Abstract
Background
The prehospital use of blood lactate measurements is increasing. However, the test’s benefits have not been methodically evaluated in non-trauma patients. This study had three aims: (1) To assess the evidence of prehospital blood lactate measurements’ prognostic value in non-trauma patients, (2) to investigate to what extent the test changed early patient treatment, and (3) to evaluate the healthcare personnel’s attitude towards the test.
Methods
MEDLINE, Embase, and Cochrane Central Register of Controlled Trials were systematically searched until Aug 26, 2023. Cohort and randomized controlled trials assessing ≥ 20 acute non-trauma patients with prehospital lactate measurements were included if they reported (1) prognostic outcomes such as short-term mortality or (2) changes in early patient treatments. All study designs were included to assess (3) the healthcare personnel’s opinion on prehospital lactate measurements. The risks of bias were assessed using the QUIPS tool, the Newcastle–Ottawa Scale, and the RoB-2. Study registration number CRD42020167169 (PROSPERO).
Results
We screened 6028 study reports. We included 15 studies on (1) the prognostic value of prehospital lactate measurements. Elevated blood lactate levels were correlated to a higher short-term mortality risk in most of the studies but not in studies with out-of-hospital cardiac arrest (OHCA) patients. The 15 prognostic studies were all cohort studies with moderate or high risks of bias. Four studies investigated (2) early treatment changes. They found that the prehospital lactate measurement may have changed early treatment in sepsis patients. However, all four studies on treatment changes were at high risk of bias. Four studies were included on (3) the healthcare personnel’s attitude towards the lactate measurement. Evidence of the healthcare personnel’s opinion on prehospital lactate measurements was scarce.
Conclusion
Most acute non-trauma patients with elevated prehospital lactate levels had increased risks of short-term mortality, except OHCA patients. Few studies suggested that measuring prehospital lactate levels could change early patient care, particularly in patients with suspected sepsis. The certainty of the evidence is low in this systematic review. The included studies were heterogeneous, and many had high risks of bias. Further studies are needed to investigate the impact of prehospital lactate measurements on patient care.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13049-024-01310-1.
Keywords: Lactate, Prehospital, Prognosis, Emergency
Introduction
Assessing acute patients can be challenging in emergency settings. Vital signs such as blood pressure, heart rate, and oxygen saturation may miss early deterioration [1]. Blood lactate measurement is a prognostic marker of poor outcomes [2]. Most research has focused on in-hospital lactate measurements [2–7]. It can be used for risk assessment in acute patients [2] and has been recommended in the SEPSIS-3 guidelines as a part of the initial patient assessment [8].
The assessment and treatment of the acute patient is initiated in the prehospital phase. Thus, a prehospital lactate measurement may support early decision-making in the prehospital setting and afterward in the emergency department. Results from in-hospital studies might not comply with acute patients in the prehospital setting, as the patients are assessed earlier in their course of disease. The in-hospital and prehospital patient populations may differ as well. As studies using prehospital lactate measurements emerge, a need for compiling the body of evidence in this field arises before a possible widespread implementation of prehospital lactate measurement is justified. The prognostic use of prehospital lactate measurements has been systematically reviewed in trauma patients in 2016 [9]. However, the evidence has not yet been assessed in other acute patient groups.
In-hospital blood lactate measurements are usually done on stationary equipment unfit for the prehospital environment. Several small point-of-care lactate meters have been approved for medical use in the last twenty years. These portable devices are better suited for use in ambulances and emergency helicopters and demonstrate good accuracy compared to lactate meters used in-hospital [10]. Still, new equipment should not be implemented without considering the pros and cons of this decision. A study suggested that lactate levels in risk stratification were superior to those of vital signs [1]. Later, another study showed that adding the lactate level to risk assessments enhanced risk stratification in acute prehospital patients [11]. Improved prognostication using prehospital lactate measurements could lead to more focused treatments being initiated earlier. In turn, this may improve patients’ outcomes. Still, it is essential that the lactate measurement is easy and fast to avoid unnecessary delays in the assessment of acute patients. Furthermore, it has not yet been shown that measuring the lactate level in acute patients increases diagnostic accuracy, e.g., in sepsis patients [12].
The healthcare personnel's attitudes toward lactate measurements could be one of the hurdles to a possible implementation in the prehospital setting, in which limited time and resources are important conditions [9].
Our systematic review aimed to assess the current evidence of prehospital blood lactate measurements in non-trauma patients. It includes three closely linked aims regarding the prognostic performance of prehospital lactate levels, possible changes in early treatment due to prehospital lactate measurements, and the healthcare personnel’s attitude toward using prehospital lactate measurements.
Methods
This study was conducted according to a study protocol using the PRISMA-P guidelines [13]. The study was prospectively registered in PROSPERO (Registration number CRD42020167169) [14]. We reported our findings according to the PRISMA 2020 statement [15].
Objectives
This systematic review identifies, summarizes, and analyzes studies of prehospital measurement of blood lactate in acute non-trauma patients. The review had the following three objectives, each resulting in the inclusion and analyses of different types of studies:
To evaluate the prognostic value of prehospital measured blood lactate on short-term mortality for acute non-trauma patients.
To evaluate whether the knowledge of the patients’ prehospital blood lactate level modified the clinicians’ early patient treatment in non-trauma patients.
To summarize to what extent the clinicians considered acute prehospital blood lactate measurement a valuable tool in early decision-making when treating non-trauma patients.
Eligibility criteria
We included studies of measurement of blood lactate (both venous, arterial, and capillary analyses) in acute (i.e., not elective) non-traumatic patients of all ages assessed in the prehospital environment (and studies of clinicians who treated such patients). In Objectives 2 and 3, a clinician was defined as any healthcare professional providing acute care to patients, including EMS personnel, nurses, and physicians working with acute patients prehospital or in-hospital.
Studies were included regardless of language, year of dissemination, and report status. Studies with less than 20 patients were excluded in Objectives 1 and 2.
Studies were excluded if data were only available for trauma patients or if study results were not separated for trauma and non-trauma patients.
Core eligibility criteria were the same for the three aims.
Objective 1: The prognostic value of prehospital blood lactate
We included cohort studies that assessed the prognosis of patients with lactate assessments, including studies of the association between high and low lactate values and good or poor outcomes. We distinguished between studies in which lactate values were blinded to the health care personnel and those with unblinded values, but they were analyzed collectively. Studies were excluded if they assessed prehospital transfer patients who had already been evaluated/treated at the hospital.
Randomized controlled trials and cohort studies were included if they compared the difference in prognosis between patients with and without lactate measurements.
We were primarily interested in short-term mortality (i.e., up to 7 days, but up to 30 days was accepted if this was the only outcome reported). In-hospital mortality was also regarded as short-term mortality despite the fact that a part of the in-hospital mortality had occurred after 30 days. We combined in-hospital and ≤ 30 days mortality as short-term mortality in the analyses. Additional outcomes related to prognoses were also assessed: length of stay in the hospital or the intensive care unit (ICU), admission to ICU, and need for mechanical ventilation or vasopressors.
Objective 2: Changes in early patient care
We included effect studies assessing how knowledge of a patient’s prehospital blood lactate level had modified the clinician’s prehospital or in-hospital early treatment in acute non-trauma patients. Randomized controlled trials and cohort studies were included if they compared the treatments of patients with known and unknown lactate values.
Regarding patient treatment, we mainly implied fluid administration, blood transfusion(s), oxygen supply, and triage (altered receiving hospital or way of transportation to hospital). Other clinically relevant outcomes were also included if reported in the studies included in this review.
Objective 3: The clinicians’ opinions about prehospital lactate measurements
We included interview and questionnaire studies that explored whether and why the clinicians (i.e., health care personnel treating acute patients in-hospital or in the prehospital area) considered acute prehospital blood lactate measurement a valuable tool in early decision-making when treating non-trauma patients. Interview studies were included regardless of the number of interviewees.
We expected that this systematic review would find limited data investigating Objective 3. To enhance the comprehensibility of the data reporting, the data from this objective will not be reported further in the manuscript. Instead, see the Supplemental Files (Sect. 3 + Table S1) for results and information regarding this objective.
Information sources
We searched three databases to find eligible studies: Medline (Ovid), Embase (Ovid), and Cochrane Central Register of Controlled Trials (Wiley). The last updated searches were done on August 26, 2023.
LHW conducted a backward citation search and searched the reference lists of three systematic review reports on blood lactate measurements in acute patients. Furthermore, a forward citation search was conducted using Google Scholar on November 29, 2023.
First authors of study reports presented as poster abstracts were contacted twice by email two weeks apart to retrieve supplemental study data. Correspondently, we contacted the authors of multiple study reports included in this systematic review to ensure that no study participants were included in our study more than once. Additionally, authors were contacted in case of any uncertainties.
Search strategy
The search strategy was developed using a comprehensive list of keywords with assistance from an information specialist. We used a PICO-style approach using the population and the intervention [16]. The three final search strategies are shown in the Supplementary Files, Figs. 1–3.
Selection process
The systematic review management software Covidence (Melbourne, Australia) was used during the selection process.
LHW and HBM conducted the first part of the selection process by independently screening the headings and abstracts. Afterward, they independently made the final decision of inclusion by reading the full texts. If the two researchers did not agree on study inclusion, they reached an agreement through discussion. If necessary, ACB was consulted and made the final decision.
The researchers were not blinded to any information about the reports (authors, publishing journal, etc.) during the selection process.
Data collection process
LHW and HBM independently extracted the data from the included studies in Objective 1, using an a-priori-developed electronic data extraction form in Excel (Microsoft, Redmond, WA). LHW extracted the data in Objective 2. Afterward, ACB verified the extracted data. Disagreements were resolved through discussion between LHW and ACB.
If more than one report covered the same study population, study outcomes were extracted from the most comprehensive report. The secondary study reports were examined to extract additional information.
Data items
Data on study outcomes were extracted according to the three objectives. We also sought information on the study setting, the number of study participants, demographic information, methodology, intervention details, financial support, etc. A list of all predefined variables extracted from the study reports is presented in the Supplementary Files, Sect. 1. In Objective 1, the first prehospital blood lactate measurement was selected in case of multiple lactate measurements in the prehospital environment.
Study risk of bias assessment
The risk of bias (RoB) was assessed in all studies included in Objectives 1 + 2 using three preselected ‘Risk of bias’ tools. We originally planned to assess the RoB at the study level. Instead, the assessments were done at the outcome level, as several of the included studies did not have our outcome of interest (the prehospital lactate level’s correlation to the risk of poor outcomes or changes in early patient care) as their primary outcome.
The cohort studies in Objective 1 were assessed using a modified version of the QUIPS (Quality In Prognosis Studies) tool [17, 18] as recommended by the Cochrane Prognosis Methods Group [19]. Our modified QUIPS tool is shown in Supplementary Files, Fig. 4. We did not attempt to assign an overall score of the RoB. LHW and HBM did the assessments independently in Objective 1. ACB made the final decision if a disagreement between the two reviewers was not resolved by discussion.
In Objective 2, the cohort studies were assessed using the ‘Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomized studies’ modified to our primary review questions [20]. Details are described in the Supplementary Files, Sect. 2. The randomized controlled trial included in Objective 2 was assessed using the revised Cochrane ‘Risk of bias’ tool for randomized trials (RoB 2) [21].
Synthesis methods
Objective 1
Instead of a meta-analysis, we planned to present the studies’ outcomes separately. We prespecified that if enough studies were included (> 4 (randomized) controlled trials or > 7 cohort studies), the studies’ outcomes would be ordered in tables according to a higher/lower risk of bias assessment. This was planned to reveal possible differences in outcomes related to the studies’ risks of bias.
The short-term mortality risks reported in the studies would be pooled to provide a total estimate of the mortality risk if two or more studies had comparable patient groups and methods for lactate measurements. We expected that the patient populations in the included cohort studies would consist of sepsis or unselected medical patients based on non-structured preliminary literature searches. If some of the included studies were restricted to specific diagnoses (for example, cardiac arrest patients, pediatric patients, etc.), these studies’ outcomes were not pooled with sepsis or non-differentiated patients.
If a study presented multiple effect measures of the same outcome, we were primarily interested in the risk ratio presented as the odds ratio. If a study did not present its results in relative risk measures, we reported the study’s lactate levels measured in the groups compared in the study. Data imputation was not allowed in our study as we did not expect the data reporting to be detailed enough to make valid changes in the data. Thus, the effect measures presented in the studies were not converted into other effect measures. Instead, the authors of a study report were asked for additional information in case of missing or unclear data presentation.
We planned to compile the results of the studies on mortality at different lactate levels. The included cohort studies were expected to divide the lactate values into intervals of low, intermediate, and high values. We planned to use the cut-off levels < 2 mmol/l, between 2 and 4 mmol/l, and > 4 mmol/l or the cut-off levels most often reported in the included studies [22, 23]. If the included cohort studies presented these data, a pooled estimation of the risk ratio of short-term mortality’s correlation to the continuous blood lactate values was also planned.
No pooled analysis was planned for outcomes other than mortality due to the anticipated few studies reporting these outcomes. Additionally, no subgroup analysis or sensitivity analysis was planned. Instead, the clinical and methodological variabilities of the studies were narratively evaluated.
Objective 2
We planned to present the outcomes from these studies as a narrative summary. More studies than expected were included in the Objective 2. Consequently, study outcomes were presented in a table instead to provide an overview.
Due to the anticipated few included studies, we did not plan any data synthesis, subgroup analysis, or sensitivity analysis for this objective. We discussed the studies’ heterogeneities in the text.
Reporting bias assessment
The presence of outcome reporting bias was evaluated by searching for published study protocols of the included studies and comparing each protocol to the respective study's report(s). If a published study protocol could not be retrieved, we compared the outcomes reported in the method and results sections.
We did not plan any analysis of the risk of publication bias.
Results
Study selection
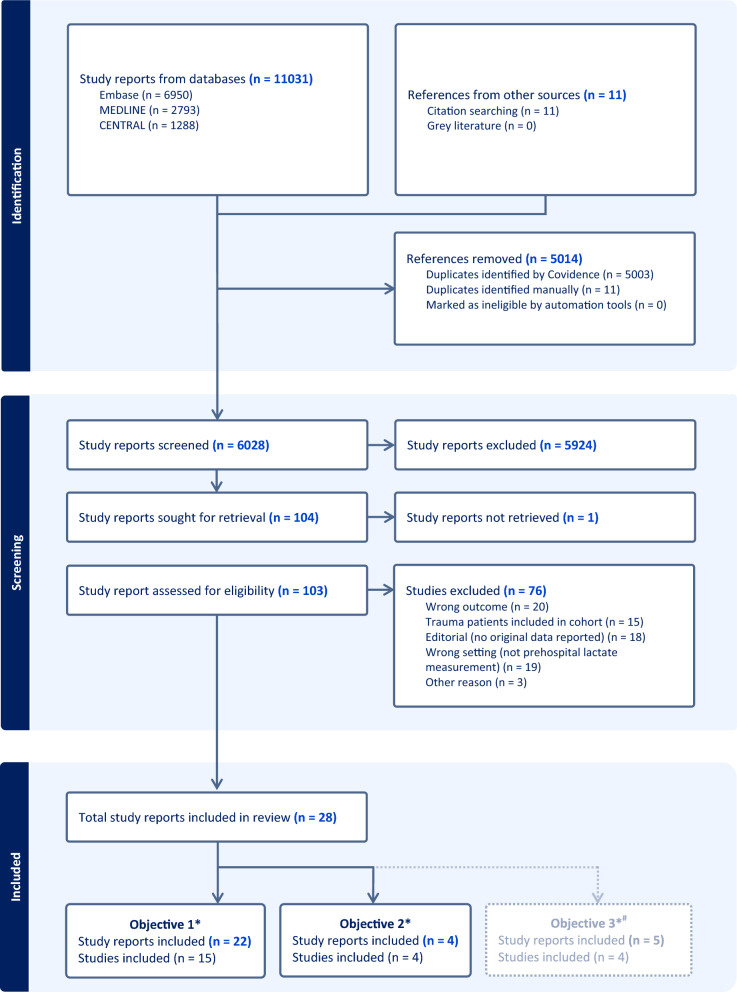
The literature search yielded 11,031 study reports with 5014 duplicates. The backward and forward citation search yielded an additional eleven study reports. A total of 6028 study reports were screened by titles and abstracts, and 104 study reports were assessed for eligibility by reading full texts. One study report (a conference abstract) had to be excluded from the full-text screening due to missing information and no response from the study report’s author. We excluded 15 study reports because data on the non-trauma patients were not separated from the trauma patients [1, 11, 23–35]. A total of 28 study reports were included in the review. See Fig. 1 for details.
Fig. 1.
Flow chart of the literature search and the study selection process. *Numbers do not add up, as three studies were included in two of the sub-studies each. #Objective 3’s data are reported in the Supplementary Files
Study characteristics and outcomes
Objective 1: The prognostic value of prehospital lactate
We included 15 studies with a total of 7456 patients [12, 36–49]. They were all cohort studies and included between 83 and 1744 patients. We excluded two studies due to overlapping patients after contact with the first authors and used their study reports as supplemental information [50, 51].
The lactate levels were blinded to all treating personnel in one study, where the prehospital lactate levels were analyzed using stationary in-hospital analyzers [36]. The remaining 14 studies used various point-of-care equipment and analyzed the lactate levels as a part of the prehospital treatment provided to the patients. Eleven studies were prospective, and four were retrospective cohort studies. Eleven studies were conducted in Europe, three in the United States, and one in Australia. Four studies investigated patients with suspected sepsis or septic shock. Three studies examined patients with out-of-hospital cardiac arrest (OHCA). All studies were conducted in adult patients ≥ 18 years old, and the inclusion periods ranged from 2010 to 2021. Eleven studies investigated the short-term mortality risks (≤ 30-day or in-hospital mortality). None of the included studies investigated adverse events related to the lactate measurements.
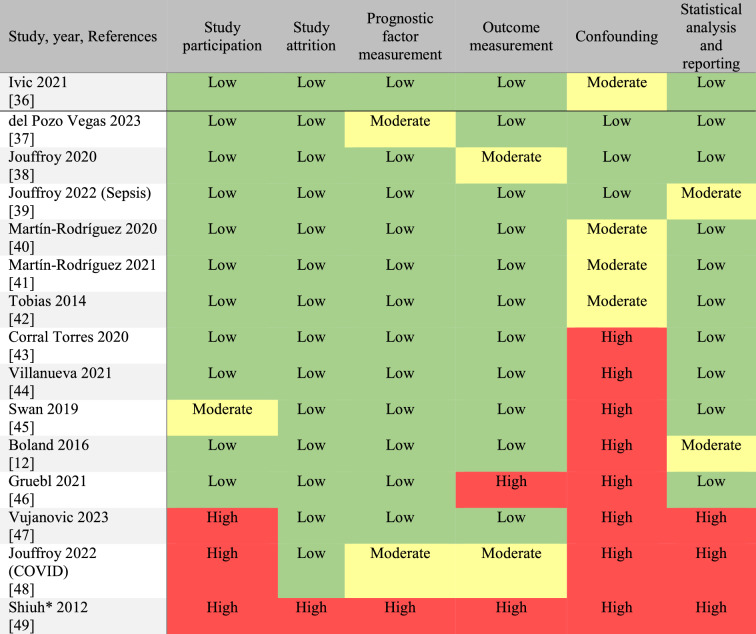
The studies’ details and outcomes are presented in Table 1. The studies’ RoB assessments are displayed in Table 2. Both tables were ordered according to the studies’ RoB, with the study with the lowest RoB at the top of both tables. Most studies had moderate or high RoB in the confounding domain. No study had low RoB in all six domains.
Table 1.
Objective 1. Cohort studies investigating the correlation between prehospital measured blood lactate levels and patients’ prognoses
| First author, year, References | Study design | Country | Study period | Sample size (N) | Patients | Age, years | Lactate levels (mmol/L), sample site, Device | Outcomes | Main findings |
|---|---|---|---|---|---|---|---|---|---|
|
Ivic 2021 [36] |
Prospective Blinded |
Sweden & Finland | 2015–2017 | 414 | Non-specific complaints |
82 (median) |
≤ 2.2 vs. ≥ 2.3 Venous, In-hospital equipment |
30-day mortality |
The median lactate level was 1.7 mmol/L (IQR 1.3–2.3) in survivors and 1.8 mmol/L (IQR 1.7–3.3) in non-survivors 10/288 patients (3.5%) died in the group with lactate ≤ 2.2 mmol/L vs. 7/109 patients (6.4%) in the group with lactate ≥ 2.3 mmol/L. p = 0.09 |
|
del Pozo Vegas 2023 [37] |
Prospective | Spain | 2019–2020 | 1744 | Acute cardiovascular disease |
62 (pt. < 75 years) 84 (pt. ≥ 75 years) (median) |
Continuous, ?, Epoc (Siemens) |
365-day mortality |
The mean lactate level was 1.99 mmol/L (SD 1.35) in survi-vors vs. 4.63 mmol/L (SD 3.79) in non-survivors. p < 0.001. Multivariate Cox regression: HR 1.25 (95% CI 1.20–1.30) p < 0.001 |
|
Jouffroy 2020 [38] |
Prospective | France | 2017–2019 | 177 | Septic shock |
70 (mean) |
Continuous & < 4.0 vs. ≥ 4.0, Venous, StatStrip (Nova Biomed.) |
30-days mortality |
The mean lactate level was 5.9 mmol/L (SD 3.5) in survivors and 7.1 mmol/L (SD 4.0) in non-survivors. P < 0.001 The OR for 30-day mortality (adjusted for confounders) = 2.95 (95% CI 1.14–9.18) in patients with lactate levels ≥ 4.0 mmol/L. P < 0.001 |
|
Jouffroy 2022 (Sepsis) [39] |
Retrospective | France | 2016–2020 | 406 | Septic shock |
69 (mean) |
Continuous, ?, StatStrip (Nova Biomed.) |
28-days mortality | The mean lactate level was 5.8 mmol/L (SD 3.5) in survivors vs. 6.9 mmol/L (SD 4.0) in non-survivors. p = 0.059 |
|
Martín-Rodríguez 2020 [40] |
Prospective | Spain | 2018–2019 | 492 | Acute cardiovascular disease |
72 (median) |
Continuous, Venous, Accutrend Plus (Roche) |
48-h mortality |
The median lactate level was 2.8 mmol/L (IQR 2.1–3.8) in survivors vs. 6.2 mmol/L (IQR 4.5–7.9) in non-survivors p < 0.001 |
|
Martín-Rodríguez 2021 [41] |
Prospective | Spain | 2018–2019 | 221 | Acute poisoning |
47 (median) |
≤ 3.9 vs. ≥ 4.0, Venous, Accutrend Plus (Roche) |
Serious adverse events including 30-day mortality | 35/183 (19.1%) had a lactate level ≥ 4.0 mmol/L in patients without serious adverse events vs. 21/38 (55.3%) in patients with serious adverse events. Only 9/221 (4.1%) died |
|
Tobias 2014 [42] |
Prospective Convenience |
USA | 2010–2011 | 673 | Unselected |
62 (grp. 1) 64 (grp. 2) (mean) |
< 2.0 (grp. 1) vs ≥ 2.0 (grp. 2), Venous, Lactate Pro (FACT) |
In-hospital mortality |
5/366 patients (1.4%) died in the group with lactate < 2.0 mmol/L vs. 16/307 patients (5.2%) in the group with lactate ≥ 2.0 mmol/L. p < 0.01 Multivariate logistic regression: OR = 3.57 (95% CI 1.10–11.60) with a lactate level ≥ 2.0 mmol/L. p = 0.03 |
|
Corral Torres 2020 [43] |
Prospective | Spain | 2012–2017 | 1552 | OHCA* |
64.8 (mean) |
Continuous, Venous, epoc (Epocal Inc.) |
Good neurological outcome (CPC I-II) after 30 days |
The mean lactate level was 7.11 mmol/L (SD 17.63) in patients with CPC I-II vs. 7.26 mmol/L (SD 6.63) in patients with CPC III-V including non-survivors OR of CPC III-V = 1.002 (0.989–1.014), p = 0.804 |
|
Villanueva 2021 [44] |
Prospective | Spain | 2018–2019 | 638 | Dyspnoea (medical) |
79 (median) |
Continuous, Venous, Accutrend Plus (Roche) |
7-day mortality | OR of 7-day mortality was 9.11 (95% CI 5.48–15.13) with a lactate level ≥ 4.1 mmol/L. p < 0.001 |
|
Swan 2019 [45] |
Retrospective | Australia | 2014–2015 | 253 | Unselected |
75 (median) |
Continuous, Venous or capillary, Lactate Pro 2 (ARKRAY) |
In-hospital mortality ICU admission |
The median lactate level was 2.4 mmol/L (IQR 1.5–3.6) in survivors vs. 3.5 mmol/L (IQR 2.75–5.85) in non-survivors p = 0.053 The median lactate level was 2.4 mmol/L (IQR 1.5–3.6) in non-ICU patients vs. 3.2 mmol/L (IQR 2.4–5.7) in ICU patients. p = 0.008 |
|
Boland 2016 [12] |
Prospective Convenience |
USA | 2011–2013 | 112 | Sepsis |
74 (mean) |
< 4.0 vs. ≥ 4.0, Capillary, Lactate Pro (KDK Corp) |
In-hospital mortality ICU admission |
In-hospital mortality was 1% (1/99) in patients with lactate levels < 4.0 mmol/L vs. 18% (2/13) in patients with lactate levels ≥ 4.0 mmol/L. P = 0.04 The ICU admission rate was 15% (15/99) in patients with lactate levels < 4.0 mmol/L vs. 23% (3/13) in patients with lactate levels ≥ 4.0 mmol/L. P = 0.44 |
|
Gruebl 2021 [46] |
Retrospective | Germany | 2015–2016 | 98 | OHCA* |
69 (mean) |
Continuous, Venous or arterial, epoc (Epocal Inc.) |
In-hospital mortality | The mean lactate level was 6.1 mmol/L in survivors vs. 8.5 mmol/L in non-survivors. p = 0.131 |
|
Vujanovic** 2023 [47] |
Prospective | Slovenia | 2020–2021 | 83 | OHCA* |
67 (median) |
Continuous, Capillary, BM-Lactate Cobas (Roche) |
7-day mortality |
The median first measured lactate level was 1.85 mmol/L (IQR 0.8–13.58) in survivors (N = 6) vs. 5.9 mmol/L (IQR 2.70–10.9) in non-survivors (N = 70) T-test comparing the mean lactate levels in the two groups: p = 0.546 |
|
Jouffroy 2022 (COVID) [48] |
Retrospective | France | 2020 | 410 | COVID-19 |
64 (mean) |
Continuous, ?, StatStrip (Nova Biomed.) |
30-day mortality ICU admission |
Multivariate logistic regression: OR = 0.87 (95% CI 0.68–1.08). p = 0.246 Multivariate logistic regression: OR = 1.14 (95% CI 0.89–1.43). p = 0.29 |
|
Shiuh*** 2012 [49] |
Prospective | USA | 2011–2012 | 183 | Sepsis | ? |
2.5–3.9 vs. ≥ 4.0, Venous, ? |
ICU admission | The ICU admission rate was 23% (22/97 patients) in the group with lactate levels 2.5–3.9 mmol/L vs. 50% (43/86 patients) with lactate levels ≥ 4.0 mmol/L |
The table is divided into studies with blinded lactate values at the top and studies with unblended lactate values at the bottom. The studies are ordered according to their risk of bias, with the study with the lowest risk displayed first in the table. The primary outcome, short-term mortality, is displayed along with the other outcomes reported from the included studies. Unless otherwise stated, all data was extracted from the primary study report of each study. Study periods are rounded off to years
*OHCA = out-of-hospital cardiac arrest
**The median lactate level data was obtained by communicating with the study report's first author
***The study report was a conference abstract, and we could not retrieve additional data
Table 2.
Risk of bias assessments in the studies included in Objective 1
The QUIPS (Quality In Prognosis Studies) tool was used for the risk of bias assessments. Studies with blinded lactate results are displayed above the black line, and studies without blinded lactate results are placed below the black line. The studies were ordered according to their risk of bias, with the study with the lowest risk of bias displayed at the top of the table. If they had equal risks of bias, the studies were listed alphabetically
*The study report was a conference abstract, and we could not retrieve additional information
Sepsis patients
Three studies presented short-term mortality data in 695 patients, of which 289 were assessed prehospital with suspected sepsis/septic shock, and 406 patients were a mix of hospital-verified and prehospital suspicion of sepsis/septic shock [12, 38, 39]. The studies’ data were not fit for pooled analysis. Two studies showed significantly higher risks of in-hospital mortality in sepsis patients with elevated lactate levels ≥ 4.0 mmol/L [12, 38]. The largest study with 406 patients did not find clear evidence of a difference in lactate levels in survivors and non-survivors (p = 0.059) [39].
Two of the included studies were conducted by the same investigators with overlapping periods [38, 39]. Thus, we expected these two cohorts to include some of the same patients. Communication with the authors did not confirm this suspicion.
Patients with OHCA
Two studies investigated short-term mortality, and one study reported good vs. poor neurological outcomes (including death) at 30 days in a total of 1733 patients with OHCA.[43, 46, 47] None of the studies found evidence of a difference in the mean lactate levels in survivors/good neurological outcomes versus non-survivors/poor neurological outcomes. The data reported from the studies were not fit for pooled analysis.
Patients with various diseases
The remaining six studies reporting short-term mortality risks did not have comparable patient populations [36, 40, 42, 44, 45, 48]. Two studies investigated unselected non-trauma patients, and one study each included patients with acute cardiovascular disease, dyspnea, non-specific complaints, and COVID-19, respectively. The studies’ data were not pooled. Two of the studies reported clear evidence of a correlation between elevated lactate levels and higher risks of short-term mortality in patients with acute cardiovascular disease and dyspnea, respectively [40, 44]. Three studies reported moderate evidence of this correlation in patients with non-specific complaints and unselected non-trauma patients [36, 42, 45]. The last study did not find evidence of any correlation between the prehospital lactate level and short-term mortality risk in COVID-19 patients [48]. This study had the highest risk of bias compared to the other five studies.
Objective 2: The prehospital lactate levels’ impact on changes/modifications in early patient care
We included four studies: two retrospective cohort studies, one prospective cohort study, and one randomized controlled trial [46, 52–54]. One study investigated the prehospital lactate level as a single test [53]. The three remaining studies investigated a panel of tests, including a lactate level, used in the treatment of prehospital patients [46, 52, 54]. Three of the studies showed changes in prehospital sepsis care in patients with prehospital lactate measurements [52–54]. One study reported additional prehospital administration of antibiotics in 42 out of 68 patients with suspected sepsis because their prehospital lactate level was > 4.0 mmol/L. This treatment was part of a treatment flow chart in this study’s setting [52]. The study by Younger et al. did not report specific changes in the care for sepsis patients, and only a few sepsis patients were included in the study by Zwisler et al. The data are presented in Table 3.
Table 3.
Studies investigating the impact of prehospital lactate levels on changes/modifications in early patient care
| First author, Year, Reference |
Study design | Setting | Patients | N | Measurement (Device) |
Change in prehospital care |
|---|---|---|---|---|---|---|
|
Collopy, 2022 [52] |
Retrospective cohort | Air/ground Emergency Service + interfacility transport, USA | All | 632 | A panel of tests including a lactate level. (Epoc) |
Antibiotics were administrated during transport in 42/68 patients (61.8%) with sepsis/ septic shock because of a lactate level > 4.0 mmol/L Patient care was altered in 10/28 patients (35.7%) with heart failure including administrating dobutamine in case of a lactate level > 4.0 mmol/L |
|
Gruebl, 2021 [46] |
Retrospective cohort | Prehospital Emergency Service, Germany | Out-of-hospital cardiac arrest | 263 | A panel of tests including a lactate level. (Epoc) | Intravenous sodium bicarbonate was administered in 61/98 patients (62%) with a point-of-care blood test vs. in 23/165 patients (14%) with no test. p < 0.001 |
|
Younger, 2014, [53] |
Prospective cohort | Prehospital Emergency Service, England | Sepsis | 109 |
Lactate level (StatStrip) |
The decision to treat the patient for sepsis was affected in 54/90 cases (60%) due to the lactate test result |
|
Zwisler, 2019, [54] |
Randomized controlled trial | Prehospital Emergency Service, Denmark | Critically ill (Glasgow Coma Score < 13) | 222 | A panel of tests including a lactate level. (ABL90) | The test results led to 159 interventions in 71/102 patients (69.6%) in the group randomized to a blood test. Sepsis treatment including prehospital antibiotics was done in 3/102 patients (2.9%) |
Studies are listed alphabetically by author name. Unless otherwise stated, all data was extracted from the primary study report of each study
Our systematic review did not find any studies reporting changes in the fluid administration, blood transfusion(s), oxygen supply, or triage, nor did we find studies investigating possible changes in early in-hospital patient care based on prehospital lactate measurements.
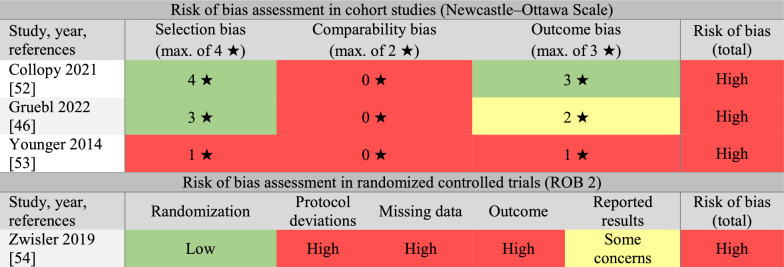
We assessed all four studies as having high risks of bias (see Table 4).
Table 4.
Risk of bias assessments in the studies included in Objective 2
The Newcastle–Ottawa Scale was used to assess the risks of biases in cohort studies. The revised Cochrane “Risk of bias” tool for randomized trials (ROB 2) was used to assess the risk of bias in the randomized controlled trial. The studies were listed in alphabetical order
Outcome reporting bias
Objective 1
We found only one published study protocol among the studies included in Objective 1 [55]. The study's results were later reported in two study reports [38, 50]. The study protocol was not complied with in all aspects, but the analysis and reporting of the primary outcome (30-day mortality) remained unaltered.
The method and results sections were compared in all the included study reports in Objective 1. No selective reporting was observed of the question addressed in this objective. One study’s results were reported as a conference abstract, and the risk of reporting bias was impossible to evaluate in this study [49].
Objective 2
We did not detect any selective reporting of results in this objective. However, only two study reports included information about their planned analyses in their method sections. It was impossible to evaluate the remaining two studies’ risks of reporting bias.
Discussion
Evidence from the studies included in our systematic review demonstrates that elevated prehospital lactate levels increased the risk of poor outcomes in most non-trauma patients but not in patients with OHCA. Limited data show changes in early prehospital care in sepsis patients, such as prehospital administration of antibiotics based on their prehospital measured lactate levels.
Cut-off lactate levels
A lactate cut-off level is relevant to efficient risk assessment in a clinical setting. A proper cut-off level may be particularly beneficial in patients at risk but without clear signs of deterioration. We expected most studies to use cut-off levels and designed this systematic review on these grounds. Surprisingly, only one-third of the included studies did so, with the majority using a cut-off of around 4 mmol/L.
Previous in-hospital studies mainly included a cut-off level of around 2.0–2.5 mmol/L, and the authors argue that an even lower threshold for serial in-hospital lactate measurements should be considered [2]. A prehospital cut-off lactate level of around 2.0 mmol/L to deem a risk of severe illness is likely to have a high sensitivity but will lead to excessive over-triage. Just two out of 13 sepsis patients with elevated prehospital lactate levels had a sustained lactate level ≥ 4.0 mmol/L at admission [12].
A prehospital lactate level cut-point around 4.0 mmol/L could be considered reasonable as it is often measured very early in the course of the disease and before initial treatment. However, this systematic review did not aim to evaluate the cut-off levels used in the included studies.
Objective 1: The prognostic value of prehospital lactate
Most of the included studies found evidence of a correlation between elevated prehospital lactate levels and poor outcomes. These results are in line with previous findings in in-hospital patients and prehospital trauma patients [2, 3, 9]. The correlation persisted in patients with various illnesses such as sepsis, cardiovascular diseases, dyspneic patients, and in unselected prehospital patients. There was a tendency to find more significant results in the studies with the lowest RoB. Thus, our confidence increased that a correlation between elevated prehospital lactate levels and poor outcomes persisted in non-trauma patients. The correlation was present in both short-term and long-term mortality and admission to the ICU.
One of the included studies blinded the patients’ lactate levels to all participants [36]. They did not find clear evidence of a correlation between lactate levels and 30-day mortality in patients with non-specific complaints (p = 0.09). The study used a low cut-off lactate level of 2.2 mmol/L, which may be the reason for no apparent difference in mortality risk between the two groups.
Sepsis patients
The Sepsis-3 Consensus Definition advocates prompt lactate measurements in suspected sepsis patients [8]. Two of three prehospital studies included in this review support this recommendation [12, 38]. One study indicated that prehospital serial lactate measurements could be superior to just one initial lactate measurement in prognostication [39]. Monitoring prehospital lactate clearance might be beneficial, particularly in long transfer times.
Patients with OHCA
The three studies on OHCA patients included in this systematic review did not find evidence of a correlation between elevated prehospital lactate levels and poor outcomes. This differs from studies examining the in-hospital lactate levels in OHCA patients with and without extracorporeal circulation [56, 57]. An explanation could be a difference in OHCA study patients’ characteristics. OHCA patients with and without transportation to the hospital are not comparable.
The difference between the studies' results may be due to the timing of the lactate measurement. The lactate levels are expected to be elevated in all OHCA patients during a period with low flow. However, studies with in-hospital cardiac arrest (IHCA) and assumed short response times have also found clear evidence of a correlation between elevated lactate levels and poor outcomes [58]. Still, patients with IHCA and patients with OHCA are not comparable in comorbidities or the reasons for cardiac arrests [59].
The increased use of prehospital advanced treatments such as extracorporeal cardiopulmonal resuscitation in OHCA calls for prehospital prognostic tools [60]. The MIRACLE-2 score has proven reliable in OHCA prognostication in patients with cardiac origin [61]. The score includes the initial pH level obtained at hospital arrival. The prehospital lactate level may assist in prehospital risk stratification before initiating advanced prehospital treatments, possibly combined with other known risk factors in a prehospital prognostic tool.
Objective 2
We found sparse evidence on the effect of prehospital lactate levels on changes/modifications in early patient care. Most changes were documented in sepsis patients. Antibiotic treatments were administered in 61.8% of septic patients during transport due to the lactate measurements (as noted in a flow chart in the study report) [52]. The only study investigating the lactate level as a sole measurement did not specify what changes were made due to the measurement [53]. The study by Zwisler et al., included in our review, reported many interventions made due to the prehospital blood analyses in patients with impaired consciousness. Unfortunately, as the lactate measurement was a part of a panel of blood tests, it is not possible to make probable correlations between the interventions and the lactate measurement alone.
Another systematic review from 2017 included eight studies to evaluate the effect of lactate measurements in sepsis patients at presentation to health care [3]. One study included in that review was from the prehospital environment and included inter-hospital aeromedical transfers, and the rest of the included studies were investigating in-hospital lactate measurements. That systematic review showed that lactate measurements reduced the time to intravenous fluid and antibiotic administration in most of the included studies. The only prehospital study included in that review did not find a difference in the number of intubations or insertion of central venous lines between patients with and without lactate measurements, but the study was probably underpowered.
Three of the four studies in Objective 2 did not report patients’ outcomes [52–54]. The fourth study did not specify the changes made due to lactate measurements alone [46]. Consequently, the impact of the treatment changes made due to the lactate measurements (as a sole analysis or as a part of a blood test panel) cannot be assessed.
Future research
This study’s findings indicate that prehospital lactate levels can be used in the early prognostic assessment of most non-trauma patients. This aligns with the evidence on the in-hospital use of lactate measurements.
Surprisingly, prehospital lactate levels did not seem to differ between survivors and non-survivors in patients with OHCA, contrary to lactate levels measured in-hospital. More studies with larger cohorts targeting this population are needed to investigate this correlation, as two out of three studies included in our systematic review had few included patients. The studies’ risks of survivor bias also need to be addressed to increase their findings’ generalizability to other prehospital settings.
Most studies on prehospital lactate measurements focus on the patients’ prognoses. Little evidence exists on the changes in early patient treatment. Future research should focus on whether prehospital lactate measurements change how we treat the patients and if these changes improve patients’ outcomes. These studies should ideally be randomized controlled trials with a lactate measurement as the intervention compared to no lactate measurement to minimize the risk of bias.
Limitations
This systematic review included studies with substantial heterogeneity. In addition, several studies were small and probably underpowered, especially in Objective 2. None of the included studies had a low risk of bias, and all studies in Objectives 2 and 3 had a high risk of bias. This induces low quality of the body of evidence, particularly in Objectives 2 and 3.
Surprisingly, most studies in Objective 1 (6/9, 67%) reported continuous lactate levels as means instead of medians. This error can introduce crucial bias to the results. This is particularly important in studies with few patients, where one or a few outliers can substantially change the mean measurement. Thus, our confidence in the evidence presented in these studies decreased.
Many large studies had to be excluded because of our predefined decision to exclude studies with both non-trauma and trauma patients unless the results were separated between groups. This decision yielded less power but potentially more precision to our systematic review. On the contrary, our decision to include in-hospital mortality as short-term mortality may have decreased the accuracy of Objective 1’s results. If the patients died after discharge but within 30 days, they were not recorded as deceased in the studies using the outcome of in-hospital mortality. Additionally, in-hospital mortality may contain patients with a survival of more than 30 days. However, as many studies use in-hospital mortality as their outcome, we decided a priori to include this outcome in our systematic review to include more potential studies.
We did not plan to assess the risk of publication bias. However, the risk of publication bias was expected to be high, as studies with down to 20 patients could be included in our review. Small studies with no difference between study groups’ outcomes are less likely to be published than small studies with significant findings.
Conclusions
Elevated prehospital lactate levels were correlated to increased short-term mortality in most acute non-trauma patients but not in patients experiencing OHCA. Few studies suggest that prehospital lactate measurements may impact interventions in septic patients. The included studies were heterogeneous, and most had high risks of bias. Further studies are needed to investigate the impact of prehospital lactate measurements on patient care and whether this affects patients’ outcomes.
Supplementary Information
Acknowledgements
The authors thank Professor Asbjørn Hróbjartsson, a specialist in systematic reviews, for his invaluable advice and help during this systematic review. The Centre for Evidence-Based Medicine Odense (CEBMO) in Denmark offered his consultancy service.
Abbreviations
- ICU
Intensive care unit
- IHCA
In-hospital cardiac arrest
- NOS
Newcastle–Ottawa scale
- OHCA
Out-of-hospital cardiac arrest
- QUIPS
Quality In Prognosis Studies
- RoB
Risk of bias
Author contributions
LHW, ATL, EFC, CBM, and SM conceived and designed the study. LHW obtained research funding and conducted the literature searches. LHW and HBM conducted the literature screening, selected the studies that were included and extracted the data. LHW, HBM, and ACB conducted the risk of bias assessments. EFC, ATL, CBM, and SM supervised the process. LHW and ACB drafted the manuscript, and all authors contributed substantially to its revision. LHW takes responsibility for the paper as a whole.
Funding
The following sources funded the corresponding author during her time as a Ph.D. student: University of Southern Denmark—Institution of Regional Health Science, The Region of Southern Denmark, Department of Anesthesiology and Intensive Care, Odense University Hospital, Hospital of Southern Jutland, The Foundation in memory of Holger & Ruth Hesse, The Foundation of Manager Kurt Bønnelycke and wife Grethe Bønnelycke, The Foundation of Professor Sophus Johansen, The A.P. Møller Foundation, The Gangsted Foundation, The Chief Physician Council’s Research Foundation in the Southern Region of Denmark, and The Danish Air Ambulance Research Foundation. None of the funders participated in the conceptualization, study design, data collection, analysis, decision to publish, or manuscript preparation.
Availability of data and materials
All additional data are available upon reasonable request by contacting the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
LHW was funded by the sources stated under ‘Funding’. All remaining authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jansen TC, van Bommel J, Mulder PG, Rommes JH, Schieveld SJ, Bakker J. The prognostic value of blood lactate levels relative to that of vital signs in the pre-hospital setting: a pilot study. Crit Care. 2008;12(6):R160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011;19:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris E, McCartney D, Lasserson D, Van den Bruel A, Fisher R, Hayward G. Point-of-care lactate testing for sepsis at presentation to health care: a systematic review of patient outcomes. Br J Gen Pract. 2017;67(665):e859–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524–8. [DOI] [PubMed] [Google Scholar]
- 5.Pal JD, Victorino GP, Twomey P, Liu TH, Bullard MK, Harken AH. Admission serum lactate levels do not predict mortality in the acutely injured patient. J Trauma. 2006;60(3):583–7 (discussion 7-9). [DOI] [PubMed] [Google Scholar]
- 6.Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, Davies A, Stachowski E, Reade MC, Bailey M, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. 2010;14(1):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen M, Brandt VS, Holler JG, Lassen AT. Lactate level, aetiology and mortality of adult patients in an emergency department: a cohort study. Emerg Med J. 2015;32(9):678–84. [DOI] [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis CT, Naumann DN, Crombie N, Midwinter MJ. Prehospital point-of-care lactate following trauma: a systematic review. J Trauma Acute Care Surg. 2016;81(4):748–55. [DOI] [PubMed] [Google Scholar]
- 10.Walther LH, Zegers F, Nybo M, Mogensen CB, Christensen EF, Lassen AT, Mikkelsen S. Accuracy of a point-of-care blood lactate measurement device in a prehospital setting. J Clin Monit Comput. 2022;36(6):1679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Rodriguez F, Vaquerizo-Villar F, Lopez-Izquierdo R, Castro-Villamor MA, Sanz-Garcia A, Del Pozo-Vegas C, Hornero R. Derivation and validation of a blood biomarker score for 2-day mortality prediction from prehospital care: a multicenter, cohort, EMS-based study. Intern Emerg Med. 2023;18(6):1797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boland LL, Hokanson JS, Fernstrom KM, Kinzy TG, Lick CJ, Satterlee PA, LaCroix BK. Prehospital lactate measurement by emergency medical services in patients meeting sepsis criteria. West J Emerg Med. 2016;17(5):648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350: g7647. [DOI] [PubMed] [Google Scholar]
- 14.Walther LH, Mieritz HB, Hróbjartsson A, Lassen AT, Christensen EF, Mogensen CB, Mikkelsen S. The use and benefits of prehospital measurements of blood lactate in acute non-traumatic patients: a systematic review. PROSPERO 2020 CRD42020167169. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020167169.
- 15.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiavenato M, Chu F. PICO: what it is and what it is not. Nurse Educ Pract. 2021;56: 103194. [DOI] [PubMed] [Google Scholar]
- 17.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6. [DOI] [PubMed] [Google Scholar]
- 18.QUIPS tool. Available from: https://methods.cochrane.org/sites/methods.cochrane.org.prognosis/files/uploads/QUIPS%20tool.pdf.
- 19.Grooten WJA, Tseli E, Äng BO, Boersma K, Stålnacke BM, Gerdle B, Enthoven P. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS-aspects of interrater agreement. Diagn Progn Res. 2019;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 21.RoB2 Development Group. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). Available from: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2. [DOI] [PubMed]
- 22.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Beest PA, Mulder PJ, Oetomo SB, van den Broek B, Kuiper MA, Spronk PE. Measurement of lactate in a prehospital setting is related to outcome. Eur J Emerg Med. 2009;16(6):318–22. [DOI] [PubMed] [Google Scholar]
- 24.Brio-Ibañez PD, López-Izquierdo R, Martín-Rodríguez F, Mohedano-Moriano A, Polonio-López B, Maestre-Miquel C, Viñuela A, Durantez-Fernández C, Villamor MÁC, Martín-Conty JL. Clinical utility of delta lactate for predicting early in-hospital mortality in adult patients: a prospective, multicentric, cohort study. Diagnostics. 2020;10(11):960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durantez-Fernandez C, Martin-Conty JL, Polonio-Lopez B, Castro Villamor MA, Maestre-Miquel C, Vinuela A, Lopez-Izquierdo R, Mordillo-Mateos L, Fernandez Mendez F, Jorge Soto C, et al. Lactate improves the predictive ability of the National Early Warning Score 2 in the emergency department. Aust Crit Care. 2022;35(6):677–83. [DOI] [PubMed] [Google Scholar]
- 26.Martín-Rodríguez F, López-Izquierdo R, Castro Villamor MA, Mangas IM, Del Brío IP, Delgado Benito JF, Martín Conty JL, Manzanares J, Mayo-Iscar A, Del Pozo VC. Prognostic value of lactate in prehospital care as a predictor of early mortality. Am J Emerg Med. 2019;37(9):1627–32. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Rodriguez F, Lopez-Izquierdo R, Del Pozo VC, Delgado Benito JF, Del Brio IP, Moro Mangas I, Martin Conty JL, Castro Villamor MA. Valor predictivo del preNEWS2-L (Pre-hospital National Early Warning Score 2 Lactate) para la deteccion de la mortalidad precoz en el ambito prehospitalario. Emergencias: revista de la Sociedad Espanola de Medicina de Emergencias. 2019;31(3):173–9. [PubMed] [Google Scholar]
- 28.Martin-Rodriguez F, Lopez-Izquierdo R, Medina-Lozano E, Ortega Rabbione G, del Pozo VC, Carbajosa Rodriguez V, Castro Villamor MA, Sanchez-Soberon I, Sanz-Garcia A. Accuracy of prehospital point-of-care lactate in early in-hospital mortality. Eur J Clin Invest. 2020;50(12): e13341. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Rodriguez F, Lopez-Izquierdo R, Sanz-Garcia A, del Pozo Vegas C, Angel Castro Villamor M, Mayo-Iscar A, Martin-Conty JL, Ortega GJ. Novel prehospital phenotypes and outcomes in adult-patients with acute disease. J Med Syst. 2022;46(7):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin-Rodriguez F, Ortega GJ, Castro Villamor MA, del Pozo Vegas C, Delgado Benito JF, Martin-Conty JL, Sanz-Garcia A, Lopez-Izquierdo R. Development of a prehospital lactic acidosis score for early-mortality: a prospective, multicenter, ambulance-based, cohort study. Am J Emerg Med. 2023;65:16–23. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Rodriguez F, Sanz-Garcia A, del Pozo Vegas C, Ortega GJ, Castro Villamor MA, Lopez-Izquierdo R. Time for a prehospital-modified sequential organ failure assessment score: an ambulance-based cohort study. Am J Emerg Med. 2021;49:331–7. [DOI] [PubMed] [Google Scholar]
- 32.Martin-Rodriguez F, Sanz-Garcia A, Martinez Fernandez FT, Otero de la Torre S, Delgado Benito JF, Del Pozo Vegas C, Garcia RP, Astorga EAI, Coalla AS, Lopez-Izquierdo R. Association between prehospital lactate categories with short- and long-term mortality. A prospective, observational multicenter study. QJM: monthly journal of the Association of Physicians. 2023. [DOI] [PubMed]
- 33.Martin-Rodriguez F, Sanz-Garcia A, Moreno LO, Vegas CP, Castro-Villamor MA, Martin-Conty JL, Lopez-Izquierdo R, Rabbione GO. Modelo de riesgo de mortalidad precoz en pacientes ancianos con enfermedad aguda atendidos por servicios de emergencias prehospitalarias. Emergencias. 2020;32(3):177–84. [PubMed] [Google Scholar]
- 34.Martín-Rodríguez F, López-Izquierdo R, Delgado Benito JF, Sanz-García A, Del Pozo Vegas C, Castro Villamor M, Martín-Conty JL, Ortega GJ. Prehospital point-of-care lactate increases the Prognostic Accuracy of National Early Warning Score 2 for early risk stratification of mortality: results of a multicenter, observational study. J Clin Med. 2020;9(4):1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usategui-Martín R, Zalama-Sánchez D, López-Izquierdo R, Delgado Benito JF, Del Pozo Vegas C, Sánchez Soberón I, Martín-Conty JL, Sanz-García A, Martín-Rodríguez F. Prehospital lactate-glucose interaction in acute life-threatening illnesses: metabolic response and short-term mortality. Eur J Emerg Med. 2024;31(3):173–80. [DOI] [PubMed] [Google Scholar]
- 36.Ivic R, Nurmi J, Kurland L, Vicente V, Lindström V, Djärv T, Kaartinen J, Castrén M, Bohm K. Soluble urokinase plasminogen activator receptor and lactate as prognostic biomarkers in patients presenting with non-specific chief complaints in the pre-hospital setting—the PRIUS-study. Scand J Trauma Resusc Emerg Med. 2021;29(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Pozo Vegas C, Zalama-Sánchez D, Sanz-Garcia A, López-Izquierdo R, Sáez-Belloso S, Mazas Perez Oleaga C, Domínguez Azpíroz I, Elío Pascual I, Martín-Rodríguez F. Prehospital acute life-threatening cardiovascular disease in elderly: an observational, prospective, multicentre, ambulance-based cohort study. BMJ Open. 2023;13(11):e078815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jouffroy R, Léguillier T, Gilbert B, Tourtier JP, Bloch-Laine E, Ecollan P, Bounes V, Boularan J, Gueye-Ngalgou P, Nivet-Antoine V, et al. Pre-hospital lactatemia predicts 30-day mortality in patients with septic shock-preliminary results from the LAPHSUS study. J Clin Med. 2020;9(10):3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jouffroy R, Gilbert B, Thomas L, Bloch-Laine E, Ecollan P, Boularan J, Bounes V, Vivien B, Gueye P-N. Association between prehospital shock index variation and 28-day mortality among patients with septic shock. BMC Emerg Med. 2022;22(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin-Rodriguez F, Lopez-Izquierdo R, Castro Villamor MA, Del Pozo VC, Delgado Benito MDP, Martinez Caballero CM, Priego Martinez V, Martin Conty JL, Mayo-Iscar A, Sanchez-Soberon I, et al. The prognostic value of prehospital blood lactate levels to predict early mortality in acute cardiovascular disease. Shock. 2020;53(2):164–70. [DOI] [PubMed] [Google Scholar]
- 41.Martin-Rodriguez F, Lopez-Izquierdo R, Castro-Villamor MA, Martin-Conty JL, Herrero-Anton RM, Del Pozo-Vegas C, Guillen-Gil D, Duenas-Laita A. A predictive model for serious adverse events in adults with acute poisoning in prehospital and hospital care. Aust Crit Care. 2021;34(3):209–16. [DOI] [PubMed] [Google Scholar]
- 42.Tobias AZ, Guyette FX, Seymour CW, Suffoletto BP, Martin-Gill C, Quintero J, Kristan J, Callaway CW, Yealy DM. Pre-resuscitation lactate and hospital mortality in prehospital patients. Prehosp Emerg Care. 2014;18(3):321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corral Torres E, Hernández-Tejedor A, Suárez Bustamante R, de Elías Hernández R, Casado Flórez I, San Juan Linares A. Prognostic value of venous blood analysis at the start of CPR in non-traumatic out-of-hospital cardiac arrest: association with ROSC and the neurological outcome. Crit Care. 2020;24(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villanueva Rabano R, Martin-Rodriguez F, Lopez-Izquierdo R. National Early Warning Score 2 Lactate (NEWS2-L) in predicting early clinical deterioration in patients with dyspnoea in prehospital care. Invest Educ Enferm. 2021;39(3):e05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swan KL, Avard BJ, Keene T. The relationship between elevated prehospital point-of-care lactate measurements, intensive care unit admission, and mortality: a retrospective review of adult patients. Aust Crit Care. 2019;32(2):100–5. [DOI] [PubMed] [Google Scholar]
- 46.Gruebl T, Ploeger B, Wranze-Bielefeld E, Mueller M, Schmidbauer W, Kill C, Betz S. Point-of-care testing in out-of-hospital cardiac arrest: a retrospective analysis of relevance and consequences. Scand J Trauma Resusc Emerg Med. 2021;29(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vujanović V, Borovnik Lesjak V, Mekiš D, Strnad M. Dynamics of capillary lactate levels in patients with out-of-hospital cardiac arrest. Medicina (Kaunas). 2023;59(11):1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jouffroy R, Brami E, Scannavino M, Daniel Y, Bertho K, Abriat A, Salome M, Lemoine S, Jost D, Prunet B, et al. Association between prehospital shock index and mortality among patients with COVID-19 disease. Am J Emerg Med. 2022;56:133–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiuh T, Sweeney T, Rupp R, Davis B, Reed J. An emergency medical services sepsis protocol with point-of-care lactate accurately identifies out-of-hospital patients with severe infection and sepsis. Ann Emerg Med. 2012;60(4):S44-S. [Google Scholar]
- 50.Jouffroy R, Leguillier T, Gilbert B, Tourtier JP, Bloch-Laine E, Ecollan P, Bounes V, Boularan J, Gueye-Ngalgou P, Nivet-Antoine V, et al. Prehospital lactate clearance is associated with reduced mortality in patients with septic shock. Am J Emerg Med. 2021;46:367–73. [DOI] [PubMed] [Google Scholar]
- 51.Martín-Rodríguez F, Del Pozo Vegas C, Mohedano-Moriano A, Polonio-López B, Maestre Miquel C, Viñuela A, Durantez Fernández C, Gómez Correas J, López-Izquierdo R, Martín-Conty JL. Role of biomarkers in the prediction of serious adverse events after syncope in prehospital assessment: a multi-center observational study. J Clin Med. 2020;9(3):651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collopy KT, Westmoreland A, Powers WF. Patient care alterations after point-of-care laboratory testing during critical care transport. Air Med J. 2022;41(4):370–5. [DOI] [PubMed] [Google Scholar]
- 53.Younger P, McClelland G. Evaluation of pre-hospital point-of-care testing for lactate in sepsis and trauma patients. J Paramed Pract. 2014;6(10):526–31. [Google Scholar]
- 54.Zwisler ST, Zincuk Y, Bering CB, Zincuk A, Nybo M, Mikkelsen S. Diagnostic value of prehospital arterial blood gas measurements—a randomised controlled trial. Scand J Trauma Resusc Emerg Med. 2019;27(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jouffroy R, Tourtier JP, Debaty G, Bounes V, Gueye-Ngalgou P, Vivien B. Contribution of the pre-hospital blood lactate level in the pre-hospital orientation of septic shock: the LAPHSUS study. Turk J Anaesthesiol Reanim. 2020;48(1):58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laurikkala J, Skrifvars MB, Bäcklund M, Tiainen M, Bendel S, Karhu J, Varpula T, Vaahersalo J, Pettilä V, Wilkman E. Early lactate values after out-of-hospital cardiac arrest: associations with one-year outcome. Shock. 2019;51(2):168–73. [DOI] [PubMed] [Google Scholar]
- 57.Debaty G, Babaz V, Durand M, Gaide-Chevronnay L, Fournel E, Blancher M, Bouvaist H, Chavanon O, Maignan M, Bouzat P, et al. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation. 2017;112:1–10. [DOI] [PubMed] [Google Scholar]
- 58.D’Arrigo S, Cacciola S, Dennis M, Jung C, Kagawa E, Antonelli M, Sandroni C. Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Resuscitation. 2017;121:62–70. [DOI] [PubMed] [Google Scholar]
- 59.Moskowitz A, Holmberg MJ, Donnino MW, Berg KM. In-hospital cardiac arrest: are we overlooking a key distinction? Curr Opin Crit Care. 2018;24(3):151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petermichl W, Philipp A, Hiller KA, Foltan M, Floerchinger B, Graf B, Lunz D. Reliability of prognostic biomarkers after prehospital extracorporeal cardiopulmonary resuscitation with target temperature management. Scand J Trauma Resusc Emerg Med. 2021;29(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sunderland N, Cheese F, Leadbetter Z, Joshi NV, Mariathas M, Felekos I, Biswas S, Dalton G, Dastidar A, Aziz S, et al. Validation of the MIRACLE(2) score for prognostication after out-of-hospital cardiac arrest. Interv Cardiol. 2023;18: e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All additional data are available upon reasonable request by contacting the corresponding author.