Abstract
Substantial therapeutic advancement has been made in the field of immunotherapy in breast cancer. The immune checkpoint inhibitor pembrolizumab in combination with chemotherapy received FDA approval for both PD-L1 positive metastatic and early-stage triple-negative breast cancer, while ongoing clinical trials seek to expand the current treatment landscape for immune checkpoint inhibitors in hormone receptor positive and HER2 positive breast cancer. Antibody drug conjugates are FDA approved for triple negative and HER2+ disease, and are being studied in combination with immune checkpoint inhibitors. Vaccines and bispecific antibodies are areas of active research. Studies of cellular therapies such as tumor infiltrating lymphocytes, chimeric antigen receptor-T cells and T cell receptor engineered cells are promising and ongoing. This review provides an update of recent major clinical trials of immunotherapy in breast cancer and discusses future directions in the treatment of breast cancer.
Keywords: Immunotherapy, Immune checkpoint inhibitor, Antibody drug conjugate, Bispecific antibody, Cellular therapy, Breast cancer
Background
One function of the immune system is to eliminate nascent malignant cells. A hallmark of malignancy is the ability of cancer cells to either actively suppress or escape detection by the immune system. Cancer cells alter their tumor immune microenvironment (TIME), creating an immunosuppressive environment through various mechanisms such as signaling changes, restriction of antigen recognition, and induction of T cell exhaustion [1]. Immune checkpoint proteins, such as programmed-death receptor (PD-1) on T cells, B cells and antigen-presenting cells, PD ligand (PD-L1) on tumor cells, or cytotoxic T-lymphocyte associated protein 4 (CTLA4) are essential components in the host immune response to tumor cells [2]. Mutations in these proteins essentially “turn off” the immune system response to malignant cells. The function of immunotherapy delivered through immune checkpoint inhibitors (ICIs), then, is to restore the normal immune response, allowing the host immune system to destroy malignant cells. ICIs include monoclonal antibodies targeting PD-L1 (atezolizumab, avelumab, and durvalumab), PD-1 (pembrolizumab and nivolumab) or CTLA-4 (Ipilumumab) [3]. Lymphocyte Activation Gene 3 (LAG-3), a recently-discovered immune checkpoint which is found on T cells that have been activated but exhausted, has also been targeted by immunotherapy [4]. In addition, cellular therapies offer a distinct mechanism of immunotherapy with the goal of inciting a durable immunologic response to cancer antigens.
The role of immunotherapy in breast cancer has expanded in the past two decades. Breast cancer was classically considered to be immunologically “cold,” unresponsive to immune manipulations. Ongoing research hopes to identify patients who might have enhanced benefit to immunotherapy based on their individual TIME.
Antibody–drug conjugates (ADCs) are another recent advancement in breast cancer. ADCs utilize monoclonal antibodies conjugated to cytotoxic agents (referred to as the “payload”) at higher concentrations into antigen-expressing tumor cells [5]. This has the benefit of targeting traditional chemotherapies to malignant cells, reducing the risk of off-target systemic toxicities. Some ADCs exhibit the bystander effect, in which cells adjacent to the antigen-expressing tumor cell are also killed by the payload [6]. Common ADC targets in breast cancer include TROP2, HER2, and HER3. While ADCs are not defined as immunotherapy, this new class of agents has been safely combined with ICIs in clinical trials [7]. Research into combinations of ADCs with ICIs is ongoing.
Cancer vaccines are being investigated for both prevention and treatment of breast cancer. A principal target of cancer vaccines are tumor-associated antigens (TAAs), which are proteins that are expressed in both normal tissue and malignant cells [8]. Examples of TAAs in breast cancer include HER2, Muc-1, and CEA [8, 9]. Cancer vaccines stimulate CD4+ T helper cells and CD8+ cytotoxic T lymphocytes to engage with TAAs to induce a specific adaptive immunologic response and to initiate immunologic memory to protect against further exposure to the antigens. A benefit of cancer vaccines is the low adverse effects associated with treatment, though studies have found minimal clinical activity. Possible mechanisms of resistance include downregulation of activated tumor antigens and antigen-presenting cells by the TIME, as well as the destruction of activated T-cells [10].
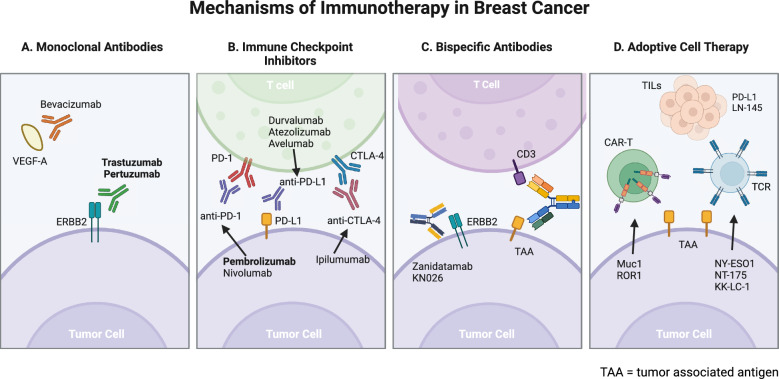
The role of immunotherapy in breast cancer has expanded in the past two decades. Breast cancer was classically considered to be immunologically “cold,” unresponsive to immune manipulations. Ongoing research hopes to identify patients who might have enhanced benefit to immunotherapy based on their individual TIME (Fig. 1).
Fig. 1.
Mechanisms of Current and Upcoming Immunotherapy in Breast Cancer. Therapies in bold are FDA approved. Created in BioRender. https://BioRender.com/t90y911
Across multiple cancer types, responsiveness to ICIs has been shown to correlate with higher PD-L1 expression on tumor cells, as determined by immunohistochemistry (IHC) [11]. Two of the most common methods of reporting levels of PD-L1 expression are the combined positive score (CPS) and immune-cell score (IC). CPS measures the proportion of PD-L1 positive cells in both tumor and immune cells out of all cells in a tumor sample, as assessed by Dako assay 22C3 and reported as a maximum score out of 100 [12]. IC is defined by the proportion of tumor area occupied by PD-L1-staining immune cells regardless of staining intensity and is assessed by Ventana assay SP142 [12, 13]. Studies of pembrolizumab typically utilize CPS, while studies of atezolizumab most commonly use IC. Concordance between assays is suboptimal, with IC typically reporting a more conservative estimate of PD-L1 positivity [14]. Standard cutoffs for PD-L1 positivity (PD-L1+) include CPS ≥ 1 and IC ≥ 1% [12–14]. PD-L1 positivity is used clinically to determine benefit of pembrolizumab in mTNBC [15].
Another biomarker that may predict response to ICIs in breast cancer is the presence of tumor infiltrating lymphocytes (TILs), such as CD4+ T cells, CD8+ T cells, NK cells, and Th1, which cause an anti-tumor effect through IFN-γ signaling [16, 17]. Recent clinical trials suggest an enhanced benefit for immunotherapy in patients with high levels of TILS, but this is not yet a standard biomarker in clinical practice. Biomarkers for immunotherapy response primarily utilized outside of breast cancer include high tumor mutational burden (TMB), defined as the number of non-inherited mutations per million base pairs, as well as deficiencies in mismatch repair genes (dMMR) which causes increase mutations in microsatellites, termed microsatellite instability-high (MSI-H), though these biomarkers are typically employed in gastrointestinal cancers [18–20]. Negative biomarkers such as TAMs, MDSc, CD4+ TRegs, B2M, HLA-A deletions and JAK1/2 mutations may decrease a cancer’s response to immunotherapy but are not used in clinical practice [21–23].
Immune-related adverse events (irAEs) are thought to be caused by disruptions in self-tolerance, or a non-specific autoinflammatory reaction [24]. The most common irAEs are cutaneous (maculopapular rash, pruritus, or lichenoid dermatitis), followed by gastrointestinal (diarrhea and colitis), then endocrine (hypothyroidism, hypophysitis or adrenal insufficiency) [25]. Rare but fatal irAEs include pneumonitis, myocarditis, hepatitis, and enteritis [25]. Management of irAE typically includes high-dose corticosteroids, with immunosuppressive therapies in severe cases [26]. Permanent discontinuation of immunotherapy is required for all grade 4 irAEs with the exception of endocrinopathies that can be controlled with hormone replacement [26].
Resistance to immunotherapy is a complex process that is possible due to both intrinsic and extrinsic tumor effects. This can occur due to multifactorial changes in the TIME. Causes of resistance include activation of immune checkpoint pathways that suppress any response from cancer cells as well adaptation within the immune recognition cascade to prevent a response by the immune system [27]. These are often referred to as “cold tumors” as they are difficult to be penetrated by immune cells. There is also the possibility of acquired resistance which occurs after an initial response to immunotherapy, and development of genetic mutations leading to resistance. There are also extrinsic factors such as patient characteristics and lifestyle that can lead to resistance as well. Strategies to overcome resistance to immunotherapy is an ongoing investigation. While there is significant improvement in responses with combination dual immunotherapy, addition of targeted therapy, or PD-L1 down regulators/ inhibitors that are being used in other cancers, there needs to be further research in breast cancer to identify ways to overcome immunotherapy resistance [28].
Triple negative breast cancer (TNBC)
TNBC is an aggressive subtype of breast cancer, accounting for 12–15% of all breast cancer in the United States [29]. In comparison to hormone receptor positive (HR+) breast cancer, TNBC is known to have higher levels of PD-L1 expression, with a median PD-L1 CPS of 7.5 and 50% expressing ≥ CPS 10 [30]. In addition, TNBC has higher levels of TILs and TMB, which are associated with an enhanced response to immunotherapy [18, 22, 23]. Over the past decade, clinical trials in TNBC have incorporated ICIs in combination with traditional cytotoxic chemotherapy with favorable results. In the metastatic setting, response to ICIs is dependent on the degree of PD-L1 expression, whereas in the neoadjuvant/adjuvant setting benefit to ICIs is seen regardless of PD-L1 positivity. This is thought to be explained, at least in part, by decreasing PD-L1 expression and TILs over the course of disease progression and with higher burden of disease [31].
Advanced and metastatic TNBC
The first studies of immunotherapy in breast cancer were conducted as monotherapy in treatment-refractory, PD-L1+ advanced/metastatic TNBC (a/mTNBC). The 2016 KEYNOTE-012 phase 1b trial showed an encouraging overall response rate (ORR) of 18.5% for patients with PD-L1 ≥ 1% mTNBC treated with single-line pembrolizumab [32]. This was followed by the phase II KEYNOTE-086 of single-agent pembrolizumab in mTNBC, which found an ORR of 21% when used in the first line for PD-L1 positive disease, and a more modest ORR of 5.7% after the first line in any level of PD-L1 expression [33, 34]. These results suggested a benefit from incorporating ICIs in PD-L1+ disease, with an enhanced benefit earlier in the treatment course. The phase III KEYNOTE-119 then compared single-agent pembrolizumab to chemotherapy of choice (capecitabine, gemcitabine, eribuilin, or vinorelbine) in 622 patients in the second- or third-line setting. While no difference in progression free survival (PFS) or overall survival (OS) was seen, a graded ORR to pembrolizumab was seen with higher PD-L1 CPS, with the most benefit seen in patients with CPS ≥ 10 [35, 36]. In a phase II trial across all solid malignancies with MSI-H or MMR disease including thirteen patients with breast cancer, single-agent pembrolizumab had a median ORR of 30.8% and PFS of 3.5 months [20].
Pembrolizumab in combination with chemotherapy is now the standard first-line therapy for PD-L1+ a/mTNBC based on the KEYNOTE-355 trial. This phase III study of 847 patients compared first-line standard chemotherapy with pembrolizumab or placebo. Patients with PD-L1 CPS ≥ 10 receiving pembrolizumab exhibited a statistically significant improvement in PFS (9.7 months vs. 5.6 months, HR (hazard ratio) 0.65) and OS (23.0 months vs. 16.1 months, HR 0.73) [15, 37], with a trend towards significance for patients with CPS ≥ 1. The PFS benefit was more pronounced in patients receiving paclitaxel or nab-paclitaxel rather than gemcitabine/carboplatin. Patients with a disease-free interval (DFI) of more than 6 months after curative treatment for early-stage TNBC were included. In an exploratory analysis, patients with early recurrence (6–12 months after curative-intent treatment) had less benefit from immunotherapy in combination with chemotherapy (HR 1.44) [15]. The FDA approved pembrolizumab for first-line advanced/metastatic TNBC in 2020 and this regimen is now the standard of care in the first line for patients with PD-L1+ a/mTNBC (Table 1).
Table 1.
Current and upcoming clinical trials of immune checkpoint inhibitors in advanced/metastatic triple negative breast cancer
| Trial Name: | Primary Author | Year | Study Design | Line of Therapy | Setting | Biomarker | # Patients | Drug regimen | Results |
|---|---|---|---|---|---|---|---|---|---|
| Pembrolizumab | |||||||||
|
KEYNOTE-012 |
Nanda | 2016 | Phase Ib | Heavily Pretreated | Metastatic | PD-L1 ≥ 1% | 32 | Pembrolizumab |
ORR 18.5% (95% CI 6.3–38.1) DCR 25.9% (11.1–46.3) |
|
KEYNOTE-086 |
Adams | 2018 | Phase II | > First line | Metastatic | Any | 170 | Pembrolizumab |
Overall ORR 5.3% (95% CI 2.7–9.9) PD-L1 + ORR 5.7% (2.4–12.2) Overall DCR 7.6% (4.4–12.7) PD-L1 + DCR 9.5% (5.1–16.8) |
|
KEYNOTE-086 |
Adams | 2018 | Phase II | First line | Metastatic | PD-L1 ≥ 1% | 84 | Pembrolizumab |
ORR 21% (95% CI 13.9–31.4) DCR 23.8% (15.9–34.0) PFS 2.1 mo (2.0–2.2) OS 18.0 mo (12.9–23.0) |
|
KEYNOTE-158 Cohort K |
Maio | 2022 | Phase II | > First line | Advanced/metastatic solid tumors, including breast | MSI-H or dMMR | 13 | Pembrolizuamb |
ORR 30.8% (95% CI 25.8–36.2) PFS 3.5 mo (2.3–4.2) OS 20.1 (14.1–27.1) |
| NCT02971761 | Yuan | 2020 | Phase II | Any | Metastatic | Androgen Receptor > 10% | 18 | Enobosarm + Pembrolizumab |
RR 13% CBR 25% PFS 2.6 mo (95% CI 1.9–3.1) OS 25.5 mo (10.4-NE) |
|
ENHANCE 1/KEYNOTE-150 |
Tolaney | 2021 | Phase Ib/II | First to third line | Metastatic | Any | 167 | Eribulin + Pembrolizumab |
Overall ORR 23.4% (95% CI 17.2–30.5) Overall PFS 4.1 mo (3.5–4.2) Overall OS 16.1 mo (13.3–18.5) First Line ORR 25.8% (15.8–38) First Line PFS 4.2 mo (3.5–5.5) First Line OS 17.4 mo (13.2–21.0) Second or Third Line ORR 21.8% (14.2–31.1) Second or Third Line PFS 4.1 mo (3.5–4.2) Second or Third Line OS 15.5 mo (12.5–18.7) |
|
KEYNOTE-162/TOPACIO |
Vinayak | 2019 | Phase II | > First line | Advanced/metastatic | Any | 55 | Niraparib + Pembrolizumab |
ORR 18% (90% CI 10–29); DCR 42% (31–54) BRCAmut ORR 47% (24–70), DCR 80% (56–94) BRCAwt ORR 11% (3–26), DCR 33% (19–51) PD-L1 CPS ≥ 1: ORR 32% (18–49), DCR 50% (33–67) |
| NCT03106415 | Chumsri | 2023 | Phase I/II | ≤ 3 Lines, no prior PD-1 or PD-L1 therapies | Advanced/metastatic | Any | 22 | Binimetinib + Pembrolizumab |
Safety: 3 patients with DLT ORR 29.4 (95% CI 10.3–55.9) with one CR and 4 PR CBR 35.3% (14.2–61.7) |
|
KEYNOTE-355 |
Cortes | 2020 | Phase III | First line | Metastatic | Any | 847 |
Arm A: Nab-Paclitaxel/Paclitaxel/Gemcitabine-Carboplatin + Pembrolizumab Arm B: Nab-Paclitaxel/Paclitaxel/Gemcitabine-Carboplatin + Placebo |
PFS CPS ≥ 10 9.7 vs. 5.6 mo, HR 0.66 (95% CI 0.50–0.88) PFS CPS ≥ 1 7.6 vs. 5.6 mo, HR 0.75 (0.62–0.91) PFS ITT 7.5 vs. 5.6 mo, HR 0.82 (0.70–0.98) OS CPS ≥ 10 23.0 vs. 16.1 mo, HR 0.73 (0.55–0.95) OS CPS ≥ 1 17.6 vs. 16.0 mo, HR 0.86 (0.72–1.04) OS ITT 17.2 vs. 15.5 mo, HR 0.89 (0.76–1.05) irAE 26.5% vs 6.4% |
| NCT03044730 | Shah | 2020 | Phase II | Any | Metastatic | Any | 16 | Capecitabine + Pembrolizumab |
ORR 13% CBR 15% PFS 4.0 mo (95% CI 1.9–12.7) |
|
KEYNOTE-119 |
Winer | 2021 | Phase III | > First line | Metastatic | Any | 622 |
Arm A: Pembrolizaumab Arm B: Physician's choice of Capecitabine, Eribuin, Gemcitabine or Vinorelbine |
ITT OS 9.9 vs. 10.8 mo, HR 0.97 (95% CI 0.82–1.15) CPS ≥ 10 OS 12.7 vs. 11.6 mo, HR 0.78 (0.57–1.06) CPS ≥ 1 OS 10.7 vs. 10.2 mo, HR 0.86 (0.69–1.06) ITT PFS 2.1 vs. 3.3 mo, HR 1.60 (1.33–1.92) CPS > 10 PFS 2.1 vs. 4.3 mo, HR 1.14 (0.82–1.59) CPS ≥ 1 PFS 2.1 vs. 3.1 mo, HR 1.35 (1.08–1.68) |
|
LEAP-005 |
Chung | 2020 | Phase Ib | > FIrst line | Advanced/metastatic | Any | 31 | Lenvatinib + Pembrolizumab |
ORR 29% (95% CI 14–47) DCR 58% (38–76) 55% of patients had grade 3–5 TRAE with one death |
| NCT03012230 | Kassi | 2023 | Phase I | Heavily pretreated | Metastatic | Any | 12 | Pembrolizumab + Ruxolitinib |
5 patients with grade 3 or higher AE; MTD not established 2 patients with stable disease lasting 6 mo |
| NCT02411656 | Iwase | 2023 | Phase II | First Line maintenance | mTNBC or Inflammatory | Any | 43 | Pembrolizumab |
4-mo DCR 58.1% (95% CI 43.4–72.9) PFS 4.8 mo (3.0–7.1) |
|
KEYLYNK-009 |
Rugo | 2020 | Phase II | First Line maintenance | Metastatic | Any | 271 |
Arm A: Olaparib + Pembrolizumab Arm B: Gemcitabine + Carboplatin + Pembrolizumab |
PFS 5.5 vs. 5.6 mo, HR 0.98 (95% CI 0.72–1.33) OS 25.1 vs. 23.4 mo, HR 0.95 (0.64–1.40) PD-L1 ≥ 10% PFS 5.7 vs. 5.7 mo, HR 0.92 (0.59–1.43) PD-L1 ≥ 10% OS NE vs. NE BRCA + PFS 12.4 vs. 8.4 mo, HR 0.7 (0.33–1.48) BRCA + OS NE vs. 23.4 mo (17.3-NE) |
| NCT02734290 | Page | 2023 | Phase Ib | First or second line | Metastatic | Any | 29 |
Arm A: Paclitaxel + Pembrolizumab Arm B: Capecitbaine + Pembrolizumab |
ORR 29% (95% CI 10–61) vs 43% (18–71) PFS 83 vs 155 days |
|
IMPRIME 1 |
O'Day | 2020 | Phase II | > First line | Metastatic | Any | 44 | Odetiglucan + Pembrolizumab |
ORR 15.9% (95% CI 4.9–29.4) DCR 54.5% (40.1–68.3) 12 mo OS 57.6% (42.4–72.8) mOS 16.4 mo (11.1–23.9) |
| Atezolizumab | |||||||||
| NCT01633970 | Adams | 2019 | Phase Ib | Any | Metastatic | Any | 33 | Atezolizumab + Nab-Paclitaxel |
ORR 39.4% (95% CI 22.9–57.9) DCR 51.5% (33.5–69.2) PFS 5.5 mo (5.1–7.7) OS 14.7 mo (10.1-NE) |
|
IMpassion130 |
Schmid | 2018 | Phase III | First line | Metastatic | PD-L1 positive | 902 |
Arm A: Atezolizumab + Nab-Paclitaxel Arm B: Placebo + Nab-Paclitaxel |
PFS 7.2 vs. 5.5 mo, HR 0.80 (95% CI 0.69–0.92) PD-L1 ≥ 1% PFS 7.5 vs. 5.0 mo, HR 0.62 (0.49–0.78) OS 21.3 vs. 17.6 mo, HR 0.84 (0.69–1.02) PD-L1 ≥ 1% OS 25.0 vs. 15.5 mo, HR 0.62 (0.45–0.86) irAE 57.3% vs 41.8% Grade 3 + irAEs 7.5% vs 4.3% |
| NCT01375842 | Emens | 2019 | Phase I | Any | Metastatic | Any | 116 | Atezolizumab |
First-Line ORR 24% (95% CI 8.2–47.2) First-Line OS 17.6 mo (10.2-NE) ≥ Second-Line ORR 6% (2.4–13.4) ≥ Second-Line OS 7.3 mo (6.1–10.8) |
|
COLET |
Brufsky | 2021 | Phase II | First line | Advanced/metastatic | Any | 153 |
Cohort I: Arm A: Paclitaxel + Cobimetinib Arm B: Paclitaxel + Placebo Cohort II: Atezolizumab + Cobimetinib + Paclitaxel Cohort III: Atezolizumab + Cobimetinib + Nab-Paclitaxel |
Cohort I PFS 5.5 vs 3.8 mo, HR 0.73 (95% CI 0.43–1.24) Cohort I Arm A ORR 38.3% (24.40–52.20) Cohort I Arm B ORR 20.9% (8.77–33.09) Cohort II ORR 34.4% (18.57–53.19) Cohort III ORR 29.0% (14.22–48.04) |
| NCT02849496 | Fanucci | 2023 | Phase II | Any | Advanced / metastatic HER2 negative | BRCA1 or BRCA2 mutant | 78 |
Arm A: Atezolizumab + Olaparib Arm B: Placebo + Olaparib |
Overall PFS 7.67 (95% CI 5.6–10) vs. 7.0 mo (5.5–11.5) (p = 0.92) OS 26.5 (19.2-NE) vs 22.4 mo (16.6–31.3) (p = 0.3) |
|
IMpassion131 |
Miles | 2021 | Phase III | First line | Metastatic | Any | 651 |
Arm A: Atezolizumab + Paclitaxel Arm B: Placebo + Paclitaxel |
ITT PFS 5.7 vs. 5.6 mo, HR 0.86 (95% CI 0.70–1.05) ITT OS 19.2 vs. 22.8 mo, HR 1.12 (0.88–1.43) PD-L1 ≥ 1% PFS 6.0 vs. 5.7 mo, HR 0.82 (0.60–1.12) PD-L1 ≥ 1% OS 22.1 vs. 28.3 mo, HR 1.11 (0.76–1.64) irAE 62% vs 53% |
| NCT03829501 | Patel | 2021 | Phase I/II | Heavily pretreated | Metastatic solid malignancy, including TNBC | Any | 69 | Atezolizumab + KY1044 | One CR, one PR |
|
IMpassion132 |
Dent | 2024 | Phase III | First relapse | Advanced, Early-Relapsing TNBC | Any | 354 |
Arm A: Atezolizumab with Gemcitabine/Carboplatin or Capecitabine Arm B: Placebo with Gemcitabine/Carboplatin or Capecitabine |
PD-L1 OS 12.1 vs. 11.2 mo, HR 0.93 (95% CI 0.73–1.20) OS 10.4 vs. 9.8 mo, HR 0.94 (0.76–1.18)* |
|
COUPLET |
Kristeleit | 2024 | Phase Ib/II | > First line | Metastatic | BRCA1 or BRCA2 mutant or BRCAwt/LOH high | 5 | Atezolizumab + Rucaparib |
Safety: 2 of 5 patients experienced grade 3 or 4 AE ORR 40% (95% CI 5–85%), two PR |
|
ENCORE-602 |
O'Shaughnessy | 2020 | Phase II | Third line | Metastatic (TNBC or HR + /HER2 +) | Any | 81 |
Arm A: Atezolizumab + Entinostat Arm B: Atezolizumab + Placebo |
PFS 1.68 vs 1.51 mo, HR 0.89 (95% CI 0.53–1.48) |
|
ATRACT1B |
Gion | 2023 | Phase II | First line | Advanced/metastatic | Any | 100 | Atezolizumab + Bevacizumab + Paclitaxel |
PFS 11.0 mo (95% CI 9.0–13.2) ORR 63% CBR 79% |
|
IPATunity170 + IPATunity130 + CO40151 |
Schmid | 2024 | Phase Ib-III | First line | Advanced/metastatic | Any | 317 | Atezolizumab + Ipatasertib + Paclitaxel/Nab-Paclitaxel + |
ORR 44%–63% mPFS 5.4–7.4 mo mDOR 5.6–11.1 mo mOS 15.7–28.3 mo |
| Avelumab | |||||||||
|
JAVELIN |
Dirix | 2018 | Phase Ib | Heavily Pretreated | Metastatic | Any | 58 | Avelumab |
Overall ORR 5.2% PD-L1 ≥ 1% ORR 22.2% |
| Camrelizumab | |||||||||
|
FUTURE Arm C |
Liu | 2023 | Phase II | Heavily pretreated | Metastatic | Immunomodulatory | 46 | Camrelizumab + Nab-Paclitaxel |
ORR 43.5% (95% CI 28.9–58.9) mPFS 4.6 mo (3.4–5.9) mOS 16.1 mo (11.7–20.5) mDOR 8.6 mo (1.2–19.7) |
|
FUTURE-C-PLUS |
Chen | 2022 | Phase II | First line | Advanced/metastatic | Immunomodulatory | 48 | Camrelizumab + Famitinib + Nab-Paclitaxel |
ORR 81.3% (95% CI 70.2–92.3) mPFS 13.6 mo (8.4–18.8) mDOR 14.9 mo (NC–NC) |
|
FUTURE-SUPER |
Fan | 2024 | Phase II | First line | Advanced/metastatic | Immunomodulatory | 139 |
Arm A: Camrelizumab + Famitinib + Nab-Paclitaxel Arm B: Nab-Paclitaxel |
PFS 15.1 vs. 6.5 mo, HR 0.46 (95% CI 0.25–0.85) |
| Durvalumab | |||||||||
|
MEDIOLA |
Domchek | 2020 | Phase I/II | > Third line | Metastatic HER2- (TNBC or HR +) | BRCA1 or BRCA2 mutant HER2-negative | 34 | Dulvalumab + Olaparib |
ORR 63.3% (95% CI: 48.9–80.1) DCR at 12 weeks 80% (90% CI: 64.3–90.0) DCR at 28 weeks 50% (90% CI: 33.9–66.1) |
|
SAFIR02-BREAST IMMUNO |
Bachelot | 2021 | Phase II | First line maintenance | Metastatic | Any | 82 |
Arm A: Durvalumab Arm B: Chemotherapy |
mOS 14.0 vs 21.1 mo, HR 0.54 (95% CI 0.30–0.97) PD-L1 ≥ 1% mOS 27.3 vs. 12.1 mo, HR 0.37 (0.12–1.13) |
|
DORA |
Tan | 2024 | Phase II | First Line maintenance | Advanced/metastatic | Any | 45 |
Arm A: Olaparib Arm B: Durvalumab + Olaparib |
PFS 4.0 (95% CI 2.6–6.1) vs 6.1 mo (3.7–10.1) CBR 44% (23–66) vs 36% (17–59) |
| Nivolumab | |||||||||
|
TONIC |
Voorwerk | 2019 | Phase I/II | Heavily pretreated | Metastatic | Any | 67 |
Arm A: waiting period then Nivolumab Arm B: irradiation then Nivolumab Arm C: Cyclophosphamide then Nivolumab Arm D: Cisplatin then Nivolumab Arm E: Doxorubicin then Nivolumab |
Overall ORR 20% Arm A ORR: 17% Arm B ORR: 8% Arm C ORR: 8% Arm D ORR: 23% Arm E ORR: 35% |
|
WJOG9917B NEWBEAT |
Ozaki | 2022 | Phase II | First line | Metastatic | Any | 17 | Bevacizumab + Nivolumab + Paclitaxel |
ORR 59% PFS 7.8 mo |
|
MARIO-1 |
Hong | 2023 | Phase I | Previously treated | Metastatic | Any | 29 | Part F: Eganelisib + Nivolumab |
ORR 7%; one CR, one PR DCR 30% |
| Toripalimab | |||||||||
|
TORCHLIGHT |
Jiang | 2024 | Phase III | First line | Advanced/metastatic | Any | 531 |
Arm A: Nab-Paclitaxel + Toripalimab Arm B: Nab-Paclitaxel + Placebo |
PD-L1 CPS ≥ 1 PFS 8.4 vs. 5.6 mo, HR 0.65 (95% CI 0.470–0.906) ITT PFS 8.4 vs. 6.9 mo, HR 0.77 (0.602–0.994) PD-L1 CPS ≥ 1 OS 32.8 vs. 19.5 mo, HR 0.62 (0.414–0.914) ITT OS 33.1 vs. 23.5 mo, HR 0.69 (0.513–0.932) |
| LAG-3 modulation | |||||||||
| NCT00349934 | Brignone | 2010 | Phase I/II | First line | Metastatic | Any | 30 | Eftilagimod + Paclitaxel | ORR 50% |
| NCT02460224 | Lin | 2024 | Phase II | > First line | Advanced/Metastatic | Any | 56 |
Arm A: Leramilimab + Spartzlizumab (q3W) Arm B: Leramilimab + Spartzlizumab (q4W) |
ORR 9.5%; response only in PD-L1 positive |
| Dual Immunotherap | |||||||||
| Santa Maria | 2018 | Pilot study | > First line | Metastatic | Any | 7 | Durvalumab + Tremelimumab |
ORR 43% Hepatotoxicity major AE |
|
|
DART/SWOG S1609 Cohort 36 |
Adams | 2022 | Phase II | Any | Metaplastic | Any | 17 | Ipilumumab + Nivolumab |
ORR 18% 3 responders all had ongoing response at 28 + mo. All responders had adrenal insufficiency |
|
NUMBUS |
Barrosa-Sousa | 2020 | Phase II | Any | Metastatic HER2- | TMB-High | 31 | Ipilumumab + Nivolumab |
ORR 13.3% PFS 1.4 mo (95% CI 1.3–9.5) OS 8.8 mo (95% CI 4.2–NE) Exploratory: TMB ≥ 14 mut/Mb ORR 60% vs 9–14 ORR 4% (p = 0.01) No grade 4/5 toxicities |
| NCT03650894 | Page | 2023 | Phase II | First or Second line | Metastatic HER2- | Any | 30 | Ipilumumab + Nivolumab + Bicalutamide |
HR+ /Androgen Receptor Negative CBR 8% HR-/AR+ CBR 33% |
| NCT02453620 | Roussos Torres | 2024 | Phase Ib | > First line | Metastatic | Any | 12 | Entinostat + Ipilumumab + Nivolumab |
No DLT ORR 40% (95% CI 12.2–73.8) CBR 60% (95% CI 26.2–87.8) |
| Selected Upcoming Clinical Trials | |||||||||
|
BELLA |
Phase II | First Relapse | Advanced, Early-Relapsing | PD-L1+ | 31 | Atezolizumab + Bevacizumab + Carboplatin + Gemcitabine | PFS | ||
|
EL1SSAAR |
Phase IIIb | First line | Advanced/metastatic | PD-L1+ | 184 | Atezolizumab + Nab-Paclitaxel | Safety | ||
|
MARIO-3 |
Phase II | First line | Advanced/metastatic | Any | 91 |
Atezolizumab + Bevacizumab + Eganelisib + Nab-paclitaxel |
CRR | ||
|
ADAGIR |
Phase II | Heavily pretreated | Metastatic solid tumors, including TNBC | Any | 247 | Atezolizumab + BDB001 + stereotactic radiation | DCR | ||
Results in bold are statistically significant
Atezolizumab as monotherapy in the first-line setting for a/mTNBC was found to have an ORR of 24% [38] and an ORR of 39.4% when combined with nab-paclitaxel after 0–2 lines of prior therapy [39]. The phase III IMpassion130 study randomized 902 patients with advanced PD-L1+ TNBC at least one year from curative-intent therapy or de novo metastatic disease to nab-paclitaxel with atezolizumab or placebo. Patients who received nab-paclitaxel with atezolizumab had a significant PFS benefit compared to patients who received nab-paclitaxel with placebo (7.2 months vs. 5.5 months, HR 0.80) in the intention-to-treat (ITT) analysis and in the PD-L1 > 1% subgroup (7.5 months vs. 5.0 months, HR 0.62) [11]. While these results did not translate into a significant OS benefit in the ITT analysis, a median 9.5-month OS benefit was seen in the PD-L1 > 1% subgroup (HR 0.62, significance not evaluated due to hierarchical testing plan) [11].
Atezolizumab in combination with other chemotherapy backbones has found less success. The IMpassion131 phase III trial investigated paclitaxel + atezolizumab or placebo in 651 patients, with no difference in PFS or OS in either the ITT to PD-L1 subgroup [40], though it is noted that the control group experienced an unprecedented OS of 22.8 months, which may have impacted the results. Overall, the reasons for discrepant results between IMpassion130 and IMpassion131 are unclear. Possible explanations include different formulations between paclitaxel and nab-paclitaxel, pretreatment with steroids in the IMpassion131 study, or differences in the tumor microenvironment in each study population [40, 41]. Though the FDA initially approved atezolizumab based on the results of IMpassion 130, the approval was removed after the IMpassion 131 results were reported.
The possible benefit of atezolizumab in early-relapsing TNBC, a high-risk population defined as relapse less than twelve months after last chemotherapy or surgery for early-stage disease, was evaluated in the phase III IMpassion132 study. In this study, 354 patients without prior immunotherapy were randomized to chemotherapy of physician’s choice plus either atezolizumab or placebo. Initially, patients with any PD-L1 status were included, which was later restricted to patients with PD-L1 > 1%. Two thirds of patients had a DFI of < 6 months. No difference in median disease-free interval or OS was seen [42]. These results, in combination with the exploratory analysis of relapse < 12 months in KEYNOTE-355, suggest that some patients with quickly relapsing TNBC may have an intrinsic resistance to immunotherapy. However, as discussed in greater depth below, the standard of care for first-line therapy in early-stage TNBC now includes immunotherapy, and as such few patients will reach the early-relapsed setting without prior immunotherapy.
The Atract1B phase II trial challenged the view that ICIs only have benefit in PD-L1 positive disease. This trial investigated paclitaxel, atezolizumab and bevacizumab (a VEGF-inhibitor) in the first line for advanced TNBC, with 97% of patients having PD-L1 negative disease. Median PFS was 11.0 months, with an ORR of 63%, including thirteen complete responses and 50 partial responses [43]. Bevacizumab in combination with nivolumab and paclitaxel was investigated in the first line of patients with metastatic HR + /HER- or TNBC in the NEWBEAT phase II trial, with an ORR of 70% (59% in TNBC, 74% in HR + /HER-) [44].
Clinical trials of dual ICI therapy in mTNBC have shown some clinical benefit but also raise concerns of higher rates of toxicity. Durvalumab in combination with tremelimumab, an anti-CTLA-4 antibody, was found to have an ORR of 43% in TNBC in a pilot study of 7 patients [45]. The DART/SWOG S1609 phase II trial of ipilumumab with nivolumab found an ORR of 18%, though all patients who had an initial response continued to respond nearly 3 years later. All responders developed adrenal insufficiency [46].
The use of Poly (ADP-ribose) polymerase inhibitors (PARPi) in combination with ICIs for patients with BRCA-mutated disease has shown promise in phase II trials. Olaparib with durvalumab had a 63.3% ORR in patients with heavily pretreated BRCA-mutant HER2-negative metastatic breast cancer (mBC) with an 80% 3-month disease control rate [47]. However, a trial of olaparib with or without atezolizumab in BRCA-mutant a/m TNBC found no PFS or OS benefit for combination therapy but did have more adverse effects [48]. The TOPACIO/KEYNOTE-162 trial evaluated patients with a/mTNBC with any PD-L1 status. Patients were treated with niraparib and pembrolizumab, with higher ORR seen in patients with BRCA-mutated disease (47%) compared to BRCA-wild type (11%), with updated PFS and OS not yet reported [49].
Optimizing maintenance regimens for patients with initial response to chemotherapy is an active area of research. The DORA phase II study evaluated the role of maintenance olaparib with or without durvalumab in patients with aTNBC who responded to platinum-based chemotherapy. Patients experienced a median PFS of 4.0 months versus 6.1 months, with benefit seen regardless of BRCA or PD-L1 status [50]. The KEYLYNK-009 phase II trial investigated the efficacy of maintenance pembrolizumab and olaparib compared to pembrolizumab and chemotherapy in patients with recurrent inoperable or mTNBC who responded to induction pembrolizumab and chemotherapy. No difference in PFS after completion of induction therapy (5.5 months vs. 5.6 months) or OS (25.1 months vs. 23.4 months) was seen, though there was a trend toward improved PFS for patients with BRCA-mutated disease [51]. Interestingly, no improvement in patient-reported outcomes was seen for patients who were maintained on a chemotherapy-free regimen in comparison with standard pembrolizumab and chemotherapy [52]. Another study of maintenance immunotherapy with durvalumab in comparison to chemotherapy found a 7.1 month OS benefit with durvalumab in an exploratory analysis of patients with TNBC [53]. Overall, these studies suggest an emerging role for chemotherapy-free maintenance for patients who have an initial response to chemotherapy.
Sacituzumab govitecan (SG), an ADC consisting of an anti-TROP2 antibody linked to a topoisomerase I inhibitor, was compared to physician’s choice of treatment in the second or third line of a/m TNBC in the phase III ASCENT trial, with a significant improvement in PFS (4.8 months vs 1.7 months, HR 0.41) and a 4.9 month absolute OS benefit (11.8 vs 6.9 mo, HR 0.51), leading to early termination for efficacy [54, 55]. SG gained FDA approval for mTNBC after two prior therapies in April 2020 [54]. SG in combination with atezolizumab is being compared to the IMPassion030 regimen of nab-paclitaxel + atezolizumab in the front line for PD-L1+ a/m TNBC in the MORPHEUS-pan BC trial, with preliminary data showing an encouraging ORR of 76.7% versus 66.7% and immature PFS data of 12.2 months versus 5.9 months (HR 0.27) [7].
Datopotamab deruxtecan (Dato-DXd) is another anti-TROP2 antibody linked to a topoisomerase inhibitor that has shown activity in TNBC. This ADC was investigated in the phase I TROPION-Pan Tumour 01 study, which found an ORR of 31.8% in patients with heavily pretreated a/m TNBC [56]. Dato-DXd is being studied in combination with an ICI in the phase Ib/II BEGONIA trial, with arm 7 of this trial investigating Dato-DXD with durvalumab in the first line for a/m TNBC. Early results found an ORR of 79% ORR, with 47% of patients having an ongoing response at 11.7 months and response seen regardless of level of PD-L1 expression [57]. Other ADCs being investigated in mTNBC include enfortumab vedotin, which found an ORR of 195 and PFD of 3.5 months in heavily-pretreated mTNBC in the phase II EV-202 trial [58]. Another anti-TROP2 ADC of note is Sacituzumab tirumotecan, which was found to significantly improved OS versus chemotherapy of physician’s choice in the second line for a/m TNBC [59].
Early Stage TNBC
Neoadjuvant treatment of early stage TNBC aims to reduce the extent of surgical excision for operable tumors or convert inoperable tumors to operable tumors. The treatment goal is to attain a pathological complete response (pCR), defined as the eradication of invasive cancer from the breast and lymph nodes (ypT0/is, ypN0) at the time of surgery [60]. Patients who achieve pCR experience significantly improved disease-free survival (DFS) and OS outcomes, thus it is a common clinical end point in neoadjuvant trials [61]. Patients failing to achieve pCR are classified according to the degree of residual cancer burden (RCB). The degree of RCB is also prognostic, with higher RCB scores prognostic for worse event-free survival (EFS) [62].
Combining chemotherapy with ICI increases the rate of pCR for patients with TNBC in comparison to standard neoadjuvant chemotherapy regimens. Unlike the metastatic setting in which PD-L1 is predictive of response to ICIs, the development of predictive biomarkers in the neoadjuvant setting is elusive. As such, there is currently no indication for PD-L1 testing outside of clinical trials as the current evidence shows benefit for ICIs for all early TNBC in the neoadjuvant setting, regardless of PD-L1 status.
Pembrolizumab is FDA approved for neoadjuvant therapy of early-stage TNBC. The phase 1b KEYNOTE-173 trial demonstrated safety and preliminary efficacy of pembrolizumab in the first line with neoadjuvant chemotherapy for early-stage, high-risk TNBC, with higher PD-L1 CPS and TILs significantly associated with higher rates of pCR [16, 63]. Data from the phase II I-SPY2 trial established the benefit of pembrolizumab added to standard neoadjuvant chemotherapy. Twenty-nine patients with TNBC > 2.5 cm and any nodal status were included. Patients in the experimental arm were treated with pembrolizumab with weekly paclitaxel followed by dose-dense (dd) doxorubicin and cyclophosphamide (AC), with estimated pCR rates of 60% vs. 22% for patients treated with paclitaxel followed by AC [64]. Another I-SPY2 regimen evaluated paclitaxel with pembrolizumab in an anthracycline-free regimen, but did not reach target pCR rates [65].
The landmark phase III, double-blind KEYNOTE-522 trial evaluated 1174 patients with cT1, N1-2 or cT2-4, N0-2 TNBC with the goal of investigating the efficacy of neoadjuvant and adjuvant pembrolizumab. Patients were randomized 2:1 to receive neoadjuvant pembrolizumab or placebo with paclitaxel and carboplatin (PC) followed by AC or epirubicin and cyclophosphamide (EC) (every-3-week dosing). After surgery, patients in the study group continued pembrolizumab to complete one total year of treatment. The pembrolizumab regimen was associated with a pCR rate of 64.8% vs. 51.2% in the placebo group, representing a treatment difference of 13.6 percent [66]. Moreover, the risk of recurrence was significantly lower in the pembrolizumab group (HR 0.63). More benefit was seen for patients with node-positive disease (treatment difference 20.6% [8.9–31.9] vs. 6.3% [− 5.3–18.2]), with no difference in response based on PD-L1 status. An updated 5-year EFS showed continued benefit for pembrolizumab with EFS rates of 81.2% versus 72.2% [67]. Improved EFS was seen even for patients with RCB-I and RCB-II after neoadjuvant chemotherapy, though patients with RCB-III after neoadjuvant therapy with pembrolizumab had worse 3-year EFS than patients who received placebo (26.2% vs. 34.6%, HR 1.24 [0.69–2.23]) [68]. This decrease in survival was driven by a higher rate of local recurrence. Five-year OS was 86.6% versus 81.7% [69]. Immune-related adverse effects occurred in 33.5% of patients receiving pembrolizumab, most commonly hypothyroidism (15.1%), skin reactions (5.7%) and adrenal insufficiency (2.6%) [70]. Based on this study, the FDA granted approval for neoadjuvant and adjuvant pembrolizumab in 2021. The KEYNOTE-522 regimen is the current standard of care for early stage TNBC.
Given the high rates of pCR and EFS seen with the Keynote-522 regimen of pembrolizumab with PC + AC, the NeoPACT trial evaluated the role of de-escalating anthracyclines in the neoadjuvant setting in an effort to decrease anthracycline toxicities. In this phase II trial, patients receiving carboplatin with docetaxel and pembrolizumab had a pCR rate of 58%. Patients achieving pCR experienced an impressive 3-year EFS of 98%, with 3-year EFS 86% overall [71]. The currently-enrolling phase III SWOG2212 / SCARLET (NCT05929768) trial will compare the KEYNOTE-522 regimen with the NeoPACT regimen in patients with T2-4, N0, M0 or T1-3, N1-2, M0 TNBC with a primary endpoint of EFS, with the goal to establish an optimal chemotherapy backbone.
Neoadjuvant atezolizumab has also shown promise in TNBC. Neoadjuvant atezolizumab demonstrated a pCR benefit when added to an anthracycline-free regimen of carboplatin and paclitaxel in a phase II trial of 67 patients [72]. The phase III IMpassion031 study of 333 patients with cT2-4, N0-3 TNBC found a significant pCR benefit for neoadjuvant atezolizumab with nab-paclitaxel followed by ddAC (pCR 58% vs. 41%). In the PD-L1 cohort, rates of pCR were significantly increased with atezolizumab (68.8% vs. 49.3%) [73]. EFS data is pending. The NeoTRIPaPDL1 / Michelangelo phase III study evaluated carboplatin and nab-paclitaxel with and without atezolizumab followed by surgery and adjuvant AC in 280 patients, without a pCR benefit seen in the atezolizumab group (48.6% vs. 44.4%) [74]. Unlike the KEYNOTE-522 study, the NeoTRIP study included patients with N3 disease, with 88% of all patients having node-positive disease, which may explain lower overall rates of pCR. A multivariate analysis found PD-L1 expression to significantly increase rates of pCR (OR 2.08, 95% CI 1.64–2.65) [74]. EFS data is pending.
Based on the OS benefit seen with maintenance durvalumab in mTNBC [53], the phase II GeparNuevo trial investigated neoadjuvant durvalumab with chemotherapy. Patients with cT2-4d, N0-3 TNBC were randomized to a window period of either durvalumab or placebo, followed by the same treatment combined with nab-paclitaxel, followed by dd EC with durvalumab or placebo. While there was no significant pCR benefit (53% vs. 44%, OR 1.45 [0.80—2.63] [22], durvalumab did show an increased 3-year DFS (85.6% vs. 77.2%, HR 0.48, [0.24–0.97]), supporting the hypothesis of long-term benefits of early ICI without adjuvant ICI [75]. In a subgroup analysis, TMB > 10% and the presence of TILs predicted treatment response, with pCR rates of 82% seen in patients with high TMB and TILs in comparison to pCR rates of 28% in patients with low TMB and TILs [18]. Data from I-SPY2 supports further investigation of neoadjuvant paclitaxel, durvalumab and olaparib followed by AC in early-stage TNBC, as this trial found pCR rates of 47% compared to 27% in the standard therapy arm [76].
Biomarkers to identify patients who are likely to respond to neoadjuvant immunotherapy are needed. Novel biomarkers such as DetermaIO utilize RNA sequencing to produce a score which predicts pCR of early stage TNBC when treated with immunotherapy [77]. A pooled analysis of 343 patients treated in one of 5 immunotherapy arms of the I-SPY2 trial identified an immune classifier, called ImPrintTN, in hopes of identifying which patients with early-stage TNBC may not benefit from immunotherapy. In the 28% of patients who were ImPrintTN+ , 74% of patients achieved a pCR. In patients who were ImPrintTN-, only 16% of patients achieved a pCR [78]. Further validations of this biomarker is needed, but it suggests that a proportion of patients with early-stage TNBC may be able to avoid immunotherapy when it is unlikely to have a benefit.
Neoadjuvant ADCs in early TNBC is an area of active research. The phase II NeoSTAR trial found a pCR rate of 30% with SG monotherapy [79]. The SOLTI TOT-HER3 trial studied neoadjuvant patritumab deruxtecan as a single dose in a window-of-opportunity phase I trial found an ORR of 35% [80].
The Neo-N phase II trial investigated the effect of lead-in vs. concurrent neoadjuvant immunotherapy. Patients with early-stage TNBC were randomized to A) lead-in nivolumab followed by nivolumab with carboplatin and paclitaxel, or B) up-front nivolumab, carboplatin and paclitaxel followed by nivolumab monotherapy. No difference in pCR was seen between the two arms (50.9% vs. 54.5%) [81]. Notably, 66.7% of patients with high TILs and 70.6% of patients with PD-L1 positive disease achieved pCR, delineating a potentially efficacious anthracycline-sparing neoadjuvant regimen [81]. EFS data is pending.
Dual neoadjuvant ICI therapy with combined PD-1 and CTLA-4 agents in early-stage TNBC has been investigated in two phase II trials. The BELLINI trial treated 31 patients with TILs ≥ 5% with neoadjuvant nivolumab ± ipilumumab followed by chemotherapy or surgery. Evidence of immune activation (defined as doubling of CD8 + T cell or IFN-γ) was seen in 58% of patients [82]. Of the three patients who underwent surgery without neoadjuvant chemotherapy, one pCR and one near-pCR was seen. All patients with radiographic response had TILs > 40% [82]. The CHARIOT trial is a phase II, single arm trial of patients with stage III TNBC with RCB ≥ 15 mm or ≥ 10 mm with node-positive disease after neoadjuvant AC. Patients were treated with neoadjuvant paclitaxel, ipilumumab and nivolumab followed by adjuvant nivolumab. In this high-risk population, overall pCR rates were 24.4%, with pCR rates of 44.4% in the PD-L1 positive subset [83]. Recently presented EFS and OS data showed a remarkable 100% 3-year EFS and OS in the PD-L1 and/or TIL high subset, even though the minority of patients achieved a pCR [83, 84].
Investigations into adjuvant ICIs in TNBC have had limited success. The phase III IMpassion030 / ALEXANDRIA study evaluated the effect of adjuvant atezolizumab with paclitaxel followed by atezolizumab with AC or EC compared with chemotherapy alone in 2199 patients with stage II-III TNBC who underwent upfront surgery. After a median follow up of 25.3 months, the study was halted after a futility analysis found the study was unlikely to meet its primary endpoint of improved iDFS vs. chemotherapy with a HR of 1.12 (0.87–1.45) [85]. When this study was designed, it was unclear if neoadjuvant vs. adjuvant chemotherapy with immunotherapy would provide better outcomes for patients with TNBC. Mounting evidence now points towards focusing on upfront systemic treatment including immunotherapy for early-stage TNBC, with a tailored approach to adjuvant immunotherapy (Table 2).
Table 2.
Current and upcoming clinical trials of immune checkpoint inhibitors in early triple negative breast cancer
| Trial name: | Primary author: | Year: | Study design: | Line of therapy | Stage | # Patients: | Drug regimen | Results |
|---|---|---|---|---|---|---|---|---|
| Pembrolizumab | ||||||||
|
KEYNOTE-173 |
Schmid | 2020 | Phase Ib | Neoadjuvant | cT1c, N1-N2; T2-T4c, N0-N2 | 60 | Nab-Paclitaxel + Pembrolizumab ± Carboplatin then AC |
Overall pCR 60% (range 49%–71%) PD-L1 CPS associated with higher rate of pCR (p = 0.0127) sTILs associated with higher rate of of pCR (p = 0.0085) |
|
I-SPY2 |
Nanda | 2020 | Phase II | Neoadjuvant | cT2-4d, N0-3 | 29 |
Arm A: Paclitaxel + Pembrolizumab then AC → ± adjuvant Pembrolizumab Control: Paclitaxel then AC |
pCR 60% (95% CI 44–75) vs 22% (13–30) |
|
I-SPY2 |
Liu | 2019 | Phase II | Neoadjuvant | T ≥ 2.5 cm; HER2 negative | 73 |
Arm A: Paclitaxel + Pembrolizumab Control: Paclitaxel then AC |
pCR 21% (95% CI 9–32) vs 20% (15–25) |
|
I-SPY2 |
Chien | 2021 | Phase II | Neoadjuvant | T ≥ 2.5 cm | 29 |
Arm A: Paclitaxel + Pembrolizumab + SD-101 then AC + Pembrolizumab Control: Paclitaxel then AC |
pCR 44% vs. 28% |
|
NCT00036488 KEYNOTE-522 |
Schmid | 2020 | Phase III | Neoadjuvant + Adjuvant | cT1N1-2, cT2-4, N0-2 | 1174 |
Arm A: Carboplatin + Paclitaxel + AC/EC + Pembrolizumab → adjuvant Pembrolizumab Arm B: Carboplatin + Paclitaxel + AC/EC + placebo → adjuvant placebo |
pCR 64.8% (95% CI 59.9–69.9) vs 51.2% (44.1–58.3) PD-L1 CPS ≥ 1 pCR 68.9% vs 54.9% Risk of Recurrence HR = 0.63 (0.48–0.82) 5-year EFS 81.2% vs 72.2%; HR 0.63 (0.49–0.81) 5-year OS 86.6% (84.0–88.8) vs 81.7% (77.5–85.2) |
|
NeoPACT |
Sharma | 2022 | Phase II | Neoadjuvant | Stage I-III | 117 | Carboplatin + Docetaxel + Pembrolizumab |
pCR 58% (95% CI: 48–67) 3-year EFS overall 86% EFS pCR subgroup: 98% EFS no pCR subgroup 68% |
|
NeoIRX |
Page | 2023 | Phase II | Neoadjuvant | Stage II/III | 12 |
Arm A: Pembrolizumab + IRX-2 then AC/T + pembrolizumab Arm B: Pembrolizumab then AC/T + Pembrolizumab |
pCR 83% vs. 33%; terminated early due to withdrawal of support for IRX-2 |
| Atezolizumab | ||||||||
|
IMpassion031 |
Mittendorf | 2020 | Phase III | Neoadjuvant | cT2-4, N0-3 | 333 |
Arm A: Atezolizumab + AC + Nab-paclitaxel Arm B: placebo + AC + Nab-paclitaxel |
ITT pCR 57.6% (95% CI 50–65) vs 41.1% (34–49), Difference 17% (6–27) PD-L1 ≥ 1 pCR 68.8% (57–79) vs 49.3% (38–61), Difference 20% (4–35) |
|
NCT002620280 NeoTRIPaPDL1/Michaelangelo |
Gianni | 2022 | Phase III | Neoadjuvant | cT1N1-3; cT2-4d, N0-3 | 280 |
Arm A: Atezolizumab + Carboplatin + Nab-paclitxel → adjuvant AC/EC Arm B: Carboplatin + Nab-paclitaxel → adjuvant AC/EC |
pCR 48.6% vs. 44.4%, OR 1.18 (95% CI 0.74–1.89) PD-L1 + expression influenced rate of pCR, OR 2.08 (1.64–2.65) |
| NCI10013 | Ademuyiwa | 2022 | Phase II | Neoadjuvant | cT2-4, N0-3 | 67 |
Arm A: Atezolizumab + Carboplatin + Paclitxel Arm B: Carboplatin + Paclitxel |
pCR 55.6 vs 18.8% |
|
IMpassion030/ALEXANDRIA |
Ignatiadis | 2023 | Phase III | Adjuvant | Stage II-III | 2199 |
Arm A: Atezolizumab + ddAC + Paclitaxel Arm B: ddAC + Paclitaxel |
iDFS HR 1.12 (95% CI 0.87—1.45) iDFS PD-L1 + 1.03 (0.75—1.42) |
| Durvalumab | ||||||||
| NCT02489448 | Foldi | 2021 | Phase I/II | Neoadjuvant | cT1-3, N0-3 | 59 | Durvalumab + AC + Nab-Paclitaxel |
pCR 44% PD-L1 ≥ 1% pCR 55% |
|
GeparNUEVO |
Loibl | 2019 | Phase II | Neoadjuvant | cT2-4d, N0-3 | 174 |
Arm A: Durvalumab window then Nab-Paclitaxel + Durvalumab then EC + Durvalumab Arm B: Placebo window then Nab-Paclitaxel + Placebo then EC + Placebo |
pCR 53% vs. 44%, OR 1.45 (95% CI 0.80–2.63) 3-year iDFS 85.6% vs. 77.2%, HR 0.48 (0.24–0.97) 3-year DDFS 91.7% vs. 78.4%, HR 0.31 (0.13–0.74) 3-year OS 95.2% vs. 83.5%, HR 0.24 (0.08–0.72) |
|
I-SPY2 |
Pusztai | 2021 | Phase II | Neoadjuvant | Stage II-III | 21 |
Arm A: Durvalumab + AC + Olaparib + Paclitaxel Arm B: AC + Paclitaxel |
pCR 47% vs 27% |
| Nivolumab | ||||||||
|
BCT1902 Neo-N |
Loi | 2023 | Phase II | Neoadjuvant | cT1cN1; cT2-4, N0-1 | 110 |
Arm A: Nivolumab Lead-In then Nivolumab + Carboplatin + Paclitaxel Arm B: Nivolumab + Carboplatin + Paclitaxel then Nivolumab alone |
pCR 50.9% vs. 54.5% sTIL high vs low: 66.7% vs 45.7% PD-L1 positive vs negative: 70.6% vs 33.3% |
| Dual Immunotherapy | ||||||||
|
EudraCT: 2018-004188-30 BELLINI |
Nederlof | 2022 | Phase II | Neoadjuvant | Stage I-III, TILs ≥ 5% | 31 |
Arm A: Nivolumab then chemotherapy or surgery Arm B: Nivolumab + Ipilumumab then chemotherapy or surgery |
Immune Activation = Doubling CD8+ T-cells or IFN-g seen in 58% of patients. Of 3 patients who went for surgery without neoadjuvant chemo, 1 pCR and 1 near-pCR |
|
BCT1702 CHARIOT |
Loi | 2022 | phase II | Neoadjuvant + Adjuvant | Stage III with ≥ 15 mm RD or 10 mm RCB + one positive lymph node after AC × 4 | 34 | Ipilimumab + Nivolumab + Paclitaxel → adjuvant Nivolumab |
pCR 24.2% PD-L1+ pCR 37.5% 3-year EFS 61.3% PD-L1+ 3-year EFS 100% 3-year OS 71.9% PD-L1+ 3-year OS 100% |
| Selected Upcoming Clinical Trials | ||||||||
|
SWOG2212/SCARLET |
Phase III | Neoadjuvant | T2-4, N0, M0 or T1-3, N1-2, M0 with high TILs,PD-L1 | 2400 |
Arm A: Carboplatin + Paclitaxel + AC + Pembrolizumab → adjuvant Pembrolizumab Arm B: Carboplatin + Docetaxel + Pembrolizumab |
EFS | ||
|
SWOG1418/NRGBR0006 |
Phase III | Adjuvant | ≥ 1 cm or N+ RCB | 1000 |
Arm A: Pembrolizumab Arm B: Observation |
iDFS in 1) all randomized patients and 2) PDL-1+ patients | ||
|
A-BRAVE |
Phase III | Adjuvant | RCB | 335 |
Arm A: Avelumab Arm B: Observation |
DFS | ||
|
Optimice-pCR/A012103 |
Phase III | Adjuvant | achieved pCR | 1295 |
Arm A: Pembrolizumab Arm B: Observation |
RFS | ||
|
GeparDouze |
Phase III | Neoadjuvant + Adjuvant | Stage II-III | 1550 |
Arm A: Atezolizumab + Carboplatin + Paclitaxel then AC or EC → adjuvant Atezolizumab Arm B: Placebo + Carboplatin + Paclitaxel then AC/EC → adjuvant Placebo |
pCR EFS |
||
|
BRE-03 |
Phase I | Neoadjuvant Window of Opportunity | Early-stage | 12 | Lenvatinib + Pembrolizumab | TILs present in biopsy | ||
| NCT05973864 | Phase III | Adjuvant | Early-stage with RCB | 418 |
Arm A: Capecitabine + Pembrolizumab Arm B: Pembrolizumab |
iDFS | ||
| NCT03036488 | Phase III | Neoadjuvant + Adjuvant | Locally Advanced | 1174 |
Arm A: Pembrolizumab + Chemotherapy → adjuvant Pembrolizumab Arm B: Placebo + Chemotherapy → adjuvant Pembrolizumab |
pCR | ||
|
NordicTrip |
Phase III | Neoadjuvant | Stage II-III | 920 |
Arm A: EC + Pembrolizumab then Carboplatin + Paclitaxel + Pembrolizumab Arm B: EC + Capecitabine + Pembrolizumab then Carboplatin + Paclitaxel + Pembrolizumab |
pCR | ||
Results in bold are statistically significant
Vaccines in TNBC and across subtypes
The majority of breast cancer vaccine clinical trials have focused on the metastatic setting. The Theratrope trial was a phase III clinical trial of 1028 patients with any subtype of mBC who were treated with sialyl-TN, a Muc1 epitope, conjugated to keyhole limpet hemocyanin (KLH) protein vs. KLH alone, with a primary endpoint of time to progression [86]. Patients were given a priming dose of cyclophosphamide 3 days prior to vaccine administration. Though the treatment group did produce anti-mucin antibodies, no difference in time to progression or overall survival was seen [86]. Adagloxad simolenin, a Globo-H epitope conjugated to KLH with cyclophosphamide, was evaluated in a phase II trial of patients with mBC [87]. No difference in overall PFS was seen in comparison to the placebo group, though patients who achieved higher anti-GloboH titers did have an improved PFS [87]. The upcoming phase III GLORIA (NCT03562637) trial will investigate this vaccine in patients with TNBC expressing Globo-H in the adjuvant setting [88].
Virus-based breast cancer vaccines have also been investigated in phase I and II studies, with an objective of infecting antigen-presenting cells to enhance the immunologic response against malignant cells. PANVAC is a poxvirus vaccine that encodes transgenes for Muc-1, CEA, and three co-stimulatory molecules [9]. Phase I trials of PANVAC demonstrated safety and immunoreactivity, with one patient with mBC achieving a complete response [9]. The phase II study combined PANVAC with docetaxel, with a nearly-significant improvement in PFS (7.9 months vs. 3.9 months, HR 0.65, p = 0.09) compared to docetaxel alone [89]. While most viral vaccines have focused on heavily pretreated patients, two vaccines have moved into the neoadjuvant space. Pelareorep, a type III reovirus, in combination with paclitaxel, was found to nearly-significantly increase overall survival in the metastatic setting (17.4 months vs. 10.4 months, HR 0.65, p = 0.1) [90]. Pelareorep is now being studied in the neoadjuvant setting in early-stage TNBC, with preliminary data demonstrating effective priming of an adaptive immune response [91]. A recent phase II study of neoadjuvant intra-tumoral talimogene iaherparepvec (T-VEC) + paclitaxel followed by AC in stage II-III TNBC found a pCR rate of 45.9%, with a 2-year DFS rate of 89%, with no recurrences in patients with RCB 0 or 1 [92].
Upcoming clinical trials in TNBC
In the a/m setting, multiple phase III studies are evaluating ADCs with ICI in the first line. For patients with PD-L1+ disease, the upcoming ASCENT-04 (NCT05382286) trial will investigate first-line SG with pembrolizumab vs. treatment of physician’s choice with pembrolizumab for PD-L1+ disease. The TROPION-Breast-05 (NCT06103864) trial will assess Dato-DXd with durvalumab against the KEYNOTE-355 regimen (chemotherapy + pembrolizumab). These studies will determine the optimal first-line therapy for patients with PD-L1+ a/mTNBC. Atezolizumab in combination with ladoratizimab vedotin (LV) is being studied in in the first line for patients with ICI-naïve a/m TNBC in another arm of the phase I/II MORPHEUS trial (NCT03424005).
For patients with PD-L1- a/m TNBC disease, upcoming phase III trials to determine the optimal first-line therapy include the ASCENT-03 (NCT05382299) trial, which is investigating SG compared to treatment of physician’s choice and TROPION-Breast 02 (NCT05374512), which investigates Dato-DXd against treatment of physician’s choice. Phase II studies such as SACI-IO TNBC (NCT04468061) study will assess SG with or without pembrolizumab in the first line while SNGLVA-002 (NCT03310957) trial will investigate first-line LV with pembrolizumab.
Deescalating neoadjuvant chemoimmunotherapy in early TNBC based on high level of TILs is being investigated in the upcoming phase II NeoTRACT (NCT05645380) trial. Patients with TILs ≥ 5% will be treated with carboplatin, docetaxel and pembrolizumab, while patients with low TILs < 5% will receive the standard Keynote-522 regimen.
Several upcoming trials will clarify the role of adjuvant ICIs, with an emphasis on tailoring therapy based on success of neoadjuvant treatment. The Optimice-pCR (NCT05812807) trial is a phase III trial that will clarify whether patients who achieve pCR can be spared adjuvant immunotherapy. Patients with early-stage TNBC who achieved pCR with combination pembrolizumab and chemotherapy will be randomized to adjuvant pembrolizumab vs. observation, with a primary outcome of recurrence-free survival.
For patients with RCB after neoadjuvant therapy, two upcoming phase III trials will evaluate the benefit of one year of adjuvant ICI vs. observation. The SWOG1418 / NRGBR0006 (NCT02954874) will study pembrolizumab in patients with > 1 cm RCB or positive lymph nodes, with co-endpoints of iDFS overall and in a PD-L1+ subgroup. The A-BRAVE (NCT02926196) trial is studying avelumab in either A) patients who underwent upfront surgery followed by adjuvant chemotherapy, or B) patients with RCB after neoadjuvant therapy.
Combinations of ICI with VEGF inhibition is undergoing further investigation, based on the success of the AtractIB study. The BELLA (NCT04739670) phase II trial is investigating atezolizumab, bevacizumab, carboplatin and gemcitabine in patient with early-relapsing PD-L1+ TNBC, while the MARIO-3 (NCT03961698) phase II trial is investigating atezolizumab, bevacizumab, nab-paclitaxel and eganelisib (A PI3Kγ inhibitor) in the first line for a/m TNBC.
Clinical trials evaluating ADCs, with or without ICIs, in the adjuvant setting are also underway. The SASCIA (NCT04595565) phase III trial is investigating adjuvant SG monotherapy in comparison to treatment of physician’s choice of therapy for patients with residual TNBC or HR+ /HER2- disease after neoadjuvant therapy and surgery. Similarly, the ASCENT-05/Optimice-RD (NCT05633654) study is testing SG with pembrolizumab against treatment of physician’s choice in the adjuvant setting. The TROPION-Breast-03 (NCT05629585) trial is investigating adjuvant Dato-Dxd with or without durvalumab versus standard of care therapy for patients with RCB, including both patients who did and did not receive neoadjuvant immunotherapy. These studies will clarify the benefit of adjuvant ICI for patients with RCB-III disease, given the poor outcomes seen in that subgroup in the KEYNOTE-522 study.
In the neoadjuvant setting, the TROPION-Breast-04 (NCT06112379) trial will compare the BEGONIA regimen of Dato-DXd with durvalumab against the KEYNOTE-522 regimen in the neoadjuvant setting for patients with stage II-III TNBC or HER2-low disease, with the hope of de-escalating toxicities of chemotherapy in the curative setting. Cohort 2 of the NeoSTAR (NCT04230109) trial will investigate SG with pembrolizumab in another de-escalated neoadjuvant regimen.
HR positive, HER2 negative breast cancer
The majority of breast cancers express estrogen and/or progesterone receptors, collectively termed hormone receptor positive (HR+). While HR+ disease has been demonstrated to respond, at least initially, to endocrine therapies, investigations into immunotherapy for the most common subtype of cancer have found less success. This may be explained, in part, by lower expression of PD-L1 and TILs, with only 15% of HR+ breast cancer expressing PD-L1 CPS > 10.[30] There are no FDA-approved immunotherapies in HR + breast cancer to date. Nonetheless, efforts are ongoing to optimize treatment regimens with some encouraging results.
Advanced and metastatic HR+ /HER2- breast cancer
The first trials of immunotherapy in HR+ /HER2- disease evaluated single-agent pembrolizumab in heavily pretreated, PD-L1 CPS ≥ 1 mBC with an ORR of 12%[93]. Combination chemotherapy and immunotherapy was investigated in a phase II trial of eribulin with or without pembrolizumab in patients who had received 0–2 prior lines of chemotherapy and at least two lines of hormonal therapy, which did not show an ORR, PFS or OS benefit.[94].
Following the success of the anti-TROP-2 ADC SG in TNBC, the TROPiCS-2 trial investigated SG vs treatment of physician’s choice in patients with endocrine-resistant HR+ /HER2- MBC in the third or later line. Patients who received SG had a significantly improved PFS (5.5 vs 4.0 months) [95] and OS (14.1 vs 11.2 months) [96], which led to FDA approval of SG in the third or later line of HR+ /HER2- MBC in February 2023 [95].
The SACI-IO HR+ phase II trial investigated SG with or without pembrolizumab in HR+ /HER2- mBC with any PD-L1 status after ≥ 1 endocrine therapy, 0–1 lines of chemotherapy and no prior immunotherapy or ADC in the metastatic setting. No PFS benefit was seen in the combination therapy arm (8.1 months vs. 6.2 months, HR 0.81) with immature OS data suggesting no difference at 12.5 months median follow-up [97]. In the PD-L1 CPS ≥ 1 subgroup, a non-significant 4.4 month increase in PFS was seen with combination SG and pembrolizumab, though OS data is immature [97].
Studies of Dato-DXd in HR+ /HER2- MBC have also shown promising results. The HR+ /HER2- arm of the phase I Pan-Tumour 01 study found an ORR of 26.8% in heavily pretreated patients who received Dato-DXd monotherapy, with a PFS of 8.3 months [56]. The phase III TROPION-Breast 01 trial compared Dato-DXd to physician’s choice of chemotherapy in the second or third line, with an improved PFS of 6.9 months versus 4.9 months (HR 0.63) but no demonstrated overall survival benefit according to a press release [98, 99]. Other ADCs in this space include enfortumab vedotin, which found an ORR of 15.6% with a PFS of 5.4 months in heavily pretreated patients in the phase II EV-202 study [58], as well as patritumab deruxtecan, which found a 3-months RR of 28.6% in the second line in the phase II ICARUS-BREAST-01 trial [100].
The AIPAC study is investigating LAG-3 modulation in breast cancer. This phase II trial randomized patients with HR+ /HER2- mBC that has developed resistance to endocrine therapy to paclitaxel with eftilagimod alpha, a LAG-3 inhibitor, or paclitaxel alone. While there was no significant difference in PFS or OS overall, patients younger than 65 did have a significant 7-month OS benefit, and patients with increased CD8 count 6 months after treatment had significantly improved OS [4].
Given the demonstrated benefit of CDK4/6 inhibition in HR+ disease, trials investigating CDK4/6 inhibitors (CDK4/6i) in combination with ICIs are of interest but have been met with safety concerns. A phase 1b study of abemaciclib with pembrolizumab with or without anastrozole in HR+ /HER2- mBC previously untreated with a CDK4/6i found an ORR of 23% in the first line and an ORR of 29% of patients who had previously received chemotherapy [101]. Rates of grade 3 adverse effects were seen in 69.2% in the untreated group and 60.7% in the previously treated group, with one case of grade-5 interstitial lung disease (ILD) in the first-line setting and higher than expected hepatotoxicity. The NEWFLAME phase II study evaluated nivolumab with abemaciclib and letrozole or fulvestrant in the first or second line of HR+ /HER2- mBC. Though the first 17 patients enrolled experienced an ORR of 54.5% in the letrozole arm and 40.0% in the fulvestrant arm, the trial was stopped early for safety [102]. Over 90% of patients experienced a grade 3 adverse effect, with one ILD-induced grade 5 event in the letrozole arm. Similarly, the Checkmate 7A8 trial of neoadjuvant nivolumab, palbociclib and anastrozole was stopped after 43% of patients discontinued treatment due to adverse events, including hepatotoxicity, neutropenia, rash and ILD [103]. The PACE phase II study of fulvestrant, fulvestrant with palbociclib, or fulvestrant with palbociclib and avelumab was studied in patients with HR + /HER2- mBC who had progressed on prior CDK4/6i and aromatase inhibitor. While it was designed to evaluate the efficacy of continuing CDK4/6i after progression, a non-significant 3.3-month PFS benefit was seen in the fulvestrant with palbociclib and avelumab arm in comparison to fulvestrant alone (HR 0.75) [104]. Grade 3 or 4 adverse events were rare, with no ILD as seen in the above trials with pembrolizumab and nivolumab.
Early-stage HR + /HER2- breast cancer
Though HR + breast cancer overall is associated with a good prognosis, treatment escalation with chemotherapy is indicated for patients with high risk of recurrence. The role for immunotherapy in combination with chemotherapy for certain high-risk subgroups is of active interest. Data from I-SPY-2 showed an improved rate of pCR with pembrolizumab concurrent with paclitaxel followed by doxorubicin and cyclophosphamide (T-AC) (pCR 30% vs. 13%) in HR + /HER2-, MammaPrint high-risk breast cancer with tumor size ≥ 2.5 cm [64]. In the phase III KEYNOTE-756 trial, the benefit of neoadjuvant and adjuvant pembrolizumab in grade 3, high-risk ER+ disease was clarified by comparing the I-SPY-2 regimen to standard T-AC in the neoadjuvant setting, followed by adjuvant endocrine therapy with pembrolizumab or placebo in the adjuvant setting. Seventy-six percent of patients were PD-L1 positive. An 8.5% improvement in pCR rates was seen in the pembrolizumab arm (24.3% vs. 15.6%, p = 0.00005) with a larger benefit seen in patients with node-positive disease, PD-L1 positivity as defined by CPS ≥ 1 (29.7% vs. 19.6%), and ER positivity < 10% [105]. Of note, in this trial clinicians were given the option of every-2-week dosing or every-3-week dosing. EFS data is pending to evaluate the benefit of adjuvant pembrolizumab.
Nivolumab in the neoadjuvant setting has also found success. The GIADA trial evaluated patients with stage II-III luminal B breast cancer, finding a pCR rate of 16.3% after therapy with EC followed by nivolumab, troptorelin and exemestane [106]. The phase III Checkmate 7Fl trial aimed to investigate neoadjuvant and adjuvant nivolumab in 1278 patients with high-risk ER+ /HER2- breast cancer. Patients were randomized to neoadjuvant paclitaxel with or without nivolumab followed by AC, followed by adjuvant endocrine therapy and nivolumab or placebo. A significant pCR advantage (24.5% vs. 13.8%) was seen, with an enhanced advantage in the 35% of patients who were PD-L1+ (44.3% vs. 20.2%). A pCR benefit was also seen in patients with ER < 50%, PR < 10%, and TILS ≥ 1%. This trial confirmed a pCR benefit with immunotherapy in ER+ disease [107]. However, the adjuvant portion of the study stopped enrollment early after adjuvant abemaciclib gained FDA approval based on data from MonarchE because CDK4/6i cannot be safely combined with ICIs due to safety concerns as discussed above. EFS data is pending.
Data from I-SPY2 also suggests that neoadjuvant durvalumab in combination with paclitaxel and olaparib may benefit patients with stage II-III HR+ /HER2- breast cancer. Patients who were high risk for recurrence by MammaPrint (either High-1 or High-2) were included. While no difference in pCR rate was seen in the high 1 group, 64% of patients with high 2 disease achieved a pCR with immunotherapy, compared to 22%s in the paclitaxel control group [76]. This finding suggests that MammaPrint High-2 could be a predictive biomarker for immunotherapy in HR+ /HER2- early breast cancer, though further validation is needed.
ADC in combination with ICI in early-stage HR+ /HER2- breast cancer has found early promising results. Neoadjuvant Dato-DXd with durvalumab for high-risk, early stage HR+ /HER2- breast cancer achieved an overall pCR rate of 50%, with rates of 79% in the immune signature subtype [108] (Table 3).
Table 3.
Current and upcoming clinical trials of immune checkpoint inhibitors in hormone receptor positive breast cancer
| Trial name | Primary author | Year | Study design | Line of therapy | Stage | # Patients | Drug regimen | Results |
|---|---|---|---|---|---|---|---|---|
| Pembrolizumab | ||||||||
|
KEYNOTE-028 |
Rugo | 2018 | Phase Ib | Heavily Pretreated | Advanced, PD-L1 CPS ≥ 1 | 25 | Pembrolizumab | ORR 12% |
|
KELLY |
Perez-Garcia | 2021 | Phase II | > First Line | Advanced | 44 | Eribulin + Pembrolizumab |
CBR 56.8% (95% CI 41.0–71.7) ORR 40.9% (26.3–56.8) |
| NCT03051659 | Tolaney | 2020 | Phase II | Two or more lines of hormonal therapy; 0–2 lines of chemotherapy | HR+ /HER2- MBC | 88 |
Arm A: Eribulin + Pembrolizumab Arm B: Eribulin |
ORR 27% (95% CI 14.9–42.8) vs 34% (20.5–39.9) PFS 4.1 (3.5–6.2) vs 4.2 mo (3.7–6.1), HR 0.80 (0.50–1.26) OS 13.4 (10.4-NE) vs. 12.5 mo (8.6-NE), HR 0.87 (0.48–1.59) |
| NCT03044730 | Shah | 2020 | Phase II | Median 1 prior therapy | Metastatic | 14 | Capecitabine + Pembrolizumab | ORR 14%; PFS 5.1 mo; OS not reached |
|
I-SPY2 |
Nanda | 2020 | Phase II | Neoadjuvant |
cT2-4d, cN0-3 HR+ /HER2- |
40 |
Arm A: Weekly paclitaxel + Pembrolizumab followed by AC Arm B: Weekly paclitaxel + placebo followed by AC |
pCR 30% (95% CI: 17–43) vs. 13% (CI 7–19) |
| NCT02395627 | Terranova-Barberio | 2020 | Phase II | Heavily pretreated, Metastatic ER+ , PD-L1 negative | Metastatic | 34 |
Arm A: Tamoxifen + Vorinostat + Pembrolizumab (C1) Arm B: Tamoxifen + Vorinostat + Pembrolizumab (C2) |
ORR 3.7% CBR 18.5% Stopped early for lack of efficacy |
|
KEYNOTE-756 |
Cardoso | 2023 | Phase III | Neoadjuvant & adjuvant | T1c-2, cN1-2 or T3-4, cN0-2; grade 3 | 1278 |
Arm A: Pembrolizumab + Paclitaxel then Doxorubicin + Cyclophosphamide → Adjuvant Pembrolizuamb + Endocrine Therapy Arm B: Placebo + Paclitaxel then Doxorubicin + Cyclophosphamide → Adjuvant Placebo + Endocrine Therapy |
pCR 24.3% (95% CI: 21.0–27.8) vs. 15.6% (12.8–18.6) (p = .00005) Stage II disease pCR 25.8% vs 16.7% Stage III disease pCR 21.6% vs. 13.6% N positive pCR 25.1% vs. 15.8% N negative pCR 16.9% vs. 13.1% PD-L1 + pCR 29.7% vs 19.6% PD-L1 + , ER + < 10% pCR 57.6% vs. PD-L1 + , ER > 10% 33.3% EFS immature |
| Atezolizumab | ||||||||
|
GELATO |
Voorwerk | 2023 | Phase II | First or second line | Metastatic, HER2- Lobular | 23 (18 with ER+ disease, 5 with TNBC) | Carboplatin + Atezolizumab |
ORR 17%; CBR 26% 4 of 6 patienst with clinical benefit had TNBC |
| Tremelimumab | ||||||||
| Vonderhiede | 2010 | Phase I | > First line | Metastatic | 26 | Tremelimumab + Exemestane | Stable disease in 42% at 12 weeks | |
| Santa Maria | 2018 | Pilot study | > First line | Metastatic | 11 | Tremelimumab + Durvalumab | ORR 0% | |
| Durvalumab | ||||||||
|
METADUR |
Taylor | 2020 | Phase II | > First line ER+ breast cancer | 9 |
Arm A: Azacitazine + Durvalumab Arm B: Azacitazine + Durvalumab + vitamin C |
no response | |
|
MEDIOLA |
Domchek | 2020 | Phase I/II | > Third line | Metastatic | 34 | Olaparib + Durvalumab |
Tolerable Safety DCR at 12 weeks 80% (90% CI: 64.3–90.9) |
|
I-SPY2 |
Pusztai | 2021 | Phase II | Neoadjuvant | Stage II-III HR+ /HER2-; MammaPrint high-risk | 65 |
Arm A: Paclitaxel + Durvalumab + Olaparib Arm B: Paclitaxel |
pCR 28% (95% CI 18–38) vs. 14% (9–19); MammaPrint MP1 pCR 9% (0–18) vs. 10% (5–18) MammaPrint MP2 pCR 64% (47—80) vs. 22% (13—32) |
|
Neo-CheckRay |
De Caluwe | 2024 | Phase II | Neoadjuvant | Luminal B, Mammaprint High-Risk | 135 |
Arm A: AC + paclitaxel followed by preoperative radiation Arm A: AC + paclitaxel + durvalumab followed by preoperative radiation Arm C: AC + paclitaxel + durvalumab + oleclumab followed by preoperative radiation |
pCR 17.8% (95% CI 6.6–28.9) vs 31.8% (18.1–45.6) vs 35.6% (21.6–49.5) |
| Nivolumab | ||||||||
|
GIADA |
Dieci | 2021 | Phase II | Neoadjuvant | Stage II-IIIA, HR+ , HER2- | 43 | EC followed by Nivolumab + Troptorelin + Exemestane |
pCR 16.3% (95% CI: 7.4—34.9) PAM50 basal pCR 50% vs. Luminal A pCR 9% vs. Luminal B 8% (p = 0.017) |
|
WJOG9917B NEWBEAT |
Ozaki | 2022 | Phase II | First line | Metastatic HR+ /HER2- | 17 | Bevacizumab + Nivolumab + Paclitaxel |
ORR 74% PFS 16.1 months |
|
CheckMate 7FL |
Loi | 2023 | Phase III | Neoadjuvant | Stage T1c-2, N1-2 or T3-4, N0-2 | 521 |
Arm A: Nivolumab + Paclitaxel then Doxorubicin + Cyclophosphamide → Adjuvant Endocrine Therapy Arm B: Placebo + Paclitaxel then Doxorubicin + Cyclophosphamide → Adjuvant Endocrine Therapy |
pCR 24.5% (95% CI 19.4–30.2) vs. 13.8% (9.8–13.7), Difference 10.5 (4.0–16.9) PD-L1 + : pCR 44.3% (33.7–55.3) vs. 20.2% (12.3–30.4), Difference 24.1 (10.7–37.5) PD-L1-: pCR 14.2% vs. 10.7% |
| Avelumab | ||||||||
|
PACE |
Mayer | 2024 | Phase II | > First line ER+ breast cancer | Metastatic | 220 |
Arm A: Fulvestrant Arm B: Fulvestrant + Palbociclib Arm C: Fulvestrant + Palbociclib + Avelumab |
PFS Arm A vs Arm B: 4.8 (90% CI 2.1–8.2) vs. 4.6 mo (3.6–5.9), HR 1.11 (0.79–1.55) PFS Arm A vs Arm C: 4.8 (2.1–8.2) vs 8.1 mo (3.2–10.7), HR 0.75 (0.50–1.12) ORR Arm A 7.3% (1.5–13.0), Arm B 9.0 (4.5–13.5), Arm C 13.0 (5.4–20.5) CBR Arm A 29.1 (19.0–39.2), Arm B 32.4 (25.1–39.7), Arm C 35.2 (24.5–45.9) |
| Combination with CDK4/6 inhibitors | ||||||||
|
WJOG11418B NEWFLAME |
Masuda | 2022 | Phase II | First or second line | Metastatic | 17 |
Cohort 1: Nivolumab + Abemaciclib + Fulvestrant Cohort 2: Nivolumab + Abemaciclib + Letrozole |
ORR 54.5% (95% CI 28.0–78.7) vs. 40.0% (11.7–76.9) Safety: Grade ≥ 3 AE: 92% vs. 100% (neutropenia, hepatotoxicity, ILD) Early termination for safety |
|
CheckMate 7A8 |
Jerusalem | 2022 | Phase Ib/II | Neoadjuvant | T ≥ 2 cm, ER+ /HER2- | 21 | Cohort 1: Nivolumab + Palbociclib + Anastrazole |
43% treatment discontinuation due to AE (hepatotoxicity, neuropenia, rash, ILD) Early termination for safety |
| NCT02779751 | Rugo | 2022 | Phase Ib | Any | Metastatic | 28 |
Cohort 1: Abemaciclib + Pembrolizumab + Anastrazole Cohort 2: Abemaciclib + Pembrolizumab |
ORR 23.1% (95% CI 9.0–43.7) vs. 28.6% (13.2–48.7) DCR 84.6% (65.1–95.6) vs. 82.1% (63.1–93.9) Safety: High rates of grade 3 neutropenia, hepatotoxicity, and diarrhea. 2 grade 5 events in cohort 1 |
| LAG-3 | ||||||||
|
AIPAC |
Wildiers | 2024 | Phase IIb | HR+ , HER2- MBC | Metastatic, ET-resistant | 226 |
Arm A: Paclitaxel + Eftilagimod Alpha Arm B: Paclitaxel + Placebo |
PFS 7.3 (95% CI 6.6–7.5) vs. 7.3 mo (5.5–7.5) OS 20.4 (14.3–25.1) vs. 17.5 mo (12.9–21.8), HR 0.88 (0.64–1.19) Age < 65, OS 22.3 mo (15.3–29.6) vs 14.8 (10.9–18.5), HR 0.66 (0.45–0.97) |
| Selected Upcoming Clinical Trials | ||||||||
|
SWOG2206 |
Phase III | Neoadjuvant | Stage II/III ER+ /HER2-, MP2/High-2 | 3680 |
Arm A: Durvalumab plus AC/T—→ Adjuvant ET Arm B: ACT → Adjuvant ET |
pCR iDFS |
||
|
AIPAC 3 |
Phase III | First line | Metastatic, endocrine-resistant HR+ /HER2- or TNBC | 771 |
Arm A: Paclitaxel + Eftilagimod Alpha Arm B: Paclitaxel + Placebo |
OS | ||
| NCT05159778 | Phase I/II | Prior CDK4/6, < 2 chemotherapies, no prior ICI | Metastatic, ET-resistant | 47 | Odetiglucan + Pembrolizumab | ORR | ||
|
KEYNOTE-B49 |
Phase III | Previously treated | Advanced, PD-L1+ | 800 |
Arm A: Pembrolizumab + Chemotherapy Arm B: Placebo + Chemotherapy |
PFS in patients with CPS ≥ 10 | ||
Results in bold are statistically significant
Overall, there may be a benefit for immunotherapy in early-stage HR+ /HER2- disease, though additional investigation of biomarkers to predict response to immunotherapy is needed given alternative treatment options in this setting.
Upcoming clinical trials in HR + /HER2- breast cancer
The upcoming SWOG S2206 (NCT06058377) phase III trial will clarify the role for neoadjuvant immunotherapy without adjuvant immunotherapy in patients with ER+ /HER2- MammaPrint High-2 disease, who will receive either durvalumab with AC-T neoadjuvant chemotherapy or AC-T alone. LAG-3 inhibition is under further investigation in the phase III trial AIPAC-003 (NCT05747794), which will investigate paclitaxel with or without eftilagimod alpha in patients with endocrine-resistant, HR+ /HER2- mBC or TNBC not eligible for PD-L1 therapy. Adjuvant ADC therapy with SG vs chemotherapy for HR+ /HER2- residual disease is being investigated in the upcoming SASCIA trial (NCT04595565), while SG with or without pembrolizumab is being evaluated in the first or second-line metastatic setting (NCT04448886).
HER2 positive breast cancer
HER2 positivity, defined as IHC 3+ , is seen in approximately 20% of breast cancer, though the majority of breast cancer express HER2 to some degree [109]. HER2+ breast cancer has higher TILs, TMB, and PD-L1 expression than HER2- disease, which may correlate with an enhanced response to immunotherapy [110]. To date, no ICI has improved outcomes in comparison to standard HER2-targeted regimens in a randomized clinical trial, though early results may suggest an improvement in PD-L1 positive disease.
Advanced and metastatic HR-/HER2 + breast cancer
The PANACEA phase Ib/II trial evaluated combination trastuzumab and pembrolizumab in patients with a/m HER2+ breast cancer who had previous progression on trastuzumab. The primary endpoint of ORR in PD-L1+ disease was 15%, with no responses in the PD-L1 negative group [111]. Median PFS did not differ by PD-L1 status (2.7 months vs. 2.5 months), though 12-month OS was numerically higher in the PD-L1+ group (65% vs. 12%) [111]. T-DM1,an ADC which consists of trastuzumab linked to DM1, a cytotoxic microtubule inhibitor, is standard therapy for patients with HER2+ residual disease after neoadjuvant chemotherapy [112].
Pembrolizumab in combination with T-DM1 was investigated in a phase Ib trial in metastatic HER2+ disease, with an ORR of 20% [113]. Atezolizumab in combination with T-DM1 in a/m HER2+ disease was associated with an ORR of 35%, while atezolizumab with trastuzumab, pertuzumab and docetaxel had an ORR of 100% in a phase Ib study [114]. The KATE2 phase II study compared T-DM1 with and without atezolizumab in a/m disease after the first line with no significant improvement in PFS in the ITT analysis, though patients in the treatment arm experienced increased toxicity requiring early unblinding of the treatment arms. However, an exploratory analysis found a trend towards improvement in PFS in patients with PD-L1+ disease [115].
Trastuzumab deruxtecan (T-DXd), a HER2-targeted ADC, has changed the treatment landscape for metastatic breast cancer that expresses HER2 and redefined classification of HER2 expression [109]. The landmark DESTINY-Breast 03 trial showed a dramatic improvement in PFS (29 vs 7.2 months) and OS (39.2 vs 26.5 months) with T-DXd vs T-DM1 in the second-line, with 21.1% of patients in the T-DXd group experiencing a complete response [116–118]. An exploratory subgroup of patients with brain metastasis found an intracranial ORR of 65.7% compared to 34.3% [119]. T-DXd is approved for HER2+ MBC in the second line.
Based on these studies, in addition to studies of T-DXd in other solid tumors such as lung and colorectal cancer, T-DXd was recently granted universal FDA approval for any HER2+ a/m solid cancer after one prior treatment [120–122].
Other ADCs under investigation in HER2+ MBC include trastuzumab duocarmazine (T-Duo), which was compared to chemotherapy in the third or greater line, or pretreated with T-DM1, for patients with HER2+ MBC in the phase III TULIP trial [123]. An improvement in PFS (7.0 vs. 4.9 months, HR 0.63) and a trend towards improved OS (21.0 vs 19.5 months, HR 0.87 (95% CI 0.68–1.12) was seen.[123].
Advanced and metastatic HER2 low breast cancer
T-DXd was also found to have significant activity in patients who were not traditionally considered to be HER2+ (IHC 3 +) [109, 124, 125]. This redefined the spectrum of HER2 expression from a binary (positive or negative) to a spectrum including HER2-low (IHC 1+ or 2+ , in-situ-hybridization (ISH) negative) and HER2-ultralow (faint, incomplete membrane staining in 10% of tumor cells, less than IHC 1+) [124–126]. The DESTINY-Breast 04 phase II trial found T-DXd to have significantly improved PFS (9.9 vs 5.1 months) and OS (23.4 vs 16.8 months) in comparison to physician’s choice of chemotherapy for HER2-low MBC in the second or third line, leading to FDA approval in this setting [127]. Recent results from DESTINY-Breast 06, which evaluated T-DXd in patients without prior chemotherapy for HER2-low or HER2-ultralow MBC, found a 5-month PFS advantage (HR 0.63) in comparison to standard chemotherapy [126]. T-DXd in combination with durvalumab in patients with HER2-low advanced or MBC was investigated in arm 6 of the BEGONIA trial, which found an ORR of 57% with mPFS 12.6 months [128].
Early Stage HR-/HER2+ Breast Cancer
A phase II trial of neoadjuvant chemotherapy followed by pembrolizumab, trastuzumab, and pertuzumab found a pCR rate of 46% [129]. The phase III trial IMPassion050 investigated atezolizumab or placebo with neoadjuvant AC followed by paclitaxel, trastuzumab and pertuzumab, followed by adjuvant atezolizumab or placebo in 454 patients with high-risk HER2+ disease. No difference in pCR was seen in the ITT or PD-L1+ subgroup, with EFS data immature [130]. Adverse events were more common in the treatment group, with two deaths in the immunotherapy arm (alveolitis, septic shock) attributed to the treatment (Tables 4 and 5).
Table 4.
Current and upcoming clinical trials of immune checkpoint inhibitors in HER2 positive breast cancer
| Trial name | Primary author | Year: | Study design | Line of therapy | Setting | Biomarker | # Patients | Drug regimen | Results |
|---|---|---|---|---|---|---|---|---|---|
| Pembrolizumab | |||||||||
|
PANACEA |
Loi | 2019 | Phase Ib/II | > First line | Advanced/metastatic | Any | 52 | Pembrolizumab + Trastuzumab |
PD-L1 + RR 15% (90% CI 7–29) PD-L1 + PFS 2.7 mo (2.6–4.0) 12-mo PD-L1 + OS 65% (50–76) PD-L1- PFS 2.5 mo (1.4–2.7) 12-mo PD-L1- OS 12% (CI 1–36) |
|
KEYRICHED-1 |
Kuemmel | 2021 | Phase II | Neoadjuvant | Early HER2+ breast cancer s/p anthracycline | Any | 48 | Pembrolizumab + Pertuzumab + Trastuzumab | pCR 46% (95% CI 0.31–0.62) |
| Atezolizumab | |||||||||
|
SOLTI-ATREZZO |
Ciruelos | 2023 | Phase Ib | Third line after trastuzumab and anti-HER2 ADC | Advanced/Metastatic | Any | 19 | Atezolizumab + Trastuzumab + Vinorelbine |
ORR 31.6% (95% CI 12.6–56.6) Phase II pending |
|
APTneo Michaelangelo |
Gianni | 2023 | Phase III | Neoadjuvant | ER+ /HER2+ or ER-/HER2+ | Any | 661 |
Arm A: Carboplatin + Paclitaxel + Pertuzumab + Trastuzumab + Placebo Arm B1: Atezolizumab + AC followed by Carboplatin + Paclitaxel + Pertuzumab + Trastuzumab Arm B2: Atezolizumab + Carboplatin + Paclitaxel + Pertuzumab + Trastuzumab |
pCR Arm A 57.8% vs Arm B 52.9 (HR 1.33, 95% CI 0.95–1.86, p = 0.091) Arm B1 vs B2 pCR 61.9% vs 53.6% (HR 1.402, 95% CI 0.95–2.07, p = 0.89) Arm B1 vs. Arm A pCR benefit 9.9% (HR 1.58, 95% CI 1.07–2.33, p = 0.022) EFS and OS pending |
|
IMpassion050 |
Huober | 2022 | Phase III | Neoadjuvant and adjuvant | T2-4, N1-3 | Any | 454 |
Arm A: Atezolizumab + AC followed by Paclitaxel + Pertuzumab + Trastuzumab → Adjuvant Atezolizumab + Pertuzumab + Trastuzumab or T-DM1 Arm B: Arm A: Placebo + AC then Paclitaxel + Pertuzumab + Trastuzumab → Adjuvant Placebo + Pertuzumab + Trastuzumab or T-DM1 |
pCR 62.7% vs 62.4%; difference = -.33% (95% CI -9.23–8.57) PD-L1 + pCR 72.5% vs. 64.2%, difference -8.26% (-20.56–4.04) |
| Durvalumab | |||||||||
|
CCTG IND 229 |
Chia | 2019 | Phase Ib | > First line | Metastatic | PD-L1 negative | 15 | Durvalumab + Trastuzumab | ORR 0% |
| Selected Upcoming Clinical Trials | |||||||||
| NCT03125928 | Phase II | First line | Advanced/metastatic | Any | 50 | Atezolizumab + Paclitaxel + Pertuzumab + Trastuzumab | Safety | ||
|
NRG-BR004 |
Phase III | First line | Metastatic | Any | 600 |
Arm A: Atezolizumab + Paclitaxel + Pertuzumab + Trastuzumab Arm B: Placebo + Paclitaxel + Pertuzumab + Trastuzumab |
PFS | ||
|
SOLTI-ATREZZO |
Phase II | Third line after trastuzumab and anti-HER2 ADC | Advanced/metastatic | Any | 55 | Atezolizumab + Trastuzumab + Vinorelbine | ORR | ||
Table 5.
Current and upcoming clinical trials of antibody drug conjugates in breast cancer
| Trial name | Primary author | Year: | Study design | Line of therapy | Setting | Biomarker: | # Patients | Drug regimen | Results |
|---|---|---|---|---|---|---|---|---|---|
| Sacituzumab govitecan (SG) | |||||||||
|
IMMU-132-01 |
Bardia | 2019 | Phase I/II | Previously treated | mTNBC | 108 | SG |
ORR 33.3% (95% CI 24.6–43.1) DOR 7.7 mo (4.9–10.8) PFS 5.6 mo (4.1–6.3) OS 13 mo (11.2–13.7) |
|
|
ASCENT |
Bardia | 2021 | Phase III | > First line | a/mTNBC | 468 |
Arm A: SG Arm B: physician's choice |
PFS 4.8 vs 1.7 mo, HR 0.41 (95% CI 0.33–0.52) OS 11.8 vs 6.9 mo, HR 0.51 (0.42–0.63) ORR 35% vs 5% |
|
|
TROPiCS-02 |
Rugo | 2022 | Phase III | Previously treated | HR+ /HER2- MBC | 543 |
Arm A: SG Arm B: physician's choice |
PFS 5.5 vs 4.0 mo, HR 0.66 (95% CI 0.53–0.83) OS 14.4 vs 11.2 mo, HR 0.79 (0.65–0.96) |
|
|
SACI-IO HR+ |
Garrido-Castro | 2024 | Phase II | Previously treated | HR+ /HER2- MBC | 110 |
Arm A: SG + Pembrolizumab Arm B: SG |
PFS 8.4 vs. 6.2 mo, HR 0.76 (95% CI 0.47–1.23) PD-L1 + PFS 11.05 vs 6.68 mo, HR 0.62 (0.29–1.36) OS data immature |
|
|
MORPHEUS |
Schmid | 2023 | Phase Ib/II | First line | Advanced/Metastatic | CD8 IHC ≥ 10% | 42 |
Arm A: SG + Atezolizumab Arm B: Nab-Paclitaxel + Atezolizumab |
ORR 76.7% (95% CI 57.8–90.1) vs. 66.7% (29.9–92.5) CBR 83.3% (65.3–94.4) vs. 66.7% (29.9–92.5) PFS 12.2 mo vs. 5.9 mo, HR 0.27 (0.11–0.70)* |
|
NeoSTAR |
Spring | 2024 | Phase II | Neoadjuvant | TNBC | 50 | Cohort 1: SG |
pCR 30% (95% CI 18%-45%) ORR 64% (77&-98%) Higher KI-67 and TILs predictive of pCR with SG |
|
| Trastuzumab emtansine (T-DM1) | |||||||||
| NCT01196052 | Krop | 2012 | Phase II | Heavily pretreated | HER2 + MBC | 110 | T-DM1 |
ORR 34.5% (95% CI: 26.1–43.9) CBR 48.2% (38.8- 57.9) mPFS 6.9 mo (4.2–8.4) mDOR 7.2 mo (4.6-NE) |
|
| NCT00509769 | Burris | 2011 | Phase II | Previously treated | HER2 + MBC | 112 | T-DM1 |
ORR 25.9% (95% CI: 18.4–34.4) mPFS 4.6 mo (3.9–8.6) |
|
| NCT03032107 | Waks | 2022 | Phase Ib | > First Line | HER2 + MBC | 20 | Pembrolizumab + T-DM1 | ORR 20% (95% CI 5.7–43.7); PFS 9.6 mo (2.8–16.0) | |
|
GO29381 |
Hamilton | 2021 | Phase Ib | Any | HER2 + MBC | 73 |
Arm A: Atezolizumab + Trastuzumab + Pertuzumab Arm B: Atezolizumab + T-DM1 Arm C: Atezolizumab + Trastuzumab, Pertuzumab, and Docetaxel |
Arm A: ORR 33% Arm B: ORR 35% Arm C: ORR 100% |
|
|
KATE2 |
Emens | 2020 | Phase II | > First line | HER2 + MBC | 202 |
Arm A: T-DM1 + Atezolizumab Arm B: T-DM1 + Placebo |
PFS 8.2 (95% CI 5.8–10.7) vs 6.8 mo (4.0–11.1), HR 0.82 (0.55–1.23) PD-L1 + PFS 8.5 mo (5.7-NE) vs. 4.1 mo (2.7–11.1), HR 0.60, (0.32–1.11) Unblinded early for futility and safety in atezolizumab arm |
|
| NCT00679341 | Hurvitz | 2011 | Phase II | First line | HER2 + MBC | 137 |
Arm A: trastuzumab + docetaxel Arm B: T-DM1 |
PFS 9.2 vs 14.2 mo, HR 0.59 (95% CI: 0.36–0.97) ORR 58.0% vs 64.2% |
|
|
EMILIA |
Verma | 2012 | Phase III | > First line | HER2 + MBC | 991 |
Arm A: T-DM1 Arm B: Lapatinib and Capecitabine |
ORR 43.6% vs 30.8% PFS 9.6 vs 6.4 mo, HR 0.65 (95% CI 0.55–0.77) OS 29.9 vs 25.9 mo, HR 0.75 (0.64–0.88) |
|
|
MARIANNE |
Perez | 2017 | Phase III | First line | HER2 + MBC | 1095 |
Arm A: Taxane + Trastuzumab Arm B: T-DM1 + Placebo Arm C: T-DM1 + Pertuzumab |
PFS: 13.7 vs 14.1 vs 15.2 mo PFS T-DM1 vs control: HR 0.91 (95% CI 0.73–1.13) PFS T-DM1 + pertuzumab vs control: HR 0.87 (0.69–1.08) ORR: 67.9% vs 59.7% vs 64.2% |
|
|
THE3RESA |
Krop | 2014 | Third line | HER2 + MBC | 602 |
Arm A: T-DM1 Arm B: treatment of physician's choice |
PFS 6.2 vs 3.3 mo, HR 0.528 (95% CI 0.422–0.661) OS 22.7 vs 15.8 mo, HR 0.68 (0.54–0.85) |
||
|
KATHERINE |
Von Minckwitz | 2019 | Phase III | Residual disease | Adjuvant HER2 + | 1486 |
Arm A: T-DM1 Arm B: Trastuzumab |
3-year iDFS 88.3% vs 77.0%, HR 0.50 (95% CI 0.39–0.64) 7-year iDFS 80.8% vs 67.1% 7-year OS 89.1% vs 84.4% |
|
| Trastuzumab deruxtecan (T-DXd) | |||||||||
| NCT02564900 | Modi | 2020 | Phase I | Heavily pretreated | HER2 low MBC | 54 | T-DXd |
ORR 37.0% (95% CI 24.3–51.3) DOR 10.4 mo (8.8-NE) |
|
|
DESTINY-Breast 01 |
Modi | 2020 | Phase II | Heavily pretreated | HER2 + MBC | 184 | T-DXd |
ORR 62.0% (95% CI 54.9–69) PFS 19.4 mo (14.5–21) OS 29.1 (24.6–36.1) |
|
|
DESTINY-Breast 02 |
André | 2023 | Phase III | Third line | HER2 + MBC | 608 |
Arm A: T-DXd Arm B: physician's choice of capecitabine with lapatinib or trastuzumab |
PFS 17.8 vs 6.9 mo,, HR 0.36 (95% CI 0.28–0.45) OS 39.2 vs 26.5 mo, HR 0.55 (0.50–0.86) |
|
|
DESTINY-Breast 03 |
Cortés Hurvitz |
2022; 2024 2023 |
Phase III | > First line | HER2 + MBC | 524 |
Arm A: T-DXd Arm B: T-DM1 |
PFS 29.0 vs 7.2 mo, HR 0.30 (95% CI 0.24–0.38) OS 52.6 vs 42.7 mo, HR 0.73 (0.56–0.94) |
|
|
DESTINY-Breast 04 |
Modi | 2022 | Phase III | > First line | HER2 low MBC | 557 |
Arm A: T-DXd Arm B: physician's choice of capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel |
PFS 9.9 vs 5.1 mo, HR 0.50 (95% CI 0.40–0.63) OS 23.4 vs 16.8 mo, HR 0.64 (0.49–0.84) |
|
|
DESTINY-Breast 06 |
Curgliano | 2024 | Phase III | Previously treated | HR+ /HER2 low or ultralow MBC | 866 |
Arm A: T-DXd Arm B: physician's choice of capecitabine or paclitaxel or nab-paclitaxel |
PFS HER2 low 13.2 vs 8.1 mo, HR 0.62 (95% CI 0.51–0.74) PFS HER2 ultralow 13.2 vs 8.3 mo, HR 0.78 (0.50–1.21) Overall PFS 13.2 vs 8.1 mo, HR 0.63 (0.53–0.75) |
|
| NCT03523572 | Hamilton | 2021 | Phase Ib | Previously treated | Metastatic HER2+ disease that progressed on T-DM1 or HER2 low that progressed on prior treatment | 52 | Nivolumab + T-DXd |
HER2 + cORR 59.4%; DCR 90.6%; PFS 8.6 mo (95% CI 5.4—NE) HER2 low cORR 37.5%; DCR 75%, PFS 6.3 mo (95% CI 2.3—NE) AE > = grade 3 in 43.8%. 5 patients with treatment-related ILD (one grade 5, 4 grade 2) |
|
|
DAISY |
Mosele | 2023 | Phase II | Heavily pretreated | HER2+ , HER2 low or HER2 negative MBC | 186 | T-DXd |
ORR HER2 + 70.6% (95% CI 58.3–81) ORR HER2 low 37.5% (26.4–49.7) ORR HER2- 29.7% (15.9–47) |
|
|
DEBBRAH cohort 5 |
Vaz Batista | 2024 | Phase II | Previously treated | HER2+ and HER2 low with leptomeningeal carcinomatosis | 41 | T-DXd |
PFS 8.9 mo (95% CI 2.1-NE) OS 13.3 mo (2.5-NE) CBR 71.4% |
|
|
TUXEDO |
Bartsch | 2022 | Phase II | Previously treated | HER2+ MBC with CNS metastasis | 15 | T-DXd | Intracranial ORR 73.3% (95% CI 48.1–89.1) | |
| Datopotamab deruxtecan (Dato-DXd) | |||||||||
|
TROPION-PanTumour 01 |
Bardia | 2024 | Phase I | Heavily pretreated | a/m HR+ /HER2- or TNBC | 85 | Dato-DxD |
ORR HR + /HER2: 26.8% (95% CI 14.2–42.9) PFS HR + /HER2- 8.3 mo ORR TNBC 31.8% (18.6–47.6) PFS TNBC 4.4 mo |
|
|
TROPION-Breast 01 |
Bardia | 2023 | Phase III | > First line | HR+ /HER2- MBC | 732 |
Arm A: Dato-DXd Arm B: physician's choice of capecitabine, eribulin, vinorelbine, or gemcitabine |
PFS: 6.9 vs 4.9 mo, HR 0.63 (95% CI 0.52–0.76) OS immature, HR 0.84 (0.62–1.14) |
|
|
BEGONIA Arm 6 |
Schmid | 2023 | Phase Ib/II | First line | HR-/HER2 low MBC | 46 | T-DXd + Durvalumab |
ORR 57% (95% CI 41–71); mDOR NE; mPFS 12.6 mo (8-NE) |
|
|
BEGONIA Arm 7 |
Schmid | 2023 | Phase Ib/II | First line | a/m TNBC | 62 | Dato-DXd + Durvalumab | ORR 79% (95% CI 67–88); mDOR 15.5 mo (9.9—NC); mPFS 13.8 mo (11—NC) | |
|
I-SPY2.2 |
Shatsky | 2024 | Phase II | Neoadjuvant | Stage II-III HER2- high-risk breast cancer | 47 | Dato-DXd + Durvalumab |
overall pCR 50% pCR in immune phenotype 79% pCR in TNBC 62% |
|
|
TUXEDO-2 |
Bartsch | 2024 | Phase II | Previously treated | TNBC with CNS metastasis | 8 | Dato-DxD | intracranial response 37.5% | |
| Disatamab vedotin | |||||||||
|
C003 CANCER |
Xu | 2020 | Phase Ib | Previously treated | HER2+ MBC | 70 | Disatamab vedotin |
no DLT ORR 31.4% CBR 38.6% PFS 5.8 months |
|
| NCT05331326 | Wu | 2024 | Phase II | Third line | HER2+ and HER2-low MBC with abnormal PAM pathway activation | 62 | Disatamab vedotin |
ORR 34.4% PFS 3.5 mo (95% CI 2.4–4.6) |
|
| Qu | 2023 | Phase II | Heavily pretreated | HER2+ or HER2 low MBC | 120 | Disatamab vedotin in combination with ICI, TKI, or chemotherapy |
ORR 38.3% (95% CI 30.0–47.3) PFS 5.7 mo (4.6–6.9) |
||
| Enfortumab vedotin | |||||||||
|
EV-202 |
Giordano | 2024 | Phase II | Heavily pretreated | TNBC or HR+ /HER2- MBC | 87 | Enfortumab vedotin |
TNBC ORR 19%, DCR 57.1%, PFS 3.5 mo (95% CI: 2.1–4.6), OS 12.9 mo (10.3-NE) HR + /HER2- ORR 15.6%, DCR 51.1%, PFS 5.4 mo (3.4–5.7), OS 19.8 mo (12.8-NE) |
|
| Ladiratuzumab vedotin | |||||||||
|
SGNLVA-001 |
Tsai | 2021 | Phase I |
HR + /HER2-: second line TNBC: third line |
LIV + HR + /HER2- and TNBC | 81 | Ladiratuzumab vedotin |
no DLT ORR TNBC: 28% (95% CI 13–47) HR + /HER2- pending |
|
|
SGNLVA-002 |
Han | 2020 | Phase Ib/II | First line | mTNBC | 51 | Ladiratuzumab vedotin + pembrolizumab | ORR 54% (95% CI: 33.4–73.4) | |
|
I-SPY2 |
Beckwith | 2021 | Phase II | Neoadjuvant | Stage II-III HER2- breast cancer | 60 | Ladiratuzumab vedotin followed by AC | predicted pCR overall: 0.16 (95% CI 0.08–0.24) | |
| Patritumab deruxtecan | |||||||||
| NCT02980341 | Krop | 2023 | Phase I/II | Heavily pretreated | MBC | HER3 + | 182 | Patritumab deruxtecan |
HR + /HER2- ORR 30.1% (95% CI 21.8–39.4), DCR 80.5% (72.0–87.4), mPFS 7.4 mo TNBC ORR 22.6% (12.3–36.2), DCR 79.2% (65.9–89.2), mPFS 5.5 mo HER2 + ORR 42.9% (17.7–71.1), DCR 92.9% (66.1–99.8), mPFS 11.0 mo |
|
ICARUS-BREAST-01 |
Pistilli | 2023 | Phase II | > First line | HR + /HER2- MBC | 56 | Patritumab deruxtecan | 3 mo RR 28.6% (95% CI 18.4–41.5), all partial response | |
|
SOLTI TOT-HER3 |
Oliveira | 2023 | Phase I | Neoadjuvant/Window of Opportunity | HR + /HER2- or TNBC early breast cancer | 37 | single dose Patritumab deruxtecan 5.6 mg/kg |
ORR 35% in TNBC, 30% in HR + /HER2- Change in cellularity and TIL associated with ORR (p = 0.049) No association between HER3 expression and ORR |
|
| Sacituzumab tirumotecan | |||||||||
|
OptiTROP-Breast01 |
Xu | 2024 | Phase III | Second line | a/m TNBC | 263 |
Arm A: Sacituzumab tirumotecan Arm B: physician's choice of capecitabine, eribulin, gemcitabine or vinorelbine |
PFS 5.7 (95% CI: 4.3–7.2) vs 2.3 mo (1.6–2.7) OS NE (11.2-NE) vs 9.4 (8.5–11.7), HR 0.53 (0.36–0.78) |
|
| Trastuzumab duocarmazine (T-Duo) | |||||||||
| NCT02277717 | Banerji | 2019 | Phase I | Metastatic | HER2 + or HER2 low MBC | 95 | T-Duo |
HER2 + ORR 33% (95% CI: 20.4–48.4) HR + /HER2 low ORR 28% (13.8–46.8) HR-/HER2 low ORR 40% (16.3–67.6) |
|
|
TULIP |
Saura Manich Aftimos |
2021 2023 |
Phase III | Previously treated | HER2 + MBC | 437 |
Arm A: T-Duo Arm B: physician's choice of capecitabine + trastuzumab, eribulin + trastuzumab, vinorelbine + trastuzumab or capecitabine + lapatinib |
PFS 7.0 vs 4.9 mo (HR 0.63, p = 0.002) OS 21.0 vs 19.5 mo, HR 0.87 (95% CI 0.68–1.12) |
|
| Selected upcoming clinical trials | |||||||||
|
ASCENT-03 |
Phase III | First line | mTNBC | PD-L1- | 540 |
Arm A: SG Arm B: physician's choice (paclitaxel, nab-paclitaxel, or gemcitabine) |
PFS | ||
|
ASCENT-04 |
Phase III | First Line | mTNBC | PD-L1 + | 440 |
Arm A: SG + pembrolizumab Arm B: physician's choice (paclitaxel, nab-paclitaxel, or gemcitabine) + pembrolizumab |
PFS | ||
|
ASCENT-05/Optimice-RD |
Phase III | Adjuvant | Early TNBC with RCB | 1514 |
Arm A: SG + Pembrolizumab Arm B: Pembrolizumab ± Capecitabine |
iDFS | |||
| NCT06393374 | Phase III | Adjuvant | Early TNBC with RCB | 1530 |
Arm A: SG + Pembrolizumab Arm B: Pembrolizumab + Capecitabine |
iDFS | |||
| NCT06393374 | Phase II | Neoadjuvant | TNBC | 260 | Cohort 2: SG + pembrolizumab | pCR | |||
|
SACI-IO TNBC |
Phase II | First Line | mTNBC | PD-L1- | 110 |
Arm A: SG Arm B: SG + pembrolizumab |
PFS | ||
|
InCITe |
Phase II | First or second line | mTNBC | 150 |
Arm A: binimetinib followed by binimetinib + avelumab + Liposomal Doxorubicin Arm B: SG followed by SG + avelumab Arm C: liposomal doxorubicin followed by liposomal doxorubicin + avelumab |
ORR | |||
|
SASCIA |
Phase III | Residual disease | HR + /HER2- or TNBC | 1332 |
Arm A: SG Arm B: physician's choice (capecitabine, carboplatin or cisplatin ± pembrolizumab) |
iDFS | |||
| NCT04448886 | Phase II | First or second line | HR + /HER2- MBC | 110 |
Arm A: SG + pembrolizumab Arm B: SG |
PFS | |||
| NCT04647916 | Phase II | Previously treated | HR + /HER2- or TNBC with CNS metastasis | 44 | SG | ORR | |||
|
SERIES |
Phase II | Previously treated | ER + /HER2 low | 75 | SG | ORR | |||
|
ASSET |
Phase I | Previously treated | a/m HER2- BC | 18 | SG + alpelisib | RP2D | |||
| NCT05675579 | Phase II | Previously treated | early-stage TNBC | 25 | SG + pembrolizumab | Safety | |||
| NCT04039230 | Phase I/II | Previously treated | mTNBC | 75 | SG + talazoparib | DLT | |||
|
ASPRIA |
Phase II | Residual disease | TNBC | 40 | Atezolizumab + SG | rate of clearance of cfDNA | |||
|
MORPHEUS |
Phase Ib/II | First line |
a/m breast cancer Cohort 1: PD-L1 + TNBC Cohort 2: ICI-naïve TNBC Cohort 3: HR + /HER2-PIK3CA + Cohort 4: HER2 + or HER2low PIK3CA + |
580 |
Cohort 1 Arm A: Atezolizumab + nab-paclitaxel Cohort 1 Arm B: Atezolizumab + Nab-Paclitaxel + Tocilizumab Cohort 1 Arm C: Atezolizumab + SG Cohort 2 Arm A: Capecitabine Cohort 2 Arm B: Atezolizumab + Ipatasertib Cohort 2 Arm C: Atezolizumab + LV Cohort 2 Arm D: Atezolizumab + Selicrelumab + Bevacizumab Cohort 2 Arm E: Atezolizumab + Chemo (Gemcitabine + Carboplatin or Eribulin) Cohort 3 Arm A: Inavolisib + Abemaciclib + Fulvestrant Cohort 3 Arm B: Inavolisib + Ribociclib + Fulvestrant Cohort 4 Arm A: Inavolisib + Trastuzumab Deruxtecan Cohort 4 Arm B: Inavolisib + Ribociclib + Letrozole Cohort 4 Arm C: Inavolisib + Ribociclib + Fulvestrant Cohort 4 Arm D: Inavolisib + Abemaciclib + Letrozole Cohort 4 Arm E: Inavolisib + Trastuzumab Deruxtecan |
ORR | |||
|
ASTEFANIA |
Phase III | Adjuvant | Early Breast Cancer with RCB | Any | 1700 |
Arm A: T-DM1 + Atezolizumab Arm B: T-DM1 + Placebo |
iDFS | ||
|
KATE3 |
Phase III | First to third line | Advanced/Metastatic | PD-L1 + | 350 |
Arm A: T-DM1 + Atezolizumab Arm B: T-DM1 + Placebo |
PFS and OS | ||
|
DESTINY-Breast 05 |
Phase III | First line | Early HER2 + Breast cancer with RCB | 1600 |
Arm A: T-DXd Arm B: T-DM1 |
iDFS | |||
|
DESTINY-Breast 07 |
Phase Ib/II |
Part 1: Previously Treated Part 2: First line |
HER2 + MBC | 245 |
Arm A: T-DXd Arm B: T-DXD + durvalumab Arm C: T-DXd + pertuzumab Arm D: T-DXd + paclitaxel Arm E: T-DXd + durvalumab + paclitaxel Arm F T-DXd + tucatinib |
Safety | |||
|
DESTINY-Breast 08 |
Phase I |
Part 1: Previously treated Part 2: First line |
HER2 low MBC | 138 |
Arm A: T-DXd + capecitabine Arm B: T-DXd + durvalumab + paclitaxel Arm C: T-DXd + capivasertib Arm D: T-DXd + anastrazole Arm E: T-DXd + fulvestrant |
Safety | |||
|
DESTINY-Breast 09 |
Phase III | First line | HER2 + MBC | 1157 |
Arm A: T-DXd + placebo Arm B: T-DXd + pertuzumab Arm C: taxane + pertuzumab + trastuzumab |
PFS | |||
|
DESTINY-Breast 11 |
Phase III | First line | Neoadjuvant HER2 + | 927 |
Arm A: T-DXd Arm B: T-DXd followed by taxane + pertuzumab + trastuzumab Arm C: doxorubicin + cyclophosphamide followed by taxane + pertuzumab + trastuzumab |
pCR | |||
|
DESTINY-Breast 12 |
Phase III | > First line | HER2 + MBC ± CNS metastasis | 506 | T-DXd | ORR and PFS | |||
|
DESTINY-Breast 15 |
Phase III | > First line | HER2 low or HER2- MBC | 250 | T-DXd | Time to Initiation of Subsequent Anticancer Treatement | |||
|
TRIO-US B-12/TALENT |
Phase II | Neoadjuvant | HR + /HER2 low | 88 |
Arm A: T-DXd Arm B: T-DXd + anastrazole |
pCR | |||
|
TRANSCENDER |
Phase II | First line | a/m HER2 + with early relapse < 12 mo | 41 | T-DXd | ORR | |||
|
BEGONIA |
Phase I/II | First line | mTNBC | 243 |
Arm 1: durvalumab + paclitaxel Arm 2: capivasertib + durvalumab + paclitaxel Arm 5: durvalumab + oleclumab + paclitaxel Arm 6: durvalumab + T-DXd Arm 7: durvalumab + Dato-DXd Arm 8: durvalumab + Datopotomab deruxtecan in PD-L1 + |
Safety | |||
|
TROPION-Breast 02 |
Phase III | First line | mTNBC | PD-L1- | 637 |
Arm A: Dato-DXd Arm B: physician's choice of paclitaxel, nab-paclitaxel, capecitabine, carboplatin or eribulin |
PFS OS |
||
|
TROPION-Breast 03 |
Phase III | Residual disease | adjuvant TNBC | 1075 |
Arm A: Dato-DXd + Durvalumab Arm B: Dato-DXd Arm C: physician's choice of capecitabine and/or pembrolizumab |
iDFS | |||
|
TROPION-Breast-04 |
Phase III | Neoadjuvant + Adjuvant | Stage II-III TNBC or HR-low, HER2 negative | 1728 |
Arm A: Dato-DXd + Durvalumab → Adjuvant Durvalumab ± Chemotherapy Arm B: Carboplatin + Paclitaxel + 4xAC/EC + Pembrolizumab → adjuvant Pembrolizumab |
pCR and EFS | |||
|
TROPION-Breast 05 |
Phase III | First Line | a/mTNBC | PD-L1 + | 635 |
Arm A: Dato-DXd + Durvalumab Arm B: physician's choice of chemotherapy (paclitaxel, nab-paclitaxel, gemcitabine or carboplatin) with pembrolizumab |
PFS | ||
|
DATO-BASE |
Phase III |
ER + /HER2-: Prior endocrine therapy TNBC or HER2-: Any |
HER2- MBC with CNS metastasis | 58 | Dato-DXd | ORR | |||
|
TROPION-PanTumour02 Cohort 2 |
Phase I/II | Third Line | a/mTNBC | 78 | Dato-DXd | ORR | |||
|
COMPASS-TNBC |
Phase I/II | First Line | mTNBC with early relapse < 12 mo | 60 |
Arm A: Dato-DXd Arm B: Dato-DXd + Durvalumab |
ORR | |||
|
TRADE-DXd |
Phase II | First to third line | HER2 low a/MBC | 357 |
Arm A: T-DXd. After progression, then Dato-DXd Arm B: Dato-DXd. After progression, then T-DXd |
ORR | |||
| NCT06157892 | Phase Ib/II | Second or third line | HER2 + or HER2 low MBC | 198 |
Arm A: Disatamab vedotin Arm B: Disatamab vedotin + tucatinib |
DLT ORR |
|||
| NCT05726175 | Phase II | Neoadjuvant | stage II-III HER2 low breast cancer | 20 | Arm A: Disatamab vedotin + penpulimab | pCR | |||
| NCT06178159 | Phase II | Neoadjuvant | HER2 + breast cancer | 80 |
Arm A: Disatamab vedotin + pertuzumab Arm B: Disatamab vedotin + pertuzumab + toripalimab |
pCR | |||
| NCT06227117 | Phase II | Neoadjuvant | stage II-III HR-/HER2 + breast cancer | 120 |
Arm A: Disatamab vedotin + toripalimab Arm B: carboplatin + disatamab vedotin + toripalimab Arm C: Disitamab Vedotin + toripalimab then EC + toripalimab |
pCR | |||
| NCT06000033 | Phase II | Third line | HR-/HER2 low MBC | 35 | Disitamab vedotin + anlotinib | ORR | |||
| NCT05831878 | Phase II | Second line | HR-/HER2 low MBC | 36 | Disatamab vedotin | ORR | |||
| NCT03500380 | Phase II/III | Previously treated | HER2 + MBC ± liver metastasis | 301 |
Arm A: Disatamab vedotin Arm B: capecitabine + lapatinib |
PFS | |||
|
Rosy |
Phase III | Previously treated | HR + /HER2 low MBC | 288 |
Arm A: Disatamab vedotin Arm B: physician's choice of endocrine therapy |
PFS | |||
| NCT04400695 | Phase III | Second line | HER2 low MBC | 366 |
Arm A: Disatamab vedotin Arm B:physician's choice of capecitabine, docetaxel, paclitaxel, or vinorelbine |
PFS | |||
| NCT04300556 | Phase I/II | > First line | Solid tumors including mTNBC | 142 | Farletuzumab ecteribulin |
ORR DLT Safety |
|||
| NCT05865990 | Phase II |
TNBC: > 1st line HER2+ : > 2nd line |
MBC and NSCLC with CNS metastasis | 60 | Patritumab deruxtecan |
Intracranial ORR OS |
|||
| NCT04699630 | Phase II |
TNBC: 2nd line HER2+ : > 2nd line including T-DXd |
MBC | 121 | Patritumab deruxtecan |
ORR PFS |
|||
|
ICARUS-BREAST-02 |
Phase Ib/II | Previously treated, including with T-DXd | MBC | 152 | Patritumab deruxtecan + olaparib | Safety, ORR, DOR, PFS, CBR | |||
|
VALENTINE |
Phase II | Neoadjuvant | HR + /HER2-; Ki67 > 20% and/or high genomic risk | 120 |
Arm A: chemotherapy Arm B: Patritumab deruxtecan + letrozole Arm C: Patritumab deruxtecan |
pCR | |||
|
TroFuse-010 |
Phase III | Previously treated | HR + /HER2- MBC | 1200 |
Arm A: Sacituzumab tirumotecan Arm B: Sacituzumab tirumotecan + pembrolizumab Arm C: physician's choice of capecitabine, liposomal doxorubicin, paclitaxel, or nab-paclitaxel |
PFS | |||
| NCT06393374 | Phase III | Adjuvant | Early TNBC with RCB | 1530 |
Arm A: Sacituzumab tirumotecan + pembrolizumab Arm B: physician's choice of capecitabine or capecitabine + pembrolizumab |
iDFS | |||
| NCT06312176 | Phase III | Previously treated | Advanced or Metastatic | 1200 | 1200 |
Arm A: Sacituzumab Tirumotecan Arm B: Sacituzumab Tirumotecan + Pembrolizumab Arm C: Treated of Physician's Choice |
PFS | ||
|
I-SPY2 |
Phase II | Neoadjuvant | Stage II-III HER2- breast cancer | T-Duo | predicted pCR | ||||
Results in bold are statistically significant
HER2+ vaccines
Several HER2-directed short peptide vaccines have been developed, with E75 being the most extensively studied. An optimal regimen of NP-S with GM-CSF monthly for 6 months was determined in a phase I/II study [131], with a trend towards increased 5-year DFS in the vaccinated group vs. control. In the phase III setting, however, no difference in 3-year DFS was seen and the trial was stopped early for futility [132]. HER2 vaccines targeting AE37 have not shown any difference in DFS [133]. GLSI-100, a HER2 vaccine targeting GP2, has been investigated in the adjuvant setting for patients with HER2+ , node-positive or otherwise high-risk disease [133]. Overall, there was no difference in 5-year DFS, but patients who had disease that was HER2 3+ by IHC did have a trend toward significant improvement in DFS [133]. This vaccine is being investigated in the upcoming phase III Flamingo-01 (NCT0523916) study in the adjuvant setting for patients with HER2+ residual disease (Table 6).
Table 6.
Current and upcoming clinical trials of vaccines in breast cancer
| Trial name | Primary author | Year | Study design | Line of Therapy | Setting | TAAs/MOA | # Patients | Drug regimen | Results |
|---|---|---|---|---|---|---|---|---|---|
| Peptide vaccines | |||||||||
|
Theratrope |
Miles | 2011 | Phase III | > First Line | Metastatic | Muc-1 | 1028 |
Arm A: sialyl-TN conjugated to keyhole limpet hemocyanin (KLH) protein + cyclophosphamide Arm B: Placebo KLH protein + cyclophosphamide |
6 mo PFS: 53% vs 33% (p = 0.011) TTP 3.4 vs 3.0 mo (p = 0.353) OS 23.1 mo vs. 22.3 (p = 0.916) |
| NCT01516307 | Huang | 2020 | Phase II | First or Second line | Metastatic with stable/responding disease | Ada-Sim / OBI-821 | 348 |
Arm A: adagloxad simolenin + cyclophosphamide Arm B: placebo + cyclophosphamide |
PFS: 7.6 mo vs 9.2 mo (HR 0.96, 95% CI 0.74–1.25) Anti-Globo-H IgG titer > = 1:160 vs < 1:160: PFS 11.1 mo (9.3–17.6) vs 5.5 mo (3.7–5.6), HR 0.52, p < 0.0001) |
| NCT01532960 | Dillon | 2017 | Phase I | First line | Adjuvant |
MAGE CEA NY-ESO-1 HER2 |
12 | Poly-ICLC vaccine |
Response Rate 0% Terminated for futility |
| NCT02364492 | Rosenbaum | 2020 | Phase I | First line | Adjuvant, HER2 negative at high risk for relapse | MAGE | 7 | MAG-Tn3/AS15 vaccine | all vaccinated patients developed high levels of Tn-specifc antibodies |
| NCT01220128 | Higgins | 2017 | Phase II | Neoadjuvant | Stage II or III breast cancer expressing WT1 | WT1 | 66 |
Arm A: Standard neoadjuvant therapy + WT1 ASCI Arm B: Standard neoadjuvant therapy + Placebo |
Terminated |
| NCT02229084 | Makhoul | 2021 | Phase I/II | Neoadjuvant | Stage I-III ER + /HER2- breast cancer | Peptide Vaccine | 25 | Standard Neoadjuvant Therapy + P10s-PADRE | Increase in CD16, NKp46 and CD94 expression on NK cells, increased IFN-γ |
| HER2 vaccines | |||||||||
|
NCT00584789 |
Mittendorf | 2014 | Phase I/II | First line | Adjuvant, HER2 IHC 1–3 + | E75/NeuVax | 195 |
Arm A: NP-S + GM-CSF Arm B: Control |
5-year DFS: 89.7% vs 80.2% (p = 0.08) |
|
PRESENT |
Mittendorf | 2019 | Phase III | First line |
Adjuvant, T1-3, N1-3, HER2 low (IHC 1 + /2 +) |
E75/NeuVax | 758 |
Arm A: NP-S + GM-CSF Arm B: placebo + GM-CSF |
Recurrence Rate at 16.8 months: 9.8% vs 6.3% (p = 0.07) 3-year DFS 77.1% vs 77.5% Stopped early for futility |
| NCT00524277 | Brown | 2020 | Phase II | First line | Adjuvant, HER2 IHC 1–3 + node-positive or high-risk node negative | GP2 | 180 | GP2 + GM-CSF |
5-year DFS 82.9% (95% CI 75–91) vs 80.4% (69–88), HR .967 (0.460–2.034) HER2 (IHC 3 +) subgroup: DFS 100% vs 87.2% (71–95), p = 0.052) |
| NCT00524277 | Brown | 2020 | Phase II | First line | Adjuvant, HER2 IHC 1–3 + node-positive of high-risk node negative | AE37 | 298 | AE37 + GM-CSF |
recurrence rate 12.4% vs 13.8%, HR 0.885 (95% CI 0.472–1.659) 5-year DFS 80.1% vs 79.3%, HR 0.989 (0.588–1.665) |
| Viral Vaccines | |||||||||
| Mohebtash | 2011 | Phase I | Heavily pretreated | Metastatic |
Muc-1 CEA co-stimulatory molecules |
12 | PANVAC monthly |
median time to progression 2.5 mo OS 13.7 mo One patient with complete response Four patients with stable disease |
|
| NCT00179309 | Heery | 2015 | Phase II | Any | Metastatic |
Muc-1 CEA co-stimulatory molecules |
48 |
Arm A: Docetaxel + PANVAC Arm B: Docetaxel |
PFS 7.9 mo vs 3.9 mo (HR 0.65 (95% CI 0.34–1.14) |
|
IND-213 |
Bernstein | 2018 | Phase II | Any | Metastatic | Type 3 Reovirus | 72 |
Arm A: Paclitaxel Arm B: Paclitaxel + Pelareorep |
PFS 3.78 mo vs 3.38 mo, HR 1.04 (80% CI 0.76–1.43) OS 17.4 mo vs 10.4 mo, HR 0.65 (0.46–0.91) |
| NCT03387085 | Nangia | 2019 | Phase Ib | Third line | Metastatic TNBC | Adenovirus CEA, MUC1, brachyury and HER2; yeast vector Ras, brachyury and CEA | 8 | chemotherapy + bevacizumab + SBRT + vaccines + avelumab |
No dose limiting toxicities One complete response, and 2 partial responses |
|
AWARE-1/REO-027 |
Manso | 2020 | Phase I | Neoadjuvant | Early- Stage TNBC Window of Opportunity | Type 3 Reovirus | 38 | Pelareorep + Atezolizumab |
Increase in caspase 3 staining (p = 0.04) Decrease in T cell diversity (p = 0.01) |
| NCT03674827 | Pfizer | 2022 | Phase I | Third line | Metastatic TNBC or NSCLC | Viral Vaccine | 36 | VBIR-2 + Tremelimumab + Sasanlimab | No DLT |
|
Bracelet-1 |
Clark | 2023 | Phase II | Any | Metastatic HR+ /HER2- | Type 3 Reovirus | 48 |
Arm A: Paclitaxel Arm B: Paclitaxel + Pelareorep Arm C: Paclitaxel + Pelareorep + Avelumab |
ORR: 20% vs 31.3% vs 17.6% PFS: 6.4 mo vs 9.6 mo vs 7.5 mo OS pending |
| NCT02779855 | Soliman | 2023 | Phase II | Neoadjuvant | Stage II-III TNBC | oncolytic herpesvirus | 37 | Intratumoral talimogene laherparepvec (T-VEC) + Paclitaxel followed by AC |
pCR 45.9% (90% CI 32–54) 2-year DFS 89% |
| NCT03256344 | Hecht | 2023 | Phase Ib | Any | Metastatic TNBC with liver metastasis | oncotypic herpesvirus | 11 | Intratumoral T-VEC + Atezolizumab |
No DLT ORR 10% (95% CI 0.3–44.5) One patient with PR |
|
KEYNOTE-890 Cohort 1 |
Telli | 2021 | Phase II | Second line | Advanced/Metastatic TNBC | Plasmid | 26 | Tavokinogene telseplasmid (TAVO-EP) + Pembrolizumab |
ORR 17.4% One patient with CR, 4 patients with PR OS 11.0 mo |
| Dendritic Cell Vaccine | |||||||||
| NCT001070211 | Sharma | 2012 | Pilot | First line | HER2 + DCIS | HER2/neu | 27 | HER2/neu DC vaccine | 5/27 patients with no residual disease at time of surgery |
| NCT02061332 | Lowenfeld | 2017 | Phase I/II | First line | HER2 + DCIS or early breast cancer | HER2/neu | 54 |
Arm A: Intralesional administration HER2/neu DC vaccine Arm B: Intranodal administration HER2/neu DC vaccine Arm C: Intralesional and intranodal administration HER2/neu DC vaccine |
IRR 84.2% (95% CI 60.4–96.6) vs 89.5% (66.9–98.7) vs 66.7% (38.4–88.2) pCR 28.6% in patients with DCIS pCR 8.3% for patients with breast cancer |
| Svane | 2007 | Phase II | Heavily pretreated | HLA-A2 + advanced breast cancer | p53 | 26 | p53-peptide loaded DC vaccine | 8/19 stable disease | |
| NCT01042535 | Soliman | 2018 | Phase I/II | Pretreated | ER + /HER2- Advanced breast cancer | p53 | 9 | p53-peptide loaded DC vaccine + indoximod | best respose stable disease |
| NCT02018458 | O'Shaughnessy | 2016 | Phase I/II | Neoadjuvant | Locally advanced TNBC |
Cyclin B1, WT1 26, CEF |
10 | ddAC followed by paclitaxel and carboplatin + DC vaccine | pCR 50% |
| Selected Upcoming Vaccine Clinical Trials | |||||||||
|
NCT0523916 FLAMINGO-01 |
Phase III | Adjuvant | Early Breast Cancer with RCB | HER2 Vaccine | 498 |
Arm A: GLSI-100 Arm B: Placebo |
iBCFS | ||
|
GLORIA |
Phase III | Adjuvant | TNBC expressing GloboH at high risk for recurrence | Ada-Sim / OBI-821 vaccine | 668 |
Arm A: adagloxad simolenin + standard of care Arm B: standard of care alone |
iDFS | ||
|
KEYNOTE-890 Cohort 2 |
Phase II | First line | Metastatic TNBC | Intratumoral IL-12 | 40 | TAVO-EP + Pembrolizumab + nab-paclitaxel | ORR | ||
| NCT04348747 | Phase II | Previously treated | Metastatic TNBC or HER2 + with untreated brain metastasis | HER2/3 Dendritic Cell Vaccine | 21 | alphaDC-1 + CKM + Pembrolizumab | RR | ||
| NCT03606967 | Phase II | First line | mTNBC | Peptide Vaccine | 70 |
Arm A: nab-paclitaxel + durvalumab + neoantigen vaccine Arm B: nab-paclitaxel + durvalumab |
PFS | ||
| NCT03362060 | Phase Ib | > First line | mTNBC HLA-A2 + | Peptide Vaccine | 20 | PVX-410 Vaccine + Pembrolizumab | PFS | ||
| NCT02826434 | Phase Ib | Prior neoadjuvant or adjuvant therapy | Stage II/III TNBC, HLA-A2 + | Peptide Vaccine | 22 | PVX-410 Vaccine + Durvalumab | DLT | ||
| NCT05269381 | Phase I | > First line | Advanced TNBC or other solid tumors | Peptide Vaccine | 36 | cyclophosphamide followed by vaccine + GM-CSF + Pembrolizumab | Safety | ||
| NCT04024800 | Phase II | First line | mTNBC | HER2 Vaccine | 29 | AE37 + Pembrolizumab | ORR | ||
| NCT05455658 | Phase II | Adjuvant | Stage IB-III TNBC | Plasmid Vaccine | 33 | STEMVAC (sargramostim) | Cellular Immune Response | ||
| NCT06435351 | Phase I | Adjuvant | Early TNBC with RCB | Dendritic Cell Vaccine | 16 | DC vaccine based on whole exon sequencing of tumor with pembrolizumab and/or capecitabine | Feasibility | ||
Results in bold are statistically significant
Upcoming clinical trials in HR-/HER2+ breast cancer
The ASTEFANIA (NCT04873362) phase III trial is investigating T-DM1 with or without atezolizumab in the adjuvant setting for patients with residual disease after neoadjuvant therapy, with the goal to enhance the current standard of care for patients with RCB in the adjuvant setting. KATE3 (NCT04740918) is a phase III trial evaluating the KATE2 regimen in patients with PD-L1+ disease in the first to third line a/m setting. Another phase III trial (NCT03199885) is investigating paclitaxel with trastuzumab, pertuzumab, and atezolizumab or placebo in the first line metastatic setting, with the goal of establishing a role for ICIs in a/m HER2+ breast cancer.
Bispecific antibodies and cellular therapies
Immunotherapies other than immune checkpoint inhibitors seek to induce a durable immunologic anti-cancer response. Areas of research in breast cancer include bispecific antibodies as well as cellular therapies including tumor infiltrating lymphocytes (TILs), chimeric antigen receptor T (CAR-T), and T cell receptor engineered (TCR) treatments.
Bispecific antibodies
Bispecific antibodies are engineered antibodies that simultaneously bind to two targets, typically a tumor-specific antigen on malignant cells and an immune cell [134]. Bringing these targets in close proximity engages the immune system, inciting apoptosis, enhanced immune activation signaling, or reducing immunosuppressive factors, all of which promote anti-tumor activity [134]. The first FDA approval for a bispecific antibody in oncology was blinatumomab, which targets CD3 and CD19, in B-cell acute lymphoblastic leukemia in 2017 [135]. Bispecifics have been an area of active research in breast cancer for over 30 years. TAAs targeted by bispecific therapies in breast cancer research include HER2, HER3, PD-L1, and CD3 [134].
HER2 is the most commonly studied bispecific TAA in breast cancer, in combination with another HER2 antibody, HER3, CD3, and CD16. Zanidatamab, a dual-HER2 bispecific antibody, is efficacious in both advanced and early-stage HER2+ BC. A phase I clinical trial of zanidatamab in advanced HER2+ solid cancers, including breast cancer, found an ORR of 37% with zanidatamab monotherapy [136]. Zanidatamab in combination with docetaxel had an 90.9% ORR in patients with advanced HER2+ mBC [137]. In HR+ /HER2+ patients with advanced disease, the combination of zanidatamab with palbociclib and fulvestrant achieved a median PFS of 11.3 months with an ORR of 34.5% [138]. In the neoadjuvant setting, patients with stage 1, node-negative HER2 + BC were treated with zanidatamab in a chemotherapy-free regimen, with preliminary data showing 36% of patients achieving pCR and 64% of patient achieving pCR or RCB-1 [139]. An upcoming phase III trial (NCT06435429) will investigate chemotherapy with zanidatamab or trastuzumab for patients with HER2+ disease who progress on T-DXd.
KN026, another dual-HER2 bispecific antibody, has found success in a phase I trial of patients with HER2+ mBC. Overall, a 28.1% ORR and median PFS of 6.8 months was found, though patients with HER2+ and CDK12 co-amplification had a significantly improved response (ORR of 50%, median PFS 8.2 months) [140]. This improved response is thought to be because both HER2 and CDK12 genes are located on chromosome 17, approximately 200 kb apart. KN026 in combination with KN046, an anti-PD-L1/CTLA-4 bispecific antibody, found an ORR of 50% in patients with HER2 + MBC [141]. In the neoadjuvant setting, KN026 with docetaxel achieved a pCR rate of 56.7% [142]. A trial of KN026 with palbociclib and fulvestrant (NCT04778982) was terminated due to low enrollment. An upcoming phase I clinical trial (NCT03842085) will investigate another dual HER2 bispecific antibody, MBS301, in HER2+ solid tumors.
Bispecific antibodies targeting HER2 and HER3 include MCLA-128/Zenocutuzumab and MM-111. A phase II trial of zenocutuzumab with endocrine therapy in patients with HR+ , HER2-low MBC previously treated with CDK4/6 therapy found an DCR of 45% [143], while zenocutuzumab with trastuzumab and vinorelbine in HER2+ MBC previously treated with T-DM1 found an DCR of 77% [144]. MM-111 has also proven to be safe in phase I clinical trials [145, 146], though without further investigation in phase II or beyond. HER2 and CD3 bispecific antibodies including ertumaxomab [147], p95HER2 [148], and GBR1302 [149] have been investigated, but are not moving forward in current clinical trials.
Bispecific antibody armed T cells targeting HER2 and CD3, referred to as HER2 BATs, were investigated in a phase II clinical trial of treatment consolidation with immunotherapy after chemotherapy in HER2- MBC [150]. Evidence of immune activation in both the adaptive and innate response were seen, with a median OS of 13.1 months [150]. The role of HER2 BATs in HER2+ disease was evaluated in a phase I clinical trial, which showed that patients with higher HER2+ expression (IHC 3+) had a longer OS in comparison to patients with HER2 0 to 2+ expression (57 months vs. 27 months) [151]. The combination of HER2 BATs with pembrolizumab will be investigated in an upcoming phase I/II study (NCT03272334) (Table 7).
Table 7.
Current and upcoming clinical trials of bispecific antibodies in breast cancer
| Trial name | Primary author | Year | Study design | Line of therapy | Setting | Mechanism of Action: immune-cell engager (ICE), T-cell engager (TCE), NK engager, dual tumor associated antigen (TAA) | TAA | # Patients | Drug regimen | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Bispecific antibody | ||||||||||
| NCT05035836 | Valero | 2023 | Phase II | Neoadjuvant | Stage I HER2 + breast cancer | dual TAA | HER2 x HER2 | 11 | Zanidatamab | pCR 36%; pCR/RCB1 64% |
| NCT04224272 | Escrivá-de-Romani | 2023 | Phase II | Previously treated | Advanced HR + /HER2 + breast cancer | dual TAA | HER2 x HER2 | 34 | Zanidatamab + Fulvestrant + Palbociclib |
DLT in one patient (neutropenia) cORR 34.5% (95% CI 17.9—54.3) mPFS 11.3 mo (CI 5.6—NE) |
| NCT02892123 | Meric-Bernstam | 2022 | Phase I | Heavily pretreated | Advanced/Metastatic HER2 + solid tumors, including MBC | dual TAA | HER2 x HER2 | 279 | Zanidatamab + chemotherapy |
DLT not reached; grade 3 + AE 3% of patients ORR 37% (95% CI 27.0–48.7) Part 3 ongoing |
| NCT04276493 | Wang | 2023 | Phase Ib/II | Previously treated | Advanced HER2 + breast cancer | dual TAA | HER2 x HER2 | 37 | Zanidatamab + Docetaxel |
ORR = 90.9% (95% CI 75.7–98.1) 67.6% with grade 3 + treatment-related AE |
| NCT03619681 | Zhang | 2022 | Phase I | Heavily pretreated | HER2 + MBC | dual TAA | HER2 x HER2 | 63 | KN026 |
ORR 28.1% PFS 6.8 mo (95% CI 4.2–8.3) CDK12 amplified subgroup ORR 50% vs 0% (p = 0.05), PFS 8.2 mo vs 2.7 mo (p = 0.04) |
| NCT04881929 | Ma | 2023 | Phase II | Neoadjuvant | Stage II-III HER2 + breast cancer | dual TAA | HER2 x HER2 | 30 | KN026 + Docetaxel |
pCR 56.7% (95% CI 37.43–74.53) ORR 90% (73.47–97.89) Grade 3 + AE 53.5% |
| NCT04521179 | Liu | 2022 | Phase II | Previously treated | HER2 + breast cancer | dual TAA | HER2 x HER2 | 36 | KN026 + KN046 |
ORR 50% (95% CI 28.2–71.8) DCR 81.8% (59.7–94.8) PFS 5.6 mo (2.5—NE) |
| NCT02912949 | Schram | 2022 | Phase II | Heavily pretreated | HER2 + breast cancer expressing NRG1 mutation | dual TAA | HER2 x HER3 | 5 | Zenocutuzumab | Response in 2/4 evaluable patients |
| NCT03321981 | Pistilli | 2020 | Phase II | Previously treated with CDK4/6i | HR + , HER2-low mBC | dual TAA | HER2 x HER3 | 48 | Zenocutuzumab + Endocrine Therapy | DCR 45% (90% CI 32–59); 2 patients with partial response |
| NCT03321981 | Hamilton | 2020 | Phase II | Previously treated with anti-HER2 ADC | HER2 + MBC | dual TAA | HER2 x HER3 | 28 | Zenocutuzumab + Vinorelbine + Trastuzumab | DCR 77% (90% CI 60–89); 1 patient with complete response and 4 patients with partial response |
| NCT01304784 | Richards | 2014 | Phase I | Previously treated | HER2 + MBC | dual TAA | HER2 x HER3 | 46 |
Arm A: MM-111 + Cisplatin + Capecitabine + Trastuzumab Arm B: MM-111 + Lapatinib ± Trastuzumab Arm C: MM-111 + Paclitaxel + Trastuzumab Arm D: MM-111 + Lapatinib + Trastuzumab + Paclitaxel Arm E: MM-111 + Docetaxel + Trastuzumab |
MTD not met |
| NCT00911898 | Beeram | 2010 | Phase I | Heavily pretreated | HER2 + advanced breast cancer | dual TAA | HER2 x HER3 | 11 | MM-111 | No DLT |
| NCT01097460 | Higgins | 2011 | Phase I/II | Heavily pretreated | HER2 + MBC | dual TAA | HER2 x HER3 | 16 | MM-111 + Herceptin | No results available |
| NCT01569412 | Haense | 2016 | Phase I | Heavily pretreated | HER2 + MBC | TCE | HER2 x CD3 | 5 | Ertumaxomab |
One partial response No dose-limiting toxicities |
| NCT02829372 | Wermke | 2018 | Phase I | Heavily pretreated | HER2 Positive solid tumor | TCE | HER2 x CD3 | 19 | GBR 1302 | Grade 1–2 CRS common; two patients with DLT. One patient with breast cancer has ongoing response at 4 mo |
| NCT00027807 | Lum | 2014 | Phase I | Heavily pretreated | MBC | TCE | BATs (HER2 x CD3) | 23 | HER2Bi + IL-2 + G-CSF |
Best response: Stable disease or better: 54.5% HER2 3 + OS 57.4 mo HER2 0–2 + OS 27.4 mo |
| NCT01022138 | Lum | 2021 | Phase II | Heavily pretreated | Metastatic HER2/HR+ and TNBC with stable disease | TCE | BATs (HER2 x CD3) | 32 | HER2Bi + IL-2 + G-CSF |
Overall OS 13.1 mo (95% CI 8.6–17.4) HER2-/HR + OS 15.2 mo (CI 8.6–19.8) TNBC OS 12.3 mo (2.1–17.8) Significant increases in interferon-γ immunospots, Th1 cytokines, Th2 cytokines, and chemokines after treatment |
| NCT02659631 | Harding | 2022 | Phase I | Heavily pretreated | Advanced Solid Tumors | TCE | CD3 x p-cadherin | 5 | PF-06671008 | Best response: stable disease |
| Selected upcoming clinical trials | ||||||||||
| NCT06435429 | Phase III | Pretreated | HER2 + MBC | dual TAA | HER2 Bispecific | 550 |
Arm A: Chemotherapy + Zanidatamab Arm B: Chemotherapy + Trastuzumab |
PFS | ||
| NCT05027139 | Phase I/II | Pretreated | HER2 + solid tumors, including MBC | dual TAA | HER2 Bispecific + Anti-CD47 | 52 | Zanidatamab + Evorpacept (ALX148) |
Safety ORR |
||
| NCT03842085 | Phase I | Metastatic | HER2 + solid tumors, including HER2 + MBC and HER2 low MBC | dual TAA | HER2x HER2 | 34 | MS301 | DLT | ||
| NCT03272334 | Phase I/II | Second line | MBC | dual TAA | HER2 Bispecific | 33 | HER2Bi + Pembrolizumab | MTD | ||
| NCT04143711 | Phase I/II | Previously treated | Advanced HER2 + solid tumors, including breast | NK cell engager | trispecific NK cell engager | 378 |
Arm A: DF1001-001 Arm B: DF1001-001 + nivolumab Arm C: DF1001-001 + nab paclitaxel Arm D: DF1001-001 + sacituzumab govitecan |
DLT Safety ORR |
||
Adoptive cell therapy: TILS, CAR-T, and TCR
While ICIs have made tremendous impact on outcomes in breast cancer, a significant subset of patients do not benefit from ICI. This is likely due, in part, to immune escape through defects in antigen presentation and other abnormal signaling pathways [152]. Mechanisms to overcome this barrier include adoptive cell transfer, which consists of infusing antigen-specific T cells into the patient, inducing an amplified immune response. Therapies such as TILs, CAR-T, and TCR cell therapies involve harvesting T cells from the patient, allowing for ex vivo expansion with or without genetic modification, and infusing these T cells back into the patient. Since the first FDA approval of CD-19-specific CAR-T therapy for B-cell acute lymphoblastic leukemia in 2017 [153], cellular therapy has changed the treatment paradigm in hematologic malignancies.
TIL therapy involves extracting T cells from within a tumor, multiplying them without genetic modification, then infusing them back into the patient. TIL therapy gained FDA approval for relapsed/refractory metastatic melanoma in February 2024 based on an ORR of 31.5% including three complete responses in seventy-three patients treated with lifeleucel, an autologous TIL therapy, in a phase II study (NCT02360579) [154, 155], with four-year follow up confirming a median OS of 13.9 months and 20.8% of patients having an ongoing response at 48 months [156]. This success has prompted investigation of TIL therapy for other solid malignancies, including breast cancer. A phase I trial of TIL therapy with IL-2 and pembrolizumab in breast cancer showed promising results, with three out of six patients having a response including 1 complete response [157]. Upcoming trials of TIL therapy in breast cancer include a phase II trial (NCT04111510) is investigating LN-145 TIL therapy in mTNBC, a phase I/II (NCT05451784) trial of PD-L1+ TILs in TNBC, a phase Ib (NCT05576077) study of TBio4101 with pembrolizumab in solid tumors including breast cancer, and a phase II (NCT03449108) trial of LN-145-S1 with ICI in metastatic TNBC.
CAR-T cell therapy engineers T-cell receptors to target a tumor-specific antigen on the cell surface. CAR-T is a staple in the treatment of hematologic malignancies, and is an area of active research in solid tumors including breast cancer. Phase I studies to date have demonstrated tolerable safety [158–160] with some patients experiencing stable disease [161]. A phase 0 trial of intratumorally injected CAR T cells against c-MET, a cell-surface molecule, in patients with mBC showed evidence of an inflammatory response within tumors, but no clinical response [158]. These results led to a phase I study of intravenous CAR-T targeting cMET, with two of four patients with TNBC experiencing stable disease at day 25 [161]. Upcoming studies of CAR-T in breast cancer include a phase I/II (NCT04020575) study targeting metastatic breast cancer expressing Muc1 growth factor receptor, called Muc1*, and a phase I study (NCT02706392) of CAR-T in liquid and solid malignancies expressing ROR1.
It is postulated that the limited efficacy of CAR-T in solid tumors to date may relate to tumor antigen modulation, insufficient specificity of target antigens, T cell exhaustion, poor cell trafficking to the tumor site, and dysregulation of effector function by the TIME [162–164]. Limited expansion of CAR-T cells after transfer has limited efficacy while also limited toxicity. Thus, concern remains for higher on-target off-tumor toxicity with greater CAR-T cell expansion, as seen in a patient treated with ERBB2-CAR-T therapy who experienced fatal cytokine release syndrome from on-target, off-tumor effect [165]. In response, it is now understood that CAR-T therapy targets should be restricted to cell-surface antigens with limited off-tumor expression, of which there are few [166]. Other toxicities from CAR-T therapy such as macrophage activation syndrome and immune cell-associated neurotoxicity syndrome also affect normal, healthy tissue.
TCR is similar to CAR-T, but expresses human leukocyte antigen (HLA)-restricted T-cell receptors, which allow for tumor-specific binding to both membrane and intracellular proteins expressed by the major histocompatibility complex (MHC), and thus limits toxicity to other healthy cells. The SPEARHEAD-1 (NCT03132922) phase II trial of afamitresgene autoleucel (“afami-cel”), an HLA-A*02 restricted TCR targeting MAGE-4 in patients with synovial sarcoma or myxoid round cell liposarcoma, found an ORR of 37% and a duration of response of 28.1 months [167]. Afami-cel received FDA approval for MAGE-4 expressing sarcoma in August 2024. Ongoing phase I trials of MAGE-A1 and MAGE-A3 have found tolerable safety and potential clinical efficacy in various solid tumors, without reported results for patients with breast cancer [168, 169]. Another TCR therapy, letetresgene autoleucel (“lete-cel”) targeting HLA-A*02 restricted NY-ESO1, found an ORR of 40% in the IGNYTE-ESO (NCT03967223) phase II trial of patients with synovial sarcoma and myxoid round cell liposarcoma [170]. These results have laid the groundwork for expansion of TCR into other cancer types. In breast cancer, an HLA-A*O2-restricted TCR targeted p53R175H therapy from peripheral blood lymphocytes was infused into a patient, who experienced a partial response lasting 6 months [171]. Upcoming trials of TCR in breast cancer include a phase Ib trial (NCT05989828) for patients with metastatic TNBC which expresses HLA-A*O2 restricted NY-ESO-1, a phase I trial (NCT05877599) of HLA-A*02 restricted NT-175 in solid tumors including breast cancer, and two phase I studies (NCT05483491, NCT05035407) of HLA-A*01-restricted KK-LC-1 in solid tumors including breast cancer (Table 8).
Table 8.
Current and upcoming clinical trials of cellular therapy in breast cancer
| Trial name | Primary author | Year | Study design | Line of therapy | Setting | TAAs/MOA | # Patients | Drug regimen | Results |
|---|---|---|---|---|---|---|---|---|---|
| TIL therapy | |||||||||
| NCT01174121 | Zacharakis | 2022 | Phase I/II | Heavily pretreated | Metastatic | 6 | TILs + pembrolizumab + IL-2 | Response in 3/6 patients, with one complete response and 2 partial responses | |
| CAR-T cell therapy | |||||||||
| NCT01837602 | Tchou | 2017 | Phase 0 | Previously treated | Metastatic | 6 | Intratumoral c-MET CAR-T cells |
No clinical responses seen six grade-3 AE; no CRS |
|
| NCT03060356 | Shah | 2023 | Phase I | Heavily pretreated | Metastatic TNBC | 4 | Intravenous C-MET CAR-T cells |
2/4 patients with stable disease No Grade 3 or higher AE |
|
| NCT04727151 | Schlecter | 2023 | Phase I | Heavily pretreated | Advanced HER2 + Solid Malignancy | T cell Antigen Coupler | 18 | TAC01-HER2 | Tolerable safety |
| TCR Therapy | |||||||||
| NCT03412877 | Kim | 2022 | Phase II | Heavily pretreated | Advanced Breast Cancer | HLA-A*02/p53R175H | 1 | HLA-A*O2-restricted TCR targeted p53R175H | Partial reponse lasting 6 mo |
| Selected Upcoming Clinical Trials | |||||||||
| NCT04111510 | Phase II | Second line | mTNBC | TIL LN-145 | 6 | TIL LN-145 | ORR | ||
| NCT05451784 | Phase I/II | Maximum 5 prior lines of therapy | a/m TNBC | TIL | 20 | PD-L1+ TILS | ORR and Safety | ||
| NCT03449108 | Phase II | Previously treated | solid tumors including TNBC | TIL LN-145-S1 | 30 | Ipilumumab + Nivolumab + cyclophosphamide + fludarabine + IL-2 + LN-145-S1 | ORR | ||
| NCT05576077 | Phase Ib | Previously treated | solid tumors including MBC | TIL TBio-4101 | 60 | TBio-4101 + Pembrolizumab | Safety | ||
| NCT02830724 | Phase I/II | Previously treated | solid tumors expressing CD70 | CD70 CAR-T cells | 124 | CD70 CAR-T cells | Safety + ORR | ||
| NCT06010862 | Phase I | Third line | solid tumors expressing CEA | CEA CAR-T cells | 36 | CEA CAR-T cells | Safety, Adverse Events | ||
| NCT06126406 | Phase I | Third line | solid tumors expressing CEA | CEA CAR-T cells | 60 | CEA CAR-T cells | Safety, Adverse Events | ||
| NCT06043466 | Phase I | Heavily pretreated | solid tumors expressing CEA | CEA CAR-T cells | 30 | CEA CAR-T cells | DLT | ||
| NCT04348643 | Phase I/II | Previously treated | solid tumors expressing CEA | CEA CAR-T cells | 40 | CEA CAR-T cells | Safety | ||
| NCT04107142 | Phase I | Previously treated | solid tumors, including TNBC | CTM-N2D CAR-T cells | 10 | CTM-N2D CAR-T cells | Safety | ||
| NCT02915445 | Phase I | Third line | solid tumors expressing EpCAM | EpCAM CAR-T cells | 30 | EpCAM CAR-T cells | DLT | ||
|
GAIL-N |
Phase I | Previously Treated | solid tumors expressing GD2 | GD2 CAR-T Cells | 94 | GD2 CAR-T Cells | Safety | ||
| NCT03696030 | Phase I | Previously treated | solid tumors expressing HER2 with brain and/or leptomeningeal metastases | HER2 CAR-T | 39 | intraventricular HER2-CAR T cells | DLT | ||
| NCT02442297 | Phase I | Previously treated | solid tumors expressing HER2 with brain metastasis | HER2 CAR-T cells | 10 | Intracranial HER2 CAR-T cells | Safety | ||
|
VISTA |
Phase I | Heavily pretreated | solid tumors expressing HER2 | HER2 CAR-T cells + oncolytic adenovirus | 45 | HER2 CAR-T cells + intratumoral CAdVEC | Safety | ||
|
TRAILBLASER |
Phase I | > First Line | Metastatic Breast Cancer (HER2 1+ , 2+ , or 3+) | HER2 and TR2 targeting CAR T cells + IL-15 | 27 |
Arm A: HTR2 T Cells (without lymphodepletion) Arm B: HTR2 T Cells (with lymphodepletion) |
DLT | ||
| NCT02414269 | Phase I/II | > First line | mesothelioma, breast or lung cancer with pleural disease | CAR-T cells expressing mesothelin | 113 |
Arm A: intrapleural iCasp9M28z CAR-T cells Arm A: intrapleural iCasp9M28z CAR-T cells + chemotherapy Arm A: intrapleural iCasp9M28z CAR-T cells + pembrolizumab |
AE and CBR | ||
| NCT02792114 | Phase I | > First line | HER2- MBC expressing mesothelin | Mesothelin-targeting CAR-T cells | 186 | Mesothelin-targeting CAR-T cells | MTD | ||
| NCT02792114 | Phase I | Previously treated | HER2- MBC expressing mesothelin | Mesothelin CAR-T cells | 186 | Mesothelin CAR-T | Safety | ||
| NCT02580747 | Phase I | Previously treated | solid tumors expressing mesothelin, including TNBC | Mesothelin CAR-T cells | 20 | Mesothelin CAR-T cells | Safety | ||
| NCT05623488 | Phase I | Previously treated | TNBC expressing mesothelin | Mesothelin CAR-T cells | 12 | huCART-meso CAR-T cells | Safety | ||
| NCT02587689 | Phase I/II | Heavily pretreated | solid tumors expressing MUC1, including TNBC | MUC1 CAR-T cells | 20 | MUC1 CAR-T cells | Safety | ||
| NCT04020575 | Phase I/II | Heavily pretreated | metastatic MUC1* + breast cancer | MUC-1 CAR-T | 69 | CAR T-targeting MUC1* | Safety | ||
| NCT05239143 | Phase I | Heavily pretreated | solid tumors expressing MUC1-c | MUC1 CAR-T cells | 100 |
Arm A: P-MUC1C-ALLO1 CAR-T cells in single ascending dose with chemo 1 Arm A: P-MUC1C-ALLO1 CAR-T cells in cyclical ascending dose with chemo 1 Arm A: P-MUC1C-ALLO1 CAR-T cells in single ascending dose with chemo 2 Arm A: P-MUC1C-ALLO1 CAR-T cells in cyclical ascending dose with chemo 2 |
Safety and Tolerability Prelininary Efficacy |
||
| NCT04430595 | Phase I/II | Previously treated | a/m breast cancer expressing GD2, CD44v6, or Her2 | Multispecific CAR-T cells | 100 | Multispecific CAR‐T cells targeting HER2, CD2 and CD44v6 | Safety | ||
| NCT02706392 | Phase I | Heavily pretreated | Metastatic TNBC expressing ROR1 | ROR1 CAR-T cells | 21 | ROR1 CAR-T cells | Safety | ||
| NCT04119024 | Phase I | Heavily pretreated | Metastatic Melanoma or solid tumors | IL13Ralpha2/CD19 | 18 | IL13Ralpha2 CAR T Cells | Safety, DLT | ||
| NCT05274451 | Phase I | Previously treated | solid tumors expressing ROR1 including TNBC | ROR1 CAR-T cells | 100 | LYL797 CAR-T cells | DLT | ||
| NCT04427449 | Phase I/II | Previously treated | advanced malignancy expressing CD44v6 | 4SCAR-CD44v6 CAR-T cells | 100 | 4SCAR-CD44v6 CAR-T cells | Safety + ORR | ||
| NCT05877599 | Phase I | Heavily pretreated | TP53 R175H mutant solid tumors including MBC | TCR | 162 | anti-HLA-A2/NT-175 | Safety | ||
| NCT05989828 | Phase Ib | Previously treated | Metastatic TNBC expressing NY-ESO-1 | TCR | 20 | cyclophosphamide + fludarabine + IL-2 + anti-HLA-A2/NY-ESO-1 TCR-T cells | MTD | ||
| NCT01967823 | Phase II | Previously treated | Metastatic solid tumors expressing NY-ESO-1, including breast cancer | TCR | 11 | cyclophosphamide + fludarabine + IL-2 + anti-HLA-A2/NY-ESO1 TCR-T cells | ORR | ||
| NCT05296564 | Phase I/II | Previously treated | Metastatic cancer expressing NY-ESO-1, including TNBC | TCR | 43 | cyclophosphamide + fludarabine + IL-2 + anti-HLA-A2/NY-ESO1 TCR-T cells | Safety, Response Rate | ||
| NCT03159585 | Phase I | Previously treated | Metastatic cancer expressing NY-ESO-1, including breast cancer | TCR | 6 | cyclophosphamide + fludarabine + anti-HLA-A2/NY-ESO1 TCR-T cells | Safety | ||
| NCT05035407 | Phase I | Previously Treated | solid tumors including MBC | TCR | 100 | cyclophosphamide + fludarabine + IL-2 + anti-HLA-A01/KK-LC-1 TCR | Safety | ||
| NCT06253520 | Phase I | Previously treated | solid tumors including breast cancer | TCR | 210 | cyclophosphamide + fludarabine + IL-2 + HLA restrcted/KRAS TCR-T cells + vaccine | Safety, Clinical Response Rate | ||
Future outlook and conclusions
The use of immunotherapy for the treatment of breast cancer has expanded over the last two decades. Pembrolizumab is a premier example of an immune checkpoint inhibitor that has changed treatment paradigms for TNBC, while ongoing clinical trials investigate the role of pembrolizumab and other immune checkpoint inhibitors in patients with HR+ and HER2+ disease. Antibody–drug conjugates such as sacitizumab govitecan and trastuzumab deruxtecan have ushered in a new generation of anti-cancer therapies, with ongoing studies evaluating new antibody–drug conjugates such as datopotamab deruxtecan as well as antibody–drug conjugates in combination with immune checkpoint inhibitors. With fourteen bispecifics approved for solid tumors to date, further studies of bispecifics such as zanidatamab and zenocutuzmab in breast cancer are needed to clarify the role for these agents in the current treatment paradigm. Future studies should investigate mechanisms to enhance the T cell response, whether through dual checkpoint inhibition and/or costimulatory signals, in both bispecifics and CAR-T therapy. Upcoming clinical trials bring hope for broadening the treatment landscape and further understanding the potential benefit of combination immune checkpoint inhibitors, antibody–drug conjugates, bispecific antibodies, and/or cellular therapies. Further research regarding biomarkers beyond PD-L1 expression and other characteristics of the tumor immune microenvironment will enhance our ability to predict which patients will respond to immune checkpoint inhibitor-containing treatments, allowing for more tailored therapy for each individual patient.
Abbreviations
- TIME
Tumor immune microenvironment
- PD-1
Programmed-death 1
- PD-L1
Programmed-death ligand 1
- CTLA4
Cytotoxic T-lymphocyte associate protein 4
- ICIs
Immune checkpoint inhibitors
- LAG-3
Lymphocyte activation gene 3
- ADC
Antibody drug conjugate
- TAA
Tumor associated antigen
- CPS
Combined positive score
- IC
Immune-cell score
- PD-L1+
Programmed-death ligand 1 positive
- TILs
Tumor infiltrating lymphocytes
- TMB
Tumor mutational burden
- dMMR
Deficiencies in mismatch repair genes
- MSI-H
Microsatellite instability-high
- irAE
Immune-related adverse events
- TNBC
Triple negative breast cancer
- HR+
Hormone receptor positive
- a/m
Advanced/metastatic
- ORR
Overall response rate
- PFS
Progression free survival
- OS
Overall survival
- HR
Hazard ratio
- DFI
Disease-free interval
- IIT
Intention-to-treat
- PARPi
Poly (ADP-ribose) polymerase inhibitors
- mBC
Metastatic breast cancer
- SG
Sacituzumab govitecan
- Dato-DXd
Datopotamab deruxtecan
- pCR
Pathologic complete response
- DFS
Disease-free survival
- RCB
Residual cancer burden
- EFS
Event-free survival
- Dd
Dose-dense
- AC
Doxorubicin and cyclophosphamide
- PC
Paclitaxel and carboplatin
- EC
Epirubicin and cyclophosphamide
- KLH
Keyhole limpet hemocyanin
- T-VEC
Talimogene iaherparepvec
- LV
Ladoratizimab vedotin
- CDK4/6i
CDK4/6 inhibitor
- ILD
Interstitial lung disease
- T-AC
Paclitaxel followed by doxorubicin and cyclophosphamide
- IHC
Immunohistochemical
- T-DXd
Trastuzumab deruxtecan
- T-Duo
Trastuzumab duocarmazine
- CAR-T
Chimeric antigen receptor
- TCR
T cell receptor engineered
- HLA
Human leukocyte antigen
- MHC
Major histocompatibility complex
- Afami-cel
Afamitresgene autoleucel
- Lete-cel
Letetresgene autoleucel
Author contributions
All three authors contributed to the preparation of the manuscript.
Funding
The authors report no funding for this research.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim SK, Cho SW. The evasion mechanisms of cancer immunity and drug intervention in the tumor microenvironment. Front pharmacol. 2022;13:868695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727–42. [PMC free article] [PubMed] [Google Scholar]
- 3.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wildiers H, Armstrong A, Cuypere E, Dalenc F, Dirix L, Chan S, et al. Paclitaxel plus Eftilagimod Alpha, a Soluble LAG-3 Protein, in Metastatic, HR+ Breast Cancer: Results from AIPAC, a Randomized, Placebo Controlled Phase IIb Trial. Clin Cancer Res. 2024;30(3):532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heater NK, Franco S, Shah A. Treatment of endocrine resistant metastatic breast cancer in the era of antibody drug conjugates. Annals of Translational Medicine. 2023;11(11):399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of <scp>DS</scp>-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmid P, Loi S, De La Cruz ML, Yerushalmi R, Im SA, Sonnenblick A, et al. 181O Interim analysis (IA) of the atezolizumab (atezo) + sacituzumab govitecan (SG) arm in patients (pts) with triple-negative breast cancer (TNBC) in MORPHEUS-pan BC: a phase Ib/II study of multiple treatment (tx) combinations in pts with locally advance. ESMO Open. 2024;9:103203. [Google Scholar]
- 8.Burke EE, Kodumudi K, Ramamoorthi G, Czerniecki BJ. Vaccine therapies for breast cancer. Surg Oncol Clin N Am. 2019;28(3):353–67. [DOI] [PubMed] [Google Scholar]
- 9.Mohebtash M, Tsang K-Y, Madan RA, Huen N-Y, Poole DJ, Jochems C, et al. A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2011;17(22):7164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. [DOI] [PubMed] [Google Scholar]
- 12.Núñez Abad M, Calabuig-Fariñas S, Lobo De Mena M, Torres-Martínez S, García González C, García García JÁ, et al. Programmed Death-Ligand 1 (PD-L1) as Immunotherapy Biomarker in Breast Cancer. Cancers. 2022;14(2):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erber R, Hartmann A. Understanding PD-L1 testing in breast cancer: a practical approach. Breast Care. 2020;15(5):481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott ML, Ratcliffe MJ, Sharpe A, Barker C, Scorer P, Rebelatto M, Al-Masri H, Walker J. Concordance of tumor cell (TC) and immune cell (IC) staining with Ventana SP142, Ventana SP263, Dako 28-8 and Dako 22C3 PD-L1 IHC tests in NSCLC patient samples. J Clin Oncol. 2017;35(15_suppl):e14503–e14503. 10.1200/JCO.2017.35.15_suppl.e14503. [Google Scholar]
- 15.Cortes J, Rugo HS, Cescon DW, Im S-A, Yusof MM, Gallardo C, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387(3):217–26. [DOI] [PubMed] [Google Scholar]
- 16.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–13. [DOI] [PubMed] [Google Scholar]
- 17.Pellegrino B, Musolino A, Llop-Guevara A, Serra V, De Silva P, Hlavata Z, et al. Homologous recombination repair deficiency and the immune response in breast cancer: a literature review. Transl Oncol. 2020;13(2):410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karn T, Denkert C, Weber KE, Holtrich U, Hanusch C, Sinn BV, et al. Tumor mutational burden and immune infiltration as independent predictors of response to neoadjuvant immune checkpoint inhibition in early TNBC in GeparNuevo. Ann Oncol. 2020;31(9):1216–22. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal C, Ben-Shachar R, Gao Y, Hyun SW, Rivers Z, Epstein C, et al. Assessment of tumor mutational burden and outcomes in patients with diverse advanced cancers treated with immunotherapy. JAMA Netw Open. 2023;6(5):e2311181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 2022;33(9):929–38. [DOI] [PubMed] [Google Scholar]
- 21.Allard B, Aspeslagh S, Garaud S, Dupont FA, Solinas C, Kok M, et al. Immuno-oncology-101: overview of major concepts and translational perspectives. Semin Cancer Biol. 2018;52:1–11. [DOI] [PubMed] [Google Scholar]
- 22.Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer JU, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30(8):1279–88. [DOI] [PubMed] [Google Scholar]
- 23.Loi S, Salgado R, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, et al. Tumor infiltrating lymphocyte stratification of prognostic staging of early-stage triple negative breast cancer. npj Breast Cancer. 2022. 10.1038/s41523-021-00362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol. 2020;17(8):504–15. [DOI] [PubMed] [Google Scholar]
- 25.Yin Q, Liuyun W, Han L, Zheng X, Tong R, Li L, et al. Immune-related adverse events of immune checkpoint inhibitors: a review. Front Immunol. 2023. 10.3389/fimmu.2023.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073–126. [DOI] [PubMed] [Google Scholar]
- 27.Bai R, Chen N, Li L, Du N, Bai L, Lv Z, et al. Mechanisms of cancer resistance to immunotherapy. Front oncol. 2020;10:1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Said SS, Ibrahim WN. Cancer resistance to immunotherapy: comprehensive insights with future perspectives. Pharmaceutics. 2023;15(4):1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LAG, et al. US Incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danziger N, Sokol ES, Graf RP, Hiemenz MC, Maule J, Parimi V, et al. Variable landscape of PD-L1 expression in breast carcinoma as detected by the DAKO 22C3 immunohistochemistry assay. Oncologist. 2023;28(4):319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacob SL, Huppert LA, Rugo HS. Role of immunotherapy in breast cancer. JCO Oncology Practice. 2023;19(4):167–79. [DOI] [PubMed] [Google Scholar]
- 32.Nanda R, Chow LQM, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34(21):2460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397–404. [DOI] [PubMed] [Google Scholar]
- 34.Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentiis M, Nanda R, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):405–11. [DOI] [PubMed] [Google Scholar]
- 35.Cortés J, Lipatov O, Im S-A, Gonçalves A, Lee KS, Schmid P, et al. KEYNOTE-119: Phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Ann Oncol. 2019;30:v859–60. 10.1093/annonc/mdz394.010. [Google Scholar]
- 36.Winer EP, Lipatov O, Im S-A, Goncalves A, Muñoz-Couselo E, Lee KS, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(4):499–511. [DOI] [PubMed] [Google Scholar]
- 37.Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im S-A, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. The Lancet. 2020;396(10265):1817–28. [DOI] [PubMed] [Google Scholar]
- 38.Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer. JAMA Oncol. 2019;5(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams S, Diamond JR, Hamilton E, Pohlmann PR, Tolaney SM, Chang C-W, et al. Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up. JAMA Oncol. 2019;5(3):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios C, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32(8):994–1004. [DOI] [PubMed] [Google Scholar]
- 41.Franzoi MA, De Azambuja E. Atezolizumab in metastatic triple-negative breast cancer: IMpassion130 and 131 trials—How to explain different results? ESMO Open. 2020;5(6):e001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dent R, André F, Gonçalves A, Martin M, Schmid P, Schütz F, et al. IMpassion132 double-blind randomised phase III trial of chemotherapy with or without atezolizumab for early relapsing unresectable locally advanced or metastatic triple-negative breast cancer. Ann Oncol. 2024;35(7):630–42. [DOI] [PubMed] [Google Scholar]
- 43.Gion M, Cortez-Castedo P, Blancas I, Cortés A, Marmé F, Blanch S, et al. Abstract PS16-02: Efficacy and safety of first-line atezolizumab + bevacizumab + paclitaxel in patients with advanced triple-negative breast cancer: the ATRACTIB phase 2 trial. Cancer Res. 2024;84(9_Supplement):PS16-02-PS16-02. 10.1158/1538-7445.SABCS23-PS16-02. [Google Scholar]
- 44.Ozaki Y, Tsurutani J, Mukohara T, Iwasa T, Takahashi M, Tanabe Y, et al. Safety and efficacy of nivolumab plus bevacizumab, paclitaxel for HER2-negative metastatic breast cancer: Primary results and biomarker data from a phase 2 trial (WJOG9917B). Eur J Cancer. 2022;171:193–202. [DOI] [PubMed] [Google Scholar]
- 45.Santa-Maria CA, Kato T, Park J-H, Kiyotani K, Rademaker A, Shah AN, et al. A pilot study of durvalumab and tremelimumab and immunogenomic dynamics in metastatic breast cancer. Oncotarget. 2018;9(27):18985–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams S, Othus M, Patel SP, Miller KD, Chugh R, Schuetze SM, et al. A multicenter phase II trial of ipilimumab and nivolumab in unresectable or metastatic metaplastic breast cancer: cohort 36 of dual Anti–CTLA-4 and Anti–PD-1 blockade in rare tumors (DART, SWOG S1609). Clin Cancer Res. 2022;28(2):271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Domchek SM, Postel-Vinay S, Im S-A, Park YH, Delord J-P, Italiano A, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020;21(9):1155–64. [DOI] [PubMed] [Google Scholar]
- 48.Fanucci KA, Pilat MJ, Derek Shyr Y, Shyr SA, Boerner DD, et al. Abstract CT145: Olaparib +/- atezolizumab in patients with BRCA-mutated (BRCAmt) locally advanced unresectable or metastatic (advanced) breast cancer: an open-label, multicenter, randomized phase II trial. Cancer Res. 2023;83(8_Supplement):CT145–CT145. 10.1158/1538-7445.AM2023-CT145. [Google Scholar]
- 49.Vinayak S, Tolaney SM, Schwartzberg L, Mita M, McCann G, Tan AR, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 2019;5(8):1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan TJ, Sammons S, Im Y-H, She L, Mundy K, Bigelow R, et al. Phase II DORA study of olaparib with or without durvalumab as a chemotherapy-free maintenance strategy in platinum-pretreated advanced triple-negative breast cancer. Clin Cancer Res. 2024;30(7):1240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rugo H, Robson M, Im SA, Dalenc F, Ruiz EY, Im YH, et al. Abstract GS01-05: Pembrolizumab + olaparib vs pembrolizumab + chemotherapy after induction with pembrolizumab + chemotherapy for locally recurrent inoperable or metastatic TNBC: randomized open-label phase 2 KEYLYNK-009 study. Cancer Res. 2024;84(9_Supplement):GS01-05-GS01-05. 10.1158/1538-7445.SABCS23-GS01-05. [Google Scholar]
- 52.Cussac AL, Rugo HS, Robson ME, Im SA, Dalenc F, Ruiz EY, et al. 198P Pembrolizumab plus olaparib vs pembrolizumab plus chemotherapy after induction with pembrolizumab plus chemotherapy for locally recurrent inoperable or metastatic TNBC: patient-reported outcomes from KEYLYNK-009. ESMO Open. 2024;9:103220. [Google Scholar]
- 53.Bachelot T, Filleron T, Bieche I, Arnedos M, Campone M, Dalenc F, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: the randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021;27(2):250–5. [DOI] [PubMed] [Google Scholar]
- 54.Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–41. [DOI] [PubMed] [Google Scholar]
- 55.Bardia A, Rugo HS, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Final results from the randomized phase III ASCENT clinical trial in metastatic triple-negative breast cancer and association of outcomes by human epidermal growth factor receptor 2 and trophoblast cell surface antigen 2 expression. J Clin Oncol. 2024;42(15):1738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bardia A, Krop IE, Kogawa T, Juric D, Tolcher AW, Hamilton EP, et al. Datopotamab deruxtecan in advanced or metastatic HR+/HER2– and triple-negative breast cancer: results from the phase I TROPION-pantumor01 study. J Clin Oncol. 2024;42(19):2281–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmid P, Wysocki PJ, Ma CX, Park YH, Fernandes R, Lord S, et al. 379MO Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): updated results from BEGONIA, a phase Ib/II study. Ann Oncol. 2023;34:S337. [Google Scholar]
- 58.Giordano A, Awan AAA, Bruce JY, Rugo HS, Diamond JR, Novik Y, et al. Enfortumab vedotin (EV) in triple-negative breast cancer (TNBC) and HR+/HER2- breast cancer (BC) cohorts of EV-202. J Clin Oncol. 2024;42(16_suppl):1005–1005. 10.1200/JCO.2024.42.16_suppl.1005. [Google Scholar]
- 59.Binghe X, Yin Y, Fan Y, Ouyang Q, Song L, Wang X, et al. Sacituzumab tirumotecan (SKB264/MK-2870) in patients (pts) with previously treated locally recurrent or metastatic triple-negative breast cancer (TNBC): results from the phase III OptiTROP-Breast01 study. J Clin Oncol. 2024;42(16_suppl):104–104. 10.1200/JCO.2024.42.16_suppl.104. [Google Scholar]
- 60.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet. 2014;384(9938):164–72. [DOI] [PubMed] [Google Scholar]
- 61.Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res. 2020;26(12):2838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yau C, Osdoit M, Van Der Noordaa M, Shad S, Wei J, De Croze D, et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022;23(1):149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmid P, Salgado R, Park YH, Muñoz-Couselo E, Kim SB, Sohn J, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31(5):569–81. [DOI] [PubMed] [Google Scholar]
- 64.Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer. JAMA Oncol. 2020;6(5):676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu MC, Robinson PA, Yau C, Wallace AM, Jo Chien A, Stringer-Reasor E, et al. Abstract P3-09-02: Evaluation of a novel agent plus standard neoadjuvant therapy in early stage, high-risk HER2 negative breast cancer: results from the I-SPY 2 TRIAL. Can Res. 2020;80(4_Supplement):P3-09-02-P3-09-02. 10.1158/1538-7445.SABCS19-P3-09-02. [Google Scholar]
- 66.Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21. [DOI] [PubMed] [Google Scholar]
- 67.Schmid P, Cortés J, Dent R, Pusztai L, McArthur H, Küemmel S, et al. Abstract LBO1-01: Neoadjuvant pembrolizumab or placebo plus chemotherapy followed by adjuvant pembrolizumab or placebo for early-stage triple-negative breast cancer: Updated event-free survival results from the phase 3 KEYNOTE-522 study. Cancer Res. 2024;84(9_Supplement):LBO1-01-LBO1-01. 10.1158/1538-7445.SABCS23-LBO1-01. [Google Scholar]
- 68.Pusztai L, Denkert C, O’Shaughnessy J, Cortes J, Dent R, McArthur H, et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: exploratory analysis from KEYNOTE-522. Ann Oncol. 2024;35(5):429–36. [DOI] [PubMed] [Google Scholar]
- 69.Schmid P, Cortes J, Dent R, (eds) (2024) LBA4: Neoadjuvant pembrolizumab or placebo plus chemotherapy followed by adjuvant pembrolizumab or placebo for high-risk early-stage TNBC: overall survival results from the phase III KEYNOTE-522 study. 2024 ESMO Congress; Barcelona, Spain.
- 70.Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556–67. [DOI] [PubMed] [Google Scholar]
- 71.Sharma P, Stecklein SR, Yoder R, Staley JM, Schwensen K, O’Dea A, et al. Clinical and biomarker findings of neoadjuvant pembrolizumab and carboplatin plus docetaxel in triple-negative breast cancer. JAMA Oncol. 2024;10(2):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ademuyiwa FO, Gao F, Street CR, Chen I, Northfelt DW, Wesolowski R, et al. A randomized phase 2 study of neoadjuvant carboplatin and paclitaxel with or without atezolizumab in triple negative breast cancer (TNBC) - NCI 10013. npj Breast Cancer. 2022. 10.1038/s41523-022-00500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 tria. The Lancet. 2020;396(10257):1090–100. [DOI] [PubMed] [Google Scholar]
- 74.Gianni L, Huang CS, Egle D, Bermejo B, Zamagni C, Thill M, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol. 2022;33(5):534–43. [DOI] [PubMed] [Google Scholar]
- 75.Loibl S, Schneeweiss A, Huober J, Braun M, Rey J, Blohmer JU, et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol. 2022;33(11):1149–58. [DOI] [PubMed] [Google Scholar]
- 76.Pusztai L, Yau C, Wolf DM, Han HS, Du L, Wallace AM, et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: results from the adaptively randomized I-SPY2 trial. Cancer Cell. 2021;39(7):989-98.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iwase T, Blenman KRM, Li X, Reisenbichler E, Seitz R, Hout D, et al. A novel immunomodulatory 27-gene signature to predict response to neoadjuvant immunochemotherapy for primary triple-negative breast cancer. Cancers. 2021;13(19):4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolf DM, Yau C, Campbell MJ, Glas AM, Mittempergher L, Kuilman MM, et al. Biomarkers predicting response to 5 immunotherapy arms in the neoadjuvant I-SPY2 trial for early-stage breast cancer (BC): evaluation of immune subtyping in the response predictive subtypes (RPS). J Clin Oncol. 2023;41(16_suppl):102–102. 10.1200/JCO.2023.41.16_suppl.102. [Google Scholar]
- 79.Spring LM, Tolaney SM, Fell G, Bossuyt V, Abelman RO, Wu B, et al. Response-guided neoadjuvant sacituzumab govitecan for localized triple-negative breast cancer: results from the NeoSTAR trial. Ann Oncol. 2024;35(3):293–301. [DOI] [PubMed] [Google Scholar]
- 80.Oliveira M, Pascual T, Ortega PT, Vila MM, Cejalvo JM, Jurado JC, et al. 124O Patritumab deruxtecan (HER3-DXd) in hormonal receptor-positive/HER2-negative (HR+/HER2-) and triple-negative breast cancer (TNBC): results of part B of SOLTI TOT-HER3 window of opportunity trial. ESMO Open. 2023;8(1):101463. [Google Scholar]
- 81.Loi S, Niman S, Zdenkowski N, Francis P, Hay SB, Fox W, et al. Abstract LBO1-03: randomized phase II study of neoadjuvant nivolumab (N) 2 week lead-in followed by 12 weeks of concurrent N+ carboplatin plus paclitaxel (CbP) vs concurrent N+ CbP in Triple negative breast cancer (TNBC):(BCT1902/IBCSG 61–20 Neo-N). Cancer Res. 2024;84:LBO1-03. [Google Scholar]
- 82.Nederlof I, Isaeva OI, Bakker N, De Graaf M, Salgado RF, Klioueva N, et al. LBA13 Nivolumab and ipilimumab in early-stage triple negative breast cancer (TNBC) with tumor-infiltrating lymphocytes (TILs): First results from the BELLINI trial. Ann Oncol. 2022;33:S1382. [Google Scholar]
- 83.Loi S, Francis PA, Zdenkowski N, Gebski V, Fox SB, White M, et al. Neoadjuvant ipilimumab and nivolumab in combination with paclitaxel following anthracycline-based chemotherapy in patients with treatment resistant early-stage triple-negative breast cancer (TNBC): A single-arm phase 2 trial. J Clin Oncol. 2022;40:602. [Google Scholar]
- 84.Loi S, Francis PA, Zdenkowski N, Gebski V, Fox SB, White M, et al. Phase II study of neoadjuvant ipilimumab and nivolumab in combination with paclitaxel following anthracycline-based chemotherapy in patients with treatment resistant stage III triple negative breast cancer (TNBC): BCT1702—survival results. J Clin Oncol. 2024;42:608. [Google Scholar]
- 85.Ignatiadis M, Bailey A, McArthur H, El-Abed S, De Azambuja E, Metzger O, et al. Abstract GS01-03: Adding atezolizumab to adjuvant chemotherapy for stage II and III triple-negative breast cancer is unlikely to improve efficacy: interim analysis of the ALEXANDRA/IMpassion030 phase 3 trial. Cancer Res. 2024;84:GS01-03. [Google Scholar]
- 86.Miles D, Roché H, Martin M, Perren TJ, Cameron DA, Glaspy J, et al. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. oncologist. 2011;16(8):1092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang C-S, Yu AL, Tseng L-M, Chow LWC, Hou M-F, Hurvitz SA, et al. Globo H-KLH vaccine adagloxad simolenin (OBI-822)/OBI-821 in patients with metastatic breast cancer: phase II randomized, placebo-controlled study. J Immunother Cancer. 2020;8(2):e000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rugo HS, Cortes J, Barrios CH, Cabrera P, Xu B, Huang C-S, et al. GLORIA: phase III, open-label study of adagloxad simolenin/OBI-821 in patients with high-risk triple-negative breast cancer. Future Oncol. 2022;18(34):3801–13. [DOI] [PubMed] [Google Scholar]
- 89.Heery CR, Ibrahim NK, Arlen PM, Mohebtash M, Murray JL, Koenig K, et al. Docetaxel alone or in combination with a therapeutic cancer vaccine (PANVAC) in patients with metastatic breast cancer. JAMA Oncol. 2015;1(8):1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bernstein V, Ellard SL, Dent SF, Tu D, Mates M, Dhesy-Thind SK, et al. A randomized phase II study of weekly paclitaxel with or without pelareorep in patients with metastatic breast cancer: final analysis of Canadian Cancer Trials Group IND.213. Breast Cancer Res Treat. 2018;167(2):485–93. 10.1007/s10549-017-4538-4. [DOI] [PubMed] [Google Scholar]
- 91.Manso L, Villagrasa P, Chic N, Cejalvo JM, Izarzugaza Y, Cantos B, et al. 41P A window-of-opportunity study with atezolizumab and the oncolityc virus pelareorep in early breast cancer (REO-027, AWARE-1). Ann Oncol. 2020;31:S30. [Google Scholar]
- 92.Soliman H, Hogue D, Han H, Mooney B, Costa R, Lee MC, et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial. Nat Med. 2023;29(2):450–7. [DOI] [PubMed] [Google Scholar]
- 93.Rugo HS, Delord J-P, Im S-A, Ott PA, Piha-Paul SA, Bedard PL, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer. Clin Cancer Res. 2018;24(12):2804–11. [DOI] [PubMed] [Google Scholar]
- 94.Tolaney SM, Barroso-Sousa R, Keenan T, Li T, Trippa L, Vaz-Luis I, et al. Effect of Eribulin with or without pembrolizumab on progression-free survival for patients with hormone receptor-positive, ERBB2-negative metastatic breast cancer. JAMA Oncol. 2020;6(10):1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rugo HS, Bardia A, Marmé F, Cortes J, Schmid P, Loirat D, et al. Sacituzumab govitecan in hormone receptor–positive/human epidermal growth factor receptor 2–negative metastatic breast cancer. J Clin Oncol. 2022;40(29):3365–76. [DOI] [PubMed] [Google Scholar]
- 96.Rugo HS, Bardia A, Marmé F, Cortés J, Schmid P, Loirat D, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. The Lancet. 2023;402(10411):1423–33. [DOI] [PubMed] [Google Scholar]
- 97.Garrido-Castro AC, Kim SE, Desrosiers J, Nanda R, Carey LA, et al. SACI-IO HR+: A randomized phase II trial of sacituzumab govitecan with or without pembrolizumab in patients with metastatic hormone receptor-positive/HER2-negative breast cancer. J Clin Oncol. 2024;42(17_suppl):LBA1004–LBA1004. 10.1200/JCO.2024.42.17_suppl.LBA1004. [Google Scholar]
- 98.Bardia A, Jhaveri K, Im SA, Pernas Simon S, De Laurentiis M, Wang S, et al. LBA11 Datopotamab deruxtecan (Dato-DXd) vs chemotherapy in previously-treated inoperable or metastatic hormone receptor-positive, HER2-negative (HR+/HER2–) breast cancer (BC): Primary results from the randomised phase III TROPION-Breast01 trial. Ann Oncol. 2023;34:S1264–5. [Google Scholar]
- 99.Datopotamab deruxtecan final overall survival results reported in patients with metastatic HR-positive, HER2-low or negative breast cancer in TROPION-Breast01 Phase III trial [press release]. AstraZeneca, Spetember, 23, 2024.
- 100.Pistilli B, Ibrahimi N, Lacroix-Triki M, D’Hondt V, Vicier C, Frenel JS, et al. 189O A phase II study of patritumab deruxtecan (HER3-DXd), in patients (pts) with advanced breast cancer (ABC), with biomarker analysis to characterize response to therapy (ICARUS-BREAST01). ESMO Open. 2023;8(1):101378. [Google Scholar]
- 101.Rugo HS, Peter Kabos J, Beck T, Jerusalem G, Wildiers H, Sevillano E, et al. Abemaciclib in combination with pembrolizumab for HR+, HER2− metastatic breast cancer: Phase 1b study. npj Breast Cancer. 2022. 10.1038/s41523-022-00482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Masuda J, Sakai H, Tsurutani J, Tanabe Y, Masuda N, Iwasa T, et al. Efficacy, safety, and biomarker analysis of nivolumab in combination with abemaciclib plus endocrine therapy in patients with HR-positive HER2-negative metastatic breast cancer: a phase II study (WJOG11418B NEWFLAME trial). J Immunother Cancer. 2023;11(9):e007126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jerusalem G, Prat A, Salgado R, Reinisch M, Saura C, Ruiz-Borrego M, et al. Neoadjuvant nivolumab + palbociclib + anastrozole for oestrogen receptor-positive/human epidermal growth factor receptor 2-negative primary breast cancer: results from CheckMate 7A8. The Breast. 2023;72:103580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mayer EL, Ren Y, Wagle N, Mahtani R, Ma C, Demichele A, et al. PACE: a randomized phase II study of fulvestrant, palbociclib, and avelumab after progression on cyclin-dependent kinase 4/6 inhibitor and aromatase inhibitor for hormone receptor–positive/human epidermal growth factor receptor-negative metastatic breast. J Clin Oncol. 2024;42(17):2050–60. [DOI] [PubMed] [Google Scholar]
- 105.Cardoso F, O’Shaughnessy J, McArthur H, et al. Abstract GS01-02: Phase 3 study of neoadjuvant pembrolizumab or placebo plus chemotherapy, followed by adjuvant pembrolizumab or placebo plus endocrine therapy for early-stage high-risk ER+/HER2− breast cancer: KEYNOTE-756. Cancer Res. 2024;84(9_Supplement):GS01-02-GS01-02. 10.1158/1538-7445.SABCS23-GS01-02. [Google Scholar]
- 106.Dieci MV, Guarneri V, Tosi A, Bisagni G, Musolino A, Spazzapan S, et al. Neoadjuvant chemotherapy and immunotherapy in luminal B-like breast cancer: results of the phase II GIADA trial. Clin Cancer Res. 2022;28(2):308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Loi S, Curigliano G, Salgado R, Díaz RIR, Delaloge S, García CIR, et al. Abstract GS01-01: Biomarker results in high-risk estrogen receptor positive, human epidermal growth factor receptor 2 negative primary breast cancer following neoadjuvant chemotherapy ± nivolumab: an exploratory analysis of CheckMate 7FL. Cancer Res. 2024;84(9_Supplement):GS01-01-GS01-01. 10.1158/1538-7445.SABCS23-GS01-01. [Google Scholar]
- 108.Trivedi MS, Shatsky RA, Nanda R, Rugo HS, Omene C, Kalinsky K, et al. LBA15 Rates of pathologic complete response (pCR) after datopotamab deruxtecan (Dato) plus durvalumab (Durva) treatment strategy in the neoadjuvant setting: results from the I-SPY 2.2 trial. Annals of Oncol. 2024;35:S1208–9. 10.1016/j.annonc.2024.08.2253. [Google Scholar]
- 109.Modi S, Tsurutani J, Tamura K, Park H, Sagara Y, Murthy R, et al. Abstract P6-17-02: Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-low expressing breast cancer: updated results of a large phase 1 study. Cancer Res. 2019;79(4_Supplement):P6-17-02-P6-17-02. 10.1158/1538-7445.SABCS18-P6-17-02. [Google Scholar]
- 110.Padmanabhan R, Kheraldine HS, Meskin N, Vranic S, Al Moustafa A-E. Crosstalk between HER2 and PD-1/PD-L1 in breast cancer: from clinical applications to mathematical models. Cancers. 2020;12(3):636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019;20(3):371–82. [DOI] [PubMed] [Google Scholar]
- 112.Loibl S, Mano M, Untch M, Huang CS, Mamounas E, Wolmark N, et al. Abstract GS03-12: Phase III study of adjuvant ado-trastuzumab emtansine vs trastuzumab for residual invasive HER2-positive early breast cancer after neoadjuvant chemotherapy and HER2-targeted therapy: KATHERINE final IDFS and updated OS analysis. Cancer Res. 2024;84(9_Supplement):GS03-12-GS03-12. 10.1158/1538-7445.SABCS23-GS03-12. [Google Scholar]
- 113.Waks AG, Keenan TE, Li T, Tayob N, Wulf GM, Richardson ET, et al. Phase Ib study of pembrolizumab in combination with trastuzumab emtansine for metastatic HER2-positive breast cancer. J Immunother Cancer. 2022;10(10):e005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hamilton EP, Kaklamani V, Falkson C, Vidal GA, Ward PJ, Patre M, et al. Impact of Anti-HER2 treatments combined with atezolizumab on the tumor immune microenvironment in early or metastatic breast cancer: results from a phase Ib study. Clin Breast Cancer. 2021;21(6):539–51. [DOI] [PubMed] [Google Scholar]
- 115.Emens LA, Esteva FJ, Beresford M, Saura C, De Laurentiis M, Kim S-B, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21(10):1283–95. [DOI] [PubMed] [Google Scholar]
- 116.Cortés J, Kim S-B, Chung W-P, Im S-A, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–54. [DOI] [PubMed] [Google Scholar]
- 117.Hurvitz SA, Hegg R, Chung W-P, Im S-A, Jacot W, Ganju V, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. The Lancet. 2023;401(10371):105–17. [DOI] [PubMed] [Google Scholar]
- 118.Cortés J, Hurvitz SA, Im S-A, Iwata H, Curigliano G, Kim S-B, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer: long-term survival analysis of the DESTINY-breast03 trial. Nat Med. 2024;30(8):2208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hurvitz SA, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer patients with brain metastases from the randomized DESTINY-Breast03 trial. ESMO Open. 2024;9(5):102924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smit EF, Felip E, Uprety D, Nagasaka M, Nakagawa K, Paz-Ares Rodríguez L, et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 2024;25(4):439–54. [DOI] [PubMed] [Google Scholar]
- 121.Raghav KPS, Siena S, Takashima A, Kato T, Van Den Eynde M, et al. Trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-overexpressing/amplified (HER2+) metastatic colorectal cancer (mCRC): primary results from the multicenter, randomized, phase 2 DESTINY-CRC02 study. J Clin Oncol. 2023;41(16_suppl):3501–3501. 10.1200/JCO.2023.41.16_suppl.3501. [Google Scholar]
- 122.Meric-Bernstam F, Makker V, Oaknin A, Oh D-Y, Banerjee S, González-Martín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol. 2024;42(1):47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aftimos PG, Turner N, O’Shaughnessy J, Van Den Tweel E, Oesterholt M, Escrivá-De-Romaní S, et al. 386MO Trastuzumab duocarmazine versus physician’s choice therapy in pre-treated HER2-positive metastatic breast cancer: Final results of the phase III TULIP trial. Ann Oncol. 2023;34:S340–1. [Google Scholar]
- 124.Mosele F, Deluche E, Lusque A, Le Bescond L, Filleron T, Pradat Y, et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: the phase 2 DAISY trial. Nat Med. 2023;29(8):2110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low–expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38(17):1887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Curigliano G, Xichun H, Dent RA, Yonemori K, Barrios CH, O’Shaughnessy J, et al. Trastuzumab deruxtecan (T-DXd) vs physician’s choice of chemotherapy (TPC) in patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2)-low or HER2-ultralow metastatic breast cancer (mBC) with prior endocrine. J Clin Oncol. 2024;42(17_suppl):LBA1000–LBA1000. 10.1200/JCO.2024.42.17_suppl.LBA1000. [Google Scholar]
- 127.Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schmid P, Wysocki P, Park YH, Jassem J, Jung KH, Lord S, et al. Abstract PD11-08: PD11-08 Trastuzumab deruxtecan (T-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic hormone receptor-negative (HR−), HER2-low breast cancer: updated results from BEGONIA, a phase 1b/2 study. Cancer Res. 2023;83(5_Supplement):PD11-08-PD11-08. 10.1158/1538-7445.SABCS22-PD11-08. [Google Scholar]
- 129.Kuemmel S, Gluz O, Reinisch M, et al. Abstract PD10-11: Keyriched-1- A prospective, multicenter, open label, neoadjuvant phase ii single arm study with pembrolizumab in combination with dual anti-HER2 blockade with trastuzumab and pertuzumab in early breast cancer patients with molecular HER2. Can Res. 2022;82(4_Supplement):PD10-11-PD10-11. 10.1158/1538-7445.SABCS21-PD10-11. [Google Scholar]
- 130.Huober J, Barrios CH, Niikura N, Jarząb M, Chang Y-C, Huggins-Puhalla SL, et al. Atezolizumab With neoadjuvant anti-human epidermal growth factor receptor 2 therapy and chemotherapy in human epidermal growth factor receptor 2–positive early breast cancer: primary results of the randomized phase III IMpassion050 trial. J Clin Oncol. 2022;40(25):2946–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mittendorf EA, Clifton GT, Holmes JP, Schneble E, Van Echo D, Ponniah S, et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol. 2014;25(9):1735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mittendorf EA, Lu B, Melisko M, Price Hiller J, Bondarenko I, Brunt AM, et al. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase III Clinical trial. Clin Cancer Res. 2019;25(14):4248–54. [DOI] [PubMed] [Google Scholar]
- 133.Brown TA, Mittendorf EA, Hale DF, Myers JW, Peace KM, Jackson DO, et al. Prospective, randomized, single-blinded, multi-center phase II trial of two HER2 peptide vaccines, GP2 and AE37, in breast cancer patients to prevent recurrence. Breast Cancer Res Treat. 2020;181(2):391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lan HR, Chen M, Yao SY, Chen JX, Jin KT. Bispecific antibodies revolutionizing breast cancer treatment: a comprehensive overview. Front Immunol. 2023. 10.3389/fimmu.2023.1266450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera J-M, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meric-Bernstam F, Beeram M, Hamilton E, Oh D-Y, Hanna DL, Kang Y-K, et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol. 2022;23(12):1558–70. [DOI] [PubMed] [Google Scholar]
- 137.Wang X, Lee KS, Zeng X, Sun T, Im YH, Li H, Wang K, Li H, et al. Zanidatamab (zani), a HER2-targeted bispecific antibody, in combination with docetaxel as first-line therapy (1L) for patients (pts) with advanced HER2-positive breast cancer (BC): Updated results from a phase 1b/2 study. J Clin Oncol. 2023;41(16_suppl):1044–1044. 10.1200/JCO.2023.41.16_suppl.1044. [Google Scholar]
- 138.Escrivá-de-Romani S, Alba E, Rodríguez-Lescure Á, Hurvitz S, Cejalvo JM, Gión M, et al. Abstract PD18-10: Treatment of HER2-positive (HER2+) hormone-receptor positive (HR+) metastatic breast cancer (mBC) with the novel combination of zanidatamab, palbociclib, and fulvestrant. Cancer Res. 2023;83(5_Supplement):PD18-10-PD18-10. 10.1158/1538-7445.SABCS22-PD18-10. [Google Scholar]
- 139.Valero V, Mouabbi J, Alonzo H, Ilheme AN, Murthy R, Huang X, et al. 132P Neoadjuvant zanidatamab for stage I node-negative HER2- positive breast cancer (BC). ESMO Open. 2023;8(1):101471. [Google Scholar]
- 140.Zhang J, Ji D, Cai L, Yao H, Yan M, Wang X, et al. First-in-human HER2-targeted bispecific antibody KN026 for the treatment of patients with HER2-positive metastatic breast cancer: results from a phase I study. Clin Cancer Res. 2022;28(4):618–28. [DOI] [PubMed] [Google Scholar]
- 141.Liu J, Song C, Wang X, Ni M, Wang X, Chen L, et al. Abstract P5-16-04: preliminary safety and efficacy results of KN046 (an anti-PD-L1/CTLA-4 bispecific antibody) in combination with KN026 (a HER2-targeted bispecific antibody) in patients with metastatic HER2-positive breast cancer: a phase II trial. Cancer Res. 2022;82(4_Supplement):P5-16-04-P5-16-04. 10.1158/1538-7445.SABCS21-P5-16-04. [Google Scholar]
- 142.Ma L, Yang B, Zhang M, Wang K, Chen Y, Fan Z, et al. 247P KN026 in combination with docetaxel as neoadjuvant treatment for HER2+ early or locally advanced breast cancer (BC): a single-arm, multicenter, phase II study. Ann Oncol. 2023;34:S282. [Google Scholar]
- 143.Pistilli B, Wildiers H, Hamilton EP, Ferreira AA, Dalenc F, Vidal M, et al. Clinical activity of MCLA-128 (zenocutuzumab) in combination with endocrine therapy (ET) in ER+/HER2-low, non-amplified metastatic breast cancer (MBC) patients (pts) with ET-resistant disease who had progressed on a CDK4/6 inhibitor (CDK4/6i). J Clin Oncol. 2020;38(15_suppl):1037–1037. 10.1200/JCO.2020.38.15_suppl.1037. [Google Scholar]
- 144.Hamilton EP, Petit T, Pistilli B, Goncalves A, Ferreira AA, Dalenc F, et al. Clinical activity of MCLA-128 (zenocutuzumab), trastuzumab, and vinorelbine in HER2 amplified metastatic breast cancer (MBC) patients (pts) who had progressed on anti-HER2 ADCs. J Clin Oncol. 2020;38(15_suppl):3093–3093. 10.1200/JCO.2020.38.15_suppl.3093. [Google Scholar]
- 145.Beeram M, Denlinger CS, Tolcher AW, Goldstein LJ, Patnaik A, Papadopoulos K, et al. Abstract P6-15-15: MM-111 - a novel bispecific antibody targeting HER-2/HER-3 Z heterodimer: safety and tolerability in a first-in human phase I/II study in patients with refractory HER2-positive (HER-2+) cancers. Cancer Res. 2010;70(24_Supplement):P6-15-15-P6-15-15. 10.1158/0008-5472.SABCS10-P6-15-15. [Google Scholar]
- 146.Richards DA, Braiteh FS, Garcia AA, Denlinger CS, Conkling PR, Edenfield WJ, et al. A phase 1 study of MM-111, a bispecific HER2/HER3 antibody fusion protein, combined with multiple treatment regimens in patients with advanced HER2-positive solid tumors. J Clin Oncol. 2014;32(15_suppl):651–651. 10.1200/jco.2014.32.15_suppl.651. [Google Scholar]
- 147.Haense N, Atmaca A, Pauligk C, Steinmetz K, Marmé F, Haag GM, et al. A phase I trial of the trifunctional anti Her2 × anti CD3 antibody ertumaxomab in patients with advanced solid tumors. BMC Cancer. 2016. 10.1186/s12885-016-2449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ruiz IR, Vicario R, Morancho B, Morales CB, Arenas EJ, Herter S, et al. p95HER2–T cell bispecific antibody for breast cancer treatment. Sci Trans Med. 2018. 10.1126/scitranslmed.aat1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wermke M, Alt J, Kauh JS, Back J, Salhi Y, Reddy V, et al. Preliminary biomarker and pharmacodynamic data from a phase I study of single-agent bispecific antibody T-cell engager GBR 1302 in subjects with HER2-positive cancers. J Clin Oncol. 2018;36(5_suppl):69–69. 10.1200/JCO.2018.36.5_suppl.69. [Google Scholar]
- 150.Lum LG, Al-Kadhimi Z, Deol A, Kondadasula V, Schalk D, Tomashewski E, et al. Phase II clinical trial using anti-CD3 × anti-HER2 bispecific antibody armed activated T cells (HER2 BATs) consolidation therapy for HER2 negative (0–2+) metastatic breast cancer. J Immunother Cancer. 2021;9(6):e002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lum LG, Thakur A, Al-Kadhimi ZS, Colvin GA, Cummings FJ, Legare RD, et al. Long-term follow up for a phase I trial HER2/neu-targeted T cells in women with advanced breast cancer. J Clin Oncol. 2014;32(15_suppl):3075–3075. 10.1200/jco.2014.32.15_suppl.3075.25092783 [Google Scholar]
- 152.Zheng Y, Li S, Tang H, Meng X, Zheng Q. Molecular mechanisms of immunotherapy resistance in triple-negative breast cancer. Front Immunol. 2023. 10.3389/fimmu.2023.1153990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sarnaik AA, Hamid O, Khushalani NI, Lewis KD, Medina T, Kluger HM, et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, metastatic melanoma. J Clin Oncol. 2021;39(24):2656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.FDA Approves First Cellular Therapy to Treat Patients with Unresectable or Metastatic Melanoma: FDA; February 26, 2024 [Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-cellular-therapy-treat-patients-unresectable-or-metastatic-melanoma.
- 156.Medina T, Chesney JA, Whitman E, Kluger H, Thomas S, Sarnaik A, et al. 119O Long-term efficacy and patterns of response of lifileucel tumor-infiltrating lymphocyte (TIL) cell therapy in patients with advanced melanoma: A 4-year analysis of the C-144-01 study. Immuno-Oncol Technol. 2023;20:100591. [Google Scholar]
- 157.Zacharakis N, Huq LM, Seitter SJ, Kim SP, Gartner JJ, Sindiri S, et al. Breast cancers are immunogenic: immunologic analyses and a phase II pilot clinical trial using mutation-reactive autologous lymphocytes. J Clin Oncol. 2022;40(16):1741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tchou J, Zhao Y, Levine BL, Zhang PJ, Davis MM, Melenhorst JJ, et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol Res. 2017;5(12):1152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Specht JM, Lee SM, Turtle C, Berger C, Balakrishnan A, Srivastava S, et al. Abstract P2-09-13: a phase I study of adoptive immunotherapy for ROR1+ advanced triple negative breast cancer (TNBC) with defined subsets of autologous T cells expressing a ROR1-specific chimeric antigen receptor (ROR1-CAR). Cancer Res. 2019;79(4_Supplement):P2-09-13-P2-09-13. 10.1158/1538-7445.SABCS18-P2-09-13. [Google Scholar]
- 160.Schlechter BL, Olson D, George MA, Saibil S, Giordano A, Bouvier R, et al. A phase I/II trial investigating safety and efficacy of autologous TAC01-HER2 in relapsed or refractory solid tumors. J Clin Oncol. 2023;41(16_suppl):2519–2519. 10.1200/JCO.2023.41.16_suppl.2519. [Google Scholar]
- 161.Shah PD, Huang AC, Xu X, Orlowski R, Amaravadi RK, Schuchter LM, et al. Phase I trial of autologous RNA-electroporated cMET-directed CAR T cells administered intravenously in patients with melanoma and breast carcinoma. Cancer Res Commun. 2023;3(5):821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Albelda SM. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat Rev Clin Oncol. 2024;21(1):47–66. [DOI] [PubMed] [Google Scholar]
- 163.Labanieh L, Mackall CL. CAR immune cells: design principles, resistance and the next generation. Nature. 2023;614(7949):635–48. [DOI] [PubMed] [Google Scholar]
- 164.Newick K, O’Brien S, Moon E, Albelda SM. CAR T cell therapy for solid tumors. Annu Rev Med. 2017;68(1):139–52. [DOI] [PubMed] [Google Scholar]
- 165.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Majzner RG, Mackall CL. Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med. 2019;25(9):1341–55. [DOI] [PubMed] [Google Scholar]
- 167.D’Angelo SP, Araujo DM, Abdul Razak AR, Agulnik M, Attia S, Blay J-Y, et al. Afamitresgene autoleucel for advanced synovial sarcoma and myxoid round cell liposarcoma (SPEARHEAD-1): an international, open-label, phase 2 trial. The Lancet. 2024;403(10435):1460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wermke M, Holderried TAW, Luke JJ, Morris VK, Alsdorf WH, Wetzko K, et al. First-in-human dose escalation trial to evaluate the clinical safety and efficacy of an anti-MAGEA1 autologous TCR-transgenic T cell therapy in relapsed and refractory solid tumors. J Immunother Cancer. 2024;12(7):e008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lu Y-C, Parker LL, Lu T, Zheng Z, Toomey MA, White DE, et al. Treatment of patients with metastatic cancer using a major histocompatibility complex class II–restricted T-Cell receptor targeting the cancer germline antigen MAGE-A3. J Clin Oncol. 2017;35(29):3322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.D’Angelo SP, Furness AJS, Thistlethwaite F, Burgess MA, Riedel RF, Haanen J, et al. Lete-cel in patients with synovial sarcoma or myxoid/round cell liposarcoma: Planned interim analysis of the pivotal IGNYTE-ESO trial. J Clin Oncol. 2024;42(16_suppl):2500–2500. 10.1200/JCO.2024.42.16_suppl.2500.38828957 [Google Scholar]
- 171.Kim SP, Vale NR, Zacharakis N, Krishna S, Yu Z, Gasmi B, et al. Adoptive cellular therapy with autologous tumor-infiltrating lymphocytes and T-cell receptor-engineered T cells targeting common p53 neoantigens in human solid tumors. Cancer Immunol Res. 2022;10(8):932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.