Abstract
Background
The Wnt pathway is involved in proliferation and tissue homeostasis. Aberrant activation promotes cancer cell proliferation and survival. Inhibition of the low-density lipoprotein receptor-related protein 5/6 (LRP5/6) coreceptors that regulate Wnt signaling could prevent cancer cell proliferation. BI 905681 is a novel LRP5 antagonist that has demonstrated potent in vivo antitumor activity.
Patients and methods
This was a phase I, dose escalation study (NCT04147247) evaluating BI 905681 in patients with advanced solid tumors over two dosing schedules (schedule A: every 3 weeks, 3-week cycles and schedule B: every 2 weeks, 4-week cycles). The primary endpoint was the maximum tolerated dose (MTD) of BI 905681 and the number of patients experiencing adverse events (AEs). Other endpoints were pharmacokinetics, pharmacodynamics, and efficacy.
Results
As a result of difficulties enrolling patients, the trial was terminated early and the MTD for schedule A could not be determined. Twenty-one patients received BI 905681 over five dose cohorts (schedule A: 1.0, 2.5, 5.0, 7.0, and 8.5 mg/kg). No patients received schedule B. No dose-limiting toxicities (DLTs) were reported during the MTD evaluation period. However, during the entire treatment period, two patients (9.5%) experienced a DLT of grade 1 C-telopeptide increase in the 5.0 and 8.5 mg/kg dose cohorts. The most frequent treatment-related AEs were diarrhea (23.8%), vomiting (23.8%), nausea (19.0%), and infusion-related reactions (IRRs; 14.3%). Despite premedication to mitigate IRRs, one patient experienced a grade 2 IRR. The pharmacokinetic profiles of BI 905681 were biphasic, with a rapid distribution phase in the beginning followed by a slower elimination phase. The objective response rate was 0%; 5 (23.8%) and 14 patients (66.7%) had a best overall response of stable disease and progressive disease, respectively.
Conclusion
BI 905681 has minimal efficacy in an unselected patient population and was generally well tolerated.
Key words: Wnt, LRP5, solid tumor, phase I, clinical trial
Highlights
-
•
BI 905681 is an LRP5 antagonist that has demonstrated preclinical antitumor activity.
-
•
This first-in-human phase I dose escalation study evaluates BI 905681 monotherapy in patients with advanced solid tumors.
-
•
Two patients experienced a DLT during the entire treatment period (C-telopeptide increase).
-
•
The MTD could not be determined.
-
•
BI 905681 had minimal efficacy in an unselected patient population.
Introduction
There is a substantial need for novel therapeutic strategies to improve outcomes for patients with advanced cancers.1 The Wnt signaling pathway is a conserved signaling axis involved in proliferation, differentiation, apoptosis, migration, invasion, and tissue homeostasis. Aberrant activation of Wnt signaling has been implicated in many human malignancies, including colorectal cancer, triple-negative breast cancer, non-small-cell lung cancer, and liver cancer.2, 3, 4, 5
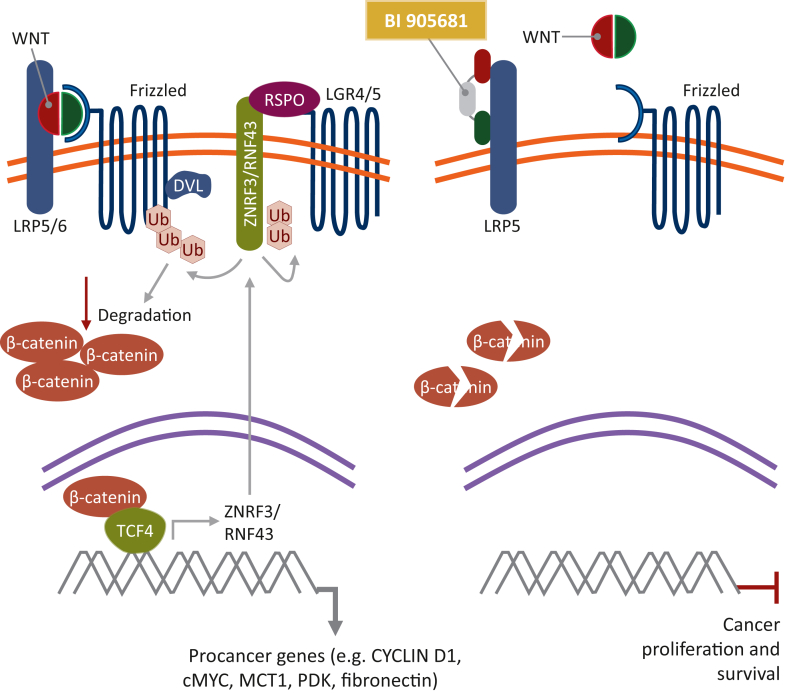
The Wnt signaling pathway is divided into canonical (β-catenin-dependent) and noncanonical (β-catenin-independent) branches, the former being the most widely studied pathway with an essential role in embryonic development, adult homeostasis, and stem cell maintenance.6 In canonical Wnt signaling, the absence of Wnt ligands leads to phosphorylation of β-catenin, which is subsequently ubiquitinated and degraded by a multiprotein destruction complex composed of Axin, adenomatous polyposis coli (APC), casein kinase 1 alpha (CK1-α), and glycogen synthase kinase-3 beta (GSK-3β).7 Upon binding of Wnt ligands to the cell surface receptor frizzled and the coreceptor low-density lipoprotein receptor-related protein 5/6 (LRP5/6), Dishevelled (Dvl) and Axin are recruited to the plasma membrane, leading to disassembly of the destruction complex and accumulation of β-catenin in the cytoplasm. β-Catenin translocates to the nucleus where it interacts with the T-cell factor/lymphoid enhancer factor family of transcription factors to promote transcription of target genes (Figure 1).6,8,9 Aberrant activation of this pathway results in β-catenin accumulation within the nucleus or cytoplasm. A frequent mechanism of Wnt pathway disruption includes APC mutations, which occur in many different cancer types, including colon, prostate, breast, and non-small-cell lung cancers. Without functional APC, β-catenin translocates into the nucleus and results in subsequent transcription of target genes.10 High-level cytoplasm expression and nuclear localization induce tumorigenic traits and promote cancer cell proliferation and survival.11
Figure 1.
Inhibition of the canonical Wnt/β-catenin signaling pathway by BI 905681. DVL, Dishevelled; LGR4/5, leucine-rich repeat-containing G-protein coupled receptor 4/5; LRP5/6, low-density lipoprotein receptor-related protein 5/6; RNF43, ring finger 43; RSPO, R-spondin; TCF4, T-cell factor 4; Ub, ubiquitin; ZNRF3, zinc and ring finger 3. Adapted from Hao H-X, et al. Cancers. 2016;8:54.9 Note: BI 905681 binds to LRP5 only to block the binding of Wnt ligands to the coreceptor complex.
Wnt pathway regulation is also provided by E3 ligase ring finger protein 43 (RNF43) and R-spondin (RSPO). RNF43 ubiquitinates LRP5/6 and frizzled receptors, which promotes their degradation and leads to reduced Wnt pathway activation. RSPO amplifies target cell sensitivity to Wnt ligands by inducing membrane clearance of RNF43 and zinc and ring finger protein 3, which increases cell surface levels of Wnt receptors. Chromosomal translocations that increase RSPO expression or mutations that inactivate RNF43 can drive cancer. RNF43 gene mutations have been reported in colorectal adenocarcinomas (∼6%), endometrial carcinomas (∼18%), and pancreatic cancers (∼13%).12, 13, 14 RSPO fusions have been shown to potentiate Wnt signaling and tumorigenesis in colon cancer (∼1%).12,13,15, 16, 17, 18 Furthermore, studies have shown that the Wnt signaling pathway may have a role in tumor immune evasion, immunotherapy resistance, and chemoresistance.19, 20, 21 As a result of this, a valid therapeutic option may involve an antagonist that inhibits LRP5, preventing the binding of Wnt ligands and subsequent proliferation of malignant cells and increasing sensitivity to immunotherapies.
BI 905681 is an intravenously (i.v.) administered agent that binds to LRP5 and blocks the binding of Wnt ligands to the coreceptor complex, leading to the inhibition of Wnt/β-catenin signaling. BI 905681 consists of three modules (nanobodies® connected by two peptide linkers); two bind to distinct epitopes of LRP5 (biparatopic) and one binds to human serum albumin for half-life extension. BI 905681 inhibited Wnt/β-catenin signaling in mechanistic Wnt-driven and disease-relevant models harboring RNF43 mutations. In RNF43-mutant pancreatic cancer cell lines and patient-derived RNF43-mutant colorectal cancer organoids, BI 905681 demonstrated effective blockade of ligand-dependent Wnt signaling in vitro. In vivo, BI 905681 reduced Axin2 and Notum gene expression, measures of β-catenin signaling, in Wnt-driven breast cancer xenografts. Furthermore, BI 905681 demonstrated in vitro inhibition of cell viability and potent in vivo antitumor activity in the aforementioned models (data on file). The strength of these data supports the investigation of BI 905681 in patients with advanced cancers.
Here we present the results of this phase I, first-in-human, dose escalation study to determine the maximum tolerated dose (MTD)/optimal biological dose (OBD) of BI 905681 in patients with advanced and/or metastatic solid tumors for whom no standard treatment options exist.
Patients and methods
Study design
This was a phase I, first-in-human, open-label, nonrandomized, dose escalation study (NCT04147247) evaluating BI 905681 monotherapy in patients with advanced and/or metastatic solid tumors. The study aimed to test two dosing schedules of BI 905681 (schedules A and B), with schedule B opening once the MTD/OBD for schedule A was determined. Schedule A involved dose escalation of BI 905681 i.v. every 3 weeks in 3-week cycles until disease progression. The planned dosing for schedule B was BI 905681 i.v. every 2 weeks in 4-week cycles. Recruitment into each dosing schedule was to occur sequentially, with the starting dose in schedule B to be determined by the Safety Monitoring Committee. Successive cohorts of patients received increasing doses of BI 905681 until the dose escalation was terminated. In each dose cohort, a minimum of two patients were treated until a first adverse event (AE) of Common Terminology Criteria for Adverse Events (CTCAE) grade ≥2 occurred during the dose-limiting toxicity (DLT) observation period (defined as the first cycle of treatment), excluding AEs related to progressive disease (PD) or concurrent illness. In that event, a minimum of three patients were to be treated in the recruiting cohort and subsequent dose cohorts.
Starting at dose cohort 2, the aim was to recruit a minimum of two patients with solid tumors harboring an RNF43 mutation or RSPO fusion. In case two patients could not be recruited to a dose cohort, additional patients harboring the RNF43 mutation or RSPO fusion could be recruited in backfill cohorts. Patients included in the backfill cohorts were treated at a dose level that was cleared by the Safety Monitoring Committee and below the dose level investigated at that time, to permit correlation between exposure and pharmacodynamic effects. The trial was conducted and reported in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, local regulations, and Boehringer Ingelheim standard operating procedures.
Patient eligibility
All patients provided written informed consent before the initiation of any trial-related procedure. Eligible patients were aged ≥18 years and had a histologically and/or cytologically confirmed diagnosis of an advanced, unresectable, and/or metastatic malignant solid tumor, who failed conventional treatment, for whom no therapy of proven efficacy exists, or who were not eligible for established treatment options. Patients were required to have an Eastern Cooperative Oncology Group performance score of 0 or 1, adequate organ function, resolution of any previous therapy-related toxicities, and a life expectancy of ≥3 months at the start of study treatment.
Patients who harbored a historically confirmed RNF43 mutation or RSPO fusion had to be willing to undergo mandatory pre- and on-treatment tumor biopsies for pharmacodynamic biomarker analyses. Patients were excluded if they had previous or concomitant malignancies other than the one treated in this study within the past 2 years (except for effectively treated nonmelanoma skin cancers, carcinoma in situ of the cervix, ductal carcinoma in situ, or another effectively treated malignancy that is considered cured by local treatment). Patients were also ineligible if they had osteoporosis CTCAE grade ≥2, chronic corticosteroid use (except for maintenance therapy of brain metastases), an osteoporotic compression fracture within 12 months before informed consent, received treatment with systemic anticancer therapy or investigational drug within 28 days of first study treatment, a history or presence of uncontrolled or symptomatic brain or subdural metastases, or had a history of grade 3 hypersensitivity reactions to monoclonal antibodies.
Endpoints
The primary endpoints were the MTD/OBD of BI 905681 and the number of patients experiencing AEs during the entire treatment period. The MTD was assessed based on the number of patients experiencing DLTs, graded according to CTCAE version 5.0, during the first cycle of treatment (the MTD evaluation period). It was defined as the highest dose with <25% risk of the true DLT rate being ≥33%. DLTs were defined as any AE that prevented a patient from starting cycle 2 within 14 days of completion of cycle 1 (schedule A), as outlined in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103730.
The secondary endpoint was the pharmacokinetic (PK) profile of BI 905681, including the maximum measured concentration of BI 905681 in serum after the first infusion (Cmax) and the area under the serum concentration–time curve over the time interval from 0 to the last measured time point (AUC0–tz).
Further efficacy endpoints included the assessment of target modulation on treatment compared with baseline, based on pharmacodynamic biomarker assessment; objective response rate, defined as complete response (CR) or partial response (PR) according to RECIST version 1.1 (investigator-assessed); disease control rate, defined as CR, PR, or stable disease (SD) according to RECIST version 1.1; and duration of disease control measured from the start of the trial treatment to the date of PD for patients who had CR, PR, or SD during treatment. Baseline imaging was carried out within 4 weeks before the first treatment with BI 905681 and tumor assessments were carried out every 6 weeks (±7 days) from the start of treatment until documented progression.
Statistical analyses
Dose escalation and cohort size were determined based on the recommendation of the Safety Monitoring Committee, guided by the Bayesian logistic regression model with overdose control. Safety analyses were descriptive and based on treatment-emergent AEs; all treated patients were included in the analyses. BI 905681 concentrations were determined by a validated immunoassay; the presence of antidrug antibodies (ADAs) in relation to BI 905681 was assessed using a validated immunoassay in a tiered approach. All patients in the treated set who received at least one dose of BI 905681 and provided at least one valid serum concentration value were included in the PK analysis and descriptive statistics were presented for Cmax and AUC0–tz. PK parameters were determined by noncompartmental analysis using Phoenix WinNonlin™ software (version Phoenix 8.1; Certara USA Inc., Princeton, NJ). Objective response was analyzed regardless of confirmation and best overall response (BOR) was analyzed descriptively. Duration of disease control was analyzed using Kaplan–Meier methods; for exploratory analyses, summary statistics of the naive duration of response without using Kaplan–Meier methods were also given.
Results
Baseline characteristics
Between 27 December 2019 and 27 May 2022, 21 patients received BI 905681 i.v. every 3 weeks (schedule A). Patients were enrolled into five dose escalation cohorts: 1.0, 2.5, 5.0, 7.0, and 8.5 mg/kg (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103730). Patient baseline characteristics are presented in Table 1. The median age was 63.0 years (range 34.0-77.0 years); 52.4% of patients were male and the majority were white (66.7%). All patients had prior anticancer therapy, including systemic therapy (n = 21, 100.0%; median prior lines 4, range 2-9), radiation (n = 11, 52.4%), and surgery (n = 16, 76.2%). Five patients (23.8%) had a history of RNF43 mutations, and no patient had a history of RSPO fusion at the time of trial enrollment.
Table 1.
Baseline characteristics
| Total (N = 21) | |
|---|---|
| Male, n (%) | 11 (52.4) |
| Age (years), median (range) | 63 (34-77) |
| Race, n (%) | |
| White | 14 (66.7) |
| Black or African American | 4 (19.0) |
| Asian | 1 (4.8) |
| Other | 2 (9.5) |
| ECOG PS 1 at baseline, n (%) | 13 (61.9) |
| Primary diagnosis, n (%) | |
| Colon and rectal | 9 (42.9) |
| Pancreas | 4 (19.0) |
| Prostate | 3 (14.3) |
| Esophageal | 1 (4.8) |
| Fallopian tube | 1 (4.8) |
| Kidney | 1 (4.8) |
| Metastatic adenocarcinoma of the GE junction | 1 (4.8) |
| Ovary | 1 (4.8) |
| Metastatic disease at diagnosis, n (%) | 12 (57.1) |
| Location of metastases at study entry, n (%) | |
| Liver | 14 (66.7) |
| Local or regional lymph nodes | 13 (61.9) |
| Lung | 14 (66.7) |
| Other | 11 (52.4) |
| Distant lymph nodes | 7 (33.3) |
| Bone | 5 (23.8) |
| Brain | 1 (4.8) |
| Median number of metastatic sites at baseline, n (range) | 3.0 (1.0-7.0) |
| History of RNF43 mutation, n (%) | 5 (23.8) |
| History of RSPO fusion, n (%) | 0 (0) |
| Previous systemic therapy, n (%) | |
| Chemotherapy | 20 (95.2) |
| Antibody | 13 (61.9) |
| Immune | 8 (38.1) |
| Other | 7 (33.3) |
| Hormone | 3 (14.3) |
| Tyrosine kinase inhibitor | 2 (9.5) |
ECOG PS, Eastern Cooperative Oncology Group performance status; GE, gastroesophageal; RNF43, ring finger protein 43; RSPO, R-spondin.
Treatment exposure
The median duration of treatment was 2.0 cycles (range 1-4 cycles) and the median dose intensity was 100.3% (range 91%-102%). One patient in the 8.5 mg/kg dose cohort experienced continued grade 2 treatment-related nausea that resulted in a dose reduction at cycle 3. Details of treatment exposure in individual dose cohorts can be found in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103730. The highest dose of BI 905681 tested was 8.5 mg/kg.
MTD/OBD
None of the patients experienced a DLT during the MTD evaluation period. Two patients (9.5%) experienced a DLT during the entire treatment period in the 5.0 and 8.5 mg/kg dose cohorts. In the 5.0 mg/kg cohort, one patient experienced a grade 1 C-telopeptide increase over twofold compared with baseline during cycle 4. One patient in the 8.5 mg/kg dose cohort experienced grade 1 C-telopeptide increase over twofold compared with baseline during cycle 2. As a result of difficulties in enrolling patients with tumors harboring either RNF43 mutations or RSPO fusions, the trial was prematurely terminated on 29 October 2021. The MTD/OBD for schedule A could not be determined; therefore schedule B was not pursued.
Adverse events
Treatment-related AEs were experienced by 13 patients (61.9%). The most frequently reported treatment-related AEs were diarrhea and vomiting (n = 5 patients, 23.8%, each), nausea (n = 4 patients, 19.0%), and infusion-related reaction (IRR; n = 3 patients, 14.3%). One patient (4.8%) in the 8.5 mg/kg dose cohort experienced grade 3 treatment-related diarrhea. No patient experienced a grade 4 or 5 treatment-related AE (Tables 2 and 3). One patient in the 8.5 mg/kg dose cohort experienced continuous grade 2 treatment-related nausea that resulted in a dose reduction at cycle 3. Two patients (9.5%) experienced an AE that resulted in permanent treatment discontinuation in the 5.0 and 8.0 mg/kg dose cohorts. One patient in the 5.0 mg/kg dose cohort experienced a grade 3 cerebrovascular accident unrelated to treatment, and one patient in the 8.5 mg/kg cohort experienced treatment-related grade 3 diarrhea, grade 2 nausea, and grade 2 vomiting, which lasted 1 day and resulted in permanent treatment discontinuation at cycle 1 day 2 due to the patient withdrawing consent. There were no AEs leading to death. Three patients (14.3%) died during the study, all of which were due to underlying disease. Five patients (23.8%) experienced serious AEs (SAEs), although none of these were defined as treatment-related by the investigator.
Table 2.
Summary of adverse events in the treated population
| Summary | BI 905681 |
|||||
|---|---|---|---|---|---|---|
| 1.0 (n = 3) | 2.5 (n = 4) | 5.0 (n = 5) | 7.0 (n = 4) | 8.5 (n = 5) | Total (N = 21) | |
| Any AE, n (%) | 2 (66.7) | 4 (100.0) | 5 (100.0) | 4 (100.0) | 4 (80.0) | 19 (90.5) |
| Treatment-related AEs (per investigator), n (%) | 2 (66.7) | 1 (25.0) | 3 (60.0) | 3 (75.0) | 4 (80.0) | 13 (61.9) |
| AESI, n (%) | 1 (33.3) | 0 (0) | 3 (60.0) | 1 (25.0) | 0 (0) | 5 (23.8) |
| AEs leading to dose reduction of BI 905681, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 1 (4.8) |
| AEs leading to permanent discontinuation of BI 905681, n (%) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 1 (20.0) | 2 (9.5) |
| DLTs, n (%) | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 1 (20.0) | 2 (9.5) |
| Serious AEs, n (%)a | 1 (33.3) | 1 (25.0) | 2 (40.0) | 1 (25.0) | 0 (0) | 5 (23.8) |
| Results in death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Life-threatening | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Persist or significant disability | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Requires hospitalization | 1 (33.3) | 1 (25.0) | 1 (20.0) | 1 (25.0) | 0 (0) | 4 (19.0) |
| Prolongs hospitalization | 1 (33.3) | 1 (25.0) | 0 (0) | 0 (0) | 0 (0) | 2 (9.5) |
| Congenital anomaly or birth defect | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other medically important serious event | 0 (0) | 1 (25.0) | 2 (40.0) | 0 (0) | 0 (0) | 3 (14.3) |
| Worst CTCAE grade, n (%) | ||||||
| Grade 1 | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 1 (20.0) | 2 (9.5) |
| Grade 2 | 1 (33.3) | 2 (50.0) | 3 (60.0) | 4 (100.0) | 2 (40.0) | 12 (57.1) |
| Grade 3b | 1 (33.3) | 2 (50.0) | 1 (20.0) | 0 (0) | 1 (20.0) | 5 (23.8) |
| Grade 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Grade 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
AE, adverse event; AESI, adverse event of special interest; CTCAE, Common Technology Criteria for Adverse Events; DLT, dose-limiting toxicity.
A patient may have serious AE(s) with multiple seriousness criteria.
All grade 3 AEs were unrelated to the trial drug, except for one event of grade 3 treatment-related diarrhea.
Table 3.
Frequency of patients with treatment-related AEs during the on-treatment period
| Preferred term, all grades | BI 905681 (mg/kg) |
|||||
|---|---|---|---|---|---|---|
| 1.0 (n = 3) | 2.5 (n = 4) | 5.0 (n = 5) | 7.0 (n = 4) | 8.5 (n = 5) | Total (N = 21) | |
| Total with treatment-related AEs, n (%) | 2 (66.7) | 1 (25.0) | 3 (60.0) | 3 (75.0) | 4 (80.0) | 13 (61.9)a |
| Diarrhea | 0 (0) | 1 (25.0) | 0 (0) | 1 (25.0) | 3 (60.0) | 5 (23.8) |
| Nausea | 0 (0) | 0 (0) | 0 (0) | 1 (25.0) | 3 (60.0) | 4 (19.0) |
| Vomiting | 0 (0) | 0 (0) | 0 (0) | 2 (50.0) | 3 (60.0) | 5 (23.8) |
| Infusion-related reaction | 1 (33.3) | 0 (0) | 1 (20.0) | 1 (25.0) | 0 (0) | 3 (14.3) |
| C-telopeptide increase | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 1 (20.0) | 2 (9.5) |
| Pruritus | 1 (33.3) | 0 (0) | 1 (20.0) | 0 (0) | 0 (0) | 2 (9.5) |
| Constipation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 1 (4.8) |
| Dyspepsia | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 0 (0) | 1 (4.8) |
| GE reflux disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 1 (4.8) |
| Chills | 0 (0) | 0 (0) | 1 (20.0) | 0 (0) | 0 (0) | 1 (4.8) |
| Influenza-like illness | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 1 (4.8) |
| Decreased appetite | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 1 (4.8) |
| Arthralgia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | 1 (4.8) |
AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; GE, gastroesophageal.
All treatment-related AEs were CTCAE grade 1 or 2, except for one case of grade 3 diarrhea in the 8.5 mg/kg cohort.
Five patients (23.8%) experienced AEs of special interest (AESIs). One patient in the 1.0 mg/kg cohort experienced a grade 2 IRR. Three patients in the 5.0 mg/kg cohort experienced AESIs: one experienced grade 2 IRR, another experienced grade 1 chills (which was determined to be an IRR), and the third had grade 1 C-telopeptide increase. β-CTX levels over time per dose level are shown in Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103730. To mitigate infusion reactions and/or hypersensitivity reactions, mandatory premedication with acetaminophen/paracetamol plus antihistamine before BI 905681 infusion was implemented. However, one patient in the 7.0 mg/kg cohort experienced a grade 2 IRR despite premedication. All AESIs were considered related to the study treatment, and none were SAEs.
Pharmacokinetics
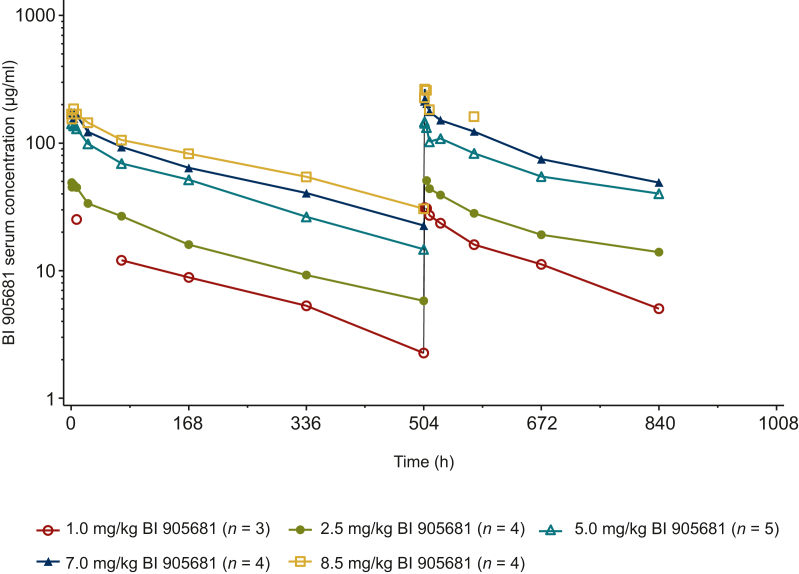
After i.v. infusion of BI 905681, serum concentrations were detectable up to 504 h after infusion, except for three patients who dropped out in the first cycle. Serum concentrations increased in proportion to the dose. The shape of the serum concentration–time profiles appeared to be similar among the different dose cohorts. The maximum serum concentrations of BI 905681 were generally observed at the end of infusion or within a few hours thereafter (Figure 2). The PK profiles of BI 905681 were biphasic, with a rapid distribution phase in the beginning followed by a slower elimination phase. After the first infusion of BI 905681 (cycle 1), geometric mean (gMean) Cmax and AUC0-tz increased in proportion to dose, except for AUC0-tz of the 8.5 mg/kg dose group due to one patient who discontinued the study 24 h after the first dose. The median tmax ranged from 1.60 to 3.99 h between the dose groups. Dose-normalized individual and gMean Cmax and AUC0-tz values were in the same range for the different dose groups (1.0-8.5 mg/kg). However, the 2.5 mg/kg dose group showed lower gMean Cmax values (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103730). After the second dose (cycle 2), the exposure of BI 905681 observed as gMean Cmax and AUC0-tz increased with increasing dose. The median tmax ranged from 1.13 to 1.57 h between the dose groups (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.103730).
Figure 2.
Geometric mean serum concentration–time profiles of BI 905681 every 3 weeks by dose group in cycle 1 (left) and cycle 2 (right).
Pharmacodynamics
Pharmacodynamic analysis was not conducted because there was only one evaluable paired tumor biopsy set.
Immunogenicity
A total of 19 patients (90.5%) were ADA-evaluable. Of these, there were a total of six patients with treatment-emergent ADA positivity (31.6%). The median time of onset of ADA development was 21 days (range 20-46 days). However, due to limited data, no conclusion about the correlation between PK and ADA could be made.
Efficacy
No patients achieved a CR or PR, and therefore the objective response rate was 0%; five patients (23.8%) had a BOR of SD and 14 patients (66.7%) had a BOR of PD. Of the five patients with SD, one patient had RNF43-mutant colorectal cancer. There were too few patients with RNF43 mutations (n = 5) in the trial to draw any conclusions. The disease control rate was 23.8% and the median duration of disease control was 80.0 days (range 35-89 days). When calculated from a Kaplan–Meier curve, the median duration of disease control was 84.5 days (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.103730).
Discussion
This first-in-human, phase I study aimed to evaluate the MTD/OBD, safety, PK profile, and efficacy of BI 905681 administered i.v. over two dosing schedules assessed sequentially, whereby a separate MTD/OBD was to be determined for each schedule, in patients with advanced and/or metastatic solid tumors. The trial was prematurely terminated on 29 October 2021 due to difficulty enrolling patients with tumors harboring either RNF43 mutations or RSPO fusions, which resulted in the MTD not being reached. As a result of this, pharmacodynamic assessment of paired tumor biopsies could not be carried out to determine a dose where a sufficient pharmacodynamic effect was achieved. Furthermore, none of the patients experienced DLTs during the MTD evaluation period. Therefore the MTD/OBD for schedule A could not be determined and consequently, schedule B was not pursued. The highest dose of BI 905681 explored was 8.5 mg/kg on day 1 of each 21-day cycle (median 2 cycles).
In this dose escalation study, two patients experienced a DLT during the entire treatment period of C-telopeptide increase of over twofold compared with baseline, which indicates increased bone resorption. This effect is not unexpected due to the role of Wnt signaling in the regulation of bone formation and remodeling and was therefore included as a specific definition to qualify as a DLT. Previous studies have shown that activation of the canonical Wnt signaling pathway in osteoblasts suppresses bone resorption.22 Furthermore, a similar study of a small-molecule inhibitor of an o-acyl transferase porcupine that blocks the secretion of Wnt revealed that acute loss of bone in treated patients could be mitigated by the coadministration of alendronate.23 Therefore it may be beneficial for future clinical studies investigating Wnt-targeting agents to administer prophylactic antiresorption treatment, such as alendronate.
BI 905681 was generally well tolerated and was independent of dose levels. Five patients experienced SAEs and three patients died during the study, though none were defined as drug-related by the investigator. After three of the first nine patients experienced IRRs, premedication with acetaminophen plus antihistamine before all BI 905681 infusions was made mandatory. However, one patient in the 7.0 mg/kg cohort experienced a grade 2 IRR despite premedication, for which no explanation could be found. The most frequent treatment-emergent AEs were gastrointestinal toxicities including diarrhea, nausea, vomiting, and constipation, which are consistent with the safety profiles of other Wnt signaling/β-catenin antagonists.24, 25, 26, 27 Most gastrointestinal toxicities were grade ≤2, with the exception of one patient who experienced grade 3 diarrhea.
Similar to many other nanobodies, the PK profile of BI 905681 is biphasic with a rapid distribution phase in the beginning followed by a slower elimination phase. However, no conclusion regarding the steady state could be drawn due to lack of data. The ADA incidence was 31.6% in this trial. As a result of limited data, no conclusions regarding the correlation between PK and ADA could be determined.
In this first-in-human study, five patients (23.8%) treated with BI 905681 had a BOR of SD, with none of the patients achieving a BOR of CR or PR. The disease control rate was 23.8%, with a median duration of disease control of 80.0 days, all together indicating that BI 905681 has minimal efficacy with regard to tumor response and disease control in an unselected patient population.
Various clinical trials have investigated agents inhibiting different components of Wnt signaling. A phase Ib study evaluated the Wnt antagonist ipafricept in combination with gemcitabine and nab-paclitaxel in patients with untreated metastatic pancreatic cancer. Of the 26 patients enrolled, 34.6% had PR and 46.2% had SD as the best response, with a clinical benefit rate of 81%. The median progression-free survival was 5.9 months (95% confidence interval 3.4-18.4 months) and the median overall survival of 9.7 months (95% confidence interval 7.0-14.0 months). However, the study was terminated due to bone-related toxicities.28 A first-in-human phase I study evaluating the anti-frizzled-10 antibody OTSA-101 in patients with synovial sarcoma resulted in three of the eight treated patients having SD.29 Vantictumab (OMP-18R5), another monoclonal antibody that binds to frizzled receptors and inhibits Wnt signaling, was investigated in combination with paclitaxel for the treatment of locally advanced or metastatic human epidermal growth factor receptor 2-negative breast cancer. The overall response rate of the 48 patients enrolled was 31.3% and the clinical benefit rate was 68.8%. However, similar to other clinical trials, the incidence of patients experiencing fractures limited the clinical development of this Wnt inhibitor.30
One explanation for the minimal efficacy observed in this study could be insufficient inhibition of the Wnt pathway. BI 905681 only blocks binding to LRP5, so Wnt can still bind to LRP6 to stimulate downstream signaling. Therefore one way to overcome this may be through inhibition of LRP5 and LRP6. BI 905677 is a novel LRP5/6 antagonist with a similar structure to BI 905681. It was also investigated in a phase I study (NCT03604445), and had a tolerable safety profile, with the MTD determined to be 2.8 mg/kg every 3 weeks. However, the study was discontinued because BI 905677 had minimal efficacy with regard to tumor response and disease control.31 Another reason for poor efficacy could be the presence of other Wnt pathway mutations. For example, APC is the most frequent Wnt-associated mutation in cancer. As this is downstream of LRP5, it could be a mechanism of resistance. Future studies investigating therapies targeting upstream Wnt signaling may want to consider downstream Wnt components when selecting patient populations.10,32
As the study was prematurely terminated due to difficulties enrolling patients with solid tumors harboring RNF43 or RSPO fusions, the MTD for schedule A could not be determined and different dosing schedules of BI 905681 (schedule B) could not be investigated to further inform the safety and antitumor efficacy in this patient population. The specific patient population, alongside the potential burden of two tumor biopsy requirements on the patient, may explain the difficulties in enrollment.
Conclusion
In conclusion, BI 905681 (schedule A only, as schedule B could not be investigated) had a generally tolerable safety profile with the majority of AEs reported being grades 1-2. One patient experienced a grade 3 treatment-related AE of diarrhea, and none of the patients experienced grade 4 or 5 treatment-related AEs. However, BI 905681 showed minimal antitumor efficacy with no patients achieving a CR or PR. The limited efficacy observed in this study may be a consequence of the inhibition of LRP5 only. Therefore future studies could continue to explore the blockade of the Wnt signaling pathway via dual inhibition of LRP5/6, which may result in a higher degree of Wnt inhibition.
Acknowledgements
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment related to the development of the manuscript. Devon Else, of Nucleus Global, provided writing, editorial support, and formatting assistance, which was contracted and funded by Boehringer Ingelheim International GmbH (BI). BI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Funding
This study was supported and funded by Boehringer Ingelheim International GmbH (BI).
Disclosures
DRS reports grants and/or other from Genentech/Roche, Novartis, Celgene, Bristol Myers Squibb, AstraZeneca, Pfizer, Boehringer Ingelheim, AbbVie, Foundation Medicine, GlaxoSmithKline, Lilly, Merck, Moderna Therapeutics, Nektar, Takeda, Amgen, TRM Oncology, Precision Oncology, Evelo Therapeutics, Illumina, PharmaMar, University of Texas Southwestern Medical Center Simmons Cancer Center, G1 Therapeutics, Neon Therapeutics, Celldex, Clovis Oncology, Daiichi Sankyo, EMD Serono, Acerta Pharma, OncoGenex, Astellas Pharma, GRAIL, Transgene, Aeglea Biotherapeutics, Tesaro, Ipsen, ARMO BioSciences, Millennium, Genzyme, Intuitive Surgical, Purdue Pharma, Spectrum Pharmaceuticals, and Sysmex. JSW reports consulting fees from Kanaph Therapeutics; speaker fees from AstraZeneca and Eisai; advisory board participation for BioNTech, Stemline/Menarini, and Janssen; and research funding to the institution from multiple biotech and pharmaceutical companies to conduct industry-sponsored oncology clinical trials. LP and MT are employees of Boehringer Ingelheim. BM is an external contractor for Boehringer Ingelheim. BB is supported by the Defense Congressionally Directed Medical Research Program # W81XWH-22-1-0208; reports advisory board participation for Merck/Eisai; and reports research support to institution from Amgen, Bicycle Therapeutics, Boehringer Ingelheim, Elucida Oncology, Gritstone Bio, Ikena Oncology, Jazz Pharmaceuticals, Kahr Medical, Lyell Immunopharma, Merck, Pionyr Immunopharma, Rascal Therapeutics, Syros, and Tarveda Therapeutics. HB is an employee of, and reports ownership of stock/shares in, HCA Healthcare and Sarah Cannon Research Institute; and reports noncompensated consulting for Daiichi Sankyo, Pfizer, Bayer, GRAIL, Novartis, Vincerx Pharma, AstraZeneca, and Incyte; and research grants or funds (paid to institution) from Roche/Genentech, BMS, Incyte, AstraZeneca, MedImmune, MacroGenics, Novartis, Boehringer Ingelheim, Lilly, Seattle Genetics, Merck, Agios, Jounce Therapeutics, Moderna Therapeutics, CytomX, GlaxoSmithKline, Verastem, Tesaro, BioMed Valley Discoveries, TG Therapeutics, Vertex, eFFECTOR Therapeutics, Janssen, Gilead Sciences, BioAtla, CicloMed, Harpoon Therapeutics, Arch, Arvinas, Revolution Medicine, Array BioPharma, Bayer, BIND Therapeutics, Kymab, miRNA Therapeutics, Pfizer, Takeda/Millennium, Foundation Medicine, EMD Serono, ARMO BioSciences, CALGB, Hengrui Therapeutics, Infinity Pharmaceuticals, XBiotech, Zymeworks, Coordination Pharmaceuticals, NGM Biopharmaceuticals, Gossamer Bio, Ryvu Therapeutics, BioTheryX, and AbbVie.
Data sharing
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data, typically, 1 year after the approval has been granted by major regulatory authorities or after termination of the development program. Researchers should use the https://vivli.org/link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Supplementary data
References
- 1.Anderson R.L., Balasas T., Callaghan J., et al. A framework for the development of effective anti-metastatic agents. Nat Rev Clin Oncol. 2019;16:185–204. doi: 10.1038/s41571-018-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y., Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:165. doi: 10.1186/s13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhan T., Rindtorff N., Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart D.J. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/djt356. [DOI] [PubMed] [Google Scholar]
- 5.Wang W., Smits R., Hao H., He C. Wnt/β-catenin signaling in liver cancers. Cancers. 2019;11:926. doi: 10.3390/cancers11070926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azbazdar Y., Karabicici M., Erdal E., Ozhan G. Regulation of Wnt signaling pathways at the plasma membrane and their misregulation in cancer. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.631623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker T.W., Neufeld K.L. APC controls Wnt-induced β-catenin destruction complex recruitment in human colonocytes. Sci Rep. 2020;10:2957. doi: 10.1038/s41598-020-59899-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J., Xiao Q., Xiao J., et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7:3. doi: 10.1038/s41392-021-00762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao H.X., Jiang X., Cong F. Control of Wnt receptor turnover by R-spondin-ZNRF3/RNF43 signaling module and its dysregulation in cancer. Cancers (Basel) 2016;8:54. doi: 10.3390/cancers8060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefanski C.D., Prosperi J.R. Wnt-independent and Wnt-dependent effects of APC loss on the chemotherapeutic response. Int J Mol Sci. 2020;21:7844. doi: 10.3390/ijms21217844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang S., Hua F., Hu Z.-W. The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget. 2017;8:33972–33989. doi: 10.18632/oncotarget.15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng J., Mo J., Zhu T., et al. Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol Cancer. 2020;19:133. doi: 10.1186/s12943-020-01250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giannakis M., Hodis E., Jasmine Mu X., et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet. 2014;46:1264–1266. doi: 10.1038/ng.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong J.Y., Cho H.J., Kim S.T., et al. Comprehensive molecular profiling to predict clinical outcomes in pancreatic cancer. Ther Adv Med Oncol. 2021;13 doi: 10.1177/17588359211038478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radaszkiewicz T., Bryja V. Protease associated domain of RNF43 is not necessary for the suppression of Wnt/β-catenin signaling in human cells. Cell Commun Signal. 2020;18:91. doi: 10.1186/s12964-020-00559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ter Steege EJ., Bakker E.R.M. The role of R-spondin proteins in cancer biology. Oncogene. 2021;40:6469–6478. doi: 10.1038/s41388-021-02059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebensohn A.M., Rohatgi R. R-spondins can potentiate WNT signaling without LGRs. Elife. 2018;7 doi: 10.7554/eLife.33126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han T., Schatoff E.M., Murphy C., et al. R-Spondin chromosome rearrangements drive Wnt-dependent tumour initiation and maintenance in the intestine. Nat Commun. 2017;8 doi: 10.1038/ncomms15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwee S.A., Tiirikainen M. Beta-catenin activation and immunotherapy resistance in hepatocellular carcinoma: mechanisms and biomarkers. Hepatoma Res. 2021;7:8. doi: 10.20517/2394-5079.2020.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong Y., Manoharan I., Suryawanshi A., et al. β-Catenin promotes regulatory T-cell responses in tumors by inducing vitamin A metabolism in dendritic cells. Cancer Res. 2015;75:656–665. doi: 10.1158/0008-5472.CAN-14-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan S., Tao F., Zhang X., et al. Role of Wnt/β-catenin signaling in the chemoresistance modulation of colorectal cancer. BioMed Res Int. 2020;2020 doi: 10.1155/2020/9390878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J.H., Liu X., Wang J., et al. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther Adv Musculoskelet Dis. 2013;5:13–31. doi: 10.1177/1759720X12466608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madan B., McDonald M.J., Foxa G.E., et al. Bone loss from Wnt inhibition mitigated by concurrent alendronate therapy. Bone Res. 2018;6:17. doi: 10.1038/s41413-018-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jimeno A., Gordon M., Chugh R., et al. A first-in-human phase I study of the anticancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin Cancer Res. 2017;23:7490–7497. doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- 25.Smith D.C., Rosen L.S., Chugh R., et al. First-in-human evaluation of the human monoclonal antibody vantictumab (OMP-18R5; anti-Frizzled) targeting the WNT pathway in a phase I study for patients with advanced solid tumors. J Clin Oncol. 2013;31:2540. [Google Scholar]
- 26.El-Khoueiry A.B., Ning Y., Yang D., et al. A phase I first-in-human study of PRI-724 in patients (pts) with advanced solid tumors. J Clin Oncol. 2013;31:2501. [Google Scholar]
- 27.Ng M., Tan D.S.P., Subbiah V., et al. First-in-human phase 1 study of ETC-159 an oral PORCN inhibitor in patients with advanced solid tumours. J Clin Oncol. 2017;35:2584. [Google Scholar]
- 28.Dotan E., Cardin D.B., Lenz H.J., et al. Phase Ib study of Wnt inhibitor ipafricept with gemcitabine and nab-paclitaxel in patients with previously untreated stage IV pancreatic cancer. Clin Cancer Res. 2020;26:5348–5357. doi: 10.1158/1078-0432.CCR-20-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giraudet A.L., Cassier P.A., Iwao-Fukukawa C., et al. A first-in-human study investigating biodistribution, safety and recommended dose of a new radiolabeled MAb targeting FZD10 in metastatic synovial sarcoma patients. BMC Cancer. 2018;18:646. doi: 10.1186/s12885-018-4544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diamond J.R., Becerra C., Richards D., et al. Phase Ib clinical trial of the anti-frizzled antibody vantictumab (OMP-18R5) plus paclitaxel in patients with locally advanced or metastatic HER2-negative breast cancer. Breast Cancer Res Treat. 2020;184:53–62. doi: 10.1007/s10549-020-05817-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenz H.-J., Argilés G., de Jonge M.J.A., et al. A phase I dose-escalation study of LRP5/6 antagonist BI 905677 in patients with advanced solid tumors. ESMO Open. 2024 doi: 10.1016/j.esmoop.2024.103729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polakis P. Wnt signaling in cancer. Cold Spring Harbor Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.