Abstract
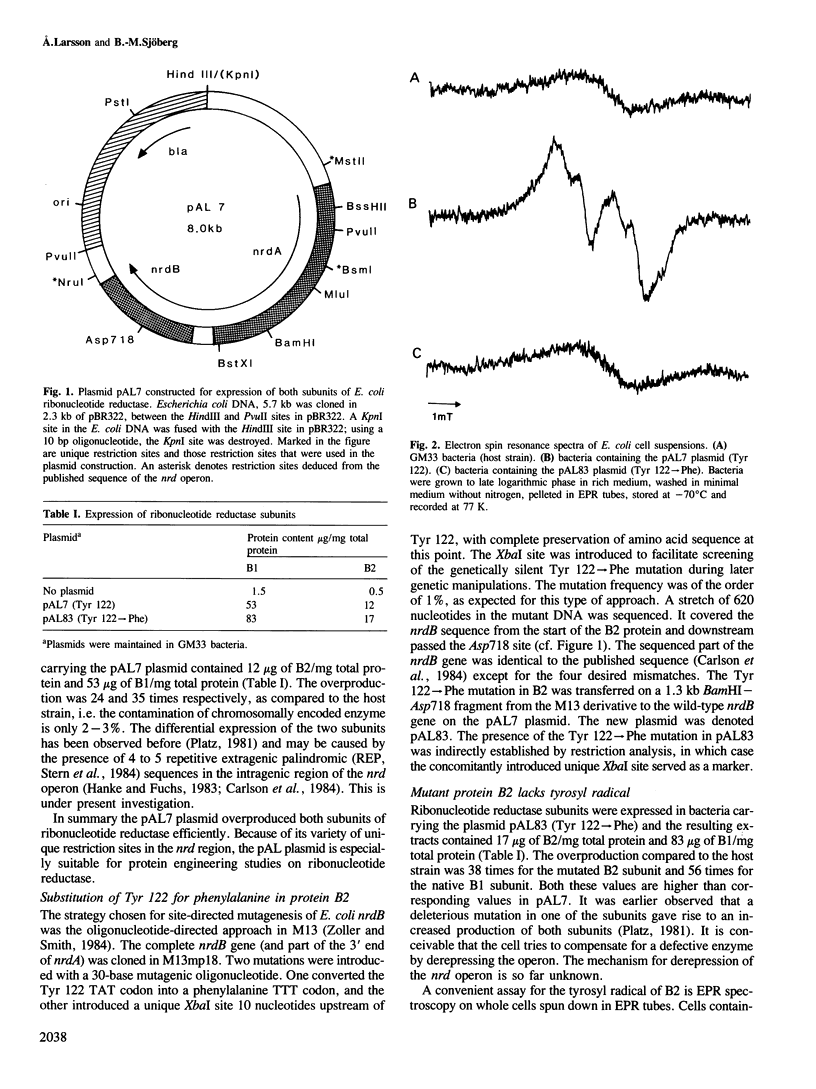
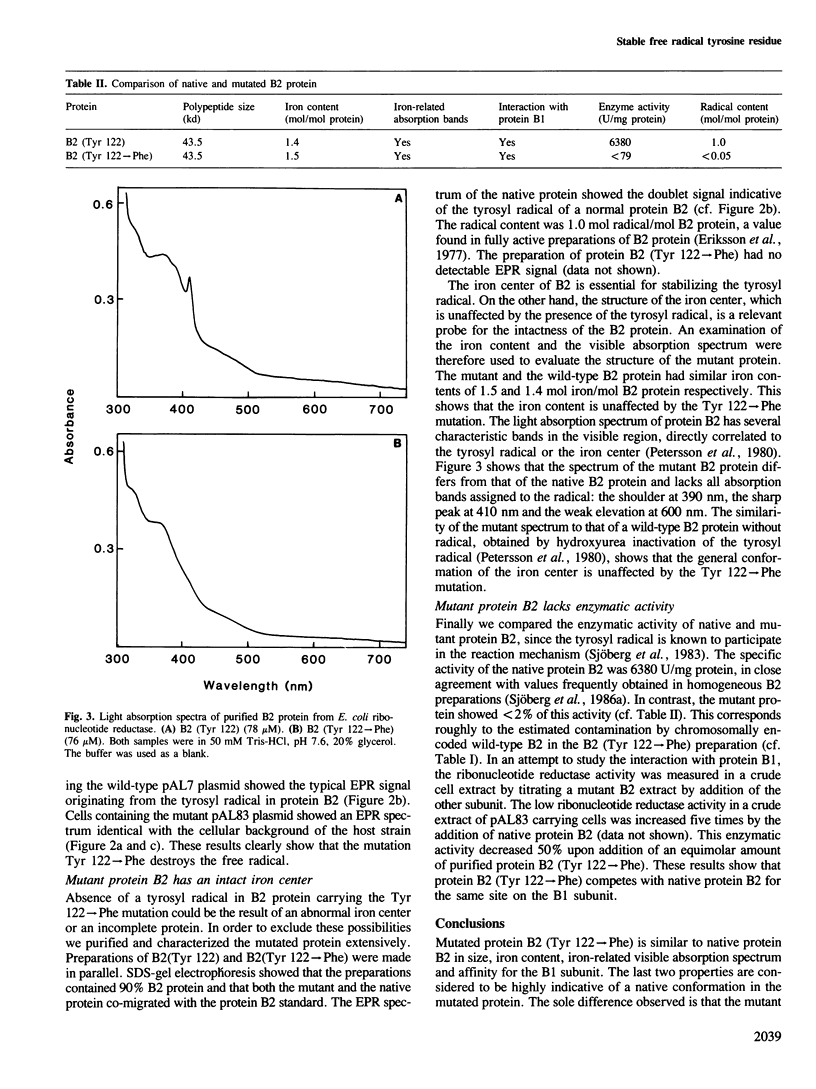
The small subunit of iron-dependent ribonucleotide reductases contains a stable organic free radical, which is essential for enzyme activity and which is localized to a tyrosine residue. Tyrosine-122 in the B2 subunit of Escherichia coli ribonucleotide reductase has been changed into a phenylalanine. The mutation was introduced with oligonucleotide-directed mutagenesis in an M13 recombinant and verified by DNA sequencing. Purified native and mutant B2 protein were found to have the same size, iron content and iron-related absorption spectrum. The sole difference observed is that the mutant protein lacks tyrosyl radical and enzymatic activity. These results identify Tyr122 of E. coli protein B2 as the tyrosyl radical residue. An expression vector was constructed for manipulation and expression of ribonucleotide reductase subunits. It contains the entire nrd operon with its own promoter in a 2.3-kb fragment from pBR322. Both the B1 and the B2 subunits were expressed at a 25-35 times higher level as compared to the host strain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin C. L., Thelander L., Reichard P., Lang G. Iron and free radical in ribonucleotide reductase. Exchange of iron and Mössbauer spectroscopy of the protein B2 subunit of the Escherichia coli enzyme. J Biol Chem. 1973 Nov 10;248(21):7464–7472. [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J., Fuchs J. A., Messing J. Primary structure of the Escherichia coli ribonucleoside diphosphate reductase operon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4294–4297. doi: 10.1073/pnas.81.14.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias L., Cervantes L., Covarrubias A., Soberón X., Vichido I., Blanco A., Kupersztoch-Portnoy Y. M., Bolivar F. Construction and characterization of new cloning vehicles. V. Mobilization and coding properties of pBR322 and several deletion derivatives including pBR327 and pBR328. Gene. 1981 Jan-Feb;13(1):25–35. doi: 10.1016/0378-1119(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Sjöberg B. M., Hahne S. Ribonucleoside diphosphate reductase from Escherichia coli. An immunological assay and a novel purification from an overproducing strain lysogenic for phage lambdadnrd. J Biol Chem. 1977 Sep 10;252(17):6132–6138. [PubMed] [Google Scholar]
- Gräslund A., Ehrenberg A., Thelander L. Characterization of the free radical of mammalian ribonucleotide reductase. J Biol Chem. 1982 May 25;257(10):5711–5715. [PubMed] [Google Scholar]
- Gräslund A., Sahlin M., Sjöberg B. M. The tyrosyl free radical in ribonucleotide reductase. Environ Health Perspect. 1985 Dec;64:139–149. doi: 10.1289/ehp.64-1568609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke P. D., Fuchs J. A. Characterization of the mRNA coding for ribonucleoside diphosphate reductase in Escherichia coli. J Bacteriol. 1983 Dec;156(3):1192–1197. doi: 10.1128/jb.156.3.1192-1197.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lankinen H., Gräslund A., Thelander L. Induction of a new ribonucleotide reductase after infection of mouse L cells with pseudorabies virus. J Virol. 1982 Mar;41(3):893–900. doi: 10.1128/jvi.41.3.893-900.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A. Overproduction of the B1 subunit of ribonucleotide reductase with gene amplification. Acta Chem Scand B. 1984;38(10):905–907. doi: 10.3891/acta.chem.scand.38b-0905. [DOI] [PubMed] [Google Scholar]
- MASSEY V. Studies on succinic dehydrogenase. VII. Valency state of the iron in beef heart succinic dehydrogenase. J Biol Chem. 1957 Dec;229(2):763–770. [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Pleiotropic effects of a DNA adenine methylation mutation (dam-3) in Escherichia coli K12. Mutat Res. 1975 Apr;28(1):15–26. doi: 10.1016/0027-5107(75)90309-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Olsson A., Moks T., Uhlén M., Gaal A. B. Uniformly spaced banding pattern in DNA sequencing gels by use of field-strength gradient. J Biochem Biophys Methods. 1984 Nov;10(1-2):83–90. doi: 10.1016/0165-022x(84)90053-8. [DOI] [PubMed] [Google Scholar]
- Petersson L., Gräslund A., Ehrenberg A., Sjöberg B. M., Reichard P. The iron center in ribonucleotide reductase from Escherichia coli. J Biol Chem. 1980 Jul 25;255(14):6706–6712. [PubMed] [Google Scholar]
- Pigiet V. P., Conley R. R. Purification of thioredoxin, thioredoxin reductase, and glutathione reductase by affinity chromatography. J Biol Chem. 1977 Sep 25;252(18):6367–6372. [PubMed] [Google Scholar]
- Platz A. Altered ribonucleotide reductase obtained by in vitro mutagenesis of cloned Escherichia coli DNA. Acta Chem Scand B. 1981;35(2):143–144. doi: 10.3891/acta.chem.scand.35b-0143. [DOI] [PubMed] [Google Scholar]
- Platz A., Sjöberg B. M. Construction and characterization of hybrid plasmids containing the Escherichia coli nrd region. J Bacteriol. 1980 Aug;143(2):561–568. doi: 10.1128/jb.143.2.561-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin M., Gräslund A., Ehrenberg A., Sjöberg B. M. Structure of the tyrosyl radical in bacteriophage T4-induced ribonucleotide reductase. J Biol Chem. 1982 Jan 10;257(1):366–369. [PubMed] [Google Scholar]
- Salowe S. P., Stubbe J. Cloning, overproduction, and purification of the B2 subunit of ribonucleoside-diphosphate reductase. J Bacteriol. 1986 Feb;165(2):363–366. doi: 10.1128/jb.165.2.363-366.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg B. M., Eklund H., Fuchs J. A., Carlson J., Standart N. M., Ruderman J. V., Bray S. J., Hunt T. Identification of the stable free radical tyrosine residue in ribonucleotide reductase. A sequence comparison. FEBS Lett. 1985 Apr 8;183(1):99–102. doi: 10.1016/0014-5793(85)80962-5. [DOI] [PubMed] [Google Scholar]
- Sjöberg B. M., Eriksson S., Jörnvall H., Carlquist M., Eklund H. Protein B1 of ribonucleotide reductase. Direct analytical data and comparisons with data indirectly deduced from the nucleotide sequence of the Escherichia coli nrdA gene. Eur J Biochem. 1985 Aug 1;150(3):423–427. doi: 10.1111/j.1432-1033.1985.tb09037.x. [DOI] [PubMed] [Google Scholar]
- Sjöberg B. M., Gräslund A., Eckstein F. A substrate radical intermediate in the reaction between ribonucleotide reductase from Escherichia coli and 2'-azido-2'-deoxynucleoside diphosphates. J Biol Chem. 1983 Jul 10;258(13):8060–8067. [PubMed] [Google Scholar]
- Sjöberg B. M., Hahne S., Karlsson M., Jörnvall H., Göransson M., Uhlin B. E. Overproduction and purification of the B2 subunit of ribonucleotide reductase from Escherichia coli. J Biol Chem. 1986 Apr 25;261(12):5658–5662. [PubMed] [Google Scholar]
- Sjöberg B. M., Hahne S., Mathews C. Z., Mathews C. K., Rand K. N., Gait M. J. The bacteriophage T4 gene for the small subunit of ribonucleotide reductase contains an intron. EMBO J. 1986 Aug;5(8):2031–2036. doi: 10.1002/j.1460-2075.1986.tb04460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. J., Ames G. F., Smith N. H., Robinson E. C., Higgins C. F. Repetitive extragenic palindromic sequences: a major component of the bacterial genome. Cell. 1984 Jul;37(3):1015–1026. doi: 10.1016/0092-8674(84)90436-7. [DOI] [PubMed] [Google Scholar]
- Thelander L., Sjöberg B. R., Eriksson S. Ribonucleoside diphosphate reductase (Escherichia coli). Methods Enzymol. 1978;51:227–237. doi: 10.1016/s0076-6879(78)51032-x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]


