Abstract
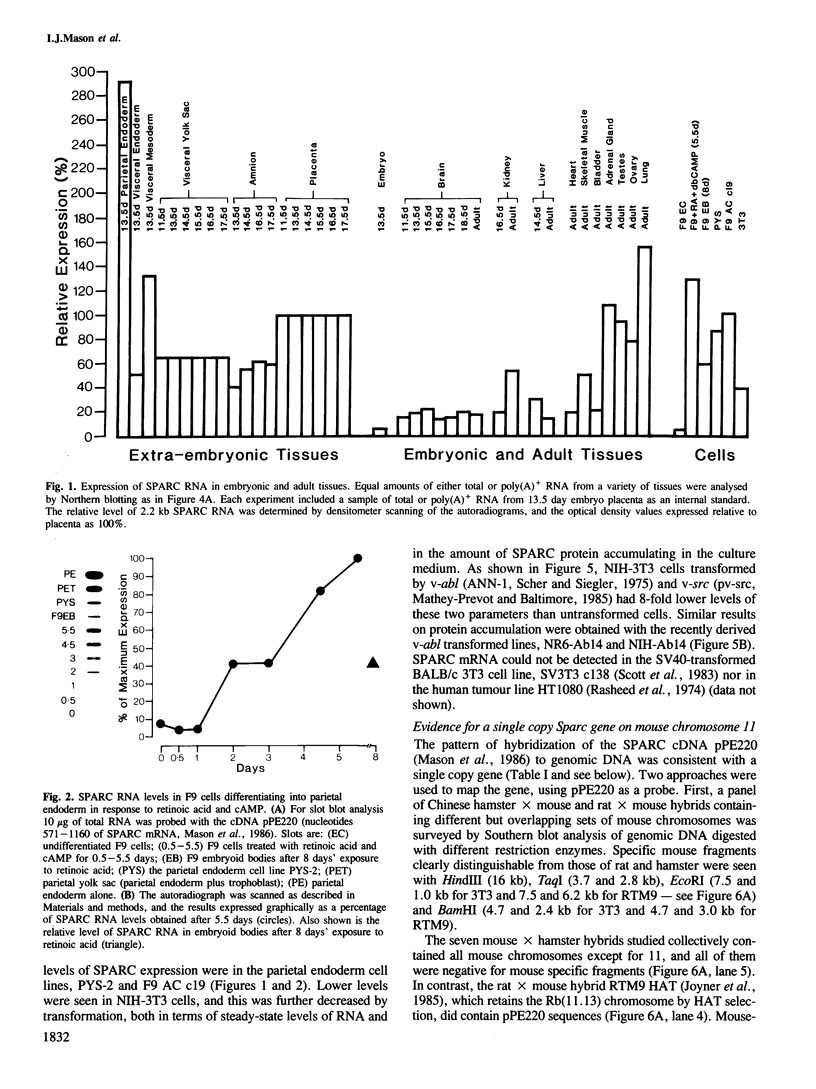
SPARC is a Mr 43,000 secreted, acidic, cysteine-rich glycoprotein homologous to 43K bovine endothelial 'culture shock' protein. We show here that it is encoded by a single gene localized to the central region of mouse chromosome 11. During development SPARC mRNA is expressed at higher levels in all the extra-embryonic tissues than in the fetus. Highest levels are found in the parietal endoderm, while visceral endoderm has approximately 6-fold less. This differential expression is also seen in F9 teratocarcinoma cells treated with retinoic acid under conditions in which they give rise to either parietal or visceral endoderm. The 20-fold increase seen during differentiation into parietal endoderm is due, at least in part, to an increase in gene transcription. We also report SPARC expression in a variety of adult tissues and cultured cells, and present evidence that a decrease accompanies the transformation of fibroblast cell lines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Keski-Oja J., Vaheri A. Extracellular matrix proteins characterize human tumor cell lines. Int J Cancer. 1981 Jun 15;27(6):755–761. doi: 10.1002/ijc.2910270605. [DOI] [PubMed] [Google Scholar]
- Andrews G. K., Janzen R. G., Tamaoki T. Stability of alpha-fetoprotein messenger RNA in mouse yolk sac. Dev Biol. 1982 Jan;89(1):111–116. doi: 10.1016/0012-1606(82)90299-8. [DOI] [PubMed] [Google Scholar]
- Baas F., Bikker H., van Ommen G. J., de Vijlder J. J. Unusual scarcity of restriction site polymorphism in the human thyroglobulin gene. A linkage study suggesting autosomal dominance of a defective thyroglobulin allele. Hum Genet. 1984;67(3):301–305. doi: 10.1007/BF00291357. [DOI] [PubMed] [Google Scholar]
- Barlow D. P., Green N. M., Kurkinen M., Hogan B. L. Sequencing of laminin B chain cDNAs reveals C-terminal regions of coiled-coil alpha-helix. EMBO J. 1984 Oct;3(10):2355–2362. doi: 10.1002/j.1460-2075.1984.tb02140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Cattanach B. M., Kirk M. Differential activity of maternally and paternally derived chromosome regions in mice. Nature. 1985 Jun 6;315(6019):496–498. doi: 10.1038/315496a0. [DOI] [PubMed] [Google Scholar]
- Cooper A. R., Kurkinen M., Taylor A., Hogan B. L. Studies on the biosynthesis of laminin by murine parietal endoderm cells. Eur J Biochem. 1981 Sep;119(1):189–197. doi: 10.1111/j.1432-1033.1981.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Linnenbach A., Huebner K., Parnes J. R., Margulies D. H., Appella E., Seidman J. G. Control of expression of histocompatibility antigens (H-2) and beta 2-microglobulin in F9 teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5754–5758. doi: 10.1073/pnas.78.9.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Levine R. A., Campisi J. c-myc regulation during retinoic acid-induced differentiation of F9 cells is posttranscriptional and associated with growth arrest. Mol Cell Biol. 1986 Feb;6(2):518–524. doi: 10.1128/mcb.6.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Francke U., Lalley P. A., Moss W., Ivy J., Minna J. D. Gene mapping in Mus musculus by interspecific cell hybridization: assignment of the genes for tripeptidase-1 to chromosome 10, dipeptidase-2 to chromosome 18, acid phosphatase-1 to chromosome 12, and adenylate kinase-1 to chromosome 2. Cytogenet Cell Genet. 1977;19(2-3):57–84. doi: 10.1159/000130799. [DOI] [PubMed] [Google Scholar]
- Francke U., Taggart R. T. Assignment of the gene for cytoplasmic superoxide dismutase (Sod-1) to a region of chromosome 16 and of Hprt to a region of the X chromosome in the mouse. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5230–5233. doi: 10.1073/pnas.76.10.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy T. E., Beaudet A. L., Yu J. A method for analyzing transcription using permeabilized cells. Anal Biochem. 1984 Dec;143(2):350–360. doi: 10.1016/0003-2697(84)90674-2. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Grover A., Andrews G., Adamson E. D. Role of laminin in epithelium formation by F9 aggregates. J Cell Biol. 1983 Jul;97(1):137–144. doi: 10.1083/jcb.97.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Taylor A., Adamson E. Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature. 1981 May 21;291(5812):235–237. doi: 10.1038/291235a0. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Lebo R. V., Kan Y. W., Tjian R., Cox D. R., Martin G. R. Comparative chromosome mapping of a conserved homoeo box region in mouse and human. Nature. 1985 Mar 14;314(6007):173–175. doi: 10.1038/314173a0. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Hammer R. E., Tilghman S. M., Brinster R. L. Developmental regulation of alpha-fetoprotein genes in transgenic mice. Mol Cell Biol. 1985 Jul;5(7):1639–1648. doi: 10.1128/mcb.5.7.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkinen M., Barlow D. P., Helfman D. M., Williams J. G., Hogan B. L. Isolation of cDNA clones for basal lamina components: type IV procollagen. Nucleic Acids Res. 1983 Sep 24;11(18):6199–6209. doi: 10.1093/nar/11.18.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman D. S., Brzeski H., Lovell-Badge R., Evans M. J. Expression of the alpha-fetoprotein gene in pluripotent and committed cells. Biochim Biophys Acta. 1984 Nov 22;783(2):130–136. doi: 10.1016/0167-4781(84)90004-6. [DOI] [PubMed] [Google Scholar]
- Liesi P., Dahl D., Vaheri A. Laminin is produced by early rat astrocytes in primary culture. J Cell Biol. 1983 Mar;96(3):920–924. doi: 10.1083/jcb.96.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P., Kaakkola S., Dahl D., Vaheri A. Laminin is induced in astrocytes of adult brain by injury. EMBO J. 1984 Mar;3(3):683–686. doi: 10.1002/j.1460-2075.1984.tb01867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotti K. R., Brown G. D., Strickland S. Two-stage hormonal control of type IV collagen mRNA levels during differentiation of F9 teratocarcinoma cells. Dev Biol. 1985 Mar;108(1):26–31. doi: 10.1016/0012-1606(85)90005-3. [DOI] [PubMed] [Google Scholar]
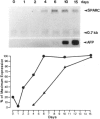
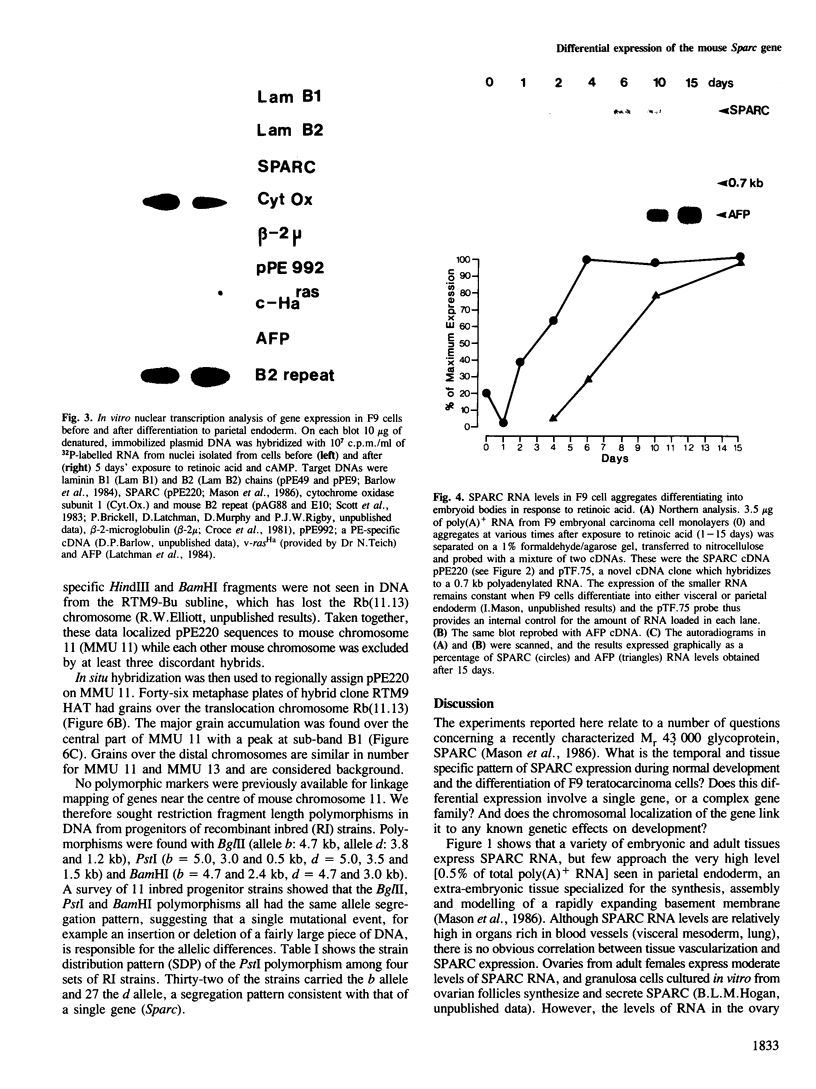
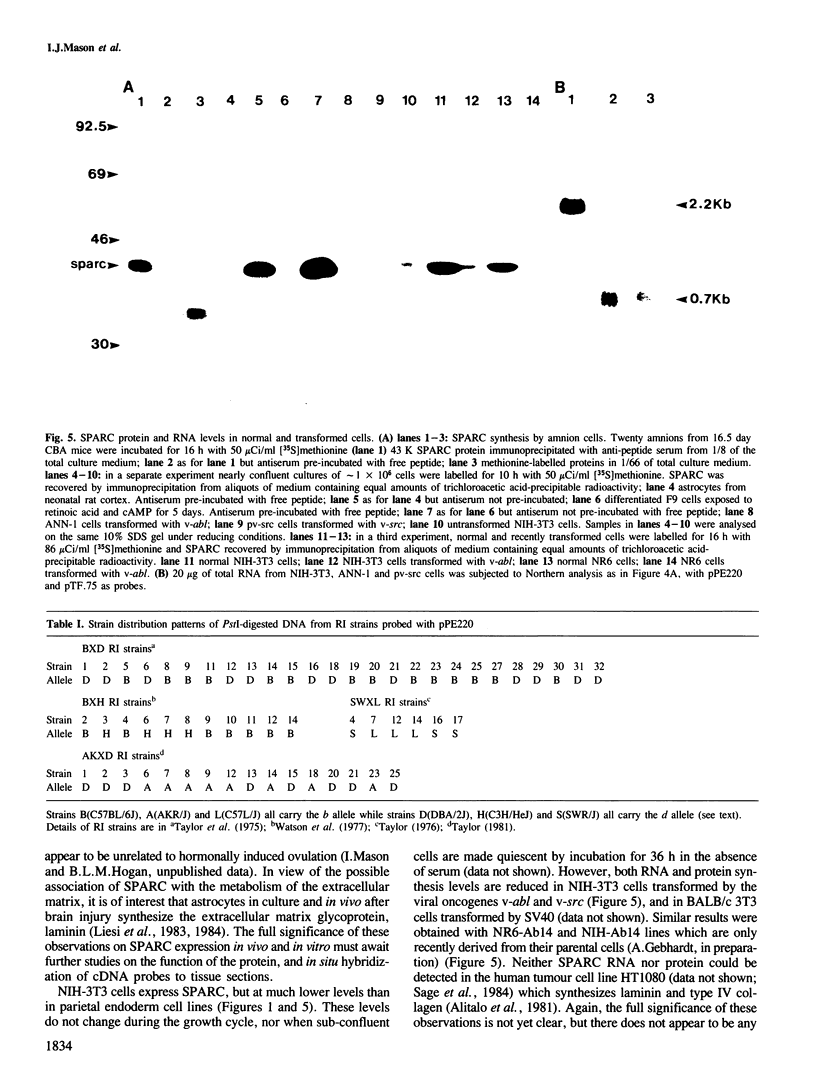
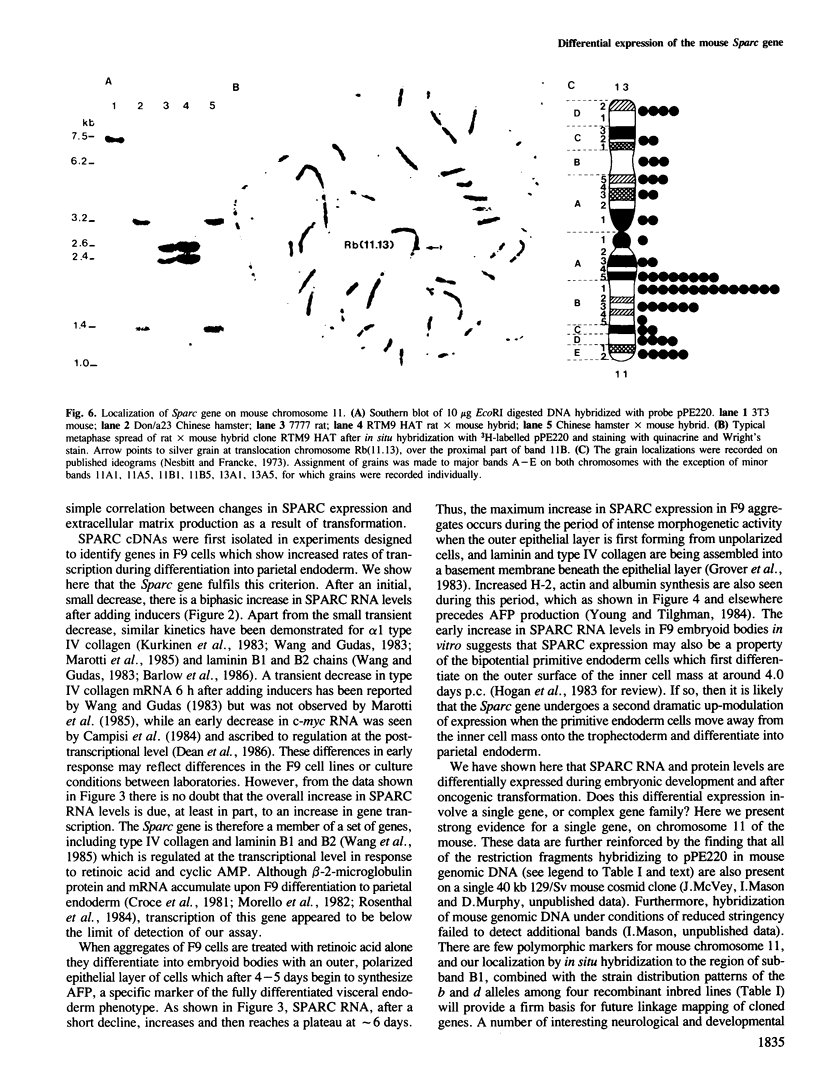
- Mason I. J., Taylor A., Williams J. G., Sage H., Hogan B. L. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell 'culture shock' glycoprotein of Mr 43,000. EMBO J. 1986 Jul;5(7):1465–1472. doi: 10.1002/j.1460-2075.1986.tb04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I., Murphy D., Hogan B. L. Expression of c-fos in parietal endoderm, amnion and differentiating F9 teratocarcinoma cells. Differentiation. 1985;30(1):76–81. doi: 10.1111/j.1432-0436.1985.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B., Baltimore D. Specific transforming potential of oncogenes encoding protein-tyrosine kinases. EMBO J. 1985 Jul;4(7):1769–1774. doi: 10.1002/j.1460-2075.1985.tb03849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello D., Daniel F., Baldacci P., Cayre Y., Gachelin G., Kourilsky P. Absence of significant H-2 and beta 2-microglobulin mRNA expression by mouse embryonal carcinoma cells. Nature. 1982 Mar 18;296(5854):260–262. doi: 10.1038/296260a0. [DOI] [PubMed] [Google Scholar]
- Münke M., Lindgren V., de Martinville B., Francke U. Comparative analysis of mouse-human hybrids with rearranged chromosomes 1 by in situ hybridization and Southern blotting: high-resolution mapping of NRAS, NGFB, and AMY on human chromosome 1. Somat Cell Mol Genet. 1984 Nov;10(6):589–599. doi: 10.1007/BF01535224. [DOI] [PubMed] [Google Scholar]
- Nesbitt M. N., Francke U. A system of nomenclature for band patterns of mouse chromosomes. Chromosoma. 1973;41(2):145–158. doi: 10.1007/BF00319691. [DOI] [PubMed] [Google Scholar]
- Noble M., Fok-Seang J., Cohen J. Glia are a unique substrate for the in vitro growth of central nervous system neurons. J Neurosci. 1984 Jul;4(7):1892–1903. doi: 10.1523/JNEUROSCI.04-07-01892.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. R. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3918–3921. doi: 10.1073/pnas.74.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Wright S., Cedar H., Flavell R., Grosveld F. Regulated expression of an introduced MHC H-2K bm1 gene in murine embryonal carcinoma cells. Nature. 1984 Aug 2;310(5976):415–418. doi: 10.1038/310415a0. [DOI] [PubMed] [Google Scholar]
- Sage H. Culture shock. Selective uptake and rapid release of a novel serum protein by endothelial cells in vitro. J Biol Chem. 1986 May 25;261(15):7082–7092. [PubMed] [Google Scholar]
- Sage H., Johnson C., Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984 Mar 25;259(6):3993–4007. [PubMed] [Google Scholar]
- Sage H., Tupper J., Bramson R. Endothelial cell injury in vitro is associated with increased secretion of an Mr 43,000 glycoprotein ligand. J Cell Physiol. 1986 Jun;127(3):373–387. doi: 10.1002/jcp.1041270305. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Bailey D. W., Cherry M., Riblet R., Weigert M. Genes for immunoglobulin heavy chain and serum prealbumin protein are linked in mouse. Nature. 1975 Aug 21;256(5519):644–646. doi: 10.1038/256644a0. [DOI] [PubMed] [Google Scholar]
- Taylor B. A. Genetic analysis of susceptibility to isoniazid-induced seizures in mice. Genetics. 1976 Jun;83(2):373–377. doi: 10.1093/genetics/83.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte B., Guertin M., Chevrette M., Bélanger L. Rat alpha 1-fetoprotein messenger RNA: 5'-end sequence and glucocorticoid-suppressed liver transcription in an improved nuclear run-off assay. Nucleic Acids Res. 1985 Apr 11;13(7):2387–2398. doi: 10.1093/nar/13.7.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., Gudas L. J. Isolation of cDNA clones specific for collagen IV and laminin from mouse teratocarcinoma cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5880–5884. doi: 10.1073/pnas.80.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., LaRosa G. J., Gudas L. J. Molecular cloning of gene sequences transcriptionally regulated by retinoic acid and dibutyryl cyclic AMP in cultured mouse teratocarcinoma cells. Dev Biol. 1985 Jan;107(1):75–86. doi: 10.1016/0012-1606(85)90377-x. [DOI] [PubMed] [Google Scholar]
- Watson J., Riblet R., Taylor B. A. The response of recombinant inbred strains of mice to bacterial lipopolysaccharides. J Immunol. 1977 Jun;118(6):2088–2093. [PubMed] [Google Scholar]
- Young P. R., Tilghman S. M. Induction of alpha-fetoprotein synthesis in differentiating F9 teratocarcinoma cells is accompanied by a genome-wide loss of DNA methylation. Mol Cell Biol. 1984 May;4(5):898–907. doi: 10.1128/mcb.4.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martinville B., Blakemore K. J., Mahoney M. J., Francke U. DNA analysis of first-trimester chorionic villous biopsies: test for maternal contamination. Am J Hum Genet. 1984 Nov;36(6):1357–1368. [PMC free article] [PubMed] [Google Scholar]