Abstract
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (EMZL) is an indolent B-cell lymphoma that can involve various anatomic sites. EMZL is derived from post-germinal center marginal zone B cells and typically lacks bcl-6 expression. Herein, we report two post-treatment cases of EMZL where unexpected bcl-6 protein expression was observed in specimens obtained following recurrence or progression. This contrasts with the primary specimens, which were negative for the bcl-6. Additionally, we confirm that the altered bcl6 expression observed in relapsed EMZL cases is independent of BCL6 gene rearrangement, as demonstrated by fluorescence in situ hybridization analysis. Relevant literature was reviewed and summarized to enhance the understanding of this phenomenon, particularly for pathologists.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13000-024-01593-z.
Keywords: Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (EMZL, MALT lymphoma); High-grade transformation (HT); Bcl6 protein expression; BCL6 gene rearrangement
Introduction
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (EMZL), also known as MALT lymphoma, is an indolent B-cell lymphoma that was first proposed in 1983 by Isaacson P and Wright DH [1]. EMZL most frequently involves the gastrointestinal tract, lung, salivary gland, ocular adnexal, and thyroid gland, to name a few. Its etiology is generally associated with continuous immune stimulation due to auto-antigens or microbial antigens induced by chronic infection. Morphologically, EMZL consists of a variety of small to medium-sized B lymphocytes, including centrocyte-like cells, monocytoid cells, and small lymphocyte-like cells. These three cell types are present individually or in mixed proportions alongside dispersed immunoblasts and centroblast-like cells. Moroever, lympho-epithelial lesions and follicular colonization are frequently seen. There may exist variable degrees of plasma cell differentiation, which can be either polyclonal or monoclonal. Immunophenotyping typically demonstrates the presence of pan-B-cell antigens such as CD20, CD79a, and PAX5, while being negative for CD10, bcl6, CD5, CD23, cyclinD1, and other markers. Consequently, EMZL remains a diagnosis of exclusion due to no distinct immunophenotypic markers. Despite its typically indolent clinical course and favorable prognosis, re-biopsy remains crucial to assess histologic changes during refractory or relapsed phases of EMZL. Herein, we present two cases where unexpected bcl-6 expression was detected within neoplastic cells of patients with EMZL who had tumor recurrence or progression after treatment. Moroever, we reviewed and summarized relevant literature to improve understanding of this phenomenon.
Case presentation
Case 1
In November 2014, a 71-year-old woman was admitted to our hospital presenting with a mass located in the right upper jaw for six months. The combined morphologic and immunohistochemical findings confirmed a diagnosis of typical EMZL. Interestingly, numerous intranuclear Dutcher bodies were also observed (Fig. 1). A bone marrow biopsy showed myeloid tri-lineage hyperplasia. Flow cytometric analysis of bone marrow revealed no abnormal antigen expression, and chromosome analysis indicated a normal karyotype (46, XX). Initially, she received eight courses of R-CHOP chemotherapeutic regimen and 6 cycles of rituximab targeted therapy and achieved complete response (CR), as assessed by CT. In November 2022, the patient was readmitted due to a mass in the right submandibular region. A PET-CT scan revealed a localized hypermetabolic right maxillary mass (SUVmax 10.6) and hypermetabolic lymph node in the bilateral submaxillary and the left anterior maxilla region (SUVmax 12.3). Notably, a mass measuring 1.6 cm × 1.2 cm was present in the right submandibular region. Histopathological findings from a fine-needle biopsy were consistent with the previous diagnosis. Subsequently, the patient received eight cycles of chemotherapy (Gazyva and CODP single-agent targeted regimen), and response evaluation using CT revealed a partial response (PR). In February 2024, the patient was readmitted due to right submandibular lymph node enlargement, reaching a maximal diameter of 3.4 cm. Subsequently, a complete surgical resection was performed. Microscopically, the specimen was composed of cells with diverse morphologies, predominantly medium to relatively large atypical lymphoid cells, arranged in a vaguely nodular to diffuse growth pattern, resembling a follicular-like structure (Fig. 2A). In addition, small to medium size typical monocytoid B cells and colonized follicles were observed (Fig. 2B). Immunohistochemistry revealed neoplastic cells diffusely positive for CD20 (Fig. 2C). The majority of neoplastic cells in the large cell-rich region were positive for bcl6 but negative for bcl2 and other germinal center markers, including CD10, LMO2 and HGAL (Fig. 2D). Clearly, the unexpected positivity for the bcl6 protein represents a significant alteration compared to the primary diagnosed immunophenotype. The number of large cells with blast-like morphology markedly increased, showing sheet-like proliferation (Fig. 2E), characterized by brisk mitotic figures and a significantly higher Ki-67 proliferation index (Fig. 2F), supporting the diagnosis of EMZL, partially transformed into a large B-cell lymphoma. Furthermore, the monoclonality with positive kappa (Fig. 2G) and negative lambda (Fig. 2H) suggested plasmacytic differentiation with cytoplasmic light chain restriction. Meanwhile, Epstein-Barr virus-encoded RNA in situ hybridization was negative, and other immunohistochemical staining results were not significant. A re-biopsy of bone marrow excluded lymphomatous involvement. The Ann Arbor staging was determined to be Stage III, Group A.
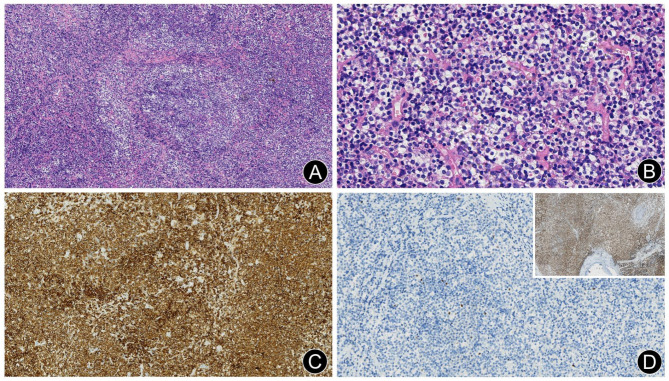
Fig. 1.
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (EMZL) in the right upper jaw specimen of case 1. (A) Small lymphoid cell diffuse infiltration with a vaguely nodular distribution (H&E, ×10). (B) Monocytoid B cells and intranuclear Dutcher bodies (H&E, ×40). (C) Diffuse positivity for CD20 (EnVision, × 20). (D) Neoplastic cells were negative for bcl6, while residual germinal center cells showed scattered positivity (En Vision, × 20). Inset: Diffuse positivity for bcl2 (En Vision, × 5)
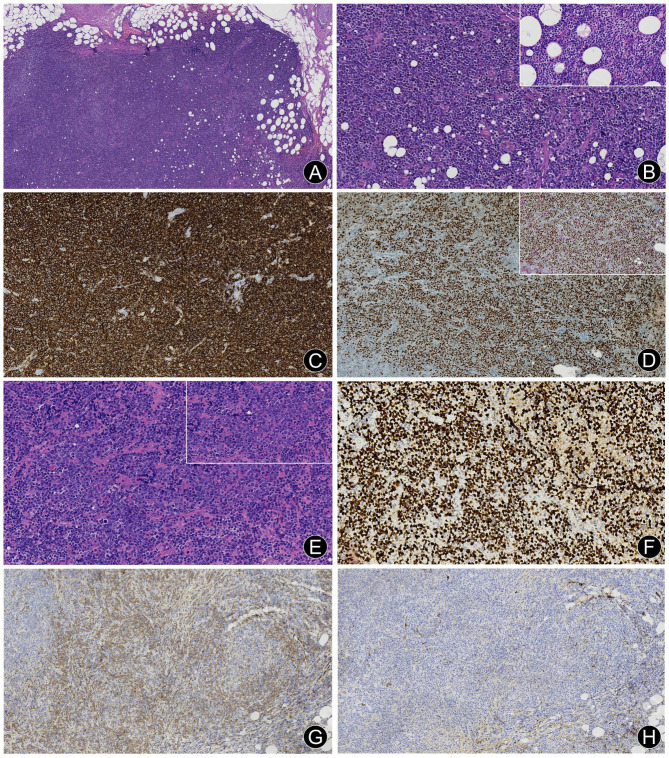
Fig. 2.
Right submandibular enlarged lymph node of case 1 revealing EMZL with partially transformed large B-cell lymphoma following recurrence. (A) Moderate to large atypical lymphoid cells with diffuse and vaguely nodular distribution (H&E, ×4). (B) Numerous moderate-sized monocytoid B cells (H&E, ×20); Inset: High-power magnification image of monocytoid B cells (H&E, ×40). (C) Diffuse positivity for CD20 (En Vision, × 10). (D) Dual immunohistochemistry for bcl6/CD10. A large number of large neoplastic cells are positive for bcl6 (brown chromogen, nuclear stain) but negative for CD10 (red chromogen, cytoplasmic stain) (En Vision, × 10). Inset: Dual immunohistochemistry for bcl6/bcl2. The bcl6 positive neoplastic cells are negative for bcl2 (red chromogen, cytoplasmic stain) (En Vision, × 20). (E) Diffuse sheets of large neoplastic cells with blast-like morphology (H&E, ×20). Inset: High-power magnification image of large cells characterized by brisk mitotic figures (H&E, ×40). (F) A high Ki-67 proliferation index was observed (En Vision, × 20). (G) Some neoplastic cells were positive for kappa (En Vision, × 10). (H) Some neoplastic cells were negative for lambda (En Vision, × 10)
Case 2
In 2021, a 64-year-old male with a self-reported history of lymphoma in the palate for nearly 20 years (details unknown) presented to a local hospital with a left intraorbital mass measuring approximately 2.5 cm in diameter. Postoperative pathological findings indicated EMZL, so the patient eventually received chemotherapy and local radiotherapy of the left eye. Further pathological analysis by our department confirmed the previous diagnosis, supported by the typical morphologic features and immunohistochemical profile most consistent with EMZL (Fig. 3A-C). In March 2024, the patient was admitted to our hospital because of low back discomfort and pain. A routine blood test revealed the following: white blood cell count 40.46 × 109/L, lymphocyte count 18.49 × 109/L, neutrophil count 18.61 × 109/L, platelet count 663.00 × 109/L. On physical examination, the patient presented with an anemic appearance, left blepharoptosis, and mild bilateral lower extremity edema; the remainder of the examination did not reveal any abnormalities. Flow cytometric analysis identified 35.21% of CD5 negative and CD10 negative monoclonal small B lymphocytes. Ultrasonography of superficial lymph nodes revealed multiple hypoechoic nodules in the bilateral cervical, axillary, and inguinal regions, with maximum diameters ranging from 1.5 cm to 2.8 cm. A whole abdominal CT scan showed extensive retroperitoneal and pelvic soft tissue masses encircling both kidneys, large retroperitoneal blood vessels, and the bladder, but no abnormalities of the spleen. A bone-marrow biopsy specimen demonstrated marrow involvement (Fig. 3D-F). Microscopically, the neoplastic cell population consisted of small lymphoid cells displaying a monocytoid B cell appearance and exhibiting a nodular and paratrabecular infiltrate. Immunohistochemical markers were consistent with the previously diagnosed specimen, except for an unexpected positivity for bcl6 protein. The Ann Arbor staging was classified as Stage IV, Group A.
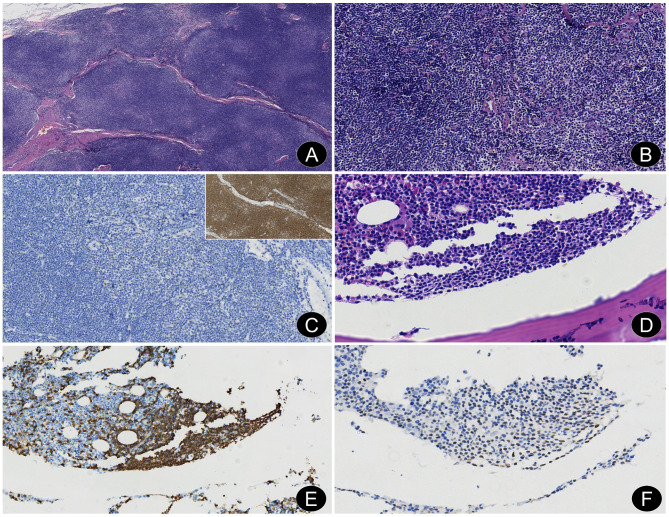
Fig. 3.
A left intraorbital EMZL and marrow involvement in case 2. (A) Small to medium-sized lymphoid cell diffuse infiltration with vaguely nodular distribution, separated by fibrous septa of varying thickness (H&E, ×4). (B) A mix of centrocyte-like cells, monocytoid B cells, and small lymphocyte-like cells (H&E, ×20). (C) Neoplastic cells were negative for bcl6 (En Vision, × 20); Inset: Diffuse positivity for CD20 (En Vision, × 10). (D) Small lymphoid cells with monocytoid B cells appearance exhibiting a paratrabecular infiltrate (H&E, ×4). (E) Neoplastic cells were strongly positive for CD20 (En Vision, × 20). (F) Neoplastic cells were weakly positive for bcl6 (En Vision, × 40)
Molecular analysis
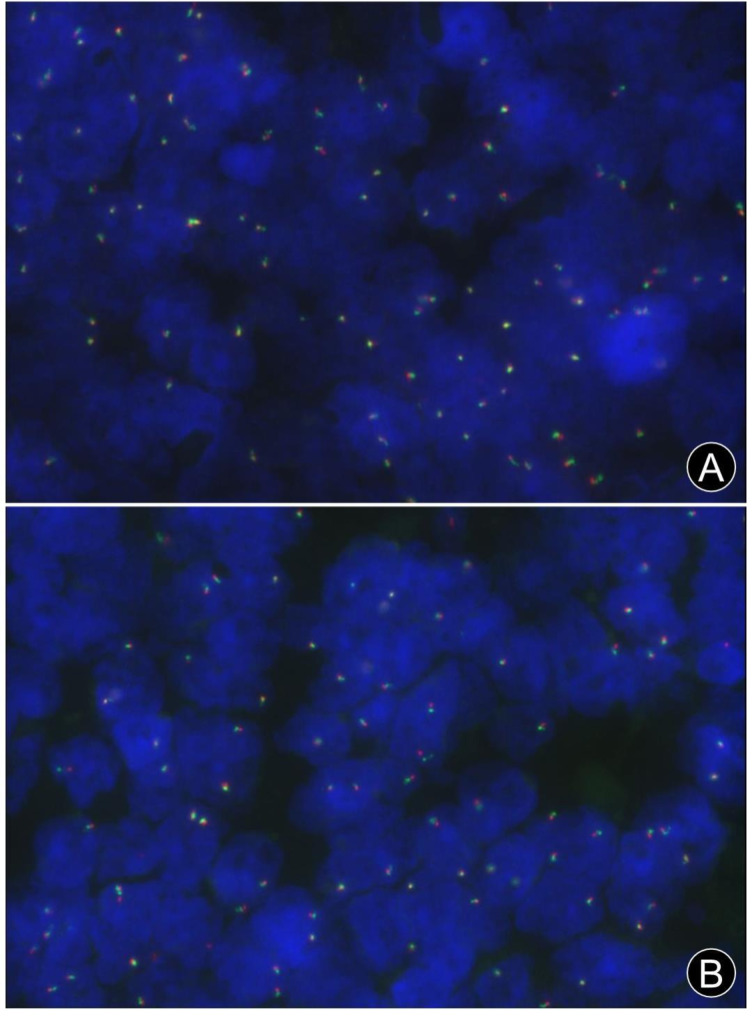
No translocations of the BCL2 (18q21) and BCL6 (3q27) genes were detected by Fluorescence In Situ Hybridization (FISH) in the two initial specimens, as well as a recurrent specimen in case 1 (Fig. 4). The bone-marrow biopsy specimen of case 2 was processed via routine decalcification, and genomic DNA was seriously degraded without any identifiable red or green fluorescent signal.
Fig. 4.
FISH analysis using break-apart probes for BCL2 (18q21) and BCL6 (3q27) genes. (A) Two red/green fusion or yellow signals indicating the absence of BCL2 gene rearrangement (FISH, × 40). (B) Two red/green fusion or yellow signals indicating the absence of BCL6 gene rearrangement (FISH, × 40)
Treatment and follow-up
Case 1 received orelabrutinib and anti-CD20 monoclonal targeted treatment, while case 2 received orelabrutinib, anti-CD20 monoclonal targeted treatment, and lenalidomide immunotherapy. As of July 2024, both patients were still alive and well.
Discussion
According to the latest 5th World Health Organization (WHO) Classification and the 2022 International Consensus Classification (ICC) of lympho-haematopoietic neoplasms [2, 3], marginal zone lymphomas (MZLs) are a heterogeneous group of low-grade B-cell lymphomas with overlapping morphology and immunophenotypes. MZLs are divided into four main entities, including extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (EMZL), nodal marginal zone lymphoma (NMZL), paediatric nodal marginal zone lymphoma (pNMZL) and primary cutaneous marginal zone lymphoma (PCMZL). EMZL is the most common subtype, accounting for nearly 70% of all MZLs. However, despite MZLs sharing some common features, there are significant differences in cytogenetic and mutational profiles among them arising in different anatomic sites [2, 3].
The BCL-6 gene, which is located at chromosome band 3q27 encoding a zinc-finger transcription repressor, functions as a nuclear transcriptional repressor required for germinal center (GC) development and T-helper 2-mediated antigen responses. The bcl6 protein is strongly expressed in B-cells and T-cells within the GC, and its down-regulation is a crucial step for the differentiation of GC lymphocytes into memory B cells or plasma cells, or for the induction of selective apoptosis following antigen stimulation [4]. EMZL originates from post-GC marginal zone B cells and mostly lacks BCL6 rearrangement, and typically does not express bcl-6. Relapsed EMZL cases can very rarely exhibit aberrant BCL6 expression following treatment, posing a significant diagnostic challenge for pathologists. To date, only three articles have been reported in PubMed [5–7]. Of note, there is significant heterogeneity among the samples reported in these three articles. The first article included composite lymphomas (EMZL with concomitant large cell components) and large cell variants of MZL. In that study, the protein expression of bcl6 and rearrangement of the BCL6 locus were exclusively observed in the large cell morphology of EMZL, rather than in the low-grade small cell components. Furthermore, the authors suggested that bcl6 could serve as an immunohistological marker for the transformation of MZBL, and that BCL6 rearrangement might indicate an association with lymphoma transformation or progression [5]. The second article studied 392 cases of EMZL, among which seven cases (1.8%, 7/392) carried a BCL6-involved chromosome translocation as determined by FISH analysis. The authors concluded that BCL6 translocation could contribute to lymphoma development [6]. Moreover, these seven cases showed the typical histologic and immunophenotypic characteristics of MALT lymphoma, and none displayed evidence of transformation [6]. Interestingly, only two of the seven cases expressed bcl6. The third paper described six cases of initially diagnosed stage I EMZL cases with positive bcl6 staining, all of which posed challenges in differentiation from Follicular lymphoma (FL). Dual bcl6/bcl2 immunostaining was performed to confirm the concurrent coexpression of bcl6 and bcl2 proteins in individual neoplastic cells within the follicular foci, and FISH analysis was used to confirm the absence of BCL2 rearrangement. Based on the overall features, these cases were ultimately diagnosed as EMZL [7]. That study also suggested that extranodal MZL cells exhibit plasticity in bcl6 protein expression patterns, which is influenced by the GC microenvironment, while BCL6 translocation status has not been determined [7].
Herein, both cases were primarily diagnosed as typical EMZLs without bcl6 expression. However, aberrant bcl6 protein expression within neoplastic cells appeared at the time of tumor recurrence or progression after treatment. One case (case 1) developed lymph nodal involvement eight years after standardized chemotherapy and was diagnosed with EMZL with partial high-grade transformation. This could be referred to as an asynchronous composite lymphoma, characterized by the occurrence of transformed aggressive B-cell lymphoma in extra-primary sites following a confirmed diagnosis of EMZL [8]. Notably, the specimens initially exhibited numerous intranuclear Dutcher bodies. However, the recurrent specimens unexpectedly exhibited strong bcl6 positivity and concurrent loss of bcl2 expression. Monoclonal plasmacytic differentiation appears to be common, often accompanied by high-grade transformation, particularly in relapse cases, which may indicate a poor prognosis or result from chemotherapy [9]. The other case (case 2), with a self-reported history of lymphoma in the palate for nearly 20 years, presented with bone marrow involvement (stage IV) two years after a confirmed diagnosis of EMZL following biopsy. The neoplastic cells showed weak positivity for bcl6, with no evidence of high-grade transformation. It is, however, unknown whether altered expression of the bcl6 protein is an early event in high-grade transformation, a consequence of chemotherapy, or others. Studies have previously reported differential upregulation or downregulation of CD10, BCL6, and MUM1 expression in cases of transformed DLBCL. Approximately 9–16% of DLBCL transformed from FL are classified as non-GCB type, whereas about 37% of DLBCL transformed from EMZL may exhibit GCB-type characteristics (CD10+ and/or bcl6+) [8]. However, the clinical significance of this rare phenomenon warrants further substantiation in a larger case series with long-term follow-up before concluding that abnormal bcl6 expression contributes to the high-grade transformation of EMZL.
In summary, what makes our cases special is that aberrant bcl6 expression were observed in recurrent or progressing EMZL specimens at new anatomic sites, whereas the primary specimens were negative for the bcl6 marker. Additionally, FISH analysis for BCL6 gene rearrangement was performed on initial and recurrent specimens in two cases, with the results being negative (though the decalcified bone marrow specimen from case 2 yielded no analyzable results). In this study, the abnormal expression of bcl6 protein in relapsed EMZL cases has been confirmed to be independent of BCL6 gene rearrangement. This finding suggests potential involvement of other mechanisms, including gene amplification, somatic mutations in promoter regions, epigenetic regulation, as well as transcriptional, post-transcriptional, and translational modifications [4, 10, 11].
The differential diagnosis of this case was performed as follows [1]. Follicular lymphoma (FL): Some cases of FL can exhibit marginal zone differentiation with loss of GC markers expression, resembling that of MZL, particularly in extranodal sites. Conversely, certain MZLs may present with a vaguely follicular nodular distribution with extensive GC colonization, mimicking the growth pattern of FL. Although typically negative, a very small subset of EMZL can express bcl6 or other GC markers, particularly in gastric EMZL. Consequently, distinguishing between FL and MZL is highly challenging for pathologists. In the present study, both primary tumors arose from extranodal sites and exhibited typical morphological and immunohistochemical features of EMZL, making the diagnosis definitive. Meanwhile, other low-grade B-cell lymphomas, such as FL, mantle cell lymphoma, and small lymphocytic lymphoma, were ruled out. The aberrant expression of bcl6 protein within neoplastic cells was observed in recurrent or progressing specimens, which raises the diagnostic pitfall of FL. Firstly, the progression of EMZL into a high-grade FL seems less likely unless this was a second malignant neoplasm occurrence. Secondly, the negative finding for BCL2 gene rearrangement helps to exclude FL in this case. Moreover, Akiko et al. also reminded us that large transformed lymphoma cells with a bcl6 + and/or CD10 + immunophenotype should not be diagnosed as FL [8] [2]. Diffuse large B cell lymphoma (DLBCL): DLBCL represents an aggressive and heterogeneous group of mature B-cell lymphomas that may arise de novo or transform from pre-existing low-grade B-cell lymphomas, for instance, MZLs or FLs. The histological features observed, including typical monocytoid B-cells with colonizing follicles, accompanied by regional diffuse proliferation of large blast-like lymphoid cells, frequent mitotic figures, and a high Ki-67 proliferation index, support the diagnosis of EMZL, partially transformed into a large B-cell lymphoma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
M.Z., J.Q. and W.Z. performed the histological examination. J.Q. and Y.L. were involved with the treatment and follow up of the patient. W.G. was responsible for performing the diagnostic imaging of the patient. M.Z. wrote the main manuscript text. W.Z. was responsible for the critical revision of the manuscript. All authors reviewed and approved the final manuscript.
Funding
No funding was received to assist with the preparation of this manuscript. The authors have no relevant financial or non-financial interests to disclose.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the ethical standards of the research committee of Yantai Yantaishan Hospital (number: 2024069). The Yantai Yantaishan Hospital ethical committee exempted the informed consent of this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52(8):1410–6. 10.1002/1097-0142(19831015)52:8 <1410::aid-cncr2820520813>3.0.co;2-3 [DOI] [PubMed] [Google Scholar]
- 2.Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720–48. 10.1038/s41375-022-01620-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campo E, Jaffe ES, Cook JR, Quintanilla-Martinez L, Swerdlow SH, Anderson KC, et al. The International Consensus classification of mature lymphoid neoplasms: a report from the Clinical Advisory Committee. Blood. 2022;140(11):1229–53. 10.1182/blood.2022015851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YW, Hu XT, Liang AC, Au WY, So CC, Wong ML, et al. High BCL6 expression predicts better prognosis, independent of BCL6 translocation status, translocation partner, or BCL6-deregulating mutations, in gastric lymphoma. Blood. 2006;108(7):2373–83. 10.1182/blood-2006-05-022517 [DOI] [PubMed] [Google Scholar]
- 5.Flossbach L, Antoneag E, Buck M, Siebert R, Mattfeldt T, Möller P, et al. BCL6 gene rearrangement and protein expression are associated with large cell presentation of extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Int J Cancer. 2011;129(1):70–7. 10.1002/ijc.25663 [DOI] [PubMed] [Google Scholar]
- 6.Ye H, Remstein ED, Bacon CM, Nicholson AG, Dogan A, Du MQ. Chromosomal translocations involving BCL6 in MALT lymphoma. Haematologica. 2008;93(1):145–6. 10.3324/haematol.11927 [DOI] [PubMed] [Google Scholar]
- 7.Poveda J, Cassidy DP, Zhou Y, Alderuccio JP, Lossos IS, Vega F, et al. Expression of germinal center cell markers by extranodal marginal zone lymphomas of MALT type within colonized follicles, a diagnostic pitfall with follicular lymphoma. Leuk Lymphoma. 2021;62(5):1116–22. 10.1080/10428194.2020.1855347 [DOI] [PubMed] [Google Scholar]
- 8.Maeshima AM, Taniguchi H, Toyoda K, Yamauchi N, Makita S, Fukuhara S, et al. Clinicopathological features of histological transformation from extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue to diffuse large B-cell lymphoma: an analysis of 467 patients. Br J Haematol. 2016;174(6):923–31. 10.1111/bjh.14153 [DOI] [PubMed] [Google Scholar]
- 9.Traverse-Glehen A, Felman P, Callet-Bauchu E, Gazzo S, Baseggio L, Bryon PA, et al. A clinicopathological study of nodal marginal zone B-cell lymphoma. A report on 21 cases. Histopathology. 2006;48(2):162–73. 10.1111/j.1365-2559.2005.02309.x [DOI] [PubMed] [Google Scholar]
- 10.Zhang TT, Gonzalez DG, Cote CM, Kerfoot SM, Deng S, Cheng Y, et al. Germinal center B cell development has distinctly regulated stages completed by disengagement from T cell help. Elife. 2017. 10.7554/eLife.19552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MK, Song JY, Koh DI, Kim JY, Hatano M, Jeon BN, et al. Reciprocal negative regulation between the tumor suppressor protein p53 and B cell CLL/lymphoma 6 (BCL6) via control of caspase-1 expression. J Biol Chem. 2019;294(1):299–313. 10.1074/jbc.RA118.004204 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.