Abstract
Objective
To clinically and laboratory characterize patients with a positive direct antiglobulin test (DAT) treated at the Hospital das Clínicas of the Federal University of Goiás (HC-UFG).
Methods
A retrospective, descriptive, cross-sectional study was carried out collecting data from medical records of patients with a positive DAT who were treated at HC-UFG between August 2021 and August 2022.
Results
Eighty-four patients with positive polyspecific DAT results were screened in the clinical laboratory. Fifty-four patients had a laboratory profile compatible with autoimmune hemolytic anemia (AIHA), however, among these, 16 patients already had a diagnosis of AIHA in their medical records. The most common symptoms present among AIHA patients were pallor, asthenia, fatigue and dyspnea. For the remaining patients, the most common symptoms were severe thrombocytopenia, anemia, renal dysfunction, fever, myalgia, headache, thrombosis, asthenia, hematuria and joint pain. Only one patient had primary AIHA, that is, he had no evident underlying disease. The majority of AIHA patients (75 %) underwent corticosteroid therapy with 60 % having a positive response. For patients without AIHA, prednisone was the most frequently prescribed medication in 17 (25 %) patients, followed by hydroxychloroquine (14 patients - 20.1 %).
Conclusion
It is essential to evaluate patients with positive DAT in detail in order to understand the real clinical case. The DAT serological result alone does not arrive at a conclusive diagnosis of AIHA, and so it must be evaluated in conjunction with both clinical data and other laboratory tests, such as hemoglobin concentration and hemolysis tests (reticulocytes, lactate dehydrogenase and/or haptoglobin).
Keywords: Autoimmune hemolytic anemia, Immunohematology, Direct antiglobulin test
Introduction
The direct antiglobulin test (DAT) consists of searching for autoantibodies, alloantibodies or complement fractions adsorbed on the erythrocyte surface in vivo. Many diseases and drugs can lead to the production of these immunoglobulins which promote early hemolysis causing anemia. Technically, it is based on the fact that the antibodies that cover red blood cells can be identified by the addition of anti-human globulin antibodies, the so-called Coombs Serum. When positive, the test indicates the presence of antibodies adhered to the red blood cells, forming bridges, leading to a visible phenomenon of agglutination.1,2
Initially produced by Coombs, Mourant and Race in 1945, human antiglobulin serum was obtained by injecting human serum into rabbits; this process was fundamental for the introduction of the antiglobulin test for blood group serology [2]. Since it, was also possible to detect several incomplete antibodies that did not cause agglutination of red blood cells in saline, for example, antibodies to the Rh(D) system involved in hemolytic disease of newborns and autoimmune hemolytic anemia (AIHA).3
Antiglobulin sera can be polyspecific, containing antibodies that bind to the crystallizable fragment portion of human immunoglobulin G (IgG) and antibodies against the complement C3 fractions (C3b and/or C3d), or monospecific, that is, anti-IgG antibodies or antibodies for specific complement components such as C3b or C3d.4
In routine immunohematology tests, the DAT is used when results are discordant or positive; for example, this test is used for positive irregular antibody testing during pre-transfusion tests, when the donor red blood cell concentrate is crossed with the patient's serum/plasma in situations of a discrepancy in Rh(D) typing. There are other situations in which the DAT is also used, such as in clinical conditions that can result in coverage of red blood cells with antibodies and/or complement system, such as in AIHA, drug-induced hemolytic anemias, hemolytic disease of the fetus and newborn and hemolytic transfusion reactions.5,6
Antibodies against various drugs and additives can also cause positive antibody test results. The drugs can result in a wide variety of hematologic abnormalities, including a positive DAT. The most common cause of these positive reactions is the formation of autoantibodies.7
The interpretation of a DAT requires knowledge of the patient's laboratory and clinical evaluations, assessment of medications being used, pregnancy and transfusion history, as well as information on the presence of AIHA. The DAT serological result alone does not give a definitive diagnosis of AIHA, and must be evaluated together with clinical data and other laboratory data, such as hemoglobin, bilirubin, haptoglobin and reticulocyte measurements. A positive DAT does not mean that the patient has hemolytic anemia. DAT is sometimes positive in hematologically normal individuals. In some situations false positive reactions occur, the causes of which are, in most cases, due to incorrect processing and interpretation of the test.1,2
Due to the high number of positive DATs observed and the fact that the results do not correspond to a characteristic picture of AIHA, the objective of this study was to clinically and laboratory characterize patients with a positive DAT treated at Hospital das Clínicas from the Federal University of Goiás (HC-UFG).
Materials and methods
Study design
This is a retrospective, descriptive, cross-sectional study carried out by collecting data from medical records of patients with a positive DAT who were treated at HC-UFG between August 2021 and August 2022.
Ethical aspects
The work was submitted to and approved by the Research Ethics Committee of the institution (# 4243,912).
Sample selection
Patients were initially identified by visiting the HC-UFG clinical analysis laboratory and accessing the electronic information system to check the positive DATs list in the evaluated period, as well as to locate each patient's medical record. The medical records were analyzed and, the following variables were extracted: age, sex, ethnicity, and municipality of residence, results of laboratory tests, underlying disease, and medications used before the positive DAT and currently in use, therapeutic approach adopted and response to treatment. Data such as the clinical picture, presented at the most recent consultation, blood transfusions, use of chemotherapy drugs and any causes of death were also noted.
Studies estimate that from 5 to 10 % of patients with AIHA have a negative DAT result, thus patients were divided into two groups for better characterization. Group A comprised patients with a laboratory profile compatible with AIHA, that is, a positive DAT, hemoglobin concentration below the reference value, a positive hemolysis test and a diagnosis confirmed by the medical team. Group B comprised patients with a laboratory profile incompatible with AIHA and/or a diagnosis not confirmed or not described in the medical record by the medical team.
Experimental procedures
The HC-UFG laboratory routinely performs only the direct polyspecific antiglobulin test. A direct monospecific DAT was also performed for patients with AIHA, to identify the type of antibody produced (IgG or IgM, or both), for patients whose blood samples were found in the HC-UFG laboratory. No additional blood was collected to carry out this research.
The monospecific DAT was performed using gel card technology (Grifols) to identify the specific class (IgG or IgM). The principle of the method is based on the gel technique described by Lapierre for detecting red blood cell agglutination reactions.8
Patients with a history of transfusion were not analyzed using monospecific DAT, in order to rule out the presence of irregular antibodies bound to the surface of recently transfused red blood cells, which could mimic a positive DAT reaction, with or without the presence of hemolysis.
Statistical analysis
The data obtained were tabulated using Microsoft Excel® software. The mean was used as a measure of central tendency, in addition to calculating standard deviation and percentage.
Results
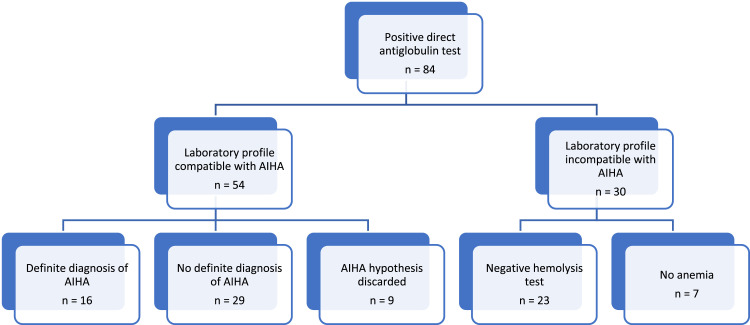
During the period from August 2021 to August 2022, 84 patients with a positive result for polyspecific DAT were registered and screened in the HC-UFG clinical laboratory. Based on the results of other laboratory tests, 54 patients had a laboratory profile compatible with AIHA, but among these, 16 patients already had a diagnosis of AIHA recorded in their medical records (Figure 1).
Figure 1.
Identification of patients with a positive direct antiglobulin test and definition of Groups A and B that constitute the participants in this study.
AIHA: autoimmune hemolytic anemia.
After the initial evaluation, Group A comprised 16 patients (19 %) and Group B included 68 (81 %) patients. The social demographic characterization of the groups is described in Table 1.
Table 1.
Social demographic characterization of Groups A and B.
| Variable | Group A |
Group B |
||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Female | 10 | 62.5 | 40 | 58.8 |
| Male | 6 | 37.5 | 28 | 41.2 |
| Age range (years) | ||||
| 10–19 | 3 | 18.75 | 6 | 8.82 |
| 20–29 | 2 | 12.5 | 13 | 19.11 |
| 30–39 | 3 | 18.75 | 11 | 16.18 |
| 40–49 | 3 | 18.75 | 9 | 13.23 |
| 50–59 | 2 | 12.5 | 12 | 17.64 |
| > 60 | 3 | 18.75 | 17 | 25 |
| Color/Ethnicity | ||||
| White | 1 | 6.25 | 28 | 41.2 |
| Mixed | 12 | 75 | 31 | 45.6 |
| Undeclared | 3 | 18.75 | 9 | 13.2 |
| Housing Municipality | ||||
| Goiânia | 8 | 50 | 33 | 48.5 |
| Aparecida de Goiânia | 2 | 12.5 | 3 | 4.4 |
| Others | 6 | 37.5 | 32 | 47.1 |
Regarding the clinical picture, the most common symptoms in Group A were pallor, asthenia, fatigue and dyspnea. Some patients also reported jaundice, splenomegaly, and hematomas, with the first two related to increased hemolysis and the last related to thrombocytopenia. For Group B, the most common symptoms were severe thrombocytopenia, anemia, renal dysfunction, fever, myalgia, headache, thrombosis, asthenia, hematuria and joint pain.
The hemolysis markers are shown in Table 2. Patients in Group A have low hemoglobin and reticulocyte levels and a high Lactate dehydrogenase (LDH) level different to all the patients in Group B.
Table 2.
Laboratorial profile of patients in Groups A and B.
| Laboratorial tests | Group A Mean ± Standard deviation |
Group B Mean ± Standard deviation |
Reference values |
|---|---|---|---|
| Hemoglobin (g/dL) | 8.9 ± 1.5 | 9.27 ± 2.34 | 12–15 |
| Reticulocytes (%) | 3.68 ± 2.5 | 3.64 ± 2.79 | 0–1.5 |
| Lactate dehydrogenase (U/L) | 433.6 ± 284.3 | 287.96 ± 142.79 | 120–240 |
Table 3 describes the underlying diseases presented by patients in both groups. Only one patient of Group A had no underlying disease and thus had primary AIHA.
Table 3.
Classification of underlying diseases and other comorbidities of patients in Groups A and B.
| Subgroups | Group A n (%) |
Group B n (%) |
|---|---|---|
| Underlying disease | ||
| SLE | 5 (31.25) | 17 (25.00) |
| Lymphoproliferative diseases | 4 (25) | 15 (22.00) |
| Other autoimmune diseases | 3 (18.75) | 1 (1.50) |
| Evans syndrome | 3 (18.75) | 0 |
| PNH | 1 (6.25) | 0 |
| Sickle cell anemia | 0 | 7 (10.30) |
| Chronic kidney disease | 0 | 6 (8.80) |
| COPD | 0 | 3 (4.40) |
| Comorbidities | ||
| Arterial hypertension | 3 (18.75) | 23 (33.90) |
| Diabetes mellitus | 3 (18.75) | 8 (11.70) |
SLE: Systemic lupus erythematosus; PNH: Paroxysmal nocturnal hemoglobinuria; COPD: Chronic obstructive pulmonary disease.
Additional to the diseases described in Table 3, Group B reported single cases of viral liver cirrhosis, refractory ascites, cardiorespiratory failure, neurosyphilis, tuberculosis, Chagas disease, inflammatory bowel disease, Wegener's granulomatosis and hairy cell leukemia.
The majority of patients in Group A (75 %) underwent treatment with corticosteroid therapy, with 60 % having a positive response. For patients refractory to the use of corticosteroids as monotherapy, second-line choices were other immunosuppressants and human immunoglobulin, which significantly increased the response rate. Corticosteroid therapy lost its effectiveness in three patients due to prolonged treatment, and splenectomy was performed. In another three cases, it was not possible to identify the therapeutic approach used.
Prednisone and methylprednisolone, as continuous use and administered as pulse therapy, respectively, were the most reported corticosteroids used to treat autoimmune diseases. Regarding other immunosuppressants, azathioprine was also mentioned, mostly in patients with systemic lupus erythematosus (SLE), as were the use of rituximab and eculizumab.
During the study period, two (12.5 %) patients received injectable chemotherapy to treat lymphoproliferative diseases and eight (50 %) patients required blood transfusions. Four (25 %) deaths were recorded with AIHA being listed as one of the causes of death in only one.
Prednisone, prescribed to 17 (25 %) patients, was the most frequently used medication in Group B followed by hydroxychloroquine for 14 (20.1 %) patients. The medical records reported prescriptions for diuretics, such as spironolactone (10 - 14.7 % patients), furosemide (10 - 14.7 % patients) and losartan (8 - 11.7 % patients). Hydroxyurea was prescribed to five (7.3 %) patients as well as metformin.
Regarding immunosuppressants, four patients took azathioprine. Other medications prescribed for Group B were propranolol, allopurinol, melphalan, thalidomide, cefepime, citoneurin, levothyroxine sodium and simvastatin. During the study period, ten (14.7 %) patients, underwent injectable chemotherapy to treat lymphoproliferative disease, 18 (26.5 %) patients required blood transfusions, six (8.8 %) patients underwent hemodialysis and five (7.3 %), underwent pulse therapy. A total of 16 (23.5 %) deaths were recorded in Group B.
Of the 16 patients with AIHA, seven (43.75 %) blood samples were found in the laboratory to perform the monospecific DAT allowing the identification of immunoglobulin isotypes in Group A (Table 4).
Table 4.
Prevalence of immunoglobulin isotypes in Group A.
| Immunoglobulin isotype | n | % |
|---|---|---|
| IgG 2+/4+ and C3d 2+/4+ | 1 | 14 |
| IgG 3+/4+ and C3d +/4+ | 1 | 14 |
| IgG 4+/4+ and C3d +/4+ | 1 | 14 |
| IgG 2+/4+ | 2 | 29 |
| IgG +/4+ | 2 | 29 |
Discussion
The results agree with previous Brazilian studies on patients diagnosed with AIHA, in relation to the predominance of females and the high rate of association of the disease with underlying causes capable of altering immune activity.9,10 Regarding the social demographic data, there was no predominant age group in this study. However, it is clear that the disease occurs more often in women between 10 and 39 years of age and that, among the six male patients, five were over 40 years old.
The associated symptoms in most cases were asthenia, fatigue and dyspnea, with jaundice and splenomegaly occurring in more severe cases. The clinical picture of AIHA is heterogeneous among those affected, and can vary between mild or compensated anemia with the patient being asymptomatic to severe hemolytic crises that are life-threatening.11
The laboratorial profile of patients diagnosed with AIHA in this study, took into account three parameters: hemoglobin, reticulocyte count and LDH. Hemoglobin is the marker of clinical severity in hemolytic diseases and may indicate anemia.12 Furthermore, increases in reticulocytes are caused by the high production of immature cells in the bone marrow in response to autoimmune destruction of circulating erythrocytes.13 In hemolytic conditions, LDH may be elevated and is used to distinguish extravascular hemolysis from intravascular hemolysis, with in the former the LDH is slightly increased and the latter it is four to five times higher than the normal value.12 Therefore, the presence of mild to moderate anemia, reticulocytosis and high LDH observed in the patients in this study (Table 2) is correlated with AIHA.
Primary AIHA was diagnosed in one of the 16 patients. This individual was diagnosed in 2009 and underwent continuous treatment with corticosteroids for around 11 years, eventually developing type 2 diabetes mellitus because of the use of corticosteroids. Due to the decreased effectiveness of this treatment and a recurrence of hemolytic crises, the medical team recommended splenectomy in 2020. The patient had a positive response to surgery and progressed with compensated disease. The main characteristic of these patients is the appearance of AIHA in isolation with the clinical picture having a good response to treatment.10,14,15
There was a predominance of AIHA secondary to other diseases in this study. SLE was the most prevalent of the underlying causes reported, characterizing a similar scenario as in other retrospective studies.10,16 AIHA occurs in approximately 10 % of patients with SLE17 and generally precedes the appearance of other clinical manifestations of the disease.18 This subgroup of patients is more likely to develop life-threatening lupus characteristics; therefore, the presence of AIHA indicates a more aggressive course of lupus.19 Although the pathophysiological mechanism that links SLE and AIHA is not yet completely understood, it is known that the anti-erythrocyte antibodies are generally hot-type IgG.20
Patients with lymphoproliferative diseases were also identified in this study. There are publications that highlight the predisposition of those affected by chronic lymphocytic leukemia (CLL) to develop autoimmune complications; around 7–10 % of these may present AIHA.21,22 The pathogenesis is mainly associated with the action of CLL B cells, which assume a role similar to antigen-presenting cells and activate autoreactive helper T cells, which can induce an imbalance of T cell subsets, favoring autoreactive B cells that produce anti-erythrocyte autoantibodies.22
Three patients were diagnosed with Evans syndrome, a condition defined as the concomitant or sequential association of AIHA with immune thrombocytopenic purpura, that is, it is an autoimmune cytopenia with decreases in red blood cells and platelets.23 Recent molecular theories explaining the pathophysiology of Evans syndrome include deficiencies of CTLA-4, LRBA, TPP2, and a decreased CD4/CD8 ratio.24
Also noteworthy is the patient with paroxysmal nocturnal hemoglobinuria (PNH), a clonal disease of hematopoietic stem cells characterized by the presence of hemolytic anemia, thrombosis and bone marrow failure.25 PNH is caused by somatic mutations that result in failure to control the process of inhibition of the complement system with the treatment of choice for these patients being eculizumab, a complement-inhibiting monoclonal antibody.26
Furthermore, even though there are no reports in our series, it is important to comment that bacterial and viral infections are also associated with secondary AIHA, with molecular mimicry being one of the main mechanisms associated with the induction of autoimmunity by these pathogens.14 This mechanism is based on the structural similarity between a pathogen or metabolite and the structure of the element in question. The similarity could be expressed as the sharing of amino acid sequences or a similar conformational structure between a pathogen and a self-antigen, causing an autoimmune reaction.27
Regarding patients adherence to treatment, the study identified that within the group treated with corticosteroids, the majority responded positively to monotherapy, however, a portion required associated second-line therapy using other immunosuppressants or human immunoglobulin. This fact can be justified by the high prevalence of patients with AIHA secondary to autoimmune diseases and lymphoproliferative diseases, since the chosen therapeutic line must be individualized in order to avoid decompensation of the underlying disease.13,14 Splenectomy was effective in controlling AIHA in the three cases in which it was performed in this study, supporting the fact that, even though it is recommended as a last resort, the procedure is effective to control AIHA.28,29
In agreement with what is described in the literature, the IgG antibody was the most frequent immunoglobulin isotype in patients with AIHA, despite the small sample size.30,31 IgG antibodies in AIHA patients suggest a warm AIHA subtype, in which autoantibodies react more easily at body temperature (37 °C). It is responsible for around 70–80 % of all cases of AIHA, which, although it can occur at any age, it is more common in adult women.
In this case, the destruction of red blood cells occurs by phagocytosis.30 However, the eluate test was not performed because patients with AIHA already had a defined diagnosis in their medical records with monospecific DAT just being a way of identifying the antibody involved. Therefore, it could not be proven that the majority of AIHA patients had the warm subtype of the disease.
AIHA is secondary in about 25 % of cases triggered by antibodies. Its association has been described in association with lymphoid neoplasms, SLE, rheumatoid arthritis and immunodeficiencies. It may also be secondary to the use of medications such as cephalosporins, levodopa, methyldopa, penicillin, quinidine and non-steroidal anti-inflammatory drugs.31
Within this immunohematological context, it is noteworthy that the choice of DAT as a screening method to select patients is related to the fact that the test highlights the presence of immunoglobulins and complement proteins linked to red blood cells, being the gold standard for the diagnosis of AIHA.32 However, DAT positivity can also be influenced by other situations that cause erythrocyte sensitization, such as transfusion reactions, drug-mediated reactions, infectious processes and other autoimmune conditions.33
The context of the COVID-19 pandemic should not be ignored either, since while this study was ongoing a significant number of individuals were hospitalized for COVID-19. Case reports demonstrate a high percentage of positive DAT among patients with COVID-19.34, 35, 36 Therefore, although the DAT is a sensitive test, its positive result does not necessarily indicate a hemolytic condition and must be interpreted appropriately within the patient's clinical condition.
The interpretation of a positive DAT requires knowledge of the patient's diagnosis, assessment of medications being used, pregnancy and transfusion history, as well as information on the presence of AIHA. The serological result of the test alone does not represent a conclusive diagnosis and must be evaluated together with both clinical data and other laboratory data, such as hematocrit, bilirubin, haptoglobin and reticulocyte count.2
Delays in carrying out the test can cause false results, as samples stored for a long time and in conditions other than ideal tend to naturally elute antibodies that were initially linked to the red blood cell. Moreover, inadequate centrifugation can promote false results.1
In view of the above, it is understood that AIHA is a rare clinical condition that may have idiopathic causes or be triggered by other pathologies, and does not have a suggestive clinical picture, requiring a precise investigation by doctors until a diagnosis is reached. There is a shortage of national publications on this topic, mainly in the State of Goiás. The characterization of patients diagnosed with AIHA is relevant for developing protocols, documenting the effectiveness of the treatments used and designing intervention strategies, therefore, this type of research provides support so that health departments can understand the situation and disseminate information to the population.
Furthermore, it is necessary to understand that the laboratory profile does not always confirm the diagnosis of AIHA, as was seen in this study. Not all patients with a positive DAT, hemoglobin below the reference value and a positive hemolysis test were diagnosed with this disease.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Feitosa E.A.M., Vizzoni E.A.G. Clinical significance of the direct Coombs test in the pre-transfusion routine. Infarma. 2009;21(11):37–46. [Google Scholar]
- 2.Silva F.R.M., Silva N.R., Neto R.P., et al. Human antiglobulin test in laboratory routine. Study Res Notebooks. 2013;17(38):57–62. [Google Scholar]
- 3.Karmo K.P.S. Course completion work (Professional Improvement Program) – State Department of Health-Fundap, prepared at the faculty of medicine of Marília. 2013. Techniques used to select packed red blood cell units in patients with autoimmune hemolytic anemia. Marília, SP: [sn] Area: Hemotherapy. [Google Scholar]
- 4.Ferraz F.L.N.S., et al. Drug-induced hemolytic anemia: a systematic review. RECIMA21. 2022;3(12):1–14. [Google Scholar]
- 5.Harmening D.M. 4th ed. Revinter; Rio de Janeiro: 2006. Modern techniques in blood banking and transfusion; p. 632. [Google Scholar]
- 6.Zantek N.D., Koepsell S.A., Tharp D.R., Jr, Cohn C.S. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87(7):707–709. doi: 10.1002/ajh.23218. [DOI] [PubMed] [Google Scholar]
- 7.Hannon J.L. Management of blood donors and blood donations from individuals found to have a positive direct antiglobulin test. Transfus Med Rev. 2012;26(2):142–152. doi: 10.1016/j.tmrv.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Lapierre Y., Rigal D., Adam J. The gel test: a new way to detect red cell antigen–antibody reactions. Transfusion. 1990;30(2):109–113. doi: 10.1046/j.1537-2995.1990.30290162894.x. [DOI] [PubMed] [Google Scholar]
- 9.Contente A.G.S., Carvalho F.G., Campos T.C.O.B., Gomes T.A. Clinical-epidemiological profile of pediatric patients with autoimmune hemolytic anemia in a reference service. Pediatr Resid. 2021;11(3):1–5. doi: 10.25060/residpediatr-2021.v11n3-233. [DOI] [Google Scholar]
- 10.Characteristics of patients with autoimmune hemolytic anemia treated at HCPA. Braz J Hematol Hemother. 2017:8–11. [Google Scholar]
- 11.Ramos A.B.A., Ribeiro A.J., Delfino K.K.S., Almeida L.S., da Silva AL. Autoimmune hemolytic anemia: an integrative review. EACAD. 2022;3(2) https://eacademica.org/eacademica/article/view/258 [cited Feb 3, 2023]. Available at. [Google Scholar]
- 12.Barcellini W., Fattizzo B. Clinical applications of hemolytic markers in the differential diagnosis and management of hemolytic anemia. Dis Markers. 2015;2015 doi: 10.1155/2015/635670. Epub 2015 Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Packman C.H. The clinical pictures of autoimmune hemolytic anemia. Transfus Med Hemother. 2015;42(5):317–324. doi: 10.1159/000440656. Epub 2015 Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barcellini W. New insights in the pathogenesis of autoimmune hemolytic anemia. Transfus Med Hemother. 2015;42(5):287–293. doi: 10.1159/000439002. Epub 2015 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira F.C., Mendes I.C., Saucer T.S., Carneiro L.C., Guillo L.A., Amaral W.N., et al. Diagnosis and treatment of autoimmune hemolytic anemia: a mini review. Minas Gerais Med Mag. 2020 [Google Scholar]
- 16.Rattarittamrong E., Eiamprapai P., Tantiworawit A., Rattanathammethee T., Hantrakool S., Chai-Adisaksopha C., et al. Clinical characteristics and long-term outcomes of warm-type autoimmune hemolytic anemia. Hematology. 2016;21(6):368–374. doi: 10.1080/10245332.2016.1138621. [DOI] [PubMed] [Google Scholar]
- 17.Kokori S.I., Ioannidis J.P., Voulgarelis M., Tzioufas A.G., Moutsopoulos H.M. Autoimmune hemolytic anemia in patients with systemic lupus erythematosus. Am J Med. 2000;108(3):198–204. doi: 10.1016/s0002-9343(99)00413-1. [DOI] [PubMed] [Google Scholar]
- 18.Beyan E., Beyan C., Turan M. Hematological presentation in systemic lupus erythematosus and its relationship with disease activity. Hematology. 2007;12(3):257–261. doi: 10.1080/10245330701214145. [DOI] [PubMed] [Google Scholar]
- 19.Jeffries M., Hamadeh F., Aberle T., Glenn S., Kamen D.L., Kelly J.A., et al. Haemolytic anemia in a multi-ethnic cohort of lupus patients: a clinical and serological perspective. Lupus. 2008;17(8):739–743. doi: 10.1177/0961203308090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein A., Molad Y. Hematological manifestations among patients with rheumatic diseases. Acta Haematol. 2021;144(4):403–412. doi: 10.1159/000511759. Epub 2020 Nov 20. [DOI] [PubMed] [Google Scholar]
- 21.Fattizzo B., Barcellini W. Autoimmune cytopenias in chronic lymphocytic leukemia: focus on molecular aspects. Front Oncol. 2020;9:1435. doi: 10.3389/fonc.2019.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Autore F., Pasquale R., Innocenti I., Fresa A., Sora' F., Laurenti L. Autoimmune hemolytic anemia in chronic lymphocytic leukemia: a comprehensive review. Cancers (Basel) 2021;13(22):5804. doi: 10.3390/cancers13225804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Audia S., Grienay N., Mounier M., Michel M., Bonnotte B. Evans' syndrome: from diagnosis to treatment. J Clin Med. 2020;9(12):3851. doi: 10.3390/jcm9123851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaime-Pérez J.C., Aguilar-Calderón P.E., Salazar-Cavazos L., Gómez-Almaguer D. Evans syndrome: clinical perspectives, biological insights and treatment modalities. J Blood Med. 2018;9:171–184. doi: 10.2147/JBM.S176144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill A., DeZern A.E., Kinoshita T., Brodsky R.A. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Prim. 2017;3:17028. doi: 10.1038/nrdp.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodsky R.A. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804–2811. doi: 10.1182/blood-2014-02-522128. Epub 2014 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angileri F., Légaré S., Marino Gammazza A., Conway de Macario E., Macario A.J.L., Cappello F. Is molecular mimicry the culprit in the autoimmune haemolytic anemia affecting patients with COVID-19? Br J Haematol. 2020;90(2):e92–e93. doi: 10.1111/bjh.16883. Epub 2020 Jun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaime-Pérez J.C., Rodríguez-Martínez M., Gómez-de-León A., Tarín-Arzaga L., Gómez-Almaguer D. Current approaches for the treatment of autoimmune hemolytic anemia. Arch Immunol Ther Exp. 2013;61(5):385–395. doi: 10.1007/s00005-013-0232-3. Epub 2013 May 21. [DOI] [PubMed] [Google Scholar]
- 29.Giudice V., Rosamilio R., Ferrara I., Seneca E., Serio B., Selleri C. Efficacy and safety of splenectomy in adult autoimmune hemolytic anemia. Open Med. 2016;11(1):374–380. doi: 10.1515/med-2016-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lima A.F. National Institute of Higher Education and Research (INESP; 2015. Autoimmune hemolytic anemia and laboratory diagnosis: a literature review. Recife. Monograph [Lato Sensu Postgraduate Course in Hematology and Laboratory Hemotherapy] [Google Scholar]
- 31.Zulfiqar A.A., Mahdi R., Mourot-Cottet R., Pennaforte J.L., Novella J.L., Andrés E., et al. Autoimmune hemolytic anemia - a short review of the literature, with a focus on elderly patients. J Hematol Thrombo. 2015;6(3):1–5. [Google Scholar]
- 32.Parker V., Tormey C.A. The direct antiglobulin test: indications, interpretation, and pitfalls. Arch Pathol Lab Med. 2017;141(2):305–310. doi: 10.5858/arpa.2015-0444-RS. [DOI] [PubMed] [Google Scholar]
- 33.Zantek N.D., Koepsell S.A., Tharp D.R., Jr, Cohn C.S. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87(7):707–709. doi: 10.1002/ajh.23218. Epub 2012 May 6. [DOI] [PubMed] [Google Scholar]
- 34.Hendrickson J.E., Tormey C.A. COVID-19 and the Coombs test. Blood. 2020;136(6):655–656. doi: 10.1182/blood.2020007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cabo J., Brochier A., Saussoy P., van Dievoet M.A., Capirchio L., Delire B., et al. Positive direct antiglobulin test in COVID-19 patients: decision-making process. Transfus Clin Biol. 2021;28(4):414–419. doi: 10.1016/j.tracli.2021.05.010. Epub 2021 Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hafez W., Ziade M.A., Arya A., Saleh H., Abdelrahman A. The significance of antiglobulin (Coombs) test reactivity in patients with COVID-19. Immunobiology. 2022;227(4) doi: 10.1016/j.imbio.2022.152240. Epub 2022 Jul 6. [DOI] [PMC free article] [PubMed] [Google Scholar]