Abstract
Background
Aberrant Wnt pathway signaling has been implicated in the development of many cancers. Targeting of low-density lipoprotein receptor-related protein 5/6 (LRP5/6) co-receptors inhibits Wnt signaling and may be a novel therapy. BI 905677 is an LRP5/6 antagonist that has demonstrated preclinical antitumor activity.
Patients and methods
This (NCT03604445) was a phase I, dose-escalation study evaluating BI 905677 for patients with advanced solid tumors over two dosing schedules (A: i.v. infusion every 3 weeks, 3-week cycles; B: i.v. infusion every 2 weeks, 4-week cycles). Adult patients were eligible if they had exhausted treatment options and had an Eastern Cooperative Oncology Group performance status of 0-1. The primary endpoints were the maximum tolerated dose (MTD) and safety. Other endpoints were pharmacokinetics, pharmacodynamics, and efficacy.
Results
In total, 37 patients received BI 905677 over nine dose cohorts (0.05-3.6 mg/kg/every 3 weeks). Dose-limiting toxicities were only reported during cycle 1 in the 3.6 mg/kg cohort and the MTD was established at 2.8 mg/kg every 3 weeks. Enrollment for schedule B was not pursued. The most frequently reported adverse events were diarrhea (35.1%), vomiting (21.6%), and C-telopeptide increase (18.9%). All patients in the 3.6 mg/kg cohort experienced a dose-limiting toxicity, suggesting a narrow therapeutic index. Paired pre-treatment and on-treatment biopsies, where available, showed decreased Axin2 expression by reverse transcriptase polymerase chain reaction with treatment, suggesting target inhibition. Best response observed was stable disease in 14 (38%) patients.
Conclusion
The MTD of BI 905677 was set at 2.8 mg/kg every 3 weeks. BI 905677 was well tolerated but a narrow therapeutic range and minimal efficacy led to early termination of the trial.
Key words: Wnt, LRP5/6, solid tumor, phase I, clinical trial
Highlights
-
•
BI 905677 is an LRP5/6 antagonist that has demonstrated preclinical antitumor activity.
-
•
This first-in-human phase I dose-escalation study evaluates BI 905677 monotherapy in patients with advanced solid tumors.
-
•
Dose-limiting toxicities: diarrhea [n = 2; grade (G)3], hyperbilirubinemia (n = 1; G4), and hyponatremia (n = 1; G4).
-
•
The maximum tolerated dose of BI 905677 was 2.8 mg/kg every 3 weeks.
-
•
BI 905677 appeared to have a narrow therapeutic index and minimal efficacy.
Introduction
The Wnt signaling pathway regulates multiple cellular functions, including proliferation, stemness, migration, development, differentiation, and tissue homeostasis.1,2 Aberrant Wnt pathway activation is associated with many cancer types, such as colorectal cancer, endometrial cancer, non-small-cell lung cancer, hepatocellular carcinoma, and a subset of triple-negative breast cancer.3, 4, 5
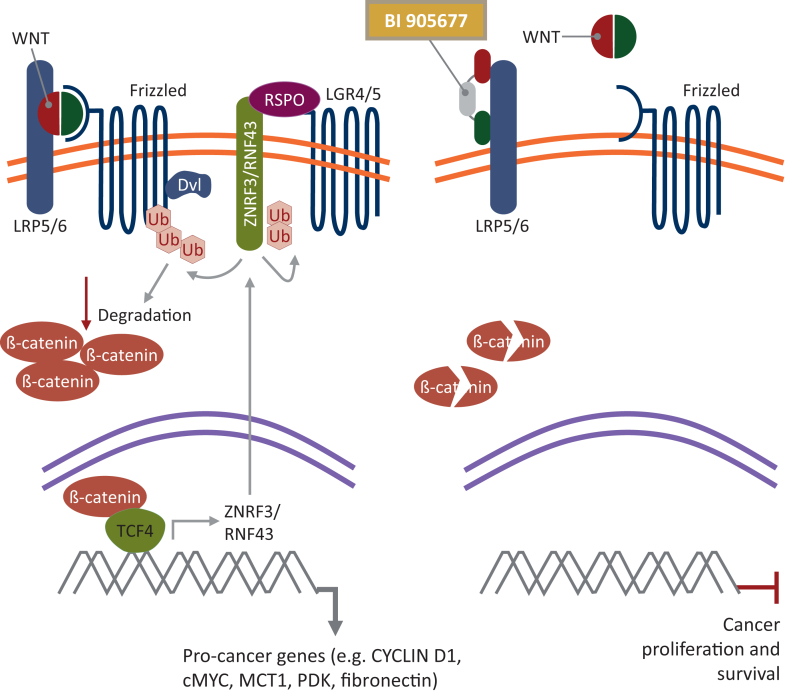
The Wnt signaling pathway is divided into two branches: β-catenin dependent (canonical) and independent (non-canonical).2 The canonical Wnt cascade is most widely studied and plays a key role in embryonic development, adult homeostasis, and stem cell maintenance.6 In canonical Wnt signaling, binding of the Wnt ligand to Frizzled (Fzd) and its co-receptor low-density lipoprotein receptor-related protein (LRP) (5 or 6) leads to recruitment of Dishevelled (Dvl) and Axin to the plasma membrane, where they polymerize and are activated. Dvl polymers inactivate the destruction complex involved in β-catenin degradation. As a result, β-catenin accumulates and translocates into the nucleus. β-catenin then forms an active complex with lymphoid enhancer factor and T-cell factor, leading to the transcription of target genes (Figure 1).2,7
Figure 1.
Inhibition of the canonical Wnt/β-catenin signaling pathway by BI 905677. Adapted from Hao et al.7Cancers. 2016;8:54. Dvl, Dishevelled; LRP5/6, low-density lipoprotein receptor-related protein 5/6; RSPO, R-spondin.
Aberrant activation of Wnt results in persistent accumulation of β-catenin in the nucleus, leading to increased expression of tumorigenic target genes such as c-myc, Jun, and Cyclin D1.8 β-catenin activation can lead to defective recruitment of dendritic cells and antigen-specific T cells, resulting in an impaired antitumor immune response.9 Furthermore, β-catenin accumulation can lead to increased secretion of interleukin 10 (IL-10), which results in immunosuppression through impairment of dendritic cells and effector T cells.10 This suggests that Wnt signaling can lead to immune checkpoint inhibitor (ICI) resistance, given that responses to ICIs are dependent on immune cell infiltration.11,12 Furthermore, Wnt signaling has been implicated in tumorigenesis and chemoresistance. Therefore, therapies that inhibit this pathway represent an appealing potential therapeutic option and may overcome ICI treatment resistance.12, 13, 14 It has, however, been challenging to effectively and safely target the LRP5/6 and Wnt pathway, particularly as inhibition of canonical Wnt signaling leads to decreased bone mass and strength.15,16
BI 905677 is a novel tri-molecular antagonistic antibody, comprising two modules binding to distinct domains of both LRP5 and LRP6 receptors, thus blocking Wnt ligand-dependent pathway activation, and one module binding to human serum albumin, to improve the pharmacokinetic profile by avoiding glomerular filtration. Modules are derived from single variable domains of camelidae heavy-chain antibodies (nanobodies). BI 905677 inhibited Wnt/β-catenin signaling in preclinical models that have ligand-dependent aberrant Wnt signaling caused by ring finger protein 43 (RNF43) mutation or R-spondin (RSPO) fusion. RNF43 and RSPO are additional regulators of the Wnt pathway. RNF43 is an E3 ligase that reduces Wnt pathway activation by ubiquitinating LRP5/6 and Fzd receptors. RSPO induces membrane clearance of RNF43 and zinc finger protein 3, leading to an increase of cell surface Wnt receptors.5,17 Patients with mutations in these upstream regulators of the Wnt pathway are expected to be more sensitive to LRP5/6 antagonists compared with those with normal RNF43 or RSPO. In contrast, BI 905677 did not show blockade of Wnt signaling or impact on cell viability in RNF43 wild-type tumor models [data on file].
Here, we present the results of a phase I, first-in-human, open-label, dose-escalation study evaluating BI 905677 (NCT03604445) for patients with advanced solid tumors.
Materials and methods
Study design
This was a phase I, multicenter, open-label, single-arm, dose-escalation study (NCT03604445) evaluating BI 905677 monotherapy for patients with advanced solid tumors. The study aimed to test two dosing schedules of i.v. BI 905677, either on day 1 of a 3-week cycle (schedule A) or on days 1 and 15 of a 4-week cycle (schedule B). The starting dose of BI 905677 for schedule A was 0.05 mg/kg. Recruitment into each dosing schedule was planned to occur sequentially, with recruitment to schedule B planned for after the maximum tolerated dose (MTD) of schedule A was determined.
Dose escalation and cohort size were determined based on the recommendation of the Safety Monitoring Committee (SMC), guided by a Bayesian logistic regression model (BLRM) with overdose control. An escalation with overdose control design was selected to increase the chance of treating patients at efficacious doses whilst reducing the risk of overdose. A minimum of three patients were treated per dose until the first adverse event (AE) of Common Terminology Criteria for Adverse Events (CTCAE) grade ≥2 occurred during the dose-limiting toxicity (DLT) evaluation period (defined as the first cycle of treatment), excluding AEs related to progressive disease (PD) or concurrent illness. Additional patients had to be entered at this dose, and subsequent dose escalations were restricted to a maximum of 50% from the previous dose until the MTD was reached. The size for the next dose-escalation cohort was recommended by the SMC. Patients continued to receive treatment with BI 905677 until disease progression according to Response Evaluation Criteria in Solid Tumors (RECIST) or until another reason requiring termination of treatment. A maximum of one dose reduction was allowed for an individual patient during the whole study and a subsequent dose increase was not permitted.
The trial was conducted and reported in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, local regulations, and Boehringer Ingelheim standard operating procedures. The study was approved by the Independent Ethics Committees and Institutional Review Boards of the participating sites and all patients provided written informed consent before initiating any trial-related procedures.
Patient eligibility
Eligible patients were ≥18 years old with a histologically or cytologically confirmed advanced, unresectable, and/or metastatic solid tumor (measurable or evaluable lesions according to RECIST v1.1). Patients were refractory to or not eligible for standard therapy and had to have exhausted treatment options. Patients had to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, adequate organ function, recovered from any previous therapy-related toxicities, and a life expectancy ≥3 months at the start of treatment. Patients had to be willing to undergo mandatory skin biopsy at the time points specified in the protocol. Genomic information, including any Wnt pathway alterations (e.g. RNF43 mutations or RSPO fusions), was collected for all patients but eligibility was not restricted by genomic alterations.
Exclusion criteria included previous or concomitant malignancies other than the one treated in this trial within the last 2 years (except for effectively treated nonmelanoma skin cancers, carcinoma in situ of the cervix, ductal carcinoma in situ, and other malignancies that were considered cured by local treatment). Patients were also excluded if they had grade ≥2 osteoporosis, chronic corticosteroid use, or osteoporotic compression fracture within 12 months before informed consent. Patients with presence or history of uncontrolled or symptomatic brain or subdural metastases were excluded, unless considered stable by the investigator and local therapy was completed.
Endpoints
The primary endpoints were the MTD of BI 905677 for schedule A and schedule B and the number of patients experiencing AEs during the entire treatment period. The MTD was assessed based on the number of patients experiencing DLTs, graded according to CTCAE Version 5.0, in the first cycle of treatment (3 weeks in schedule A and 4 weeks in schedule B). The MTD was defined as the highest dose with <25% risk of the true DLT rate being ≥33%. The definition of AEs classified as DLTs following review by the SMC, unless unequivocally due to underlying malignancy or an extraneous cause, are presented in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103729. The DLT definition also include any AEs, unless unequivocally due to underlying malignancy or an extraneous cause, that prevented a patient from starting cycle 2 within 14 days of completion of cycle 1 for schedule A, and any AE that prevented a patient from starting cycle 2 within 7 days of completion of cycle 1 for schedule B.
The secondary endpoint was the pharmacokinetic profile of BI 905677, measured by the maximum concentration of BI 905677 in serum after first infusion (Cmax) and area under the serum concentration-time curve over the time interval from 0 to the last measured time point (AUC0-tz).
Further endpoints included pharmacodynamics and efficacy. Pharmacodynamics were assessed via tissue biopsies. A total of two skin biopsies were required. The first skin biopsy had been obtained any time before the first dose. A second skin biopsy sample was obtained within 48-96 h of receiving treatment in cycle 2. Efficacy measures included objective response (OR), defined as complete response (CR) or partial response (PR) according to RECIST v1.1 as assessed by the investigator; disease control rate, defined as CR, PR, or stable disease (SD) according to RECIST v1.1; and duration of disease control, measured from start of trial treatment to the date of disease progression for patients who had CR, PR, or SD during treatment.
Immunogenicity was assessed by the presence of anti-drug antibodies (ADAs) to BI 905677. Blood samples were taken on day 1 and at end of treatment. Presence of ADAs was assessed via a tiered approach using a validated electrochemiluminescence assay. Patients were categorized as positive or negative per immunogenicity guidelines.
Statistical analyses
Dose escalation and cohort size were determined based on the recommendation of the SMC, guided by a BLRM with overdose control. The screened set included all patients who had signed the informed consent form and was used for patient disposition. The treated set included all patients who received at least one dose of BI 905677 and was used for both safety and efficacy analyses, and for baseline characteristics and demographic summaries. The MTD evaluation set included all patients in the treated set who were not replaced for the MTD determination and was used for the primary analyses of DLTs and MTD determination. The pharmacokinetic analysis set included all patients in the treated set who provided at least one valid serum concentration value and was used for all pharmacokinetic analyses. Safety analyses were descriptive and based on treatment-emergent AEs. Descriptive statistics were presented for Cmax and AUC0-tz using non-compartmental analysis (Phoenix WinNonlin™ software version Phoenix 8.1, Certara USA Inc., Princeton, NJ). All secondary endpoints were analyzed descriptively.
Results
Baseline characteristics
A total of 59 patients were screened between 10 August 2018 and 27 July 2022, and 37 patients were treated with i.v. BI 905677 every 3 weeks (schedule A; treated set) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103729). At the time of the database lock (28 October 2022), all patients were off treatment. There was one patient in the 0.4 mg/kg dose cohort who had a protocol deviation due to missing safety information from cycle 2 onward. Enrollment for schedule B was not started based on the SMC recommendation.
The median age was 56.0 years (range: 32.0-77.0 years), 64.9% of patients were male, and the majority were Caucasian (64.9%) (Table 1). Most patients (54.1%) had an ECOG performance status of 0. Patients had a variety of different tumor types, with the most frequent being colorectal (56.8%) and pancreatic (13.5%). All patients showed distant metastatic disease at the time of screening, with most (56.8%) having three or more metastatic sites. All patients had prior anticancer therapy, with most patients having previously received chemotherapy [89.2%; median of prior lines: 4 (range: 1-8)] and/or radiotherapy (51.4%). RNF43 mutations were assessed in 11 patients, 9 of whom had an RNF43 abnormality. Similarly, RSPO fusions were evaluated in seven patients, two of whom were positive (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103729).
Table 1.
Baseline characteristics
| Total N = 37 |
|
|---|---|
| Male, n (%) | 24 (64.9) |
| Race, n (%) | |
| White | 24 (64.9) |
| Asian | 11 (29.7) |
| Black or African American | 1 (2.7) |
| Native Hawaiian or other Pacific Islander | 1 (2.7) |
| Median age, years (range) | 56 (32-77) |
| ECOG performance status 0 at baseline, n (%) | 20 (54.1) |
| Tumor type, n (%) | |
| Colorectal | 21 (56.8) |
| Pancreas | 5 (13.5) |
| Anal | 2 (5.4) |
| Melanoma | 2 (5.4) |
| Othera | 7 (18.9) |
| Number of metastatic sites at screening, n (%) | |
| 1 | 5 (13.5) |
| 2 | 11 (29.7) |
| ≥3 | 21 (56.8) |
| Mutations, n (%) | |
| RNF43 mutation | 9 (24.3) |
| RSPO fusion | 2 (5.4) |
| Previous anticancer therapies, n (%) | |
| Chemotherapies | 33 (89.2) |
| Radiotherapy | 19 (51.4) |
| Immunotherapy | 8 (21.6) |
| Molecular targeted therapies | 7 (18.9) |
| Other | 5 (13.5) |
| Median prior lines of chemotherapy, n (range) | 4 (1-8) |
ECOG, Eastern Cooperative Oncology Group.
The histologies for the seven patients with tumor type classified as ‘other’ were gastrointestinal stromal tumor, neuroendocrine tumor, esophageal tumor, head and neck cancer, non-small-cell lung cancer, skin cancer other than melanoma, and small intestine cancer, respectively.
Treatment exposure
The median duration of treatment with BI 905677 was 2 cycles (range: 1-10 cycles) (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103729). A total of three patients (8.1%) had a dose reduction at doses 1.6 mg/kg, 2.8 mg/kg, and 3.6 mg/kg.
Maximum tolerated dose and dose-limiting toxicities
All patients across dose-escalation cohorts were evaluable for DLTs except for one patient in the 2.8 mg/kg dose cohort; as per protocol, this patient was excluded from the MTD analysis set as their cycle 1 day 15 visit was not carried out due to hospitalization. The patient was replaced but not excluded for evaluation of DLTs in the treated set. No patients in the dose cohorts ranging from 0.05 to 2.8 mg/kg experienced DLTs during the MTD evaluation period. All patients (n = 3) in the 3.6 mg/kg dose cohort experienced DLTs during cycle 1 (Table 2): diarrhea [n = 2 (66.7%); grade 3], hyperbilirubinemia [n = 1 (33.3%); grade 4], and hyponatremia [n = 1 (33.3%); grade 4]. Therefore, the MTD of BI 905677 was determined to be 2.8 mg/kg every 3 weeks. During the entire treatment period, seven patients (18.9%) experienced DLTs in the 1.6-3.6 mg/kg dose cohorts. Most DLTs reported beyond cycle 1 consisted of changes in bone biomarkers, including four patients (10.8%) with increased C-telopeptide and one (2.7%) with decreased bone density.
Table 2.
Summary of adverse events during the on-treatment period
| BI 905677, mg/kg |
Total N = 37 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 n = 3 | 0.1 n = 3 | 0.2 n = 3 | 0.4 n = 3 | 0.8 n = 3 | 1.6 n = 6 | 2.4 n = 6 | 2.8 n = 7 | 3.6 n = 3 | ||
| Any AE, n (%) | 3 (100.0) | 3 (100.0) | 2 (66.7) | 3 (100.0) | 3 (100.0) | 6 (100.0) | 6 (100.0) | 7 (100.0) | 3 (100.0) | 36 (97.3) |
| Treatment-related AEs, n (%) | 2 (66.7) | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 6 (100.0) | 5 (83.3) | 5 (71.4) | 3 (100.0) | 27 (73.0) |
| AEs leading to permanent treatment discontinuation, n (%) | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 2 (28.6) | 3 (100.0) | 6 (16.2) |
| AEs leading to dose reduction, n (%) | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (14.3) | 1 (33.3) | 3 (8.1) |
| Serious AEs, n (%) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 2 (33.3) | 4 (66.7) | 7 (100.0) | 3 (100.0) | 23 (62.2) |
| AEs leading to death, n (%) | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 2 (5.4) |
| DLTs, n (%) | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 1 (16.7) | 2 (28.6) | 3 (100.0) | 7 (18.9) |
| DLTs during the MTD evaluation period, n (%) | ||||||||||
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (66.7) | 2 (5.6) |
| Hyperbilirubinemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (2.8) |
| Hyponatremia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (2.8) |
| DLTs during the on-treatment period, n (%) | ||||||||||
| Increased C-telopeptide | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 1 (16.7) | 1 (14.3) | 1 (33.3) | 4 (10.8) |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (66.7) | 2 (5.4) |
| Decreased bone density | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (14.3) | 0 | 1 (2.7) |
| Hyperbilirubinemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (2.7) |
| Hyponatremia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (2.7) |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (2.7) |
AE, adverse event; DLT, dose-limiting toxicity; MTD, maximum tolerated dose.
Safety
AEs of any grade were reported in 36 patients (97.3%) (Table 2). Treatment-related AEs were reported in 27 patients (73.0%); the most frequently reported treatment-related AEs were diarrhea [n = 13 (35.1%)], vomiting [n = 8 (21.6%)], increased C-telopeptide [n = 7 (18.9%)], and decreased appetite and nausea [n = 6 (16.2%) each] (Table 3; Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.103729). Grade 3 treatment-related AEs were reported in three patients (8.1%): diarrhea and vomiting [n = 2 (5.4%) each], abdominal pain, increased alanine aminotransferase, increased aspartate aminotransferase, increased alkaline phosphatase, dehydration, increased gamma-glutamyl transferase, and hypokalemia [n = 1 (2.7%) each]. Grade 4 treatment-related AEs were reported in two patients (5.4%): hyponatremia, blood electrolytes decreased, and hyperbilirubinemia [n = 1 (2.7%) each]. There were no grade 5 treatment-related AEs reported.
Table 3.
Treatment-related adverse events of any grade occurring in ≥5% of all patients during the treatment period or any grade 3 or 4 event
| Total N = 37 |
|||
|---|---|---|---|
| Treatment-related AE, n (%) | Any grade | Grade 3 | Grade 4 |
| Diarrhea | 13 (35.1) | 2 (5.4) | 0 |
| Vomiting | 8 (21.6) | 2 (5.4) | 0 |
| Increased C-telopeptide | 7 (18.9) | 0 | 0 |
| Decreased appetite | 6 (16.2) | 0 | 0 |
| Nausea | 6 (16.2) | 0 | 0 |
| Asthenia | 3 (8.1) | 0 | 0 |
| Abdominal pain | 2 (5.4) | 1 (2.7) | 0 |
| Dry mouth | 2 (5.4) | 0 | 0 |
| Hyponatremia | 2 (5.4) | 0 | 1 (2.7) |
| Infusion-related reaction | 2 (5.4) | 0 | 0 |
| Increased alanine aminotransferase | 1 (2.7) | 1 (2.7) | 0 |
| Increased aspartate aminotransferase | 1 (2.7) | 1 (2.7) | 0 |
| Increased alkaline phosphatase | 1 (2.7) | 1 (2.7) | 0 |
| Increased gamma-glutamyl transferase | 1 (2.7) | 1 (2.7) | 0 |
| Dehydration | 1 (2.7) | 1 (2.7) | 0 |
| Hypokalemia | 1 (2.7) | 1 (2.7) | 0 |
| Decreased blood electrolytes | 1 (2.7) | 0 | 1 (2.7) |
| Hyperbilirubinemia | 1 (2.7) | 0 | 1 (2.7) |
AE, adverse event.
A total of three patients (8.1%) experienced AEs that required a dose reduction, and six patients (16.2%) had AEs resulting in permanent treatment discontinuation of BI 905677. Of those who permanently discontinued treatment due to AEs, five received a dose ≥2.8 mg/kg.
Bone mineral density change of >5% from baseline was observed in seven patients (18.9%). Of these, four received treatment for 3 weeks, and the other three patients received treatment for 1.5, 2.1, and 7.2 months, respectively. The bone mineral density decrease after an interval of at least 2 months after the first observation of the bone density change could only be confirmed for one patient.
Pharmacokinetics
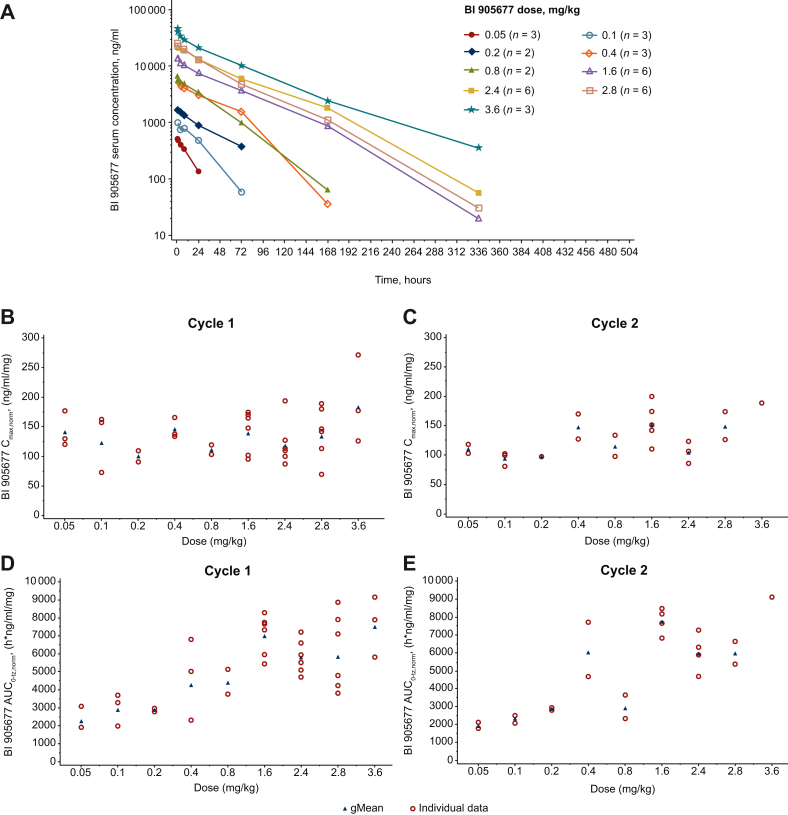
Serum concentrations were detectable up to 336 h after i.v. infusion and increased in proportion to dose. The shape of the serum concentration-time profiles was similar among the different dose cohorts. Maximum serum concentrations (Cmax) of BI 905677 were generally observed at the end of infusion or within a few hours thereafter (Figure 2A). The inter-individual variability of BI 905677 serum concentrations was low to moderate (cycle 1 range: 2.14%-40.1%; cycle 2 range: 2.85%-30.9%), with the exception of a geometric coefficient of variation value of 158% in the 0.05 mg/kg dose cohort (cycle 1, 24 h after dose). After the first infusion of BI 905677, geometric mean (gMean) Cmax and AUC0-tz increased in line with dose. The median time to reach maximum serum concentration (tmax) ranged from 1.01 to 1.58 h across dose cohorts (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.103729).
Figure 2.
Pharmacokinetics. (A) Geometric mean drug serum concentration-time profiles of BI 905677 after single i.v. infusion; (B and C) individual and geometric mean Cmax,norm after one or two cycles; (D and E) AUC0-tz,norm after one or two cycles. AUC0-tz,norm, area under the serum concentration-time curve over the time interval from 0 to the last measured time point normalized values; Cmax,norm, maximum concentration of BI 905677 in serum normalized values; gMean, geometric mean.
Dose-normalized individual and gMean Cmax and AUC0-tz values increased with increasing dose. The last measurable time point, however, differed across dose groups (Figure 2B and C; Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.103729). As expected for a nanobody, the gMean volume of distribution during the terminal phase and clearance (CL) were small and the gMean terminal half-life (t1/2) generally increased in line with dose. The t1/2 was, however, shorter for 2.8 mg/kg (35.9 h) compared with that observed for 2.4 and 3.6 mg/kg (40.4 and 52.0 h, respectively). After the second dose, the exposure of BI 905677, observed as gMean Cmax and AUC0-tz, increased in line with dose (Figure 2B and C). The AUC0-tz values in the 0.8 and 2.8 mg/kg dose cohorts, however, were lower than the preceding dose cohort in each case. The median tmax ranged from 1.05 to 2.75 h across dose groups. The gMean volume of distribution at steady state, CL, and t1/2 were in the same range as in cycle 1. Exposure in cycle 2 was similar to or slightly lower than in cycle 1, as shown by the accumulation ratios (RA, Cmax and RA, AUC0-504) presented in Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2024.103729.
Pharmacodynamics
Assessment of on-target modulation was planned as an exploratory study. Axin2 expression by reverse transcription polymerase chain reaction was reduced in paired skin biopsies but no dose dependency was observed.
Immunogenicity
The majority of patients (83.8%) were evaluable for ADAs. ADA classification was not possible for six patients (16.2%) due to the availability of only one sample at baseline (negative sample in all cases). Of those who were evaluable for ADAs, 1 patient was ADA positive only at baseline (3.2%), 12 patients (38.7%) were ADA positive-induced, 1 patient (3.2%) was ADA positive non-boosted, and 17 patients (54.8%) were ADA negative. The 12 patients who were ADA positive-induced were further divided into two subcategories: persistent [n = 1 (3.2%)] or potentially persistent [n = 11 (35.5%)]. The median time to ADA development was 52.5 days (range: 21-155 days). Due to limited data, no conclusion about the correlation between pharmacokinetics and ADAs could be made.
Efficacy
No patient had a best overall response of CR or PR. Therefore, the OR rate was 0%. The best response achieved across dose cohorts was SD [n = 14 (37.8%)]. The disease control rate was 37.8%. Median duration of disease control was 79.5 days (range: 36-213 days). A total of 10 patients had SD duration of longer than 4 months.
All nine patients with RNF43 mutations experienced disease progression. Of two patients with an RSPO fusion, one had SD.
Discussion
This first-in-human, phase I study aimed to determine the MTD of BI 905677 and investigate its safety, pharmacokinetics, and efficacy for patients with advanced solid tumors. BI 905677 was well tolerated at dose levels up to 2.8 mg/kg every 3 weeks. All patients (n = 3) at the 3.6 mg/kg dose, however, experienced DLTs. The SMC decided not to explore an intermediate dose level of 3.1 mg/kg every 3 weeks, which was the maximum dose recommended according to the BLRM. Pharmacokinetic analysis had shown that the half-life of the molecule was longer than expected for a nanobody, possibly as a result of the albumin binding ability. There was an overlap in exposure between the 2.4 mg/kg and 3.6 mg/kg cohorts. Therefore, it was not expected that a 10% increase in dose (from 2.8 to 3.1 mg/kg) would be meaningful from a pharmacokinetic perspective. Consequently, the MTD was determined to be 2.8 mg/kg every 3 weeks. Gastrointestinal AEs and changes in bone biomarkers and bone densitometry were the most frequently reported toxicities associated with BI 905677, which is consistent with other Wnt signaling/β-catenin antagonists.18, 19, 20, 21 There were some reports of asymptomatic bone mineral density changes during the trial. This is expected given the role of the Wnt pathway in bone formation and remodeling.22 These results must, however, be interpreted with caution due to the limited follow-up measurements.
The trial was prematurely discontinued after the MTD was determined for schedule A. Enrollment for schedule B was not started based on the SMC recommendation. With AEs and DLTs occurring between days 12 and 14 of cycle 1 (schedule A), patients would not have sufficient time to recover if receiving doses more frequently, such as in the 2-week schedule planned for schedule B.
The complexity of Wnt signaling and its redundancy has been a challenge for the development of drugs targeting this pathway with a favorable risk–benefit profile. This study is no exception; BI 905677 had minimal efficacy with regards to tumor response and disease control in this overall population as a single agent. Wnt inhibitors have had limited clinical results, as seen in the recent phase I and phase Ib/II studies evaluating WNT974 and BI 905681.16,23,24 One explanation for the limited efficacy may be how heavily pretreated the population was and the need to enrich the patient population using specific biomarkers. Of patients included in the trial, 89.2% received prior chemotherapy [median of prior lines: 4 (range: 1-8)], with 62.2% having received ≥3 prior lines, and 51.4% received prior radiotherapy. Further, it may be that targeting this pathway is best done in combination with other therapies rather than as a single agent. Recent Wnt-targeting methods have focused on combinations with existing anticancer therapies considering the heterogeneity and numerous pathways involved in cancer and disease progression and in order to compensate for the low efficacy observed with safe-dose Wnt-targeting monotherapy.25 Numerous preclinical studies combining Wnt signaling inhibitors with other conventional chemotherapy agents or targeted inhibitors have revealed synergistic efficacy, including in patient-derived colorectal, ovarian, pancreatic, and non-small-cell lung cancer xenograft models.26, 27, 28 Furthermore, studies have advanced into phase I and II clinical trials investigating the safety and efficacy of Wnt signaling modulator combination therapies in various cancer types.29, 30, 31, 32, 33, 34, 35, 36, 37
BI 905677 had preclinical efficacy in a tumor line driven by an RNF43 mutation, but not in those with wild-type RNF43. Therefore, BI 905677 may be more potent in patients whose tumors have an RNF43 mutation. This compound was not tested in a population in whom all tumors presented an RNF43 mutation or RSPO fusion as this could present challenges for patient enrollment. Furthermore, it was beyond the scope of this study to confirm whether the RNF43 mutations detected were associated with loss of function and thus enhanced Wnt signaling. Of those with mutational information available, however, there was only one reported case of SD in a patient with an RSPO fusion; all those with a known RNF43 mutation experienced PD.38 This suggests that relying on individual genomic markers to evaluate the functional state of a signaling pathway as complex as this might be an oversimplified endeavor. A possible way forward could be the combination of RNF43 mutations and RSPO fusion with functional biomarkers for Wnt activation, such as LGR5 expression.
Wnt signaling remains an important target across different cancer types, particularly in colorectal cancer, in which a large proportion of tumors carry a Wnt pathway alteration. Although targeted therapies have been studied in small, genomically defined subsets of patients with colorectal cancer, there is a great unmet need for treatment options. Further to this, studies suggest that LRP5 is an important target.39 Therefore, the development of Wnt pathway inhibitors remains important to broadly target this disease.
Conclusion
The MTD of BI 905677 was set at 2.8 mg/kg every 3 weeks with an acceptable safety profile. Despite BI 905677 having a generally tolerable safety profile, there was minimal antitumor activity, which resulted in the trial being terminated early.
Acknowledgements
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment related to the development of the manuscript. Devon Else, and Amber Wood, PhD, of Nucleus Global provided writing, editorial support, and formatting assistance, which was contracted and funded by Boehringer Ingelheim. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. The authors would like to acknowledge the instrumental contributions of Serge Nazabadioko, Clinical Trial Leader of the trial, who sadly passed away during the development of this manuscript.
Funding
This study was supported by Boehringer Ingelheim International GmbH (BI). The study was also supported by MSK’s Cancer Center Core grant [grant number P30 CA008748].
Disclosure
GA has served as an advisor for Gadeta B.V., has received research support from Bayer, and travel grants from Amgen.
AEK reports honoraria from ABL Bio, Agenus, AstraZeneca/MedImmune, Bayer, Bristol Myers Squibb, CytomX Therapeutics, EISAI, EMD Serono, Exelixis, Gilead Sciences, Merck, Pieris Pharmaceuticals, QED Therapeutics, Roche/Genentech, Servier, and Tallac Therapeutics; consulting or advisory board roles with BL Bio, Agenus, AstraZeneca/MedImmune, Bayer, Bristol Myers Squibb, CytomX Therapeutics, Eisai, EMD Serono, Exelixis, Gilead Sciences, Merck, Pieris Pharmaceuticals, QED Therapeutics, Roche, Servier, and Tallac Therapeutics; and institutional research funding from Astex Pharmaceuticals, AstraZeneca, Fulgent Genetics, and GlaxoSmithKline (GSK).
RY has served as an advisor for Amgen, Natera, Array BioPharma/Pfizer, Mirati Therapeutics, and Zai Lab and has received research support to her institution from Array BioPharma/Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Mirati Therapeutics.
YK has received research funding from Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Genmab, GSK, Incyte, Lilly, ONO, Taiho, Takeda; honoraria from Bayer, Bristol Myers Squibb, Lilly, Merck Serono, ONO, and Taiho.
JB, SN, and LP are employees of Boehringer Ingelheim.
JT reports personal financial interest in the form of a scientific consultancy role for Alentis Therapeutics, AstraZeneca, Aveo Oncology, Boehringer Ingelheim, Cardiff Oncology, CARSgen Therapeutics, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech Inc., hC Bioscience, Ikena Oncology, Immodulon Therapeutics, Inspirna Inc., Lilly, Menarini, Merck Serono, Merus, Merck Sharp & Dohme (MSD), Mirati, Neophore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Scandion Oncology, Scorpion Therapeutics, Seattle Genetics, Servier, Sotio Biotech, Taiho, Takeda Oncology, and Tolremo Therapeutics. Stocks: Oniria Therapeutics, Alentis Therapeutics, Pangaea Oncology, and 1TRIALSP, and also educational collaboration with Medscape Education, PeerView Institute for Medical Education, and Physicians Education Resource (PER).
TD has received institutional research funding from Lilly, MSD, Daiichi Sankyo, Sumitomo Dainippon, Taiho, Novartis, Merck Serono, Janssen, Boehringer Ingelheim, Pfizer, BMS, AbbVie, IQVIA, and Eisai. He has also received personal funding from MSD, Daiichi Sankyo, Amgen, Novartis, Janssen, Boehringer Ingelheim, AbbVie, Bayer, and Astellas as member of an advisory board; from Sumitomo Dainippon, Taiho, Takeda, Chugai, Rakuten Medical, Otsuka, and Oncolys BioPharma for an advisory role; and from Bristol Myers Squibb, Ono, and Taiho for his role as an invited speaker.
HJL has received consultancy fees from Bristol Myers Squibb, Merck, Merck KG, Fulgent, 3T Bioscience, Jazz Therapeutics, Oncocyte, AbbVie, Invitae, G1 Therapeutics, Affini-T, Adagene, and Agenus for advisory boards; meeting and travel support from Bristol Myers Squibb and Replimune. He has participated on Data Safety Monitoring or advisory boards for Veloxis, Kisima, and UCB. He holds stock or stock options with Fugent and BreakBio.
All other authors have declared no conflicts of interest.
Data sharing
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Supplementary data
References
- 1.Zhang Y., Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:165. doi: 10.1186/s13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhan T., Rindtorff N., Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart D.J. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/djt356. [DOI] [PubMed] [Google Scholar]
- 4.Wang W., Smits R., Hao H., He C. Wnt/β-catenin signaling in liver cancers. Cancers. 2019;11:926. doi: 10.3390/cancers11070926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannakis M., Hodis E., Jasmine Mu X., et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat Genet. 2014;46:1264–1266. doi: 10.1038/ng.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azbazdar Y., Karabicici M., Erdal E., Ozhan G. Regulation of Wnt signaling pathways at the plasma membrane and their misregulation in cancer. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.631623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao H.X., Jiang X., Cong F. Control of Wnt receptor turnover by R-spondin-ZNRF3/RNF43 signaling module and its dysregulation in cancer. Cancers. 2016;8:54. doi: 10.3390/cancers8060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang S., Hua F., Hu Z.-W. The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget. 2017;8:33972–33989. doi: 10.18632/oncotarget.15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz de Galarreta M., Bresnahan E., Molina-Sánchez P., et al. β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Disc. 2019;9:1124–1141. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaguchi T., Goto Y., Kido K., et al. Immune suppression and resistance mediated by constitutive activation of Wnt/β-catenin signaling in human melanoma cells. J Immunol. 2012;189:2110–2117. doi: 10.4049/jimmunol.1102282. [DOI] [PubMed] [Google Scholar]
- 11.Kümpers C., Jokic M., Haase O., et al. Immune cell infiltration of the primary tumor, not PD-L1 status, is associated with improved response to checkpoint inhibition in metastatic melanoma. Front Med (Lausanne) 2019;6:27. doi: 10.3389/fmed.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y., Xu J., Luo H., et al. Wnt signaling pathway in cancer immunotherapy. Cancer Lett. 2022;525:84–96. doi: 10.1016/j.canlet.2021.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Hong Y., Manoharan I., Suryawanshi A., et al. β-catenin promotes regulatory T-cell responses in tumors by inducing vitamin A metabolism in dendritic cells. Cancer Res. 2015;75:656–665. doi: 10.1158/0008-5472.CAN-14-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan S., Tao F., Zhang X., et al. Role of Wnt/β-catenin signaling in the chemoresistance modulation of colorectal cancer. BioMed Res Int. 2020;2020 doi: 10.1155/2020/9390878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron R., Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 16.Rodon J., Argilés G., Connolly R.M., et al. Phase 1 study of single-agent WNT974, a first-in-class porcupine inhibitor, in patients with advanced solid tumours. Br J Cancer. 2021;125:28–37. doi: 10.1038/s41416-021-01389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ter Steege E.J., Bakker E.R.M. The role of R-spondin proteins in cancer biology. Oncogene. 2021;40:6469–6478. doi: 10.1038/s41388-021-02059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimeno A., Gordon M., Chugh R., et al. A first-in-human phase I study of the anticancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin Cancer Res. 2017;23:7490–7497. doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- 19.Smith D.C., Rosen L.S., Chugh R., et al. First-in-human evaluation of the human monoclonal antibody vantictumab (OMP-18R5; anti-Frizzled) targeting the WNT pathway in a phase I study for patients with advanced solid tumors. J Clin Oncol. 2013;31:2540. [Google Scholar]
- 20.El-Khoueiry A.B., Ning Y., Yang D., et al. A phase I first-in-human study of PRI-724 in patients (pts) with advanced solid tumors. J Clin Oncol. 2013;31:2501. [Google Scholar]
- 21.Ng M., Tan D.S.P., Subbiah V., et al. First-in-human phase 1 study of ETC-159 an oral PORCN inhbitor in patients with advanced solid tumours. J Clin Oncol. 2017;35:2584. [Google Scholar]
- 22.Chen Y., Alman B.A. Wnt pathway, an essential role in bone regeneration. J Cell Biochem. 2009;106:353–362. doi: 10.1002/jcb.22020. [DOI] [PubMed] [Google Scholar]
- 23.Tabernero J., Van Cutsem E., Garralda E., et al. A phase Ib/II study of WNT974 + encorafenib + cetuximab in patients with BRAF V600E-mutant KRAS wild-type metastatic colorectal cancer. Oncologist. 2023;28:230–238. doi: 10.1093/oncolo/oyad007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spigel D.R., Wang J.S., Pronk L., et al. A phase I dose-escalation study of the LRP5 antagonist BI 905681 in patients with advanced and metastatic solid tumors. ESMO Open. 2024 doi: 10.1016/j.esmoop.2024.103730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park W.-J., Kim M.J. A new wave of targeting ‘undruggable’ Wnt signaling for cancer therapy: challenges and opportunities. Cells. 2023;12:1110. doi: 10.3390/cells12081110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong Z., Virshup D.M. Wnt signaling and drug resistance in cancer. Mol Pharmacol. 2020;97:72–89. doi: 10.1124/mol.119.117978. [DOI] [PubMed] [Google Scholar]
- 27.Chen G., Gao C., Gao X., et al. Wnt/β-catenin pathway activation mediates adaptive resistance to BRAF inhibition in colorectal cancer. Mol Cancer Therap. 2018;17:806–813. doi: 10.1158/1535-7163.MCT-17-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chartier C., Raval J., Axelrod F., et al. Therapeutic targeting of tumor-derived R-spondin attenuates β-catenin signaling and tumorigenesis in multiple cancer types. Cancer Res. 2016;76:713–723. doi: 10.1158/0008-5472.CAN-15-0561. [DOI] [PubMed] [Google Scholar]
- 29.ClinicalTrials.gov CGX1321 in Subjects With Advanced Solid Tumors and CGX1321 With Pembrolizumab or Encorafenib + Cetuximab in Subjects With Advanced GI Tumors (Keynote 596) https://clinicaltrials.gov/study/NCT02675946 Available at.
- 30.ClinicalTrials.gov Study to Evaluate the Safety and Tolerability of RXC004 in Advanced Malignancies. https://clinicaltrials.gov/study/NCT03447470 Available at.
- 31.ClinicalTrials.gov WaKING: Wnt and checKpoint INhibition in Gastric Cancer. https://clinicaltrials.gov/study/NCT04166721 Available at.
- 32.ClinicalTrials.gov A Study of LGK974 in Patients With Malignancies Dependent on Wnt Ligands. https://clinicaltrials.gov/study/NCT01351103 Available at.
- 33.ClinicalTrials.gov Combination Chemotherapy and Bevacizumab With or Without PRI-724 in Treating Patients With Newly Diagnosed Metastatic Colorectal Cancer (PRIMIER) https://clinicaltrials.gov/study/NCT02413853 Available at.
- 34.ClinicalTrials.gov Study of WNT974 in Combination With LGX818 and Cetuximab in Patients With BRAF-mutant Metastatic Colorectal Cancer (mCRC) and Wnt Pathway Mutations. https://clinicaltrials.gov/study/NCT02278133 Available at.
- 35.ClinicalTrials.gov DKN-01 Inhibition in Advanced Liver Cancer. https://clinicaltrials.gov/study/NCT03645980 Available at.
- 36.ClinicalTrials.gov A Study of DKN-01 as a Monotherapy or in Combination With Paclitaxel in Patients With Recurrent Epithelial Endometrial or Epithelial Ovarian Cancer or Carcinosarcoma (P204) https://clinicaltrials.gov/study/NCT03395080 Available at.
- 37.ClinicalTrials.gov Study of Docetaxel Combined With Cirmtuzumab in Metastatic Castration Resistant Prostate Cancer. https://clinicaltrials.gov/study/NCT05156905 Available at.
- 38.Yu J., Yusoff P.A.M., Woutersen D.T.J., et al. The functional landscape of patient-derived RNF43 mutations predicts sensitivity to Wnt inhibition. Cancer Res. 2020;80:5619–5632. doi: 10.1158/0008-5472.CAN-20-0957. [DOI] [PubMed] [Google Scholar]
- 39.Li J., Ma X., Chakravarti D., et al. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021;35:787–820. doi: 10.1101/gad.348226.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.