Abstract
Microtubule-associated protein tau is essential for microtubule assembly and stabilization. Hyperphosphorylation of the microtubule-associated protein tau plays an important pathological role in the development of Alzheimer’s disease and other tauopathies. In vivo studies using kinase inhibitors suggest that reducing tau phosphorylation levels has therapeutic potential; however, such approaches showed limited benefits. We sought to develop further our Phosphorylation Targeting Chimera (PhosTAC) technology to specifically induce tau dephosphorylation. Herein, we use small molecule based PhosTACs to recruit tau to PP2A, a native tau phosphatase. PhosTACs induced the formation of a stable ternary complex, leading to rapid, efficient, and sustained tau dephosphorylation, which also correlated the enhanced down regulation of tau protein. Mass spectrometry data validated that PhosTACs downregulated multiple phosphorylation sites of tau. We believe that PhosTAC possess several advantages over current strategies to modulate tau phosphorylation and represent a new avenue for disease-modifying therapies for tauopathies.
Graphic abstract
Introduction
Tau, encoded by the microtubule-associated protein tau (MAPT) gene, is one of the most well-studied proteins in neurodegenerative diseases. MAPT comprises 16 exons, with alternative splicing producing 6 main tau isoforms. Each isoform contains 0 to 2 copies of 29-residue near-amino-terminal inserts (named as 0N, 1N and 2N), along with 3 or 4 carboxy-terminal repeat domains (named as 3R or 4R).1 Tau is predominantly expressed in neurons, where it plays important roles in microtubule assembly and stabilization via its carboxy-terminal repeat domains.2 Tau is soluble and flexible in its natural form, characterized as an intrinsically disordered protein. Aggregation of tau into higher-order oligomers is a hallmark of many neurodegenerative diseases, including Alzheimer’s disease (AD),3 Pick disease (PiD),4 and progressive supranuclear palsy (PSP),4 and thus is thought to play a critical role in disease pathogenesis and pathology.5
Tau is heavily regulated by post-translational modifications, most notably phosphorylation. Tau hyperphosphorylation is frequently observed in AD brains,6 and correlates with tau aggregate formation7 and cognitive decline8. Several mechanisms by which hyperphosphorylated tau contributes to tauopathies have been proposed. For example, tau phosphorylation reduces its binding affinity for microtubules,2, 9 leading to its dissociation and subsequent microtubule disassembly, resulting in axonal transport deficits. Specifically, Thr231-phosphorylated tau undergoes a trans-to-cis isomerization, leading to a conformational change that reduces its microtubules affinity.10 Also, hyperphosphorylated tau was reported to be aggregation-prone, assembling into paired helical filaments (PHF) in vitro11, while dephosphorylation has been shown to inhibit tau polymerization and restore its ability to stabilize microtubules.12 Tau hyperphosphorylation may also hinder its degradation: phosphorylation at Ser262 or Ser356 prevents its recognition by the C-terminus of HSP70-interacting protein–heat shock protein 90 (CHIP-HSP90) and subsequent proteasomal degradation.13 Tau phosphorylation also regulates its cellular localization, with hyperphosphorylated tau mislocalizing from axons to the somatodendritic compartment of hippocampal neurons.14 Thus, while the dominant mechanisms by which phosphorylated tau causes neuronal toxicity remain under active investigation, hyperphosphorylation is widely considered to be a potential therapeutic target for tauopathies.
An imbalance between kinases and phosphatases may underlie tau hyperphosphorylation. Many kinases have been shown to phosphorylate tau, such as GSK-315, CDK5 16 AMPK17, CK118, MARKs19, PKA20, dyrk-1a21, Fyn22, Abl23, and Syk24. Tau is likewise regulated by a variety of phosphatases, such as PP1, PP2A, PP2B, PP2C and PP5.25 Among reported phosphatases, PP2A is the major phosphatase and has been shown to be responsible for approximately 70% of tau dephosphorylation.25 This suggests an important role of PP2A in AD and its potential as a target for tauopathies treatment.
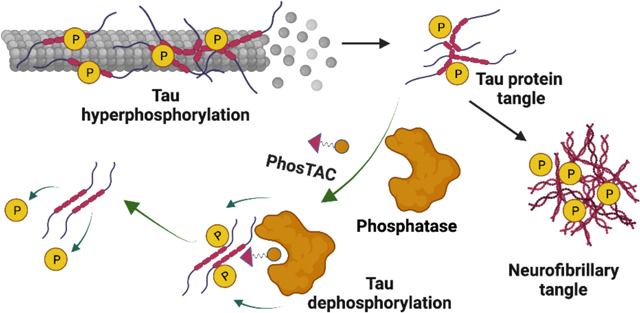
Our lab has previously reported the development of Phosphorylation Targeting Chimeras (PhosTACs).26 This new class of heterobifunctional molecules recruits a phosphatase to a target protein to achieve targeted protein dephosphorylation (Fig. 1A). We have successfully shown targeted dephosphorylation of PDCD4 and FOXO3a. Here, we sought to adapt this technology to induce the targeted dephosphorylation of tau. We showed that tau PhosTACs can efficiently dephosphorylate tau protein, which correlates with the tau down regulation. This study presents the first instance of PhosTAC-induced tau dephosphorylation, demonstrating PhosTACs’ potential as a possible new modality for the treatment of AD and other tauopathies.
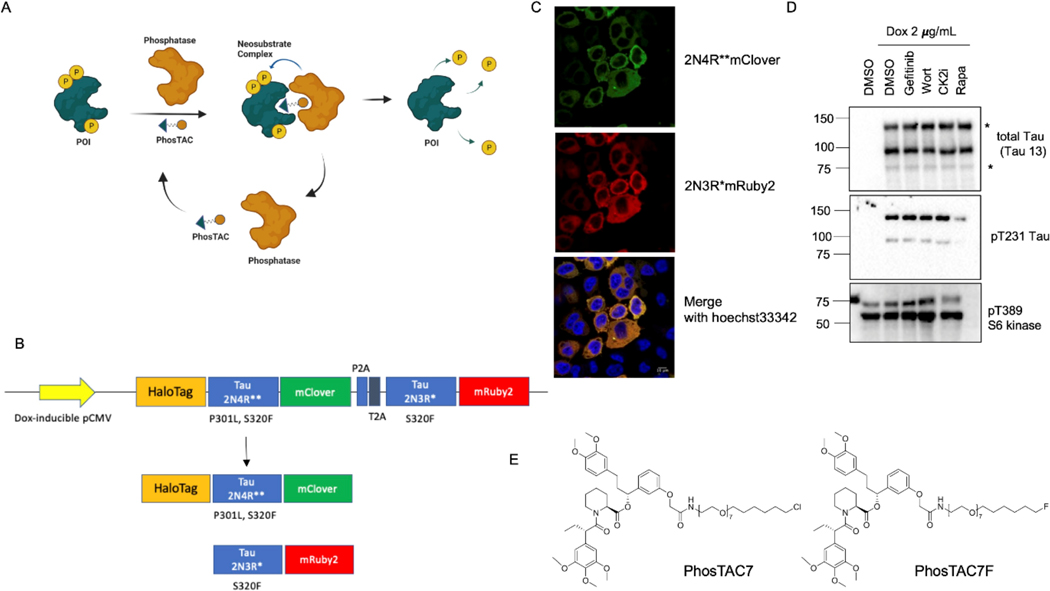
Figure 1.
Design of Tau-targeting Phosphorylation TArgeting Chimeras (PhosTACs) A, scheme of PhosTAC action mechanism. B, Design of inducible tau expression construct. C, fluorescent microscopy image of tau-expressing Hela cells after doxycycline induction. D, validation of tau expression and tau phosphorylation in Hela cells. Tau-expressing Hela cells were treated with or without doxycycline for 24h. Induced cells were then treated with gefitinib (200 nM), wortmannin (1 uM), CK2i (1 uM), and rapamycin (500 nM), respectively for another 24 h. Cell lysates were collected and analyzed by WB using indicated antibodies. E, structures of PhosTAC7 and PhosTAC7F.
Results and Discussion
Design of an inducible 2N3R and 2N4R Tau expressing system and Tau PhosTACs
To study tau hyperphosphorylation, we first engineered a mammalian cell system consisting of doxycycline-inducible expression of full-length 2N4R (P301L and S320F, denoted as 2N4R**) and 2N3R (S320F, denoted as 2N3R*) tau variants. P301L and S320F variants were introduced as they are reported to accelerate AD-type neurofibrillary tangles formation.27 Tau proteins were expressed on a single bicistronic vector separated by P2A and T2A elements to generate 2N4R** and 2N3R* tau.28 To visualize and quantify tau levels, 2N4R** tau was fused with the fluorescent protein mClover and 2N3R* tau fused with mRuby2 (expected molecular weight: 70 kDa). Lastly, 2N4R**-mClover tau was fused with HaloTag7 (expected molecular weight: 112 kDa), which has a highly specific chloroalkane ligand, making it amenable to induced proximity systems such as PhosTAC7 (Fig. 1B, Fig. 1E).
We used confocal microscopy to confirm induction of both fluorescently tagged tau proteins after 24h treatment with 2 μg/mL doxycycline (dox) (Fig. 1C). Protein expression was further confirmed via immunoblot, with both tau variants at the correct molecular weight (indicated by asterisks) appearing following dox treatment (Fig. 1D). Notably, total tau antibodies detected additional bands, which could correspond to tau proteolysis products or alternatively post-translationally modified species29. Importantly, we observed robust tau phosphorylation at Thr231 (pT231), supporting its use as a model to study targeted dephosphorylation. As the expression of 2N4R** was more abundant than 2N3R* in our cell line, we focused on 2N4R** isoform to evaluate induced tau dephosphorylation in subsequent experiments.
We next tested whether tau phosphorylation can be repressed by kinase inhibitors in our system. While inhibitors such as gefitinib (epidermal growth factor receptor inhibitor), wortmannin (phosphoinositide 3-kinase inhibitor), and casein kinase 2 inhibitor showed no significant effects on tau phosphorylation, rapamycin, a mTOR kinase inhibitor, dramatically reduced tau phosphorylation at Thr231 (Fig. 1D). Rapamycin activity was confirmed by a robust decrease in Thr389 phosphorylation of the mTORC1 downstream target S6 Kinase (Fig. 1D). This was consistent with a previous study demonstrating rapamycin can modulate tau phosphorylation levels.30
Given that PP2A is one of the most abundance phosphatases in mammalian cells31 and, importantly, is the major tau phosphatase in the human brain25, we selected PP2A for testing in a PhosTAC system. Of note, we have previously demonstrated the ability of PP2A-targeting PhosTACs to specifically dephosphorylate PDCD4 and FOXO3a.26 As there are not yet specific PP2A small molecule ligands available, we engineered a FKBP12F36V-PP2A fusion protein to allow recruitment using the FKBP ligand and co-expressed it in cells expressing dox-inducible 2N4R**-mClover-HaloTag7 and 2N3R*-mRuby2 tau variants (Fig. 1D). In this proof-of-concept system, we can use our previously reported PhosTAC7 and the inactive PhosTAC7F (Fig. 1E),26 to investigate targeted tau dephosphorylation by PP2A.
PhosTAC-induced tau dephosphorylation in a PP2A-dependent manner
As ternary complexes are required for bifunctional molecules (such as PROTACs) to execute their functions, pull-down assay was performed to exam the formation of a tau-PP2A complex. We used HaloTrap, consisting of a high affinity HaloTag nanobody coupled to agarose resin, to pulldown HaloTag7-2N4R**-tau from cells treated with PhosTAC7, PhosTAC7F, or DMSO. This approach successfully enriched 2N4R**-tau (Fig. 2A, identified with double asterisks), 2N3R*-tau (identified with single asterisk), and truncated tau isoforms, suggesting the formation of tau self-assemblies in cells. Upon treatment with PhosTAC7, both the FKBP12F36V-PP2A A subunit and endogenous PP2A C subunit coprecipitated with tau, demonstrating the successful formation of a ternary complex with an intact PP2A holoenzyme. No coprecipitation was observed following treatment with DMSO vehicle or PhosTAC7F, which possesses a fluorine substitution that abolishes HaloTag7 binding, demonstrating the PhosTAC dependence of the observed ternary complex.
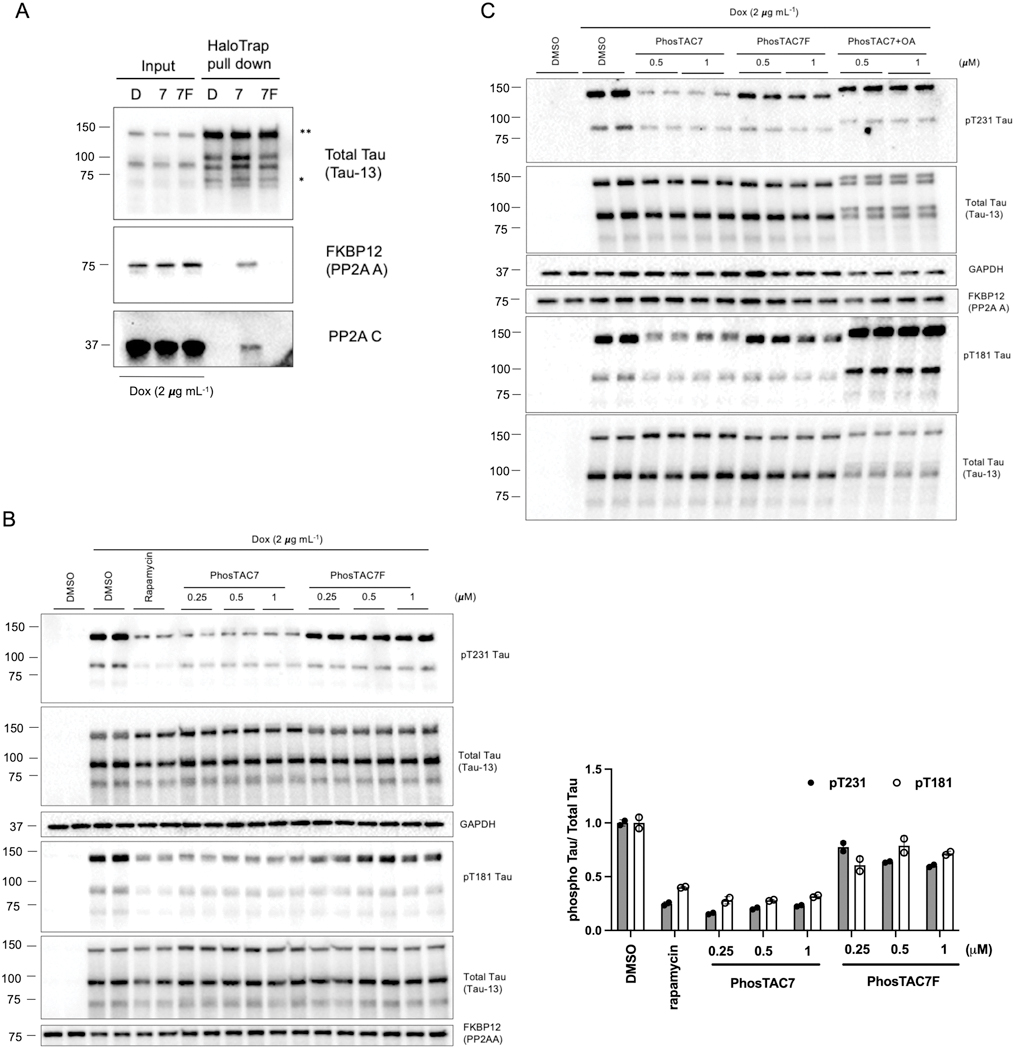
Figure 2.
PhosTAC7 dephosphorylates Tau in a PP2A-dependent manner. A. PhosTAC induced stable ternary complex with tau and PP2A A subunit and C subunit. Tau/FKBP12(F36V)-PP2A A HeLa cells were treated with doxycycline (dox) for 24 h and then incubated with PhosTACs (1μM, 24h) and lysed for HaloTrap pulldown and Western blot using indicated antibodies. B. PhosTAC7 but not the inactive PhosTAC7F induced tau dephosphorylation. Tau/FKBP12(F36V)-PP2A A HeLa cells were treated with dox for 24 h, followed by treatment with indicated concentrations of PhosTAC7, PhosTAC7F, DMSO, or rapamycin (0.5μM) for 24 h. Cell lysates were collected and analyzed by western blot using indicated antibodies. Data were quantified from the phosphorylated or total 2N4R** tau species with two replicates and summarized as mean and standard deviation. C. PhosTAC7 induced tau dephosphorylation via PP2A. Tau/FKBP12(F36V)-PP2A A HeLa cells (dox-induction for 24 h) were treated with indicated concentrations of PhosTAC7, PhosTAC7F, or PhosTAC7 – OA (20 nM, 24 h) cotreatment. Cell lysates were collected 24 h after treatment and analyzed by western blot using indicated antibodies.
We next assessed whether PhosTAC7-induced tau-PP2A ternary complex formation modulated tau phosphorylation levels. Indeed, starting at 0.25 μM, PhosTAC7 triggered robust dephosphorylation at Thr231 and Thr181 (one of the most elevated tau phosphorylation sites in AD32), with a maximal dephosphorylation at 24 hours (DPmax_24h) of approximately 75%. The inactive PhosTAC7F had minimal effects on tau phosphorylation (Fig. 2B), demonstrating that PhosTAC7 acts via complex formation. Importantly, PhosTAC7-induced tau dephosphorylation at Thr231 and Thr181 was abolished upon cotreatment with okadaic acid (OA), a potent PP2A inhibitor (Fig. 2C). Collectively, these data demonstrate PhosTAC7 can induce targeted dephosphorylation of tau via ternary complex formation and PP2A phosphatase activity.
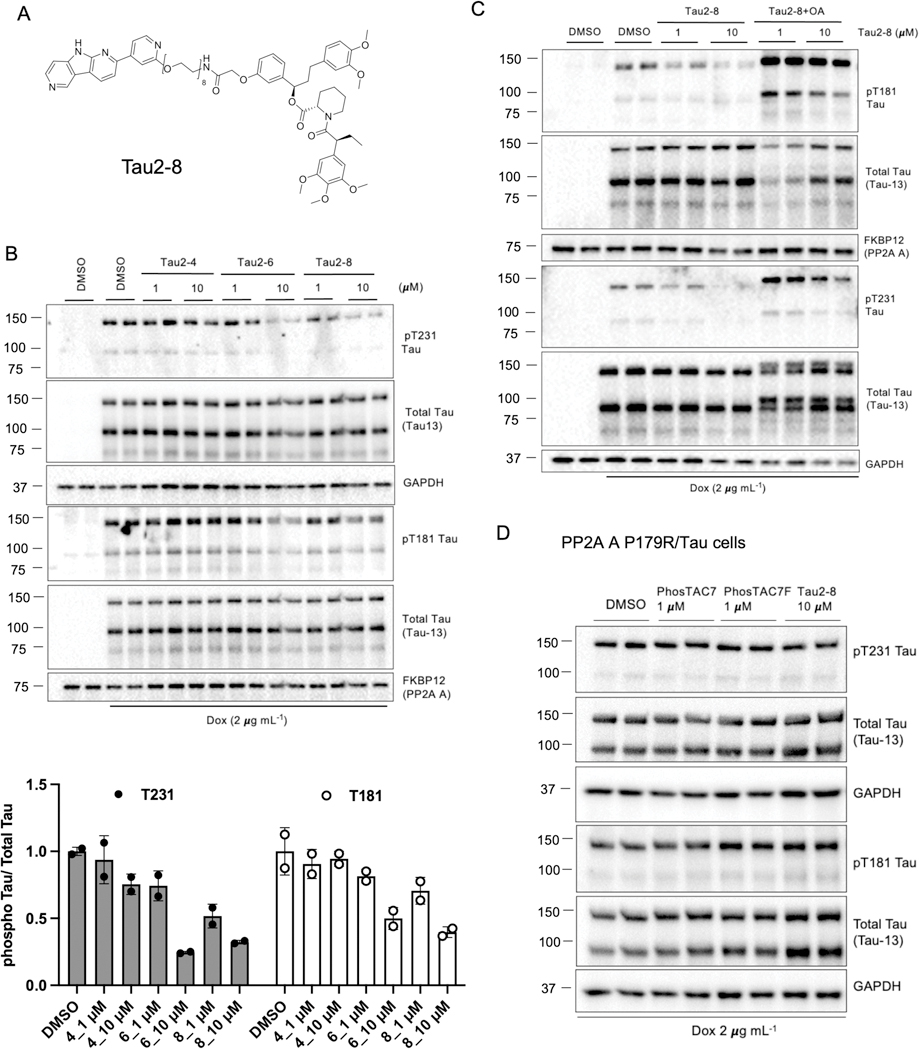
To improve upon this proof-of-concept system, we next sought to generate PhosTACs that directly bind tau rather than via a fused Halotag domain. To this end, we replaced the HaloTag7 chloroalkane warhead with one based on a positron emission tomography (PET) tau tracer, which has proven a valuable clinical tool to specifically detect pathological tau in patients,33 and as a warhead for tau-targeting PROTACs.34 PI-2620 (Fig. S1) is a newly reported derivative of the T807 PET probe that has been shown to possess higher affinity and selectivity for 4R and 3R/4R tau isoforms.35 We thus employed PI-2620 as the targeting warhead and coupled it to the FKBPF36V ligand using a range of polyethylene glycol (PEG) linkers, yielding a new series of tau-targeting PhosTACs (Fig. 3A, Tau2–8 as an example; Fig. S1). As commonly observed during PROTAC development,36 we found PEG linker length affected the efficacy of PhosTAC-mediated tau dephosphorylation; “Tau2–8” with an 8 PEG linker demonstrated better efficacy compared with the 4 PEG linker-based “Tau2–4” and 6 PEG linker-based “Tau2–6” (Fig. 3B). We found Tau2–8 induced approximately 50% pThr231 dephosphorylation at 1 μM and 65% dephosphorylation at 10 μM, with similar trends for pThr181 dephosphorylation (Fig. 3B). Cotreatment with the PP2A inhibitor, OA, significantly enhanced phosphorylation at Thr231 and Thr181 and prevented Tau2–8-mediated dephosphorylation, confirming an on-target mechanism (Fig. 3C). To further demonstrate our tau-targeting PhosTACs act via PP2A activity, we introduced a P179R variation in PP2A A subunit, which is reported to cause a conformational shift that impairs holoenzyme assembly,37 and expressed it as a FKBPF36V fusion in cells expressing inducible mClover-2N4R**/mRuby2–2N3R* tau. In this PP2AP179R cell line, both PhosTAC7 and Tau2–8 were unable to induce substantial tau dephosphorylation (Fig. 3D). Thus, the developed PhosTACs with the PI-2620 targeting warhead successfully promote tau dephosphorylation through induced proximity with the active PP2A holoenzyme.
Figure 3.
Tau2–8 dephosphorylates Tau. A. Structures of Tau2–8. B. Tau2–8 induced Tau dephosphorylation. Tau/FKBP12(F36V)-PP2A A HeLa cells were treated with dox for 24 h, followed by treatment with indicated concentrations of Tau2–4, Tau2–6 or Tau2–8. Cell lysates were collected 24 h after treatment and analyzed by WB using indicated antibodies. Data were quantified from the phosphorylated or total 2N4R** Tau species with two replicates and summarized as mean ± sd. C. Tau2–8 induced tau dephosphorylation via PP2A. Tau/FKBP12(F36V)-PP2A A HeLa cells were treated with dox for 24 h, followed by treatment with indicated concentrations of Tau2–8 with or without OA (20 nM) cotreatment. Cell lysates were collected 18 h after treatment and analyzed by WB using indicated antibodies. D. PP2A Pro179 is critical for PhosTAC-mediated dephosphorylation. Tau/FKBP12(F36V)-PP2A A (P179R) HeLa cells were treated with dox for 24 h, followed by treatment with indicated concentrations of PhosTAC7, PhosTAC7F, or Tau2–8. Cell lysates were collected 24 h after treatment and analyzed by WB using indicated antibodies.
Tau dephosphorylation kinetics mediated by PhosTACs
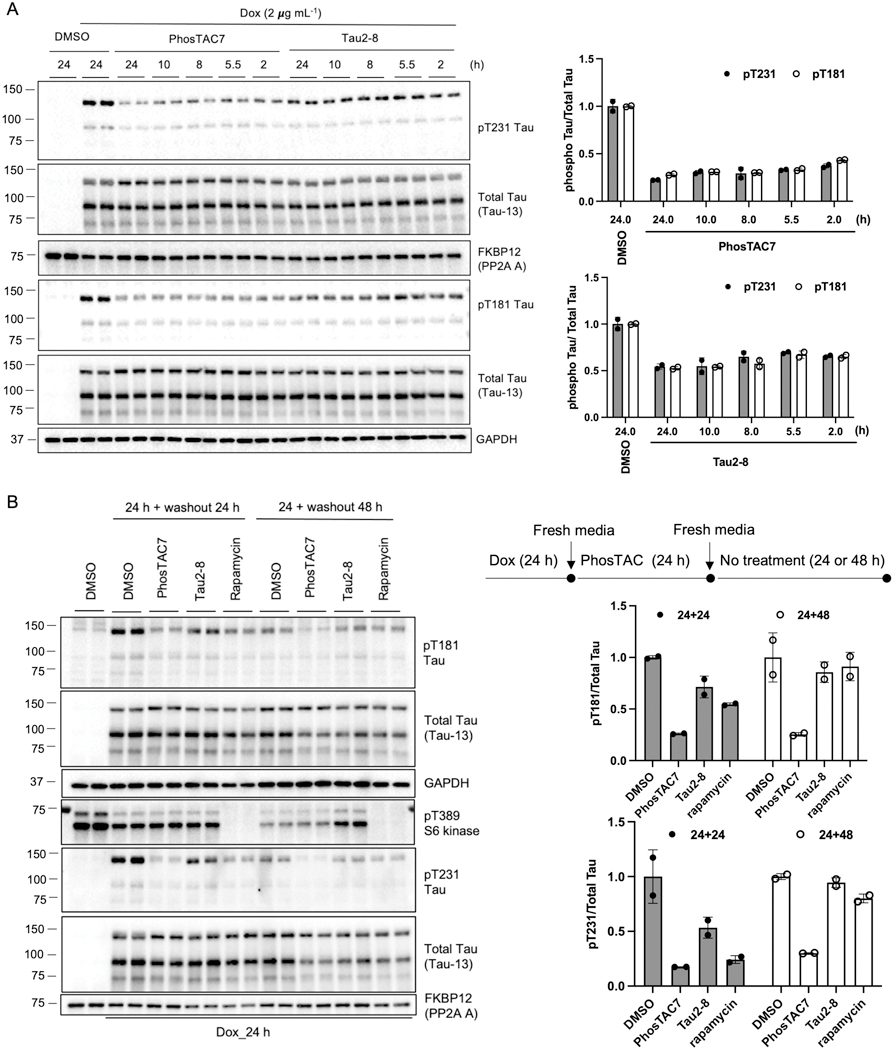
We next investigated the kinetics of PhosTAC-mediated tau dephosphorylation. Both PhosTAC7 and Tau2–8 induced rapid tau dephosphorylation at Thr181 and Thr231, with approximately 50% dephosphorylation observed as early as 2 hours after treatment at 1 μM of PhosTAC7 (Fig. 4A). Maximal dephosphorylation was observed following 24 hours of treatment (Fig. 4A), with 75% pT231 dephosphorylation at the end of treatment of PhosTAC7 (Fig. 4A). A similar phenomenon was observed with Tau2–8. These results indicated that tau PhosTAC can induce rapid dephosphorylation, which is in accordance with the rapid enzymatic profiles of phosphatases.38
Figure 4.
Rapid and long-lasting effects of Tau-PhosTACs. A. PhosTACs induced rapid tau dephosphorylation. Doxycycline induced Tau/FKBP12(F36V)-PP2A A HeLa cells (dox-induction for 24 h) were treated with PhosTAC7 (1μM) or Tau2–8 (1 μM). Cell lysates were collected after treatment of indicated time and analyzed by WB using indicated antibodies. Data were quantified from two biological samples and summarized as mean and standard deviation. B. PhosTACs induced long-lasting tau dephosphorylation. Flow chart of experiment design was shown at top right panel. Doxycycline induced Tau/FKBP12(F36V)-PP2A A HeLa cells were treated with PhosTAC7 (0.5μM), Tau2–8 (10 μM) or rapamycin (0.5 μM) for 24h, the media were then changed to remove any treatment. Cell lysates were collected after indicated time and analyzed by WB using indicated antibodies. Data were quantified from two biological samples and summarized as mean and standard deviation.
Bifunctional molecules such as PROTACs utilize an event-driven mechanism that allows for sub-stoichiometric, catalytic activity and prolonged cellular activity compared with conventional small molecule compounds39. Thus, we next tested the effect of PhosTACs on a prolonged period, cells were stimulated with dox for 24 h to induce tau expression, followed by wash out dox and treatment with PhosTAC7 or Tau2–8 for another 24 h (Fig. 4B top right). Compounds were then removed and replaced with fresh media, and cells were cultured for an additional 24 or 48 h without PhosTACs. PhosTAC7 induced potent and sustained tau dephosphorylation at Thr181 and Thr231 for as long as 48 h post-washout (Fig. 4B). Sustained dephosphorylation was apparent 24 h post-washout of Tau2–8, which returned to DMSO levels by 48 h, possibly due to its lower potency compared to PhosTAC7. It should be noted that the covalent nature of HaloTag may pose limitation on the catalytic effect of PhosTAC and render PhosTAC7 resistant to washout. Although rapamycin was as potent as PhosTAC7 in inducing tau dephosphorylation after 24 h of incubation (Fig. 1B and 2B), rapamycin-mediated tau dephosphorylation was only modest after 24 h washout and did not persist to 48 h (Fig. 4B). As a critical negative control, we found neither PhosTAC7 nor Tau2–8 reduced pT389 of S6 kinase compared with DMSO, while rapamycin dramatically repressed S6K phosphorylation (Fig. 4B). These data suggested that PhosTACs and rapamycin exhibited different kinetics and mechanisms in mediating tau dephosphorylation in our current system.
Proteomics approaches validated tau dephosphorylation and enrichment of PP2A holoenzymes by PhosTAC
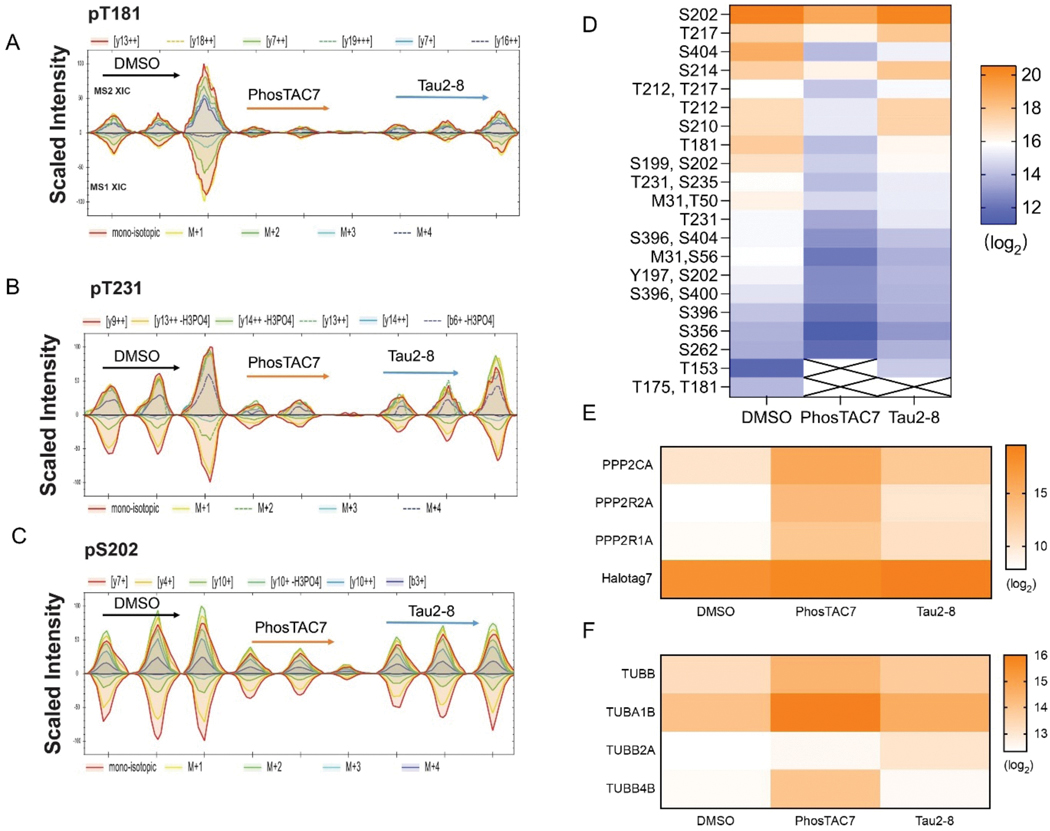
Next, we used a data-independent acquisition mass spectrometry (DIA-MS) based proteomic approach40 to validate tau dephosphorylation and identify potential tau interacting proteins. We used HaloTrap to pulldown HaloTag7–2N4R**-tau from cells treated with dox for 24 h, followed by an additional 24 h treatment with either PhosTAC7 (1 μM), Tau2–8 (10 μM), or DMSO. Tau and interacting proteins were eluted from HaloTrap agarose, digested, and subjected to mass spectrometric (MS) analysis. We found tau peptides were successfully enriched, identified 24 out of 85 possible phosphorylation sites on tau.41 MS analysis confirmed PhosTAC7 and Tau2–8 both cause robust tau dephosphorylation at Thr181 and Thr231 compared with DMSO (Fig. 5A and 5B). In addition, many canonical AD-related tau phosphorylation sites such as Ser202, Ser210, Thr217, Ser356, and Ser40442 were found to be reduced by PhosTAC7, and to a lesser extent by Tau2–8 (Fig. 5C, 5D, S2A-E), consistent with our findings (Fig. 2 to 4).
Figure 5.
Proteomic approaches validated tau dephosphorylation and PP2A enrichment by PhosTACs. A-C. Validation of tau dephosphorylation on tau pT181, pT231, and pS202 by PhosTACs. HaloTag fusion tau and its interactome were pulled down by HaloTrap after PhosTAC7 or Tau2–8 treatment. The eluted tau and interacting proteins were analyzed by mass spectrometry. Data were collected from three biological samples for each condition. D. Heatmap of tau phosphorylation level of 24 sites measured by mass spectrometry. E. Heatmap of enrichment level for PP2A C subunit (PPP2CA), PP2A B55 subunit (PPP2R2A), PP2A A subunit (PPP2R1A) and Halotag7 (tau) proteins. F. Heatmap of enrichment level for tubulin beta chain (TUBB), tubulin alpha-1B chain (TUBA1B), tubulin beta-2A chain (TUBB2A) and tubulin beta-4B chain (TUBB4B) proteins.
DIA-MS data further validated PhosTAC-mediated ternary complex formation between tau and a functional PP2A holoenzyme. Enhanced ternary complex formation was observed with PhosTACs treatments, with PhosTAC7 showed significant enrichment of PP2A A (PPP2R1A) and C (PPP2CA) subunits (Fig. 5D). Of note, the PP2A B subunit, B55α (PPP2R2A), was significantly enriched by both PhosTACs (Fig. 5E and S2F), indicating that B55 might be the major regulatory unit for PhosTACs mediated tau dephosphorylation. This is consistent with an earlier report demonstrating that PP2A/B55α regulates tau phosphorylation.43 In accordance with its role as cytoskeleton regulator,44 DIA-MS analysis of HaloTrap pulldowns also identified several structural proteins as tau interactors. In fact, one of the well-known tau interacting proteins, tubulin, was enriched in PhosTAC-treated samples relative to DMSO (Fig. 5F), suggesting that PhosTAC-mediated tau dephosphorylation may enhance its interaction with tubulin.
PhosTAC-induced dephosphorylation correlates with accelerated tau degradation
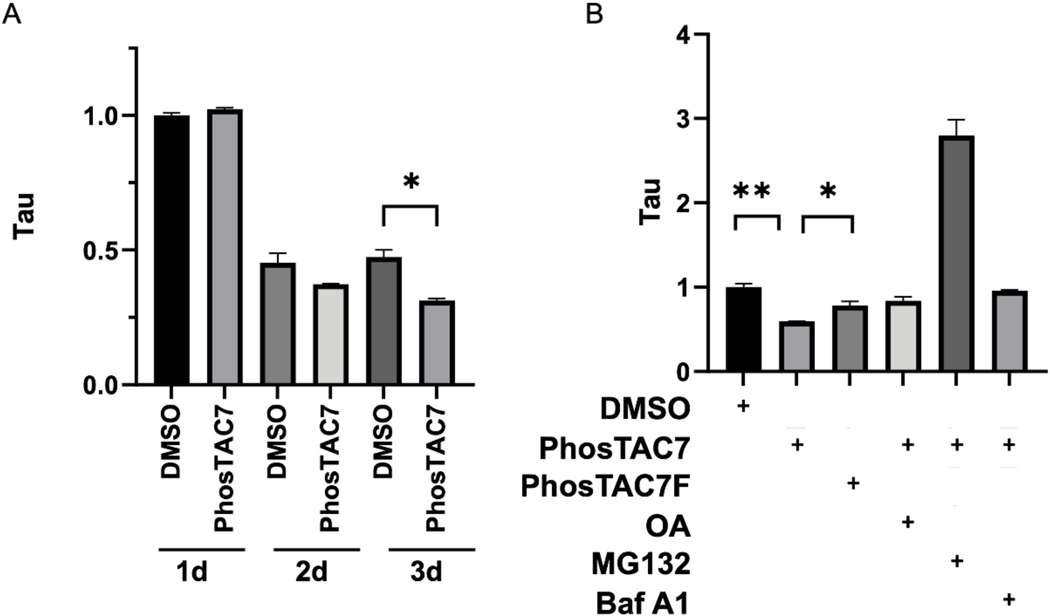
Given that phosphorylation of tau has been reported to hinder its degradation,13 we next investigated the effect of PhosTACs on tau degradation, which could be monitored by flow cytometry via measuring the fused florescent proteins in our design (Figure 1B). After induction with dox for 24 hours, dox was removed and cells were treated with 1 μM PhosTAC7 for 1 to 3 days. Although we did not observe a significantly accelerated down regulation of tau protein expression with treatment of Tau2–8, which may correlate with its lower potency in tau dephosphorylation, PhosTAC7 treatment enhanced tau degradation. As measured by mClover mean fluorescence intensity by flow cytometry, PhosTAC7 induced a 34% reduction relative to DMSO observed at prolonged day 3 treatment (Fig. 6A, S3A–D, Table S1). To probe which degradation pathway may be contributing to reduced tau levels, we treated cells with MG132, a proteasome inhibitor, Bafilomycin A1 (BafA1), an inhibitor of autophagosome-lysosome fusion, or OA as a control. While BafA1 and OA had minimal effects, MG132 dramatically increased tau levels, suggesting tau is generally degraded via the proteasome in our system (Fig. 6B, Table S2).
Figure 6.
The biological effects on Tau protein expression by PhosTAC. A. PhosTAC induced dephosphorylation correlated with accelerated tau protein down regulation. Tau/FKBP12(F36V)-PP2A A HeLa cells were treated with dox for 24 h, after which dox was removed and cells were treated with DMSO or PhosTAC7 (1μM) for the indicated times. Tau levels were assessed by mClover mean fluorescence intensity by flow cytometry. Data were quantified from two biological samples and summarized as mean ± standard deviation. B. PhosTAC-mediated tau degradation correlated with phosphatase and proteasome activity. Doxycycline induced Tau/FKBP12(F36V)-PP2A A HeLa cells were treated with DMSO, PhosTAC7 (1μM), PhosTAC7F (1μM) for 2 days, then treated with OA (10 nM), MG132 (10 μM) or Bafilomycin A1 (500 nM) for 24h. Tau protein levels was monitored by measuring mClover fluorescence intensity with flow cytometry. Data were quantified from two biological samples and summarized as mean and standard deviation, t tests were performed with Prism 9.
Conclusion
Despite nearly a century of investigation and an estimated 416 million patients globally45, developing successful disease-modifying therapies for AD remains a critical challenge. Tau aggregation in neurons is thought to be a primary driver of AD. Accordingly, extensive efforts have been placed on developing therapeutics that remove or disaggregate these pathological structures.34a
Tau hyperphosphorylation promotes its aggregation in mouse models of AD,46 thus strategies aimed at specifically reducing tau phosphorylation may disfavor its aggregation and provide therapeutic benefit. Attempts to ameliorate pathology in mouse models of tauopathies using kinase inhibitors have been mostly unsuccessful.47 This may be due to the fact that tau phosphorylation is controlled by several kinases, including GSK-315, CDK516, and AMPK.17 Therefore, inhibition of a single kinase in vivo may be insufficient to significantly attenuate tau hyperphosphorylation,48 whereas combined kinase inhibition comes at the expense of increased off-target activity and toxicity. Thus, targeted tau dephosphorylation provides a new and significant modality, with possible more specificity and less side effects.
Our recently described PhosTAC technology builds upon the framework of PROTACs and targeted protein degradation, an attractive therapeutic modality designed to eliminate pathological proteins by co-opting cellular degradation systems. Early work by Zheng et al.49 and Yamazone et al.50 utilized peptide based bifunctional molecules to achieve dephosphorylation of tau and AKT, representing exploratory and pioneering work in targeted protein dephosphorylation. In our system, small-molecule based PhosTACs recruit cellular phosphatases to phosphorylated substrates, resulting in ternary complex formation and dephosphorylation by induced proximity. Owing to their event-driven mechanism, PhosTACs display favorable kinetic profiles and sustained cellular effects. Importantly, unlike kinase and phosphatase inhibitors, PhosTACs offer the advantage of being able to tune the phosphorylation status of a single protein of interest.
In this proof-of-concept study, we applied our PhosTAC technology to target tau. We found that recruitment of PP2A, a primary native tau phosphatase, to tau using genetically-encoded tags and chemical recruiting elements resulted in ternary complex formation and robust dephosphorylation. Notably, tau dephosphorylation was sustained, lasting up to 24–48 hours post-PhosTAC removal. In addition, we used MS to identify the PhosTAC-induced tau interactome, which may provide insights into the cellular processes affected by forced tau dephosphorylation. Importantly, PhosTAC treatment enhanced tau degradation, which is in accordance with PhosTAC-induced dephosphorylation of the degradation hindering pS356 (Fig. S2),13 highlighting the potential for this strategy. While our current study for proof-of-concept is based on a synthetic system, future efforts will focus on targeting endogenous tau and phosphatases using of specific ligands for PP2A and other phosphatases, we believe the data described herein lay an important foundation for the development of targeted therapies for tauopathies.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH R35 CA197589 to CMC. This study does not reflect the sponsor’s position or the policy of the Government, and no official endorsement was inferred. We acknowledge critical discussion from members of the Crews Lab: Dr. Saul Jaime-Figueroa, Dr. Ke Li, Dr. Mack Krone, Dr. Jacques Saarbach and Dr. Cesar De Leon. Figure abstract was created with Biorender.com.
Footnotes
ASSOCIATED CONTENT
The Supporting Information is available free of charge at:
Complete contact information is available at:
Conflict of interests
The authors declare no competing financial interest.
References
- 1.Lee G; Cowan N; Kirschner M The primary structure and heterogeneity of tau protein from mouse brain. Science 1988, 239 (4837), 285–8. [DOI] [PubMed] [Google Scholar]
- 2.Kellogg EH; Hejab NMA; Poepsel S; Downing KH; DiMaio F; Nogales E Near-atomic model of microtubule-tau interactions. Science 2018, 360 (6394), 1242–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer A; Stelzmann RA; Schnitzlein HN; Murtagh FR An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat 1995, 8 (6), 429–31. [DOI] [PubMed] [Google Scholar]
- 4.Vaquer-Alicea J; Diamond MI; Joachimiak LA Tau strains shape disease. Acta Neuropathol 2021, 142 (1), 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naseri NN; Wang H; Guo J; Sharma M; Luo W The complexity of tau in Alzheimer’s disease. Neurosci Lett 2019, 705, 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. (a).Hanger DP; Betts JC; Loviny TL; Blackstock WP; Anderton BH New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer’s disease brain using nanoelectrospray mass spectrometry. J Neurochem 1998, 71 (6), 2465–76; [DOI] [PubMed] [Google Scholar]; (b) Tavares IA; Touma D; Lynham S; Troakes C; Schober M; Causevic M; Garg R; Noble W; Killick R; Bodi I; Hanger DP; Morris JD Prostate-derived sterile 20-like kinases (PSKs/TAOKs) phosphorylate tau protein and are activated in tangle-bearing neurons in Alzheimer disease. J Biol Chem 2013, 288 (21), 15418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vega IE; Cui L; Propst JA; Hutton ML; Lee G; Yen SH Increase in tau tyrosine phosphorylation correlates with the formation of tau aggregates. Brain Res Mol Brain Res 2005, 138 (2), 135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boekhoorn K; Terwel D; Biemans B; Borghgraef P; Wiegert O; Ramakers GJ; de Vos K; Krugers H; Tomiyama T; Mori H; Joels M; van Leuven F; Lucassen PJ Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci 2006, 26 (13), 3514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bramblett GT; Goedert M; Jakes R; Merrick SE; Trojanowski JQ; Lee VMY Abnormal tau phosphorylation at Ser396 in alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 1993, 10 (6), 1089–1099. [DOI] [PubMed] [Google Scholar]
- 10. (a).Kondo A; Shahpasand K; Mannix R; Qiu J; Moncaster J; Chen CH; Yao Y; Lin YM; Driver JA; Sun Y; Wei S; Luo ML; Albayram O; Huang P; Rotenberg A; Ryo A; Goldstein LE; Pascual-Leone A; McKee AC; Meehan W; Zhou XZ; Lu KP Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 2015, 523 (7561), 431–436; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lu PJ; Wulf G; Zhou XZ; Davies P; Lu KP The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 1999, 399 (6738), 784–8. [DOI] [PubMed] [Google Scholar]
- 11.Alonso A; Zaidi T; Novak M; Grundke-Iqbal I; Iqbal K Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A 2001, 98 (12), 6923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iqbal K; Alonso AC; Gong CX; Khatoon S; Pei JJ; Wang JZ; Grundke-Iqbal I Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles. J Neural Transm Suppl 1998, 53, 169–80. [DOI] [PubMed] [Google Scholar]
- 13.Dickey CA; Kamal A; Lundgren K; Klosak N; Bailey RM; Dunmore J; Ash P; Shoraka S; Zlatkovic J; Eckman CB; Patterson C; Dickson DW; Nahman NS Jr.; Hutton M; Burrows F; Petrucelli L The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest 2007, 117 (3), 648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoover BR; Reed MN; Su J; Penrod RD; Kotilinek LA; Grant MK; Pitstick R; Carlson GA; Lanier LM; Yuan LL; Ashe KH; Liao D Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010, 68 (6), 1067–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. (a).Lovestone S; Hartley CL; Pearce J; Anderton BH Phosphorylation of tau by glycogen synthase kinase-3 beta in intact mammalian cells: the effects on the organization and stability of microtubules. Neuroscience 1996, 73 (4), 1145–57; [DOI] [PubMed] [Google Scholar]; (b) Lovestone S; Reynolds CH; Latimer D; Davis DR; Anderton BH; Gallo JM; Hanger D; Mulot S; Marquardt B; Stabel S; et al. Alzheimer’s disease-like phosphorylation of the microtubule-associated protein tau by glycogen synthase kinase-3 in transfected mammalian cells. Curr Biol 1994, 4 (12), 1077–86; [DOI] [PubMed] [Google Scholar]; (c) Noble W; Planel E; Zehr C; Olm V; Meyerson J; Suleman F; Gaynor K; Wang L; LaFrancois J; Feinstein B; Burns M; Krishnamurthy P; Wen Y; Bhat R; Lewis J; Dickson D; Duff K Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A 2005, 102 (19), 6990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. (a).Baumann K; Mandelkow EM; Biernat J; Piwnica-Worms H; Mandelkow E Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett 1993, 336 (3), 417–24; [DOI] [PubMed] [Google Scholar]; (b) Cruz JC; Tseng HC; Goldman JA; Shih H; Tsai LH Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 2003, 40 (3), 471–83. [DOI] [PubMed] [Google Scholar]
- 17. (a).Thornton C; Bright NJ; Sastre M; Muckett PJ; Carling D AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid beta-peptide exposure. Biochem J 2011, 434 (3), 503–12; [DOI] [PubMed] [Google Scholar]; (b) Mairet-Coello G; Courchet J; Pieraut S; Courchet V; Maximov A; Polleux F The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Abeta oligomers through Tau phosphorylation. Neuron 2013, 78 (1), 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanger DP; Byers HL; Wray S; Leung KY; Saxton MJ; Seereeram A; Reynolds CH; Ward MA; Anderton BH Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. J Biol Chem 2007, 282 (32), 23645–54. [DOI] [PubMed] [Google Scholar]
- 19.Drewes G; Trinczek B; Illenberger S; Biernat J; Schmitt-Ulms G; Meyer HE; Mandelkow EM; Mandelkow E Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem 1995, 270 (13), 7679–88. [DOI] [PubMed] [Google Scholar]
- 20.Andorfer CA; Davies P PKA phosphorylations on tau: developmental studies in the mouse. Dev Neurosci 2000, 22 (4), 303–9. [DOI] [PubMed] [Google Scholar]
- 21.Sheppard O; Plattner F; Rubin A; Slender A; Linehan JM; Brandner S; Tybulewicz VL; Fisher EM; Wiseman FK Altered regulation of tau phosphorylation in a mouse model of down syndrome aging. Neurobiol Aging 2012, 33 (4), 828 e31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson R; Scales T; Clark BR; Gibb G; Reynolds CH; Kellie S; Bird IN; Varndell IM; Sheppard PW; Everall I; Anderton BH Rapid tyrosine phosphorylation of neuronal proteins including tau and focal adhesion kinase in response to amyloid-beta peptide exposure: involvement of Src family protein kinases. J Neurosci 2002, 22 (1), 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancino GI; Perez de Arce K; Castro PU; Toledo EM; von Bernhardi R; Alvarez AR c-Abl tyrosine kinase modulates tau pathology and Cdk5 phosphorylation in AD transgenic mice. Neurobiol Aging 2011, 32 (7), 1249–61. [DOI] [PubMed] [Google Scholar]
- 24.Lebouvier T; Scales TM; Hanger DP; Geahlen RL; Lardeux B; Reynolds CH; Anderton BH; Derkinderen P The microtubule-associated protein tau is phosphorylated by Syk. Biochim Biophys Acta 2008, 1783 (2), 188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong CX; Singh TJ; Grundke-Iqbal I; Iqbal K Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem 1993, 61 (3), 921–7. [DOI] [PubMed] [Google Scholar]
- 26.Chen PH; Hu Z; An E; Okeke I; Zheng S; Luo X; Gong A; Jaime-Figueroa S; Crews CM Modulation of Phosphoprotein Activity by Phosphorylation Targeting Chimeras (PhosTACs). ACS Chem Biol 2021, 16 (12), 2808–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. (a).Koller EJ; Gonzalez De La Cruz E; Machula T; Ibanez KR; Lin WL; Williams T; Riffe CJ; Ryu D; Strang KH; Liu X; Janus C; Golde TE; Dickson D; Giasson BI; Chakrabarty P Combining P301L and S320F tau variants produces a novel accelerated model of tauopathy. Hum Mol Genet 2019, 28 (19), 3255–3269; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Strang KH; Croft CL; Sorrentino ZA; Chakrabarty P; Golde TE; Giasson BI Distinct differences in prion-like seeding and aggregation between Tau protein variants provide mechanistic insights into tauopathies. J Biol Chem 2018, 293 (7), 2408–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z; Chen O; Wall JBJ; Zheng M; Zhou Y; Wang L; Vaseghi HR; Qian L; Liu J Systematic comparison of 2A peptides for cloning multi-genes in a polycistronic vector. Sci Rep 2017, 7 (1), 2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinn JP; Corbett NJ; Kellett KAB; Hooper NM Tau Proteolysis in the Pathogenesis of Tauopathies: Neurotoxic Fragments and Novel Biomarkers. J Alzheimers Dis 2018, 63 (1), 13–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caccamo A; Magri A; Medina DX; Wisely EV; Lopez-Aranda MF; Silva AJ; Oddo S mTOR regulates tau phosphorylation and degradation: implications for Alzheimer’s disease and other tauopathies. Aging Cell 2013, 12 (3), 370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seshacharyulu P; Pandey P; Datta K; Batra SK Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett 2013, 335 (1), 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanmechelen E; Vanderstichele H; Davidsson P; Van Kerschaver E; Van Der Perre B; Sjogren M; Andreasen N; Blennow K Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett 2000, 285 (1), 49–52. [DOI] [PubMed] [Google Scholar]
- 33.Xia CF; Arteaga J; Chen G; Gangadharmath U; Gomez LF; Kasi D; Lam C; Liang Q; Liu C; Mocharla VP; Mu F; Sinha A; Su H; Szardenings AK; Walsh JC; Wang E; Yu C; Zhang W; Zhao T; Kolb HC [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement 2013, 9 (6), 666–76. [DOI] [PubMed] [Google Scholar]
- 34. (a).Silva MC; Ferguson FM; Cai Q; Donovan KA; Nandi G; Patnaik D; Zhang T; Huang HT; Lucente DE; Dickerson BC; Mitchison TJ; Fischer ES; Gray NS; Haggarty SJ Targeted degradation of aberrant tau in frontotemporal dementia patient-derived neuronal cell models. Elife 2019, 8; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Silva MC; Nandi G; Donovan KA; Cai Q; Berry BC; Nowak RP; Fischer ES; Gray NS; Ferguson FM; Haggarty SJ Discovery and Optimization of Tau Targeted Protein Degraders Enabled by Patient Induced Pluripotent Stem Cells-Derived Neuronal Models of Tauopathy. Front Cell Neurosci 2022, 16, 801179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. (a).Kroth H; Oden F; Molette J; Schieferstein H; Gabellieri E; Mueller A; Berndt M; Sreenivasachary N; Serra AM; Capotosti F; Schmitt-Willich H; Hickman D; Pfeifer A; Dinkelborg L; Stephens A PI-2620 Lead Optimization Highlights the Importance of Off-Target Assays to Develop a PET Tracer for the Detection of Pathological Aggregated Tau in Alzheimer’s Disease and Other Tauopathies. J Med Chem 2021, 64 (17), 12808–12830; [DOI] [PubMed] [Google Scholar]; (b) Kroth H; Oden F; Molette J; Schieferstein H; Capotosti F; Mueller A; Berndt M; Schmitt-Willich H; Darmency V; Gabellieri E; Boudou C; Juergens T; Varisco Y; Vokali E; Hickman DT; Tamagnan G; Pfeifer A; Dinkelborg L; Muhs A; Stephens A Discovery and preclinical characterization of [(18)F]PI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer’s disease and other tauopathies. Eur J Nucl Med Mol Imaging 2019, 46 (10), 2178–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bond MJ; Chu L; Nalawansha DA; Li K; Crews CM Targeted Degradation of Oncogenic KRAS(G12C) by VHL-Recruiting PROTACs. ACS Cent Sci 2020, 6 (8), 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor SE; O’Connor CM; Wang Z; Shen G; Song H; Leonard D; Sangodkar J; LaVasseur C; Avril S; Waggoner S; Zanotti K; Armstrong AJ; Nagel C; Resnick K; Singh S; Jackson MW; Xu W; Haider S; DiFeo A; Narla G The Highly Recurrent PP2A Aalpha-Subunit Mutation P179R Alters Protein Structure and Impairs PP2A Enzyme Function to Promote Endometrial Tumorigenesis. Cancer Res 2019, 79 (16), 4242–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoermann B; Kokot T; Helm D; Heinzlmeir S; Chojnacki JE; Schubert T; Ludwig C; Berteotti A; Kurzawa N; Kuster B; Savitski MM; Kohn M Dissecting the sequence determinants for dephosphorylation by the catalytic subunits of phosphatases PP1 and PP2A. Nat Commun 2020, 11 (1), 3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Z; Crews CM Recent Developments in PROTAC-Mediated Protein Degradation: From Bench to Clinic. Chembiochem 2022, 23 (2), e202100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao E; Li W; Wu C; Shao W; Di Y; Liu Y Data-independent acquisition-based proteome and phosphoproteome profiling across six melanoma cell lines reveals determinants of proteotypes. Mol Omics 2021, 17 (3), 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goedert M; Spillantini MG; Jakes R; Rutherford D; Crowther RA Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3 (4), 519–26. [DOI] [PubMed] [Google Scholar]
- 42.Xia Y; Prokop S; Giasson BI “Don’t Phos Over Tau”: recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer’s disease and other tauopathies. Mol Neurodegener 2021, 16 (1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Y; Chen Y; Zhang P; Jeffrey PD; Shi Y Structure of a protein phosphatase 2A holoenzyme: insights into B55-mediated Tau dephosphorylation. Mol Cell 2008, 31 (6), 873–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grundke-Iqbal I; Iqbal K; Tung YC; Quinlan M; Wisniewski HM; Binder LI Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A 1986, 83 (13), 4913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gustavsson A; Norton N; Fast T; Frolich L; Georges J; Holzapfel D; Kirabali T; Krolak-Salmon P; Rossini PM; Ferretti MT; Lanman L; Chadha AS; van der Flier WM Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement 2022, 1–13. [DOI] [PubMed] [Google Scholar]
- 46.Andorfer C; Kress Y; Espinoza M; de Silva R; Tucker KL; Barde YA; Duff K; Davies P Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem 2003, 86 (3), 582–90. [DOI] [PubMed] [Google Scholar]
- 47.Eldar-Finkelman H; Martinez A GSK-3 Inhibitors: Preclinical and Clinical Focus on CNS. Front Mol Neurosci 2011, 4, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tolosa E; Litvan I; Hoglinger GU; Burn D; Lees A; Andres MV; Gomez-Carrillo B; Leon T; Del Ser T; Investigators T A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord 2014, 29 (4), 470–8. [DOI] [PubMed] [Google Scholar]
- 49.Zheng J; Tian N; Liu F; Zhang Y; Su J; Gao Y; Deng M; Wei L; Ye J; Li H; Wang JZ A novel dephosphorylation targeting chimera selectively promoting tau removal in tauopathies. Signal Transduct Target Ther 2021, 6 (1), 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamazoe S; Tom J; Fu Y; Wu W; Zeng L; Sun C; Liu Q; Lin J; Lin K; Fairbrother WJ; Staben ST Heterobifunctional Molecules Induce Dephosphorylation of Kinases-A Proof of Concept Study. J Med Chem 2020, 63 (6), 2807–2813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.