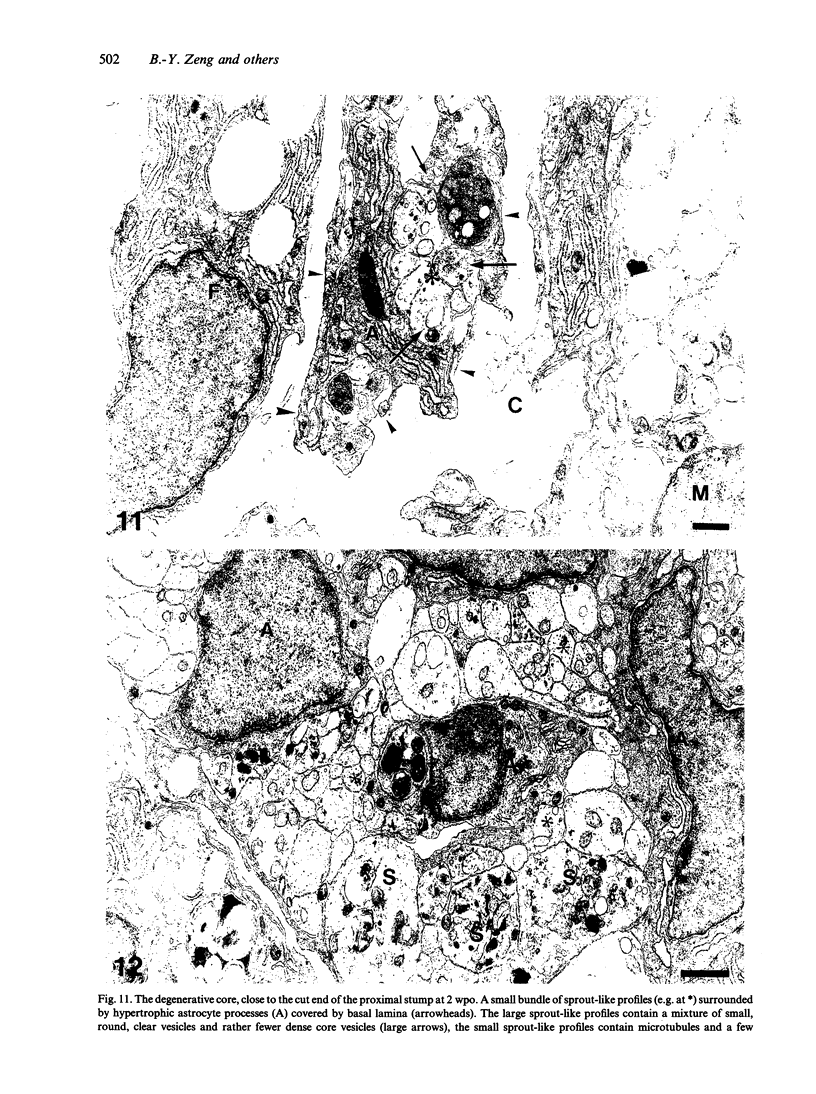
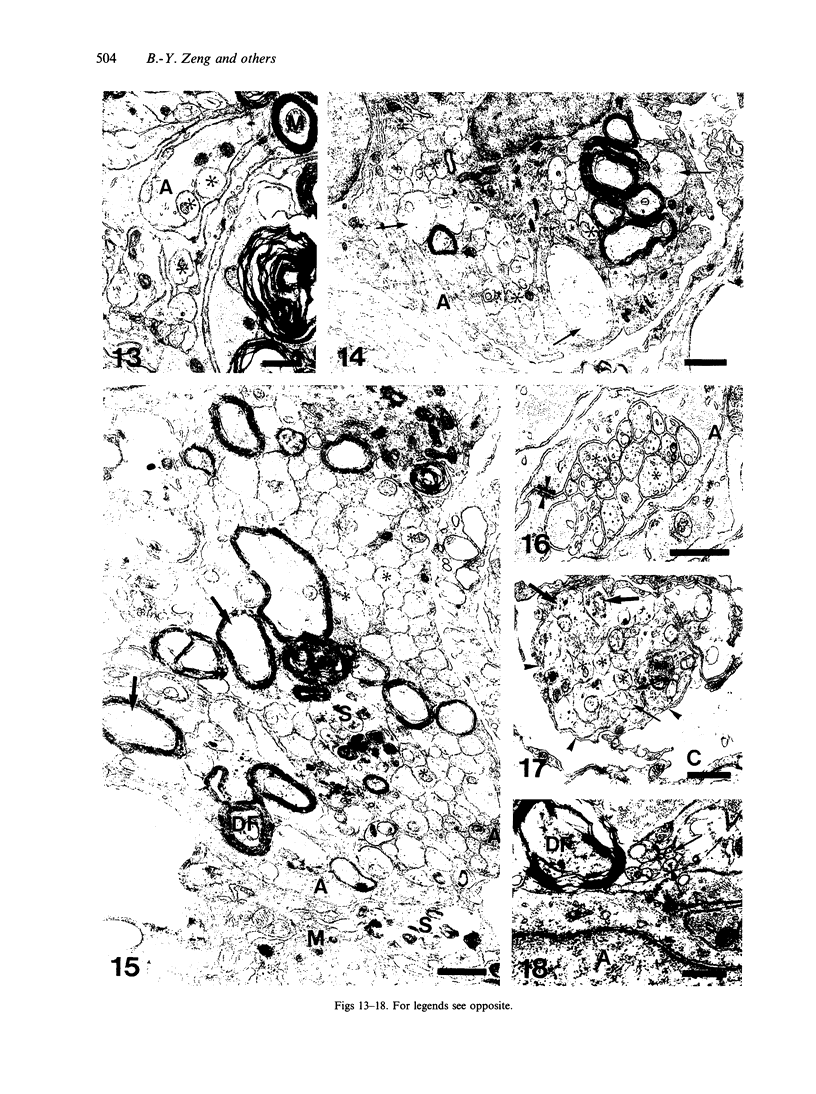
The response to injury of the proximal (retinal) stump of the intracranially transected optic nerve in adult rats has been studied by electron microscopy. The central part of the retinal stump of the optic nerve underwent severe ischaemic damage resulting in the formation by 3 days postoperation (dpo) of a cone-shaped region of necrotic tissue which extended from a base occupying most of the cross-sectional area of the nerve at the cut end to an apex within the intraorbital part of the nerve and only 2-3 mm from the eyeball. A mixture of apparently viable and dead or dying cells and axons was present in an intermediate zone surrounding the ischaemic core. Apparently intact nerve fibres occupied most of the periphery of the optic nerve. Small bundles of sprout-like axons were seen in the intermediate zone at 3 dpo, and by 5 dpo such sprouts were present at the periphery of the degenerative core. By 7 dpo, the sprouts were also found in the centre of the degenerative core, accompanied by astrocyte processes. The number of axonal sprouts present in the degenerative core and intermediate zone was much higher at 2 and 4 wk postoperation (wpo) than at 7 dpo, then declined gradually by 6 and 8 wpo. These results show that intracranial transection of the rat optic nerve produces extensive degeneration in the proximal stump and effectively produces an intraorbital axotomy of many retinal ganglion cells. Nevertheless, surviving axons display the ability to produce regenerative sprouts which persist for considerably longer than those produced after intraorbital injury.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allcutt D., Berry M., Sievers J. A quantitative comparison of the reactions of retinal ganglion cells to optic nerve crush in neonatal and adult mice. Brain Res. 1984 Nov;318(2):219–230. doi: 10.1016/0165-3806(84)90027-0. [DOI] [PubMed] [Google Scholar]
- Anderson D. R. Vascular supply to the optic nerve of primates. Am J Ophthalmol. 1970 Sep;70(3):341–351. doi: 10.1016/0002-9394(70)90093-0. [DOI] [PubMed] [Google Scholar]
- Anderson P. N., Woodham P., Turmaine M. Peripheral nerve regeneration through optic nerve grafts. Acta Neuropathol. 1989;77(5):525–534. doi: 10.1007/BF00687255. [DOI] [PubMed] [Google Scholar]
- Banker G. A. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980 Aug 15;209(4458):809–810. doi: 10.1126/science.7403847. [DOI] [PubMed] [Google Scholar]
- Campbell G., Lieberman A. R., Anderson P. N., Turmaine M. Regeneration of adult rat CNS axons into peripheral nerve autografts: ultrastructural studies of the early stages of axonal sprouting and regenerative axonal growth. J Neurocytol. 1992 Nov;21(11):755–787. doi: 10.1007/BF01237903. [DOI] [PubMed] [Google Scholar]
- Doster S. K., Lozano A. M., Aguayo A. J., Willard M. B. Expression of the growth-associated protein GAP-43 in adult rat retinal ganglion cells following axon injury. Neuron. 1991 Apr;6(4):635–647. doi: 10.1016/0896-6273(91)90066-9. [DOI] [PubMed] [Google Scholar]
- Forrester J., Peters A. Nerve fibres in optic nerve of rat. Nature. 1967 Apr 15;214(5085):245–247. doi: 10.1038/214245a0. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Sugimoto T., Shirokawa T. Strain differences in quantitative analysis of the rat optic nerve. Exp Neurol. 1982 Feb;75(2):525–532. doi: 10.1016/0014-4886(82)90181-9. [DOI] [PubMed] [Google Scholar]
- Gage F. H., Olejniczak P., Armstrong D. M. Astrocytes are important for sprouting in the septohippocampal circuit. Exp Neurol. 1988 Oct;102(1):2–13. doi: 10.1016/0014-4886(88)90073-8. [DOI] [PubMed] [Google Scholar]
- Giftochristos N., David S. Laminin and heparan sulphate proteoglycan in the lesioned adult mammalian central nervous system and their possible relationship to axonal sprouting. J Neurocytol. 1988 Jun;17(3):385–397. doi: 10.1007/BF01187860. [DOI] [PubMed] [Google Scholar]
- Grafstein B., Ingoglia N. A. Intracranial transection of the optic nerve in adult mice: preliminary observations. Exp Neurol. 1982 May;76(2):318–330. doi: 10.1016/0014-4886(82)90212-6. [DOI] [PubMed] [Google Scholar]
- Hall S., Berry M. Electron microscopic study of the interaction of axons and glia at the site of anastomosis between the optic nerve and cellular or acellular sciatic nerve grafts. J Neurocytol. 1989 Apr;18(2):171–184. doi: 10.1007/BF01206660. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M. Adult rat brain astrocytes support survival of both NGF-dependent and NGF-insensitive neurones. Nature. 1979 Nov 1;282(5734):80–82. doi: 10.1038/282080a0. [DOI] [PubMed] [Google Scholar]
- Madison R., Moore M. R., Sidman R. L. Retinal ganglion cells and axons survive optic nerve transection. Int J Neurosci. 1984 Mar;23(1):15–32. doi: 10.3109/00207458408985342. [DOI] [PubMed] [Google Scholar]
- Mathewson A. J., Berry M. Observations on the astrocyte response to a cerebral stab wound in adult rats. Brain Res. 1985 Feb 18;327(1-2):61–69. doi: 10.1016/0006-8993(85)91499-4. [DOI] [PubMed] [Google Scholar]
- Misantone L. J., Gershenbaum M., Murray M. Viability of retinal ganglion cells after optic nerve crush in adult rats. J Neurocytol. 1984 Jun;13(3):449–465. doi: 10.1007/BF01148334. [DOI] [PubMed] [Google Scholar]
- Noble M., Fok-Seang J., Cohen J. Glia are a unique substrate for the in vitro growth of central nervous system neurons. J Neurosci. 1984 Jul;4(7):1892–1903. doi: 10.1523/JNEUROSCI.04-07-01892.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V. H., Henderson Z., Linden R. Postnatal changes in retinal ganglion cell and optic axon populations in the pigmented rat. J Comp Neurol. 1983 Sep 20;219(3):356–368. doi: 10.1002/cne.902190309. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Issa V. M., Shemie S. Regeneration and retrograde degeneration of axons in the rat optic nerve. J Neurocytol. 1982 Dec;11(6):949–966. doi: 10.1007/BF01148310. [DOI] [PubMed] [Google Scholar]
- Rudge John S., Alderson Ralph F., Pasnikowski Elizabeth, McClain Joyce, Ip Nancy Y., Lindsay Ronald M. Expression of Ciliary Neurotrophic Factor and the Neurotrophins-Nerve Growth Factor, Brain-Derived Neurotrophic Factor and Neurotrophin 3-in Cultured Rat Hippocampal Astrocytes. Eur J Neurosci. 1992;4(6):459–471. doi: 10.1111/j.1460-9568.1992.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Schreyer D. J., Skene J. H. Fate of GAP-43 in ascending spinal axons of DRG neurons after peripheral nerve injury: delayed accumulation and correlation with regenerative potential. J Neurosci. 1991 Dec;11(12):3738–3751. doi: 10.1523/JNEUROSCI.11-12-03738.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small R. K., Riddle P., Noble M. Evidence for migration of oligodendrocyte--type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987 Jul 9;328(6126):155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- So K. F., Aguayo A. J. Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rats. Brain Res. 1985 Mar 4;328(2):349–354. doi: 10.1016/0006-8993(85)91047-9. [DOI] [PubMed] [Google Scholar]
- Vidal-Sanz M., Bray G. M., Villegas-Pérez M. P., Thanos S., Aguayo A. J. Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J Neurosci. 1987 Sep;7(9):2894–2909. doi: 10.1523/JNEUROSCI.07-09-02894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Pérez M. P., Vidal-Sanz M., Bray G. M., Aguayo A. J. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988 Jan;8(1):265–280. doi: 10.1523/JNEUROSCI.08-01-00265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Pérez M. P., Vidal-Sanz M., Rasminsky M., Bray G. M., Aguayo A. J. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993 Jan;24(1):23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- Weinberg E. L., Raine C. S. Reinnervation of peripheral nerve segments implanted into the rat central nervous system. Brain Res. 1980 Sep 29;198(1):1–11. doi: 10.1016/0006-8993(80)90339-x. [DOI] [PubMed] [Google Scholar]
- Zeng B. Y., Anderson P. N., Campbell G., Lieberman A. R. Regenerative and other responses to injury in the retinal stump of the optic nerve in adult albino rats: transection of the intraorbital optic nerve. J Anat. 1994 Dec;185(Pt 3):643–661. [PMC free article] [PubMed] [Google Scholar]