Abstract
Background
Poor sleep quality is one of the prevalent manifestations experienced by cancer patients. There is a lack of research focusing specifically on sleep quality and affecting factors in Gastrointestinal (GI) cancer patients. This study aimed to assess the potential interaction between dietary, comorbid conditions, demographic, and socioeconomic determinants of sleep quality in GI cancer patients.
Methods
In a cross-sectional study, the Pittsburg Sleep Quality Index (PSQI) was completed for 875 adult patients suffering from GI cancer in a referral hospital. We conducted structural equation modeling analyses to evaluate the potential interaction between dietary and socioeconomic determinants of sleep quality in GI cancer patients.
Results
This study demonstrated that the PSQI encompasses two factors (perceived sleep quality and sleep disturbances) in GI cancer patients in Iran. Based on the standardized coefficients for the structural paths, the wealth index (WI) partially mediated the effect of food insecurity (FI) on the sleep quality index. There was a direct predictive effect of the WI on the PSQI (β = 0.10, P = 0.01). In addition, WI indirectly through FI (effect of WI on FI: β = -0.21, P = 0.01 & effect of FI on PSQI: β = 0.07, P = 0.03) had a negative effect on PSQI. Our finding suggested the full mediation effect of age on PSQI through the number of comorbidities (effect of age on number of comorbidities: β = 0.25, P < 0.001 & the effect of number of comorbidities on PSQI: β = 0.13, P < 0.001). Structural path outputs for gender-model indicated the full mediation effect of age on PSQI through the number of comorbidities among males and through the FI among females.
Conclusion
These findings highlight the importance of considering the bi-dimensional construct of the PSQI for assessing sleep quality among GI cancer patients. The current study demonstrated that food insecurity and comorbidity prevalence mediated the relationship between socio-demographic determinants of sleep quality in patients with GI cancer.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-13347-7.
Keywords: Gastrointestinal (GI) cancer, Pittsburg Sleep Quality Index, Food insecurity, Structural equation model
Introduction
Gastrointestinal (GI) cancer is one of the most common malignancies worldwide caused by both environmental and genetic factors, which make it a multifactorial disease [1]. It is responsible for 35.4% of global deaths [2]. Along with the medical management of these patients, considering the factors that influence general health and consequently, treatment adherence is significant. In this context, sleep quality is crucial to overall well-being and health outcomes, especially for patients with GI cancer. Sleep disturbance is common among these patients [3, 4]. Despite its prevalence, sleep disturbance remains a missing issue in the development and treatment of cancers [5]. This problem may associated with low quality of life, fatigue, and possibly a reduced survival rate [6, 7].
The Pittsburgh Sleep Quality Index (PSQI) is a widely recognized tool for assessing sleep quality across various populations, including cancer patients [8]. The PSQI, developed by Buysse et al., offers a comprehensive evaluation of sleep quality by capturing different dimensions over a month. While some studies support a unidimensional application of the instrument, a systematic review concluded that it is more appropriately understood as a multidimensional tool [9]. A study examining the factor structure of the PSQI within Singapore's general population indicated that two-and three-factor models were superior to the unidimensional model initially proposed by Buysse et al. [10]. The factor structure of the PSQI may vary based on the population it is used with, highlighting the necessity of investigating its dimensionality across different groups to ensure its suitability and optimal use. Given that sleep quality can differ between specific clinical populations and the general population [11], further research in this area is needed.
Sleep is affected by biological, and psychosocial issues, that influence mental and physical status [12]. In this context, some factors are known to affect the quality and quantity of sleep, including demographic factors and chronic diseases [13, 14]. Gender and age need to be more considered particularly in cancerous patients. Earlier studies have revealed that sleep disturbance is more common among females than males [15, 16]. In a meta-analysis, Zhang and Wing [17] found that women are 1.41 times more likely to suffer from insomnia. Regarding age, Madrid-Valero in a Spanish study demonstrated that age has a significant association with sleep quality [14]. In this context, developing cancer at a young age may be related to higher psychological distress and fear of cancer progression and its consequences [16].
Furthermore, among affected factors on sleep quality, socioeconomic status is a key predictor [18, 19]. Higher income is consistently linked with superior education and job status, resulting in increased food security. Also, variables such as access to health care and food security may be influenced by socioeconomic status. All these factors could impact an individual's sleep quality [20]. Decreased socioeconomic status can result in longer work hours or night shifts, as well as limited food access, which can negatively affect sleep quality either alone or in conjunction with other factors [21]. Xue et al., in a study on 3250 adult persons demonstrated that socioeconomic status as well as poor sleep quality had a significant impact on the development and presentation of multi-morbidities [22]. Grandner et al., in a large-scale study, revealed that lower socioeconomic status is associated with sleep complications as lower income and educational attainment were associated with more sleep complaints, particularly among patients with cancers [23]. Food insecurity (FI) is defined as the limited or uncertain availability of safe and nutritious foods or access to food in a socially acceptable manner. It is a worldwide health problem that influences one-third of the world population [24, 25]. FI is often used as a proxy for individuals’ low socioeconomic status [26]. Association of FI in mild to severe levels with sleep quality was reported. This issue may multiply among cancerous patients who suffer from cancer-related chronic fatigue and psychological problems that may cause limitations in performance and doing their work [19, 27, 28].
A major behavioral risk factor for human health is thought to be nutrition and eating habits. Growing scientific data points to a possible connection between diet and sleep [29]. Previous studies have revealed that some eating behaviors may affect nighttime sleep and daytime alertness [30, 31]. Additionally, a link between energy consumption and sleep quality was found [31, 32]. Grimaldi et al. in a meta-analysis of 28 longitudinal studies demonstrated that lower BMI and fat percentage are associated with longer sleep duration, better sleep quality, and lower insomnia symptoms. Also, sleep duration of less than seven hours and low sleep quality were associated with a higher risk of obesity and its consequences. Although, anthropometric indices were not related to sleep over time [33].
In summary, despite a large number of studies about sleep quality in different populations, there is a lack of research focusing specifically on sleep and affecting factors and the link between these factors in GI cancer patients. Investigating this aspect could unravel the distinct dimensions of sleep quality that are most affected in this patient group. Moreover, identifying determinants could pave the way for tailored interventions to improve sleep quality and subsequently enhance patients' overall quality of life and treatment outcomes.
This research endeavors to unravel the factors affecting sleep quality among patients with GI cancers and elucidate the determinants contributing to sleep disturbances within this unique population. Therefore, based on the above-mentioned evidence, the following hypothesized model was proposed (Fig. 1).
Fig. 1.
Proposed hypothesized model of Pittsburgh Sleep Quality Index and its determinants among GI cancer patients
Methods
Study design and sample
This cross-sectional investigation was carried out as part of the Firoozgar Gastrointestinal Cancer Cohort Study (FGCCS) in Tehran, Iran.
The recommended sample size was calculated according to the number of observed (n = 20) and latent variables (n = 2) in the model, the anticipated effect size equal to 0.1, and the desired probability and statistical power levels equal to 0.8 and 0.05 respectively, using an online calculator:
https://www.danielsoper.com/statcalc/calculator.aspx?id=89.
Within the FGCCS framework, a total of 948 previously untreated adult patients diagnosed with gastrointestinal (GI) cancer at Firoozgar Hospital from 2015 to 2018 were included in the study. These patients were consequently selected for those who were referred to the clinic due to suspected GI cancer and need to be evaluated more carefully.
Exclusion criteria for this study encompassed: 1) individuals who were not of Iranian nationality, 2) those below 18 years of age, and 3) questionnaires with over 20% missing data. Furthermore, individuals currently undergoing anti-cancer treatments were also excluded from the study. A consent form according to the Declaration of Helsinki obtained of each patient.
Measurements
Sleep quality assessment
Sleep quality and sleep duration were evaluated using the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Previous research has demonstrated the reliability and validity of the PSQI-P [34, 35]. Beck's study also confirmed the reliability and validity of the PSQI in cancer patients [36]. In a study conducted by Khorrami-Rad et al., in breast cancer patients receiving chemotherapy, Intraclass Correlation Coefficient (ICC) was 0.87 and the reliability of the questionnaire was verified with a Cronbach’s alpha of 0.82 [37]. The PSQI is a commonly used tool for assessing sleep quality, consisting of seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medication, and daytime dysfunction [38]. In our study, these components were subjectively assessed using 19 self-rating items. Each component was assigned a score from 0 to 3, resulting in a global PSQI score ranging from 0 to 21. A global PSQI score greater than 5 indicates poor sleep quality. This cutoff score demonstrates a sensitivity of 89.6% and a specificity of 86.5% [8].
Lifestyle information
Lifestyle information including nutrition, physical activity, smoking, and alcohol consumption was recorded through a validated questionnaire [39, 40].
Food insecurity assessment
Food insecurity was measured using the validated six-item Short Questionnaire of Household Food Security Scale (SQHFSS) [41]. The questionnaire consisted of six items related to anxiety about food supply, compromised food quality and variety, insufficient food quantity, and experiencing hunger. Participants were classified as food insecure if they answered yes to two or more of the questions [42]. A dichotomized food insecurity indicator was created, with a value of one indicating food insecurity and zero indicating food security.
Wealth Index (WI)
The Wealth Index is considered a valid indicator of affluence and income, especially in countries where reliable income and expenditure data are lacking [43]. It provides an alternative measure of socioeconomic status and helps avoid multi-collinearity problems in regression models. To calculate the Wealth Index, the authors used Principal Component Analysis (PCA) to analyze different assets possessed by individuals, such as electrical appliances and sanitation facilities [44–46]. The assets were weighted based on their distribution among households. Items that were more unequally distributed were given greater weight, while items that were owned by all households were given zero weight. These scores were then used to determine wealth quintiles, dividing households into five categories, including poorest, poor, middle, rich, and richest.
Other variables
Socio-demographic characteristics (age, sex, education, marital status, area of living, and ethnicity), and history of comorbidities were measured. Weight and height were measured under standard protocols using calibrated instruments [40]. BMI was calculated as weight (kg) divided by squared height (m2).
Lifestyle and socioeconomic data were collected using the WHO-STEP questionnaire and the ultra-short Socioeconomic Status (SES-Iran) assessments. The STEP questionnaire had two sections: the first collected demographic information (age, gender, education, household size, ethnicity, comorbidities, family history of diseases), while the second included 12 questions about lifestyle factors such as smoking, fruit and vegetable intake, dietary habits, and physical activity. The validity of the questionnaire (Kappa coefficient = 0.94) and its reliability were confirmed (Cronbach-Alpha = 0.98, ICC = 0.94; CI: 0.87–0.97) [47]. All assessments were conducted simultaneously through patient interviews before the commencement of treatment.
Statistical analysis
The student’s t-test was applied for the continuous variable (age), and χ2 test was used for the categorical variables, including age, sex, marital status, family local residency, years of education, ethnicity (fars, other), wealth index, physical activity, abdominal obesity, BMI, food security, number of comorbidities (no comorbidities, 1 comorbidity, ≥ 2 comorbidities), family history of GI cancer, chronic disease, smoking, alcohol consumption, GI cancer diagnosis by sleep quality status.
We used both unadjusted and multivariable-adjusted logistic regression models for the binary outcome of sleep quality. Results were reported as crude or adjusted ORs (AORs) with 95% CI by controlling for potential confounders, including age, gender (male, female), marital status (Single/Divorced/Widowed, married), ethnicity (Fars, other), smoking (no, yes), education (< 12 yrs, ≥ 12 yrs), number of comorbidities, food insecurity (no, yes), wealth index (poorest, poorer, moderate, richer, richest). Additionally, Principal Component Analysis (PCA) was used to calculate the Wealth Index.
We conducted Explanatory Factor Analysis (EPA) using maximum likelihood estimation with orthogonal rotation, and the scree plot and eigenvalues (≥ 1) were used to determine the required number of factors. A rotated factor loading threshold of 0.4 was considered meaningful in identifying items with common characteristics within each PSQI component [33, 34]. A Confirmatory Factor Analysis (CFA) was then conducted using maximum likelihood estimation approaches, and various statistical measures were used to assess model fit, including χ2, the ratio of the χ2 to degrees of freedom (CMIN/DF)/chi-squared statistics, root mean square error of approximation (RMSEA), comparative fit index (CFI), standardized root mean square residual (SRMR), goodness-of-fit index (GFI), adjusted goodness-of-fit index (AGFI), and Tucker Lewis index (TLI). The fit indices of models were evaluated against predefined criteria [48].
To provide an overall view of the relationships between various variables and PSQI in Model I, demographic and SES factors, eating behaviors, food insecurity, BMI, and the total PSQI score (as Sleep Quality) were included. In Model II, PSQI was included as the two-factor model (Fig. 2) to provide a more detailed understanding of the mechanistic relationships among these variables. Gender-specific models were developed because the effects of socio-demographic factors and eating behaviors on sleep quality have been shown to differ between males and females [49–51].
Fig. 2.
Confirmatory factor analysis for the two-factor model of sleep quality. Model fit indices: χ2 = 33.463, χ2/df = 3.042, GFI = 0.99, AGFI = 0.974, CFI = 0.982, IFI = 0.982, TLI = 0.965 SRMR = 0.026 and RMSEA = 0.048. The numbers on the paths represent standardized effects. The bold-face arrows represent significant values. The significance level of the comparison of each effect is depicted by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). Red arrows refer to females whereas blue arrows refer to males
Results
Characteristics of study participants
Table 1 shows the baseline characteristics of the population. The mean age of participants was 62.07 (± 13.16) years, with a large proportion aged more than ≥ 65 years (44.5%); the majority of participants were male (61.2%), married (83.8%), urban residency (61.9%), educated lower than 12 years (73.1%). The prevalence of general and central obesity was 61.4% and 37.7%, respectively, without any significant difference between normal and insomnia sleepers/normal and impaired sleep quality.
Table 1.
Characteristics of study participants
| Variables | Total n (%) |
Good sleep quality n (%) |
Poor sleep quality n (%) |
p-value | |
|---|---|---|---|---|---|
| Sleep durationa | |||||
| < 5 h | 359(41.0) | 11(3.7) | 348(60.2) | < 0.001 | |
| ≥ 5- ≤ 6 h | 255(29.1) | 91(30.6) | 164(28.4) | ||
| > 6- < 9 h | 229(26.2) | 174(58.6) | 55(9.5) | ||
| ≥ 9 h | 32(3.7) | 21(7.1) | 11(1.9) | ||
| Age (years) | |||||
| < 50 | 139(15.9) | 52(17.5) | 87(15.1) | 0.501 | |
| 50- < 65 | 347(39.7) | 111(37.4) | 236(40.8) | ||
| ≥ 65 | 389(44.5) | 134(45.1) | 255(44.1) | ||
| Sex | |||||
| Male | 534(61.0) | 195(65.7) | 339(58.7) | 0.044 | |
| Female | 341(39.0) | 102(34.3) | 239(41.3) | ||
| Marital Statusb | |||||
| Single/widow/divorce | 142(16.2) | 44(14.8) | 98(17.0) | 0.416 | |
| Married | 733(83.8) | 253(85.2) | 480(83.0) | ||
| Family local residency | |||||
| Urban | 179(61.9) | 77(64.7) | 102(60.0) | 0.417 | |
| Rural | 110(38.1) | 42(35.3) | 68(40.0) | ||
| Years of education (years) | |||||
| < 12 | 640(73.1) | 215(72.4) | 425(73.5) | 0.719 | |
| ≥ 12 | 235(26.9) | 82(27.6) | 153(26.5) | ||
| Ethnicity | |||||
| Fars | 359(41.1) | 118(40.0) | 241(41.7) | 0.630 | |
| Other | 514(58.9) | 177(60.0) | 337(58.3) | ||
| WI | |||||
| Poorest | 154(19.9) | 53(19.1) | 101(20.4) | 0.065 | |
| Poor | 161(20.9) | 48(17.3) | 113(22.9) | ||
| Moderate | 148(19.2) | 49(17.6) | 99(20.0) | ||
| Rich | 155(20.1) | 59(21.2) | 96(19.4) | ||
| Richest | 154(19.9) | 69(24.8) | 85(17.2) | ||
| Physical activity level | |||||
| Level 1 | 197(22.7) | 67(22.8) | 130(22.7) | 0.233 | |
| Level 2 | 211(24.3) | 77(26.2) | 134(23.4) | ||
| Level 3 | 314(36.2) | 94(32.0) | 220(38.4) | ||
| Level 4 | 145(16.7) | 56(19.0) | 89(15.5) | ||
| BMI | |||||
| < 25 | 484(61.4) | 178(65.0) | 306(59.5) | 0.136 | |
| ≥ 25 | 304(38.6) | 96(35.0) | 208(40.5) | ||
| Abdominal obesity† | |||||
| No | 492(62.3) | 173(63.4) | 319(61.7) | 0.646 | |
| Yes | 298(37.7) | 100(36.6) | 198(38.3) | ||
| Food security | |||||
| Food secure | 537(67) | 192(69.3) | 345(65.8) | 0.320 | |
| Food insecure | 264(33) | 85(30.7) | 179(34.2) | ||
| Number of comorbidities | |||||
| No comorbidities | 506(57.8) | 191(64.3) | 315(54.5) | 0.016 | |
| 1 comorbidity | 254(29.0) | 76(25.6) | 178(30.8) | ||
| ≥ 2 comorbidities | 115(13.1) | 30(10.1) | 85(14.7) | ||
| Comorbidities | |||||
| Heart attack | Yes | 39(4.5) | 13(4.4) | 26(4.5) | 1 |
| No | 836(95.5) | 284(95.6) | 552(95.5) | ||
| High blood pressure | Yes | 241(27.5) | 67(22.6) | 174(30.1) | 0.018 |
| No | 634(72.5) | 230(77.4) | 404(69.9) | ||
| Diabetes | Yes | 197(22.5) | 56(18.9) | 141(24.4) | 0.063 |
| No | 678(77.5) | 241(81.1) | 437(75.6) | ||
| Rheumatic | Yes | 27(3.1) | 6(2.0) | 21(3.6) | 0.221 |
| No | 848(96.9) | 291(98) | 557(96.4) | ||
| Family history of GI cancer | |||||
| No | 767(87.7) | 263(88.6) | 504(87.2) | 0.589 | |
| Yes | 108(12.3) | 34(11.4) | 74(12.8) | ||
| Chronic disease | |||||
| No | 253(28.9) | 116(39.1) | 137(23.7) | < 0.001 | |
| Yes | 622(71.1) | 181(60.9) | 441(76.3) | ||
| Smoking status | |||||
| Never | 597(68.2) | 195(65.7) | 402(69.6) | 0.350 | |
| Former | 138(15.8) | 54(18.2) | 84(14.5) | ||
| Current | 140(16.0) | 48(16.2) | 92(15.9) | ||
| Addiction | |||||
| No | 766(87.5) | 257(86.5) | 509(88.1) | 0.516 | |
| Yes | 109(12.5) | 40(13.5) | 69(11.9) | ||
| Alcohol consumption | |||||
| No | 842(96.2) | 287(96.6) | 555(96.0) | 0.712 | |
| Yes | 33(3.8) | 10(3.4) | 23(4.0) | ||
| GI Cancer diagnosis | |||||
| Esophagus | 75(8.6) | 29(9.8) | 46(8.0) | 0.425 | |
| Gastric | 237(27.1) | 89(30.0) | 148(25.6) | ||
| Pancreas | 268(30.6) | 84(28.3) | 184(31.8) | ||
| Biliary | 169(19.3) | 51(17.2) | 118(20.4) | ||
| Colorectal | 92(10.5) | 30(10.1) | 62(10.7) | ||
| liver | 34(3.9) | 14(4.7) | 20(3.5) | ||
| Stage | |||||
| I | 132(15.3) | 45(15.5) | 87(15.2) | 0.145 | |
| II | 151(17.5) | 49(16.8) | 102(17.8) | ||
| III | 201(23.3) | 81(27.8) | 120(21.0) | ||
| IV | 334(38.7) | 99(34.0) | 235(41.1) | ||
| Unknown | 45(5.4) | 17(5.8) | 28(4.9) | ||
Abbreviations: BMI body mass index, WI wealth index, GI Gastrointestinal
†Abdominal obesity: waist circumference > 102 cm for men and > 88 cm for women
The association between the study variable and sleep quality index
As presented in Table 2, except for the association between sugary drinks and sleep quality index in the unadjusted model among men, no other significant associations were identified. Higher intake of sugary drinks disturbed the quality of sleep (OR 1.502, 95% CI 1.023–2.204). However, it was no longer significant after the adjustment for potential confounders.
Table 2.
Multivariable linear and multinomial logistic regression results of outcome variable with sleep quality status
|
Poor sleep quality (Mean ± SD) |
Good sleep quality (Mean ± SD) |
Unadjusted | Adjusted | |||
| β | 99% CI* | β | 99% CI* | |||
| BMI (kg/cmb)c | 24.44 ± 5.17 | 24.05 ± 5.02 | 0.037 | (−0.353; 1.148) | 0.018 | (−0.609; 0.990) |
| WC (cm)a | 93.01 ± 14.75 | 93.65 ± 14.54 | −0.021 | (−2.792; 1.510) | −0.046 | (−3.312; 0.760) |
|
Poor sleep quality n (%) |
Good sleep quality n (%) |
OR | 99% CI* | OR | 99% CI* | |
| Fruit and vegetablesc | ||||||
| ≥ 400 g/day | 17(3.1) | 9(3.2) | 1.007 | (0.442; 2.293) | 0.730 | (0.476; 1.118) |
| ≥ 200& < 400 g/day | 91(16.5) | 43(15.1) | 0.898 | (0.605; 1.335) | 0.661 | (0.268; 1.630) |
| < 200 g/day | 443(80.4) | 233(81.8) | Ref | Ref | ||
| Dietary fiberc | ||||||
| ≥ 17 g/day | 16(2.9) | 11(3.9) | 1.350 | (0.618; 2.949) | 1.213 | (0.497; 2.958) |
| < 17 g/day | 534(97.1) | 272(96.1) | Ref | Ref | ||
| Red and processed meata | ||||||
| Low amount of meat$ | 45(8.1) | 29(10.1) | 1.283 | (0.785; 2.100) | 1.172 | (0.687; 2.01) |
| Moderate amount of meat$ | 470(84.8) | 236(82.5) | Ref | Ref | ||
| High amount of meat$ | 39(7.0) | 21(7.3) | 1.072 | (0.617; 1.864) | 0.850 | (0.473; 1.530) |
| Alcohol (drinks/day)c | ||||||
| ♂: ≤ 2 and ♀: ≤ 1 drinks | 556(96.2) | 287(96.6) | Ref | Ref | ||
| ♂: > 2 and ♀: > 1 drinks | 22(3.8) | 10(3.4) | 0.881 | (0.411; 1.885) | 0.689 | (0.275; 1.729) |
| Sugary drinks (ml/day)c | ||||||
| Men | ||||||
| 0 ml | 95(30.7) | 72(40.0) | 1.502 | (1.023; 2.204) | 1.280 | (0.823;1.988) |
| > 0 ml | 214(69.3) | 108(60.0) | Ref | Ref | ||
| Women | ||||||
| 0 ml | 92(42.2) | 38(41.3) | 0.964 | (0.588; 1.580) | 0.670 | (0.366; 1.229) |
| > 0 ml | 126(57.8) | 54(58.7) | Ref | Ref | ||
aRegression coefficients and odds ratios (OR) based on multivariable linear and multivariable multinomial logistic regression analyses of independent variable with poor (PSQI ≥ 8) versus good (PSQI < 8, reference) sleep quality (dependent variable)
bAdjusted for age, sex, educational level, WI, FI, number of comorbidities, physical activity, BMI, and site of cancer/cancer diagnosis
cAdjusted for age, educational level, WI, FI, number of comorbidities, physical activity, BMI, and site of cancer/cancer diagnosis
$ Low: red/processed meat < 500 g/week of which processed meat < 3 g/day;
$ Moderate: red/processed meat < 500 g/week of which processed meat ≥ 3– < 50 g/day;
$ High: red/processed meat ≥ 500 g/week of which processed meat ≥ 50 g/day
*Statistical significance was set at p < 0.01 to avoid the inflated type I errors associated with multiple testing
Exploratory factor analysis of sleep quality
The EFA was used to explore the underlying construct of the PSQI (Pittsburgh Sleep Quality Index). The analysis resulted in a two-factor model, with the first factor representing "perceived sleep quality" and the second factor representing "sleep disturbances". The first factor included subjective sleep quality, sleep latency, sleep duration, and habitual sleep efficiency, while the rest of the components had good loadings on the second factor. Table 3 shows the factor matrix for the two-factor solutions by gender. The variance accounted for by the factor perceived sleep quality was 33.96%, 33.75%, and 33.94% for the whole sample, female and male, respectively. The variance accounted for by the second factor, sleep disturbances, was 56.23%, 55.17%, and 56.93% for the whole sample, female and male, respectively. Since the results from the sensitivity analysis involving both genders produced similar results, the two factors extracted by EFA were combined for the whole sample. The scree plot results for the factor matrix according to the whole sample and two genders are provided in the Supplementary files.
Table 3.
Factor loadings for sleep quality components extracted from factor analysis
| Total | Female | Male | ||||
|---|---|---|---|---|---|---|
| Components | Sleep quality & efficiency | Sleep disturbances | Sleep quality & efficiency | Sleep disturbances | Sleep quality & efficiency | Sleep disturbances |
| sleep duration | 0.844 | 0.019 | 0.853 | −0.055 | 0.834 | 0.075 |
| subjective sleep quality | 0.797 | 0.188 | 0.820 | 0.133 | 0.776 | 0.230 |
| habitual sleep efficiency | 0.691 | 0.108 | 0.643 | 0.100 | 0.734 | 0.043 |
| sleep latency | 0.636 | 0.164 | 0.632 | 0.211 | 0.626 | 0.204 |
| use of sleep medication | −0.025 | 0.769 | −0.069 | 0.754 | −0.001 | 0.774 |
| daytime dysfunction | 0.147 | 0.756 | 0.133 | 0.740 | 0.149 | 0.763 |
| sleep disturbance | 0.356 | 0.567 | 0.358 | 0.555 | 0.354 | 0.570 |
| Total variance (%) | 33.96 | 56.23 | 33.75 | 55.17 | 33.94 | 56.93 |
Factor loading ≥ 0.4 are in bold font
Confirmatory factor analysis of sleep quality measurement model
Confirmatory factor analysis for the two-factor model of sleep quality revealed a desirable fit with χ2/df = 3.042, GFI = 0.99, AGFI = 0.974, CFI = 0.982, TLI = 0.965, RMSEA = 0.048, SRMR = 0.026. There was a small positive correlation (r = 0.59) between the two factors in the whole model (see Fig. 2).
Determinant variables of PSQI
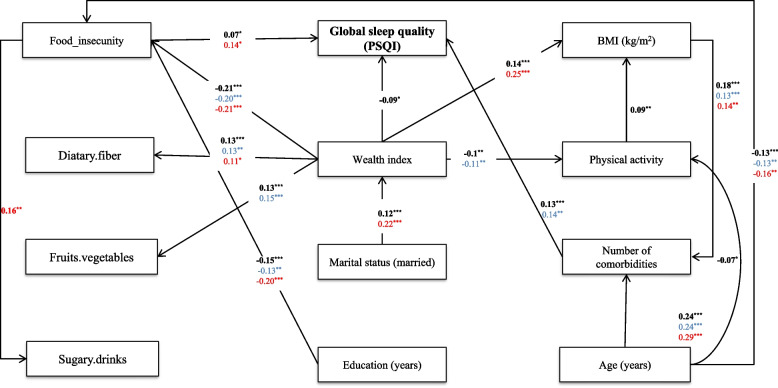
The path analysis output of full and gender-model I is presented in Fig. 3. Model 1 fitted the sample data very well, as indicated by the selected absolute goodness of-fit statistics: χ2 = 72.98, χ2/df = 1.738, P = 0.002, GFI = 0.988, AGFI = 0.971, CFI = 0.980, IFI = 0.980, TLI = 0.956 SRMR = 0.028 and RMSEA = 0.029.
Fig. 3.
Full-and gender-model of Pittsburgh Sleep Quality Index and its determinants among GI cancer patients. Model fit indices: χ2 = 89.84, χ2/df = 1.99, GFI = 0.986, AGFI = 0.967, CFI = 0.967, IFI = 0.968, TLI = 0.934 SRMR = 0.032 and RMSEA = 0.034. The numbers on the paths represent standardized effects. The bold-face arrows represent significant values. The significance level of the comparison of each effect is depicted by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). Red arrows refer to females whereas blue arrows refer to males
Based on the standardized coefficients for the structural paths, food insecurity partially mediated the effect of WI on the sleep quality index. There was a direct predictive effect of the WI on the PSQI (β = −0.09, P = 0.01). In addition, WI indirectly through FI (effect of WI on FI: β = −0.21, P = 0.01 & effect of FI on PSQI: β = 0.07, P = 0.03) had a negative effect on PSQI. Our finding was suggestive of the full mediation effect of age on PSQI through the number of comorbidities (effect of age on number of comorbidities: β = 0.25, P < 0.001 & the effect of number of comorbidities on PSQI: β = 0.13, P < 0.001). Structural path outputs for gender-model 1, indicated the full mediation effect of age on PSQI through the number of comorbidities among males (effect of age on number of comorbidities: β = 0.24, P < 0.001 & the effect of number of comorbidities on PSQI: β = 0.14, P = 0.002), and through the FI among females (effect of age on FI: β = 0.16, P = 0.007 & the effect of FI on PSQI: β = 0.14, P = 0.01). In addition, WI fully mediated the effect of marital status on PSQI (effect of marital status on wealth index: β = 0.12, P < 0.001 & the effect of wealth index on PSQI: β = −0.09, P < 0.001).
Determinant variables of sleep disturbances and perceived sleep quality
The SEM output of full and gender-model II is presented in Fig. 4. Model II fitted the sample data very well, as indicated by the selected absolute goodness of fit statistics: χ2 = 228.670, χ2/df = 1.84, P < 0.0001, GFI = 0.975, AGFI = 0.958, CFI = 0.963, IFI = 0.964, TLI = 0.943 SRMR = 0.032, and RMSEA = 0.031.
Fig. 4.
Full-and gender-model of two latent variables of PSQI and its determinants among GI cancer patients. Model fit indices: χ2 = 226.783, χ2/df = 1.81, GFI = 0.975, AGFI = 0.958, CFI = 0.962, IFI = 0.963, TLI = 0.942, SRMR = 0.031 and RMSEA = 0.030. The numbers on the paths represent standardized effects. The bold-face arrows represent significant values. The significance level of the comparison of each effect is depicted by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). Red arrows refer to females whereas blue arrows refer to males
Based on the standardized coefficients for the SEM, food insecurity partially mediated the effect of WI on the latent variable of sleep disturbance. There was a direct predictive effect of the WI on sleep disturbance (β = −0.12, P = 0.009). In addition, WI indirectly through FI (effect of WI on FI: β = −0.23, P < 0.001 & effect of FI on sleep disturbance: β = 0.11, P = 0.01) had a negative effect on sleep disturbance. Additionally, marital status through WI indirectly (effect of marital status on WI: β = 0.11, P < 0.001 & effect of WI on sleep quality: β = −0.12, P = 0.009) effect on sleep quality.
Our finding was suggestive of the full mediation effect of age on two latent variables of PSQI through the number of comorbidities (effect of age on the number of comorbidities: β = 0.24, P < 0.001, the effect of the number of comorbidities on sleep disturbance: β = 0.18, P < 0.001, and the effect of number of comorbidities on sleep disturbance: β = 0.12, P = 0.003). SEM outputs for gender-model II indicated that in men, only the age factor indirectly through the number of comorbidities had a predictive effect on sleep quality with a similar pattern (effect of age on number of comorbidities: β = 0.24, P < 0.001, the effect of number of comorbidities on sleep disturbance: β = 0.18, P < 0.001). In females, age and BMI were predictors of sleep disorder, which was fully mediated by the number of comorbidities (effect of age on the number of comorbidities: β = 0.29, P < 0.001, effect of BMI on number of comorbidities: β = 0.14, P = 0.005, the effect of number of comorbidities on sleep disturbance: β = 0.29, P < 0.001). Also, the wealth index with a negative effect on food insecurity had a good prognosis in improving the sleep quality among cancer patients (effect of WI on FI: β = −0.26, P < 0.001 & effect of FI on sleep quality: β = 0.15, P = 0.01). Our results suggested that better-educated patients had lower sleep disturbance than low-educated females (effect of education on sleep disturbance: β = −0.19, P = 0.029). Further, older patients had more sleep disturbance due to less education (effect of age on education: β = −0.39, P < 0.00).
Discussion
Exploratory factor analysis of sleep quality
The finding of our study explored two latent variables for PSQI, including sleep disturbance and sleep quality. Although the PSQI is a well-validated instrument for assessing sleep quality, the current findings consistent with previous studies [10], suggest that sleep quality rather than its single unidimensional measure, may be better assessed using the multiple factors identified within the PSQI. Different factors such as medical conditions, sleep disorders, mental/psychological factors, behavioral issues, and environmental factors can impact sleep quality in different ways and affect the magnitude of sleep quality through different components [52, 53]. This suggests that the global PSQI score alone may not provide a complete picture of sleep quality.
Determinant variables of PSQI
Despite its widespread use in clinical and research settings, a growing body of literature advocates for reconstructing the PSQI. A systematic review by Manzar et al. (2018) found that the PSQI can be best explained by two-factor models, followed by one-factor models and three-factor models [54]. This finding is consistent with other studies that have reported similar two-factor structures as the most appropriate model fit for the PSQI in different populations, including breast cancer survivors, the general population, and students from various countries [55–57].
This study adds to the growing body of literature on FI as a possible factor influencing sleep problems. The link between FI and sleep problems may be explained by multiple biological, psychosocial, and contextual pathways. FI can lead to sleep problems due to the coexistence of hunger and wakefulness, as well as chronic stress and increased cortisol levels that are associated with sleep disturbance [58]. However, we did not assess the psychological components in the current study, evidence from other studies shows that mental health problems could mediate the association between FI and poor sleep quality [59].
Gender differences in sleep quality and the potential pathways of influencing factors were the study's novel findings. The association between FI and poor sleep quality was more pronounced in women. Food insecurity has been linked more strongly with depression and poor sleep quality in women, due to the complex interplay of stressors like financial strain and caregiving responsibilities [60].
The number of comorbidities was a determinant factor of poor sleep quality in men. Consistent with our results, several studies found that men suffering from chronic conditions such as obesity, diabetes, and cardiovascular disease were more likely to be diagnosed with obstructive sleep apnea and report higher rates of poor sleep quality [61, 62]. These findings underscore the importance of addressing comorbid chronic conditions to improve sleep quality in men. Interventions focusing on managing both physical and mental health conditions could lead to better sleep health outcomes.
The relationship between wealth index and marital status shows a complex dynamic influenced by social and economic factors. Kapelle and Lersch emphasize that marriage often leads to increased household income and shared financial responsibilities, which can increase wealth accumulation over time [63]. Similarly, other studies also emphasize that marital stability contributes to better financial planning and investment opportunities and further increases the level of wealth [64, 65]. A study conducted in Ethiopia identified low income as a significant factor associated with poor sleep quality, with an adjusted odds ratio of 5.36, indicating a strong correlation between financial status and sleep disturbances [66]. Similar to previous studies, our findings suggest the possibility of comorbid conditions as a cause of poor sleep quality. A study of non-small cell lung cancer patients identified comorbidities as a strong predictor of poor sleep quality with an odds ratio of 2.57 for those with additional health problems [67]. Furthermore, a systematic review revealed that a greater occurrence of poor sleep quality is linked to depression and anxiety, conditions that are frequently associated with chronic illnesses [68]. These findings underscore the importance of addressing medical conditions and economic factors to improve sleep quality among cancer patients.
Determinant variables of sleep disturbances and perceived sleep quality
As for the contextual factors, food insecurity is often caused by poverty. Unemployment, tight household budgets, and rising living costs can make it difficult for the population to afford enough food to meet their basic needs [69], which may lead to poor sleep due to safety concerns, and other poor housing conditions [70]. The current study also showed that WI was the main factor influencing FI [71].
The results showed that better-educated patients experienced less sleep disturbance than low-educated females. High-educated female patients may have more knowledge about their disease (cancer) and its consequences, which could explain their better situation of sleep quality [72]. In the current study, obese and old patients with GI cancer had significantly worse sleep through positive effects on food insecurity and the number of commodities than their young counterparts. This corroborates previous results that reported a trend of worsening sleep in older adults, as well as in subjects with obesity and multiple comorbid conditions [73]. However, older adult patients in our study were less-educated and this issue could be the reason.
In our study, gender differences in social determinants of sleep disturbance were more obvious in women than in men. In corroborating our results, previous studies have shown that women have lower sleep quality scores than men [74]. Shaib et al. suggested distinct sex and gender differences in the pathophysiology, clinical semiology, therapeutic implications, and complications of sleep disorders. They reported sleep disorders twice as frequent in women as in men [51]. these gender differences may be due to a matter of perception (female patients experience higher rates of sleep disturbances than men, whereas male patients are in coping with sleep problems) [51, 75]. Research has also shown that women with comorbidities, such as chronic pain, depression, or anxiety, are more likely to experience sleep disturbances. These conditions can significantly impact women's health, including their ability to sleep properly [76]. Furthermore, hormonal fluctuations during the menstrual cycle, pregnancy, and menopause can also contribute to sleep disturbances in women with comorbidities [77].
On the other hand, although men with comorbidities may not directly experience sleep disturbances like women do because of these conditions, their overall sleep quality may be affected. Certain comorbidities, such as cardiovascular disease or obstructive sleep apnea, can lead to poor overall sleep quality in men [78]. Chronic diseases often lead to physiological changes that further impede sleep. For example, inflammation associated with both chronic pain and cancer can disrupt the sleep–wake cycle, impairing the ability to regulate sleep stages [79].
The present study has several limitations. First, the cross-sectional nature of the study makes it uncertain whether FI drives sleep disturbance in GI cancer patients. Therefore, longitudinal research is warranted to determine the direction of the association. Second, this study only involves GI cancer patients, so the results cannot infer all cancer patients. Third, the eating behavior checklist relied on self-reporting, which could have introduced a certain degree of recall bias and desirability bias. Fourth, the study mainly focused on worry-induced sleep disturbance, so it is important to be careful when applying the findings to other types of sleep disturbances. Finally, errors in the measurement of confounders will lead to residual confounding [80]. Despite these limitations, an obvious advantage of this study is using the SEM method, which has been the favored approach to testing mediation hypotheses to evaluate social determinants of sleep quality in GI cancer patients. The current study complements the literature in this field and contributes to a broader understanding of the social determinants of sleep.
Conclusion
These findings highlight the importance of considering the bi-dimensional construct of the PSQI for assessing sleep quality among GI cancer patients. The current study demonstrated that food insecurity and comorbidity prevalence mediated the relationship between socio-demographic determinants of sleep quality in patients with GI cancer. These findings emphasize the importance of solving FI and social inequality to improve sleep quality and disturbance, especially in women with GI cancer. Furthermore, by acknowledging these differences and exploring how they intersect with gender-specific experiences, a multidisciplinary approach to managing both cancer and chronic conditions is essential for improving sleep quality and overall well-being in this population. Effective management should include screening for depression and anxiety, implementing pain management strategies, and executing supportive policies and community programs. Further research and longitudinal studies are warranted to understand the underlying mechanisms driving these gender-specific differences.
Supplementary Information
Acknowledgements
We sincerely appreciate the participants and the GILDRC staff (https://gildrc.iums.ac.ir), without whom the study would not have been possible.
Authors’ contributions
MS, FZ, and AGH conceived and designed the research. SH and PH collected the data. AD and PH analysed and/or interpretation of data. MS and AD drafted the manuscript. FZ, AD, MS, PH, and HA revised the manuscript critically for important intellectual content. All authors read and approved the final paper.
Funding
No funding.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The principles of the Declaration of Helsinki were followed to conduct this study. The Ethics Committee of the Iran University of Medical approved the study protocol (IR.IUMS.REC.1397.870). The participants agreed to participate in the study by confirming informed consent after being enlightened about the study objectives.
Consent for publication
The authors affirm that human research participants provided informed consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Farhad Zamani, Email: zamani.farhad@gmail.com.
Azam Doustmohammadian, Email: mohammadian.az@iums.ac.ir.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335-349. e315. 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legg M, Meertens RM, van Roekel E, Breukink SO, Janssen ML, Keulen ET, Steindorf K, Weijenberg MP, Bours M. The association between sleep quality and fatigue in colorectal cancer survivors up until two years after treatment: a cross-sectional and longitudinal analysis. Cancers. 2022;14(6):1527. 10.3390/cancers14061527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lagergren P, Johar A, Rosenlund H, Arnberg L, Haglund L, Ness-Jensen E, Schandl A. Severe reflux, sleep disturbances, and health-related quality of life after esophageal cancer surgery. J Cancer Survivorship. 2021:1–7. 10.1007/s11764-020-00974-9. [DOI] [PMC free article] [PubMed]
- 5.Kwak A, Jacobs J, Haggett D, Jimenez R, Peppercorn J. Evaluation and management of insomnia in women with breast cancer. Breast Cancer Res Treat. 2020;181:269–77. 10.1007/s10549-020-05635-0. [DOI] [PubMed] [Google Scholar]
- 6.Bach L, Kalder M, Kostev K. Depression and sleep disorders are associated with early mortality in women with breast cancer in the United Kingdom. J Psychiatr Res. 2021;143:481–4. 10.1016/j.jpsychires.2020.11.036. [DOI] [PubMed] [Google Scholar]
- 7.Hofmeister D, Schulte T, Mehnert-Theuerkauf A, Geue K, Zenger M, Esser P, Götze H, Hinz A. The association between sleep problems and general quality of life in cancer patients and in the general population. Front Psychol. 2022;13: 960029. 10.3389/fpsyg.2022.960029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 9.Manzar MD, BaHammam AS, Hameed UA, Spence DW, Pandi-Perumal SR, Moscovitch A, Streiner DL. Dimensionality of the Pittsburgh Sleep Quality Index: a systematic review. Health Qual Life Outcomes. 2018;16:1–22. 10.1186/s12955-018-0915-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunleavy G, Bajpai R, Comiran Tonon A, Chua AP, Cheung KL, Soh C-K, Christopoulos G, de Vries H, Car J. Examining the factor structure of the Pittsburgh sleep quality index in a multi-ethnic working population in Singapore. Int J Environ Res Public Health. 2019;16(23):4590. 10.3390/ijerph16234590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinz A, Glaesmer H, Brähler E, Löffler M, Engel C, Enzenbach C, Hegerl U, Sander C. Sleep quality in the general population: psychometric properties of the Pittsburgh Sleep Quality Index, derived from a German community sample of 9284 people. Sleep Med. 2017;30:57–63. 10.1016/j.sleep.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Pourmotabbed A, Ghaedi E, Babaei A, Mohammadi H, Khazaie H, Jalili C, Symonds ME, Moradi S, Miraghajani M. Sleep duration and sarcopenia risk: a systematic review and dose-response meta-analysis. Sleep and Breathing. 2020;24:1267–78. 10.1007/s11325-019-01965-6. [DOI] [PubMed] [Google Scholar]
- 13.Mazloomi SN, Talebi S, Kazemi M, Ghoreishy SM, Moosavian SP, Amirian P, Mohammadi H, Nouri-Majd S, Marx W, Kermani MAH. Food insecurity is associated with the sleep quality and quantity in adults: a systematic review and meta-analysis. Public Health Nutr. 2022:1–31. 10.1017/S1368980022002488. [DOI] [PMC free article] [PubMed]
- 14.Madrid-Valero JJ, Martínez-Selva JM. Couto BRd, Sánchez-Romera JF, Ordoñana JR: Age and gender effects on the prevalence of poor sleep quality in the adult population. Gac Sanit. 2017;31:18–22. 10.1016/j.gaceta.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Schulte T, Hofmeister D, Mehnert-Theuerkauf A, Hartung T, Hinz A. Assessment of sleep problems with the Insomnia Severity Index (ISI) and the sleep item of the Patient Health Questionnaire (PHQ-9) in cancer patients. Support Care Cancer. 2021;29(12):7377–84. 10.1007/s00520-021-06282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santoso AM, Jansen F, Lissenberg-Witte BI. Baatenburg de Jong RJ, Langendijk JA, Leemans CR, Smit JH, Takes RP, Terhaard CH, van Straten A: Poor sleep quality among newly diagnosed head and neck cancer patients: prevalence and associated factors. Support Care Cancer. 2021;29:1035–45. 10.1007/s00520-020-05577-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B, Wing Y-K. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 18.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12(2):110–8. 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Ding M, Keiley MK, Garza KB, Duffy PA, Zizza CA. Food insecurity is associated with poor sleep outcomes among US adults. J Nutr. 2015;145(3):615–21. 10.3945/jn.114.199919. [DOI] [PubMed] [Google Scholar]
- 20.Jehan S, Myers AK, Zizi F, Pandi-Perumal SR, Jean-Louis G, Singh N, Ray J, McFarlane SI. Sleep health disparity: the putative role of race, ethnicity and socioeconomic status. Sleep Med Disord. 2018;2(5):127. 10.15406/smdij.2018.02.00057. [PMC free article] [PubMed] [Google Scholar]
- 21.Komleva Y, Gollasch M, König M. Nocturia and frailty in older adults: a scoping review. BMC Geriatr. 2024;24(1):498. 10.1186/s12877-024-05049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue B, Xue Y, Dong F, Zheng X, Shi L, Xiao S, Zhang J, Ou W, Wang Q, Zhang C. The impact of socioeconomic status and sleep quality on the prevalence of multimorbidity in older adults. Front Public Health. 2022;10: 959700. 10.3389/fpubh.2022.959700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandner MA, Patel NP, Gehrman PR, Xie D, Sha D, Weaver T, Gooneratne N. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Med. 2010;11(5):470–8. 10.1016/j.sleep.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q. Food insecurity and sleep disturbance among 223,561 adolescents: a multi-country analysis of cross-sectional surveys. Front Public Health. 2021;9: 693544. 10.3389/fpubh.2021.693544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith MD, Rabbitt MP, Coleman-Jensen A. Who are the world’s food insecure? New evidence from the Food and Agriculture Organization’s food insecurity experience scale. World Dev. 2017;93:402–12. 10.1016/j.worlddev.2017.01.006. [Google Scholar]
- 26.Moradi S, Mirzababaei A, Mohammadi H, Moosavian SP, Arab A, Jannat B, Mirzaei K. Food insecurity and the risk of undernutrition complications among children and adolescents: a systematic review and meta-analysis. Nutrition. 2019;62:52–60. 10.1016/j.nut.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Isaura ER, Chen Y-C, Su H-Y, Yang S-H. The relationship between food security status and sleep disturbance among adults: a cross-sectional study in an Indonesian population. Nutrients. 2020;12(11):3411. 10.3390/nu12113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Noh J-W, Kim A, Kwon YD. Demographic and socioeconomic influences on sleep patterns among adolescent students. Int J Environ Res Public Health. 2020;17(12):4378. 10.3390/ijerph17124378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godos J, Grosso G, Castellano S, Galvano F, Caraci F, Ferri R. Association between diet and sleep quality: A systematic review. Sleep Med Rev. 2021;57:101430. 10.1016/j.smrv.2021.101430. [DOI] [PubMed] [Google Scholar]
- 30.Katagiri R, Asakura K, Kobayashi S, Suga H, Sasaki S. Diets TgSoWo, Group HS: Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle-aged female Japanese workers. J Occup Health. 2014;56(5):359–68. 10.1539/joh.14-0051-OA. [DOI] [PubMed] [Google Scholar]
- 31.Hemiö K, Lindström J, Peltonen M, Härmä M, Viitasalo K, Puttonen S. High need for recovery from work and sleep problems are associated with workers’ unhealthy dietary habits. Public Health Nutr. 2021;24(8):2185–94. 10.1017/S1368980020000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boushey C, Ard J, Bazzano L, Heymsfield S, Mayer-Davis E, Sabaté J, Snetselaar L, Van Horn L, Schneeman B, English LK. Dietary Patterns and Growth, Size, Body Composition, and/or Risk of Overweight or Obesity: A Systematic Review, USDA Nutrition Evidence Systematic Reviews: Alexandria. USA: VA; 2020. [PubMed] [Google Scholar]
- 33.Grimaldi M, Bacaro V, Natale V, Tonetti L, Crocetti E. The Longitudinal Interplay between Sleep, Anthropometric Indices, Eating Behaviors, and Nutritional Aspects: A Systematic Review and Meta-Analysis. Nutrients. 2023;15(14):3179. 10.3390/nu15143179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nazifi M, Mokarami H, Akbaritabar A, Kalte HO, Rahi A: Psychometric properties of the Persian translation of Pittsburgh sleep quality index. Health Scope. 2014;3(2). 10.17795/jhealthscope-15547.
- 35.Farrahi Moghaddam J, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep and Breathing. 2012;16:79–82. 10.1007/s11325-010-0478-5. [DOI] [PubMed] [Google Scholar]
- 36.Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27(2):140–8. 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Rad AK, Noroozi M, AhmariTehran H, Rahmani A. Quality of sleep and related factors in Breast Cancer Patients Receiving Chemotherapy in Qom 2011. Iranian Quarterly Journal of Breast Diseases. 2012;4(4):51–60. [Google Scholar]
- 38.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. 10.1016/j.smrv.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Djalalinia S, Saeedi Moghaddam S, Sheidaei A, Rezaei N, Naghibi Iravani SS, Modirian M, Zokaei H, Yoosefi M, Gohari K, Kousha A. Patterns of obesity and overweight in the iranian population: findings of steps 2016. Front Endocrinol. 2020;11:42. 10.3389/fendo.2020.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Djalalinia S, Modirian M, Sheidaei A, Yoosefi M, Zokaiee H, Damirchilu B, Mahmoudi Z, Mahmoudi N, Hajipour MJ, Peykari N. Protocol design for large–scale cross–sectional studies of surveillance of risk factors of non–communicable diseases in Iran: STEPs 2016. Arch Iranian Med. 2017;20(9):608–16. [PubMed] [Google Scholar]
- 41.Dastgiri S, Tutunchi H, Ostadrahimi A, Mahboob S. Sensitivity and specificity of a short questionnaire for food insecurity surveillance in Iran. Food Nutr Bull. 2007;28(1):55–8. 10.1177/156482650702800106. [DOI] [PubMed] [Google Scholar]
- 42.Blumberg SJ, Bialostosky K, Hamilton WL, Briefel RR. The effectiveness of a short form of the Household Food Security Scale. Am J Public Health. 1999;89(8):1231–4. 10.2105/AJPH.89.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferguson BD, Tandon A, Gakidou E, Murray CJ. Estimating permanent income using indicator variables. Health systems performance assessment: debates, methods and empiricism. Geneva: World Health Organization. 2003. p. 747–760.
- 44.Hargreaves JR, Morison LA, Gear JS, Kim JC, Makhubele MB, Porter JD, Watts C, Pronyk PM. Assessing household wealth in health studies in developing countries: a comparison of participatory wealth ranking and survey techniques from rural South Africa. Emerg Themes Epidemiol. 2007;4(1):1–9. 10.1186/1742-7622-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Booysen F, Van Der Berg S, Burger R, Von Maltitz M, Du Rand G. Using an asset index to assess trends in poverty in seven Sub-Saharan African countries. World Dev. 2008;36(6):1113–30. 10.1016/j.worlddev.2007.10.008. [Google Scholar]
- 46.Balen J, McManus DP, Li Y-S, Zhao Z-Y, Yuan L-P, Utzinger J, Williams GM, Li Y, Ren M-Y, Liu Z-C. Comparison of two approaches for measuring household wealth via an asset-based index in rural and peri-urban settings of Hunan province. China Emerging themes in epidemiology. 2010;7(1):1–17. 10.1186/1742-7622-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golestani M, Sadeghi-Bazargani H, Saadati M, Farahbakhsh M, Dalal K. Lifestyle risk factor assessment through WHO STEP approach in Tabriz, Iran. ClinicoEconomics Outcomes Res. 2021:487–492. 10.2147/CEOR.S304189. [DOI] [PMC free article] [PubMed]
- 48.Hooper D, Coughlan J, Mullen M. Structural equation modelling: guidelines for determining model fit. Electron J Bus Res Methods. 2008;6:53–60. [Google Scholar]
- 49.Ahn S, Lobo JM, Logan JG, Kang H, Kwon Y, Sohn M-W. A scoping review of racial/ethnic disparities in sleep. Sleep Med. 2021;81:169–79. 10.1016/j.sleep.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 50.Zeng L-N, Zong Q-Q, Yang Y, Zhang L, Xiang Y-F, Ng CH, Chen L-G, Xiang Y-T. Gender difference in the prevalence of insomnia: a meta-analysis of observational studies. Front Psych. 2020;11: 577429. 10.3389/fpsyt.2020.577429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaib F, Attarian H. Sex and gender differences in sleep disorders: an overview. Principles Gender-specific Med. 2023:661–679. 10.1016/B978-0-323-88534-8.00036-5.
- 52.Sochal M, Małecka-Panas E, Gabryelska A, Talar-Wojnarowska R, Szmyd B, Krzywdzińska M, Białasiewicz P. Determinants of sleep quality in inflammatory bowel diseases. J Clin Med. 2020;9(9):2921. 10.3390/jcm9092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez BD, Eisel SL, Qin B, Llanos AA, Savard J, Hoogland AI, Jim H, Lin Y, Demissie K, Hong C-C. Prevalence, risk factors, and trajectories of sleep disturbance in a cohort of African-American breast cancer survivors. Support Care Cancer. 2021;29:2761–70. 10.1007/s00520-020-05786-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manzar MD, BaHammam AS, Hameed UA, Spence DW, Pandi-Perumal SR, Moscovitch A, Streiner DL. Dimensionality of the Pittsburgh Sleep Quality Index: a systematic review. Health Qual Life Outcomes. 2018;16(1):1–22. 10.1186/s12955-018-0915-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Otte JL, Rand KL, Carpenter JS, Russell KM, Champion VL. Factor analysis of the Pittsburgh Sleep Quality Index in breast cancer survivors. J Pain Symptom Manage. 2013;45(3):620–7. 10.1016/j.jpainsymman.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magee CA, Caputi P, Iverson DC, Huang X-F. An investigation of the dimensionality of the Pittsburgh Sleep Quality Index in Australian adults. Sleep Biol Rhythms. 2008;6:222–7. 10.1111/j.1479-8425.2008.00371.x. [Google Scholar]
- 57.Gelaye B, Lohsoonthorn V, Lertmeharit S, Pensuksan WC, Sanchez SE, Lemma S, Berhane Y, Zhu X, Vélez JC, Barbosa C. Construct validity and factor structure of the pittsburgh sleep quality index and epworth sleepiness scale in a multi-national study of African, South East Asian and South American college students. PLoS ONE. 2014;9(12): e116383. 10.1371/journal.pone.0116383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagata JM, Palar K, Gooding HC, Garber AK, Whittle HJ, Bibbins-Domingo K, Weiser SD. Food insecurity is associated with poorer mental health and sleep outcomes in young adults. J Adolesc Health. 2019;65(6):805–11. 10.1016/j.jadohealth.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jordan ML, Perez-Escamilla R, Desai MM, Shamah-Levy T. Household food insecurity and sleep patterns among Mexican adults: results from ENSANUT-2012. J Immigr Minor Health. 2016;18:1093–103. 10.1007/s10903-015-0246-5. [DOI] [PubMed] [Google Scholar]
- 60.Madigan KE, Leiman DA. Food Insecurity Is an Independent Risk Factor for Depressive Symptoms in Survivors of Digestive Cancers. 2021;30(6):1122–8. 10.1158/1055-9965.EPI-20-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopez A, Simburger D, Zajacova A, Sheehan C. Unraveling the link between chronic pain and sleep quality: Insights from a national study. Sleep Epidemiology. 2024;4: 100079. 10.1016/j.sleepe.2024.100079. [Google Scholar]
- 62.Edéll-Gustafsson UM, Gustavsson G, Yngman Uhlin P. Effects of sleep loss in men and women with insufficient sleep suffering from chronic disease: a model for supportive nursing care. Int J Nurs Pract. 2003;9(1):49–59. 10.1046/j.1440-172X.2003.00402.x. [DOI] [PubMed] [Google Scholar]
- 63.Kapelle N, Lersch PM. The accumulation of wealth in marriage: Over-time change and within-couple inequalities. Eur Sociol Rev. 2020;36(4):580–93. 10.1093/esr/jcaa006. [Google Scholar]
- 64.Grinstein-Weiss M, Zhan M, Sherraden M. Saving performance in Individual Development Accounts: Does marital status matter? J Marriage Fam. 2006;68(1):192–204. 10.1111/j.1741-3737.2006.00241.x. [Google Scholar]
- 65.Wilmoth J, Koso G. Does marital history matter? Marital status and wealth outcomes among preretirement adults. J Marriage Fam. 2002;64(1):254–68. 10.1111/j.1741-3737.2002.00254.x. [Google Scholar]
- 66.Abebe E, Giru BW, Boka A. Sleep quality and associated factors among adult cancer patients on treatments at tikur anbessa specialized hospital oncology unit, Addis Ababa, Ethiopia, 2021. Cancer Control. 2023;30:10732748231160128. 10.1177/10732748231160129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen X, Hu Y, Fan J, Li Y. Post-Operative Poor Sleep Quality and Its Associated Factors Among Non-Small Cell Lung Cancer Patients: A Cross-Sectional Study. Cancer Manage Res. 2023:1283–1295. 10.2147/CMAR.S430436. [DOI] [PMC free article] [PubMed]
- 68.Chen M-Y, Zheng W-Y, Liu Y-F, Li X-H, Lam MI, Su Z, Cheung T, Ungvari GS, Tang L, Ng CH. Global prevalence of poor sleep quality in cancer patients: A systematic review and meta-analysis. Gen Hosp Psychiatry. 2023. 10.1016/j.genhosppsych.2023.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Negriff S, Brensilver M, Trickett PK. Elucidating the mechanisms linking early pubertal timing, sexual activity, and substance use for maltreated versus nonmaltreated adolescents. J Adolesc Health. 2015;56(6):625–31. 10.1016/j.jadohealth.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Y, Njai RS, Greenlund KJ, Chapman DP, Croft JB. Peer Reviewed: Relationships Between Housing and Food Insecurity, Frequent Mental Distress, and Insufficient Sleep Among Adults in 12 US States, 2009. Preventing Chronic Disease. 2014;11. 10.5888/pcd11.130334. [DOI] [PMC free article] [PubMed]
- 71.Sisha TA. Household level food insecurity assessment: Evidence from panel data. Ethiopia Scientific African. 2020;7: e00262. 10.1016/j.sciaf.2019.e00262. [Google Scholar]
- 72.Ji Y-B, Bo C-L, Xue X-J, Weng E-M, Gao G-C, Dai B-B, Ding K-W, Xu C-P. Association of Inflammatory Cytokines With the Symptom Cluster of Pain, Fatigue, Depression, and Sleep Disturbance in Chinese Patients With Cancer. J Pain Symptom Manage. 2017;54(6):843–52. 10.1016/j.jpainsymman.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 73.Stein MB, Belik S-L, Jacobi F, Sareen J. Impairment associated with sleep problems in the community: relationship to physical and mental health comorbidity. Psychosom Med. 2008;70(8):913–9. 10.1097/PSY.0b013e3181871405. [DOI] [PubMed] [Google Scholar]
- 74.Phillips BA, Collop NA, Drake C, Consens F, Vgontzas AN, Weaver TE. Sleep disorders and medical conditions in women. J Womens Health. 2008;17(7):1191–9. 10.1089/jwh.2007.0561. [DOI] [PubMed] [Google Scholar]
- 75.Hofmeister D, Schulte T, Hinz A. Sleep problems in cancer patients: a comparison between the Jenkins Sleep Scale and the single-item sleep scale of the EORTC QLQ-C30. Sleep Med. 2020;71:59–65. 10.1016/j.sleep.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 76.Garrett K, Dhruva A, Koetters T, West C, Paul SM, Dunn LB, Aouizerat BE, Cooper BA, Dodd M, Lee K, et al. Differences in Sleep Disturbance and Fatigue Between Patients with Breast and Prostate Cancer at the Initiation of Radiation Therapy. J Pain Symptom Manage. 2011;42(2):239–50. 10.1016/j.jpainsymman.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng W, Luo X-N, Li H-Y, Ke X-Y, Dai Q, Zhang C-J, Cassidy RM, Zhang X-Y, Ning Y-P. Gender differences in the prevalence and clinical correlates of sleep disturbance in general hospital outpatients. Psychiatry Res. 2018;269:134–9. 10.1016/j.psychres.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 78.Huang B-H, Duncan MJ, Cistulli PA, Nassar N, Hamer M, Stamatakis E. Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br J Sports Med. 2022;56(13):718–24. 10.1136/bjsports-2021-104046. [DOI] [PubMed] [Google Scholar]
- 79.Bean DJ, Horne J, Lee AC, Johnson MH. Pre-sleep cognitive arousal exacerbates sleep disturbance in chronic pain: an exploratory daily diary and actigraphy study. Scand J Pain. 2021;21(4):724–31. 10.1515/sjpain-2020-0185. [DOI] [PubMed] [Google Scholar]
- 80.Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol. 2007;166(6):646–55. 10.1093/aje/kwm165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.