Abstract
Objectives
This study compared the clinical outcomes of minimally invasive surgery (MIS) and open surgery (OS) for patients with intraspinal tumors.
Methods
A systematic search of PubMed, Cochrane Library, EMBASE, Web of Science, China National Knowledge Infrastructure (CNKI), and Wanfang databases was conducted to identify relevant studies. Continuous variables, including estimated blood loss, surgery duration, time to mobilization, length of hospitalization, visual analog scale (VAS) score, and incision length, were reported as mean differences (MD) with 95% confidence intervals (95% CIs). Dichotomous variables, such as gross total resection, blood transfusion, cerebrospinal fluid (CSF) leakage, and overall complications, were presented as risk ratios (RR) with 95% CIs. Meta-analyses were performed using RevMan 5.3.
Results
Fifteen studies, comprising a total of 943 patients (488 in the MIS group and 455 in the OS group), met the inclusion criteria. The meta-analysis indicated that MIS significantly reduced estimated blood loss (MD = -76.73, 95% CI -102.56 to -50.91, P < 0.01), incision length (MD = -4.09, 95% CI -5.20 to -2.97, P < 0.01), VAS score (MD = -0.79, 95% CI -1.48 to -0.11, P = 0.02), time to mobilization (MD = -4.27, 95% CI -5.12 to -3.43, P < 0.01), length of hospitalization, (MD = -3.94, 95% CI -5.05 to -2.84, P < 0.01), and overall complications (RR = 0.40, 95% CI 0.25 to 0.64, P < 0.01) compared with OS. No significant differences were observed in surgery duration (MD = -28.67, 95% CI -58.58 to 1.23, P = 0.06), gross total resection (RR = 1.00, 95% CI 0.94 to 1.07, P = 0.92), blood transfusion (RR = 0.23, 95% CI 0.05 to 1.04, P = 0.06), or CSF leakage (RR = 0.50, 95% CI 0.24 to 1.04, P = 0.07).
Conclusion
Findings from this analysis suggest that MIS offers clinical advantages over OS in reducing blood loss, incision length, pain, time to mobilization, length of hospitalization, and overall complication rates.
Keywords: Minimally invasive surgery, Open surgery, Intraspinal tumors, Meta-analysis
Introduction
Intraspinal tumors, encompassing primary and metastatic growths within the spinal cord, meninges, nerve roots, and adjacent tissues, present a significant concern in spinal surgery. These tumors are classified based on anatomical location as extradural, intradural-extramedullary, or intramedullary. The incidence of intraspinal tumors is approximately 0.96–0.99 per 100,000 individuals, with benign tumors such as schwannomas, meningiomas, and neurofibromas being the most common [1, 2]. Early symptoms may be subtle; however, as the tumors advance, they frequently lead to spinal cord and nerve root compression, resulting in progressive neurological impairments [3, 4].
Surgical resection is the most effective treatment for intraspinal tumors. Traditional approaches, such as laminectomy and modified hemilaminectomy, have long been the standard of care. However, with advancements in precision medicine and minimally invasive surgical techniques, surgeons are increasingly aiming to reduce surgical trauma. In the past decade, minimally invasive surgery (MIS) using tubular retractors has gained popularity for resecting intraspinal tumors [5–7].
Although numerous studies [6, 8–13] have compared the outcomes of MIS with traditional open surgery (OS), findings have often been inconsistent due to clinical heterogeneity, small sample sizes, and limited statistical power. This study aims to synthesize the existing evidence and evaluate the clinical efficacy of MIS versus OS for treating intraspinal tumors.
Materials and methods
Literature search
We systematically searched PubMed, Cochrane Library, EMBASE, Web of Science, China National Knowledge Infrastructure (CNKI), and Wanfang databases for studies published from database inception through March 10, 2024. The search strategy involved combining keywords such as “minimally invasive surgery,” “keyhole surgery,” “tubular retractor,” “traditional open surgery,” and “intraspinal tumor.” Reference lists of selected studies were also reviewed. When full texts or original data were unavailable, authors were contacted via email. Two independent authors (CX and HL) conducted the search to maintain objectivity, with a third author consulted to resolve any disagreements.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (i) patients had a confirmed diagnosis of intraspinal tumors; (ii) the study compared MIS with OS; (iii) MIS was defined as using tubular retractors for lesion exposure, followed by laminectomy; (iv) OS involved subperiosteal lamina exposure and laminectomy or hemilaminectomy; (v) at least one of the following outcomes was reported: surgery duration, estimated blood loss, time to mobilization, length of hospitalization, visual analog scale (VAS) score, gross total resection, blood transfusion, cerebrospinal fluid (CSF) leakage, or overall complications; and (vi) the study included at least five patients per group. Studies lacking full texts or original data, duplicate publications, reviews, commentaries, conference abstracts, case series, or those focusing on biomechanical or cadaveric analyses were excluded.
Literature screening and data extraction
Two authors (CX and HL) independently screened the titles and abstracts to identify eligible studies. Full texts of selected studies were reviewed in detail based on inclusion and exclusion criteria. Extracted data included the first author, publication year, country, study design, tumor type, sample size, and reported outcomes. Discrepancies were resolved by consulting a third author.
Quality assessment
The methodological quality of observational studies was assessed using the Newcastle-Ottawa Scale (NOS), which evaluates selection, comparability, and outcome assessment. Studies scoring 6–9 were considered to be of good quality, while those scoring 0–5 were rated as poor quality. Quality assessment was performed independently by two authors (CX and HL), with a third author consulted to resolve any disagreements.
Sensitivity analysis and publication bias
A sensitivity analysis was conducted by sequentially excluding each study to evaluate the stability of the overall results. Publication bias was assessed using funnel plots, with asymmetry suggesting potential bias.
Statistical analysis
Data were analyzed using RevMan 5.3 software. Heterogeneity was evaluated using I² and P-values; if I² ≤ 50% and P ≥ 0.1, a fixed-effect model was applied; otherwise, a random-effect model was used. Continuous variables, including estimated blood loss, surgery duration, time to mobilization, length of hospitalization, visual analog scale (VAS) score, and incision length, were reported as mean differences (MD) with 95% confidence intervals (95% CIs). Dichotomous variables, including gross total resection, blood transfusion, cerebrospinal fluid (CSF) leakage, and overall complications, were reported as relative risks (RR) with 95%CIs. Statistical significance was set at α = 0.05 for all tests.
Results
Literature retrieval results
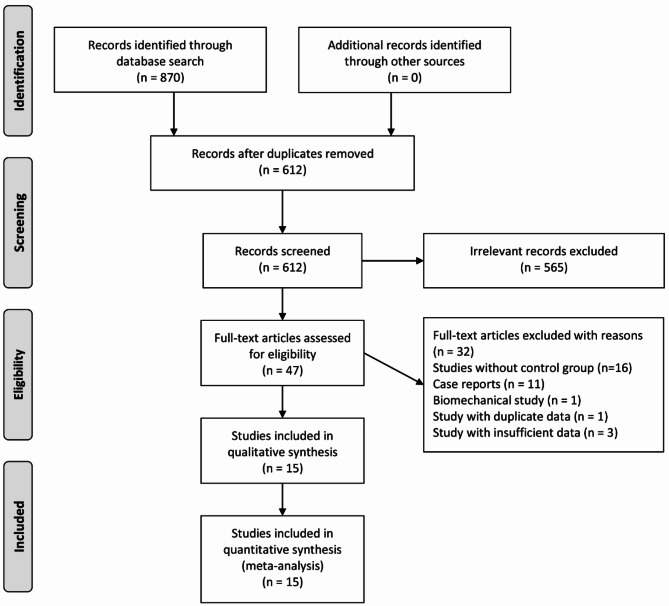
A comprehensive search of six databases yielded 870 relevant articles: 316 from PubMed, 369 from Web of Science, 123 from EMBASE, 1 from the Cochrane Library, 26 from CNKI, and 35 from Wanfang Data. No additional studies were identified through manual reference checks. One study, published in Russian, could not be accessed, and attempts to contact the author via email were unsuccessful. After removing duplicates and screening the titles and abstracts, the remaining articles underwent a full-text evaluation. Ultimately, 15 articles [6, 8–21] met the inclusion criteria and were included in the meta-analysis. The detailed literature screening process is presented in Fig. 1.
Fig. 1.
Flowchart of literature selection
Basic characteristics of included studies
Fifteen cohort studies were included, with 11 published in English and 4 in Chinese. In total, 943 participants were involved: 488 patients in the MIS group and 455 in the OS group. Among these, the study by Jiang et al. [18] was a prospective cohort, while the others were retrospective. The studies were conducted across several countries, including China, the United States, South Korea, France, Germany, and Portugal. The study quality evaluation showed that all studies scored at least 6 points, indicating good quality. Detailed characteristics of the included studies are summarized in Table 1.
Table 1.
Main characteristics of included studies
| Author | Year | Country | Publication language | Study design | Tumor type | Sample size | MIS/OS | Outcomes | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Kelly et al. | 2022 | America | English | RCS | Multiple | 21 | 11/10 | ①②④⑦⑧ | 7 |
| He et al. | 2022 | China | English | RCS | Meningioma, nerve sheath tumor | 42 | 9/33 | ①②④⑦⑧⑩ | 7 |
| Dauleac et al. | 2022 | France | English | RCS | Meningioma | 48 | 18/30 | ①②④⑦⑧⑩ | 7 |
| Xie et al. | 2020 | China | Chinese | RCS | Multiple | 69 | 31/38 | ①②③④⑦⑧⑨ | 7 |
| Wang et al. | 2020 | China | English | RCS | Multiple | 125 | 73/52 | ②③④⑤ | 6 |
| Li et al. | 2020 | China | Chinese | RCS | Multiple | 102 | 50/52 | ①②④⑤⑥⑨ | 7 |
| Jiang et al. | 2020 | China | Chinese | PCS | Nerve sheath tumor | 30 | 15/15 | ①②④⑧⑨ | 8 |
| Mende et al. | 2017 | Germany | English | RCS | Multiple | 245 | 156/89 | ④⑦⑧ | 7 |
| Chen et al. | 2016 | China | Chinese | RCS | Multiple | 33 | 16/17 | ①②③④⑤⑨ | 6 |
| Fontes et al. | 2016 | America | English | RCS | Multiple | 35 | 17/18 | ⑧⑩ | 8 |
| Wong et al. | 2015 | America | English | RCS | Multiple | 45 | 27/18 | ①②④⑥⑦⑧⑩ | 7 |
| Raygor et al. | 2015 | America | English | RCS | Multiple | 51 | 25/26 | ①②⑥⑦⑧⑩ | 8 |
| Lee et al. | 2015 | South Korea | English | RCS | Nerve sheath tumor | 24 | 6/18 | ①②④⑩ | 6 |
| Lu et al. | 2011 | America | English | RCS | Multiple | 27 | 18/9 | ①②④⑧⑨⑩ | 7 |
| Kitumba et al. | 2023 | Portugal | English | RCS | Multiple | 46 | 16/30 | ② | 7 |
RCS, retrospective cohort study; PCS, prospective cohort study; MIS, minimally invasive surgery; OS, open surgery; ① Estimated blood loss; ② surgery duration; ③ Incision length; ④ Length of hospitalization; ⑤ Time to mobilization; ⑥ Blood transfusion; ⑦ Cerebrospinal fluid leakage; ⑧ Overall complications; ⑨VAS; ⑩ Gross total resection
Results of meta-analysis
Surgery duration
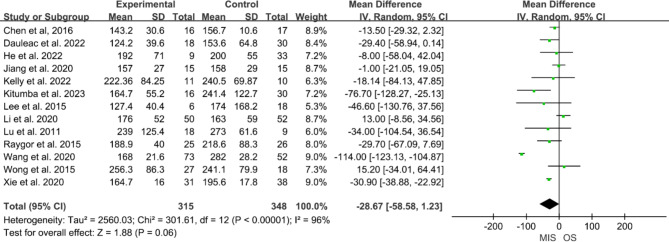
Thirteen studies [8–15, 17–21] reported surgery duration, involving 315 participants in the MIS group and 348 in the OS group. Significant heterogeneity was noted among these studies (P < 0.01, I² = 96%), necessitating the use of a random-effects model. The pooled data suggested that the MIS group had a shorter surgery duration than the OS group, but the difference was not statistically significant (MD = -28.67, 95% CI -58.58 to 1.23, P = 0.06, Fig. 2).
Fig. 2.
Forest plot of minimally invasive surgery versus open surgery for surgery duration
Estimated blood loss
Eleven studies [8–12, 14, 15, 17–19, 21] reported estimated blood loss, involving 226 participants in the MIS group and 266 in the OS group. High heterogeneity was present (P < 0.01, I² = 89%), so a random-effects model was applied. The results indicated that the MIS group experienced significantly less blood loss than the OS group (MD = -76.73, 95% CI -102.56 to -50.91, P < 0.01, Fig. 3).
Fig. 3.
Forest plot of minimally invasive surgery versus open surgery for estimated blood loss
Incision length
Three studies [17, 20, 21] reported incision length, involving 121 participants in the MIS group and 107 in the OS group. Due to substantial heterogeneity (P < 0.01, I² = 82%), a random-effects model was used. The MIS group had significantly shorter incisions than the OS group (MD = -4.09, 95% CI -5.20 to -2.97, P < 0.01).
VAS score
Six studies [14, 17–21] reported VAS pain scores, including 203 participants in the MIS group and 183 in the OS group. The analysis revealed considerable heterogeneity (P < 0.01, I² = 89%), prompting the use of a random-effects model. The pooled results demonstrated that the MIS group had significantly lower VAS scores than the OS group (MD = -0.79, 95% CI -1.48 to -0.11, P = 0.02).
Time to mobilization
Three studies [17, 19, 20] reported the time from surgery to first mobilization, including 139 participants in the MIS group and 121 in the OS group. High heterogeneity was noted (P < 0.01, I² = 94%), necessitating the use of a random-effects model. The analysis showed that the MIS group had a significantly shorter time to mobilization than the OS group (MD = -4.27, 95% CI -5.12 to -3.43, P < 0.01).
Length of hospitalization
Twelve studies [6, 9–12, 14, 15, 17–21] reported the length of hospitalization, involving 430 participants in the MIS group and 381 in the OS group. Considerable heterogeneity across studies was detected (P < 0.01, I2 = 88%), and a random-effects model was applied. The pooled data indicated that the MIS group had a significantly shorter hospital stay than the OS group (MD = -3.76, 95% CI -4.82 to -2.70, P < 0.01).
Gross total resection
Seven studies [8–11, 14–16] reported the gross total resection, involving 120 participants in the MIS group and 152 participants in the OS group. No between-study heterogeneity was detected (P < 0.99, I² = 0), and a fixed-effects model was applied. The pooled data indicated that there was no significant difference in the gross total resection rate between two groups (RR = 1.00, 95% CI 0.94 to 1.07, P = 0.92).
Blood transfusion
Three studies [8, 9, 19] examined the blood transfusion, including 102 participants in the MIS group and 96 in the OS group. With no observed heterogeneity (P = 0.88, I² = 0), a fixed-effects model was applied. Although the pooled results suggested a lower incidence of blood transfusion in the MIS group, the difference was not statistically significant (RR = 0.23, 95% CI 0.05 to 1.04, P = 0.06).
CSF leakage
Seven studies [6, 8–12, 21] reported the incidence of CSF leakage, involving 227 participants in the MIS group and 244 in the OS group. No heterogeneity was observed (P = 0.67, I² = 0), and a fixed-effects model was therefore used. The pooled data indicated a reduced incidence of CSF leakage in the MIS group, though the difference did not reach statistical significance (OR = 0.50, 95% CI 0.24 to 1.04, P = 0.07).
Total complications
Ten studies [6, 8–12, 14, 16, 18, 21] reported the overall complication rates, including 327 participants in the MIS group and 286 in the OS group. With no heterogeneity detected (P = 0.49, I² = 0), a fixed-effects model was utilized. The findings revealed a significantly lower overall complication rate in the MIS group than the OS group (RR = 0.49, 95% CI 0.33 to 0.73, P < 0.01).
Sensitivity analysis and publication bias
Sensitivity analyses confirmed that most results were stable, supporting their reliability. However, VAS scores proved sensitive to the inclusion of the study by Lu et al. [14], which showed notably larger difference between MIS and OS groups than other studies. Excluding this study led to stabilized VAS results, suggesting it was an outlier impacting the initial findings. Funnel plots for all outcomes showed no significant asymmetry, indicating no major publication bias (Fig. 4).
Fig. 4.
Funnel plot of minimally invasive surgery versus open surgery for surgery duration
Discussion
Traditional laminectomy for treating intraspinal tumors requires stripping the paraspinal muscles and removing posterior elements such as the lamina, spinous process, and interspinous ligament. This extensive removal can compromise spinal stability, increasing the risk of iatrogenic spinal deformity and often necessitating pedicle screw fixation for stabilization [22, 23]. In contrast, the hemilaminectomy technique, which preserves the interspinous and supraspinous ligaments, intervertebral joints, and paravertebral muscle on the contralateral side, offers enhanced stability and fewer postoperative complications. This approach is particularly suited for unilateral intraspinal tumors [24]. Microscope-assisted hemilaminectomy has further refined the precision of this approach, reducing the risk of complications [25]. However, limitations such as restricted exposure and a narrower surgical field persist, as the ipsilateral muscles are still removed.
Laminoplasty offers an alternative with increased structural resistance to buckling, shear, and rotation. A study by Song et al. [22] found that laminoplasty (replanting the resected lamina with micro-plate fixation) resulted in shorter operation time, lower blood loss, and reduced incidence of CSF leakage compared to laminectomy. The laminoplasty group also exhibited better neurological function improvement at 12-month follow-up. However, other studies have not demonstrated a consistent benefit in neurological outcomes or reduction in postoperative deformity risks [26]. For instance, Park et al. [27] used hollow screws to reattach the lamina and reconstruct ligaments in 10 patients, achieving favorable outcomes. Despite these improvements, risks remain; for example, the use of a T-saw to cut the lamina carries a risk of damaging the spinal cord or nerve roots, particularly in patients with larger tumors or reduced spinal canal space. A study by Wang et al. [28] on unilateral laminoplasty in 38 patients with cervical intraspinal tumors demonstrated significant neurological improvement, stable cervical curvature, and no severe complications over a 2-year follow-up.
In 2006, Tredway et al. [29] introduced channel-based techniques to facilitate tumor resection, which allow for gross total resection with significant symptom improvement. Lu et al. [14] further compared minimally invasive expandable channels for tumor resection against traditional open surgery. Results indicated that minimally invasive approaches led to reduced trauma, less blood loss, faster recovery, and fewer complications. Since then, comparative studies of MIS and OS for intraspinal tumors have emerged, although many have small sample sizes and inconsistent findings. Meta-analysis offers a solution to these limitations, combining data from multiple studies to enhance statistical power and reliability.
In this analysis, we reviewed 15 studies from China, South Korea, the United States, France, Portugal, and Germany, comparing MIS and OS for intraspinal tumors. Our findings showed that MIS significantly reduced intraoperative blood loss, incision length, postoperative pain (measured by VAS), length of hospitalization, and complication rates compared to OS. No significant differences were observed in surgical duration, CSF leakage, gross total resection, or blood transfusion. The analyses revealed high heterogeneity in surgery duration, estimated blood loss, incision length, VAS, time to mobilization, and length of hospitalization. This variability likely stems from differences in surgical methods, instrumentation, and inclusion criteria, as well as tumor location, type, size, and surgeon expertise. Due to the limited number and small size of included studies, subgroup analyses based on lesion type, site, and surgical approach were not feasible.
To assess the stability of our findings, we performed sensitivity analyses, which indicated good stability across indices except for the VAS score. Differences in pain assessment timing and baseline pain levels may have contributed to the VAS score variability. The study by Lu et al. [14] emerged as an outlier, showing large preoperative VAS score differences between MIS and OS groups. Excluding this study stabilized the results, indicating that MIS was associated with significantly lower postoperative pain. Variability in VAS score data limits further conclusions, suggesting that pain reduction may be a more consistent measure of procedural differences.
OS remains the standard approach for intraspinal tumor resection, providing wide exposure, clear visualization, and ample space for safe and complete tumor removal with minimized neurological injury risks. However, the removal of the spinal lamina in OS disrupts muscle and ligament attachments, which can compromise spinal stability and potentially lead to deformities [26, 30]. For ventral tumors, OS may involve cutting the dentate ligament and retracting the spinal cord, which heightens the risk of neurological injury. Subchannel resection with precise incision placement and channel adjustment could mitigate some of these risks, though OS is also associated with larger incisions, increased blood loss, greater postoperative pain, and prolonged recovery. In a study by Xie et al. [21], patients undergoing MIS showed lower creatine kinase levels on the first postoperative day than those undergoing OS, suggesting reduced muscle injury in MIS. Financially, MIS was 24.5% less expensive than OS in the study by Fontes et al. [16], although cost analysis was not performed in this study due to limited data.
MIS is also technically challenging, requiring high skill and a steep learning curve due to the limited field of view and narrow operating space. Additionally, MIS is not suitable for all tumors, particularly those spanning multiple segments. Frequent fluoroscopy during channel establishment can increase radiation exposure for both patients and staff. The constrained space in MIS makes dural suturing challenging and necessitates accurate preoperative and intraoperative tumor localization.
Despite this study systematically reviewed evidence on OS and MIS for intraspinal tumors, several limitations remain to be overcome. First, this meta-analysis lacks of randomized controlled trial studies, and except for one prospective study by Jiang et al. [18], all included studies were retrospective, which might reduce the overall strength of the evidence. Second, all studies were single-center with small sample sizes, which might introduce selection bias. Future studies should prioritize multi-center and large-sample prospective randomized controlled trials to strengthen the findings. Third, the included studies primarily focused on short-term intraoperative outcomes, long-term outcomes such as tumor recurrence and spinal alignment could not be evaluated. Fourth, the literature search was limited to English and Chinese databases, potentially excluding relevant studies in other languages, and incurring potential publication bias. Fifth, heterogeneity in MIS channel selection, record method of blood loss, inclusion/exclusion criteria, and tumor size across studies also influence the pooled outcomes.
Conclusion
MIS for intraspinal tumors offers several advantages over OS, including smaller incisions, reduced trauma and blood loss, shorter hospital stays, faster mobilization, less postoperative pain, and fewer complications. However, MIS is limited in applicability to larger tumors, requires advanced technical skill and equipment, and entails a steep learning curve. Due to the limitations of this meta-analysis, including small sample sizes and retrospective study designs, further multi-center, large-sample prospective randomized controlled trials are essential to validate these findings.
Abbreviations
- MIS
Minimally invasive surgery
- OS
Open surgery
- CNKI
China national knowledge infrastructure
- VAS
Visual analog scale
- MD
Mean differences
- CSF
Cerebrospinal fluid
- RR
Risk ratios
- CI
Confidence intervals
- NOS
Newcastle-ottawa scale
Author contributions
Haonan Li, Chuanhui Xun and Weibin Sheng wrote the main manuscript text; Yukun Hu, Shutao Gao, Jianlin Xu and Yanlong Wang prepared Figs. 1, 2, 3 and 4; Ting Wang prepared Table 1. All authors reviewed the manuscript.
Funding
This work was funded by the Key Program of Natural Science Foundation of Xinjiang Uygur Autonomous Region (2021D01D18), the National Natural Science Foundation of China (82360257), the Program of Technological leading talent of Tianshan talent (2023TSYCLJ0031), the Youth Foundation of Research and Development (2023YFY-QKMS-06).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chuanhui Xun and Haonan Li contributed equally to this work.
References
- 1.Duong LM, McCarthy BJ, McLendon RE, Dolecek TA, Kruchko C, Douglas LL, Ajani UA. Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004–2007. Cancer 2012 118(17):4220–7. [DOI] [PMC free article] [PubMed]
- 2.Weber C, Gulati S, Jakola AS, Habiba S, Nygaard ØP, Johannesen TB, Solheim O. Incidence rates and surgery of primary intraspinal tumors in the era of modern neuroimaging: a national population-based study. Spine. 2014;39(16):E967–973. [DOI] [PubMed] [Google Scholar]
- 3.Tsai CY, Tsai TH, Su YF. Surgical treatment of intraspinal tumors in Southern Taiwan: the 30-year experience of a single institution. J Clin Neurosci. 2020;75:52–4. [DOI] [PubMed] [Google Scholar]
- 4.Nemeiko I, Borgstedt-Bakke JH, Wichmann TO, Gudmundsdottir G, Rasmussen MM. Characteristics and outcomes in patients with primary intraspinal tumours. Dan Med J. 2019;66(3):A5534. [PubMed] [Google Scholar]
- 5.Formo M, Halvorsen CM, Dahlberg D, Brommeland T, Fredø H, Hald J, Scheie D, Langmoen IA, Lied B, Helseth E. Minimally invasive microsurgical resection of primary, Intradural Spinal Tumors is feasible and safe: a consecutive series of 83 patients. Neurosurgery. 2018;82(3):365–71. [DOI] [PubMed] [Google Scholar]
- 6.Mende KC, Krätzig T, Mohme M, Westphal M, Eicker SO. Keyhole approaches to intradural pathologies. Neurosurg Focus. 2017;43(2):E5. [DOI] [PubMed] [Google Scholar]
- 7.Soriano Sánchez JA, Soto García ME, Soriano Solís S, Rodríguez García M, Trejo Huerta P, Sánchez Escandón O, Flores Soria ER, Romero-Rangel JAI. Microsurgical Resection of Intraspinal Benign tumors using Non-expansile Tubular Access. World Neurosurg. 2020;133:e97–104. [DOI] [PubMed] [Google Scholar]
- 8.Raygor KP, Than KD, Chou D, Mummaneni PV. Comparison of minimally invasive transspinous and open approaches for thoracolumbar intradural-extramedullary spinal tumors. Neurosurg Focus. 2015;39(2):E12. [DOI] [PubMed] [Google Scholar]
- 9.Wong AP, Lall RR, Dahdaleh NS, Lawton CD, Smith ZA, Wong RH, Harvey MJ, Lam S, Koski TR, Fessler RG. Comparison of open and minimally invasive surgery for intradural-extramedullary spine tumors. Neurosurg Focus. 2015;39(2):E11. [DOI] [PubMed] [Google Scholar]
- 10.Dauleac C, Leroy HA, Karnoub MA, Obled L, Mertens P, Assaker R. Minimally invasive surgery for intradural spinal meningioma: a new standard? A comparative study between minimally invasive and open approaches. Neurochirurgie. 2022;68(4):379–85. [DOI] [PubMed] [Google Scholar]
- 11.He Z, Li CY, Mak CH, Tse TS, Cheung FC. Minimally invasive tubular retractor surgery for Intradural Extramedullary spinal tumor reduces postoperative degeneration of paraspinal muscle. Asian J Neurosurg. 2022;17(1):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly R, Fayed I, Conte A, Rock M, Nair N, Voyadzis JM, Sandhu F, Anaizi A. Minimally invasive surgery for intradural extramedullary spinal cord pathologies: a case series and technical note. J Clin Neurosci. 2022;97:108–14. [DOI] [PubMed] [Google Scholar]
- 13.Kitumba D, Reinas R, Pereira L, Pinto V, Alves OL. Spinal Intradural Extramedullary tumors. A retrospective analysis on ten-years’ experience of minimally invasive surgery and a comparison with the Open Approach. Acta Neurochir Suppl. 2023;135:357–60. [DOI] [PubMed] [Google Scholar]
- 14.Lu DC, Chou D, Mummaneni PV. A comparison of mini-open and open approaches for resection of thoracolumbar intradural spinal tumors. J Neurosurg Spine. 2011;14(6):758–64. [DOI] [PubMed] [Google Scholar]
- 15.Lee SE, Jahng TA, Kim HJ. Different Surgical approaches for spinal Schwannoma: a single surgeon’s experience with 49 consecutive cases. World Neurosurg. 2015;84(6):1894–902. [DOI] [PubMed] [Google Scholar]
- 16.Fontes RB, Wewel JT, OʼToole JE. Perioperative cost analysis of minimally invasive vs Open Resection of Intradural Extramedullary spinal cord tumors. Neurosurgery. 2016;78(4):531–9. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Bao G, He BX. Comparison of the efficacy of intraspinal tumor resection under quadrant channel and hemilaminectomy surgery. J Xi’an Jiaotong Univ. 2016;37(02):183–6. [Google Scholar]
- 18.Jiang WJ, Wang YZ, Yang J, Jia WQ. Comparison between minimally invasive surgery and traditional laminectomy in the resection of schwannoma of lumbar vertebra. Zhonghua Yi Xue Za Zhi. 2020;100(4):274–8. [DOI] [PubMed]
- 19.Li Y, Xu J, Zheng W. Comparison of the safety and outcomes between keyhole surgery and total laminectomy for resection of intradural extramedullary spinal cord tumors. Zhonghua Yi Xue Za Zhi. 2020;100(39):3093–8. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Zhao HY, Lei DQ, Zhu WD, Zhou YC. An analysis of clinical efficacy of Microsurgical Resection of Intradural Neoplasm by Unilateral Approach with Caspar Retractors. Med Princ Pract. 2020;29(3):231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie R, Shou JJ, Chen G, Che XY, Dong YH, Li JQ, Che XM. Surgical strategy of intraspinal tumors using minimal invasive channels. Zhonghua Yi Xue Za Zhi. 2020;100(4):265–9. [DOI] [PubMed]
- 22.Song Z, Zhang Z, Ye Y, Zheng J, Wang F. Efficacy analysis of two surgical treatments for thoracic and lumbar intraspinal tumours. BMC Surg. 2019;19(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang P, Ma K, Chen T, Xue X, Ma D, Wang S, Chen X, Meng H, Cui G, Gao B, et al. Risk factor analysis for progressive spinal deformity after resection of intracanal tumors: a retrospective study of 272 cases. BMC Neurol. 2020;20(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei D, Zhou Y, Yao D, Zhang F, Wang X, Jiang X, Xiong N, Zhao H. Efficacy of unilateral hemilaminectomy for intraspinal tumor resection: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(2):984–99. [DOI] [PubMed] [Google Scholar]
- 25.Yan X, Wang H, Li C, Lin Y, Lin L, Zhu S, Wang C, Lin Z, Jiang C, Kang D. Endoscopically controlled surgery with open hemilaminectomy for the treatment of intradural extramedullary tumors: an operative technique and short-term outcomes of 20 consecutive cases. Chin Neurosurg J. 2021;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGirt MJ, Garcés-Ambrossi GL, Parker SL, Sciubba DM, Bydon A, Wolinksy JP, Gokaslan ZL, Jallo G, Witham TF. Short-term progressive spinal deformity following laminoplasty versus laminectomy for resection of intradural spinal tumors: analysis of 238 patients. Neurosurgery. 2010;66(5):1005–12. [DOI] [PubMed] [Google Scholar]
- 27.Park YJ, Kim SK, Seo HY. Ligament-Saving Laminoplasty for Intraspinal Tumor Excision: a technical note. World Neurosurg. 2019;128:438–43. [DOI] [PubMed] [Google Scholar]
- 28.Wang ZC, Li SZ, Qu XF, Yin CQ, Sun YL, Wang YL, Wang J, Liu CJ, Cao ZL, Wang T. Application of open-door laminoplasty with ARCH plate fixation in cervical intraspinal tumors. BMC Surg. 2021;21(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tredway TL, Santiago P, Hrubes MR, Song JK, Christie SD, Fessler RG. Minimally invasive resection of intradural-extramedullary spinal neoplasms. Neurosurg 2006;58(1 Suppl):ONS52-58; discussion ONS52-58. [DOI] [PubMed]
- 30.Joaquim AF, Riew KD. Management of cervical spine deformity after intradural tumor resection. Neurosurg Focus. 2015;39(2):E13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.