Abstract
Polycystic ovary syndrome (PCOS) is associated with several mild metabolic disorders, including insulin resistance (IR), obesity, and dyslipidemia, as well as with some more severe ones, including type 2 diabetes mellitus, non-alcoholic fatty liver disease (NAFLD), and cardiovascular disease. Clinically, mild metabolic complications of PCOS such as IR or lipid metabolism disorders are the predictors of these more severe ones. So far, there is no reliable single marker that enables defining metabolic risk in patients with PCOS. Therefore, novel independent markers of metabolic disturbances are needed. Most reports have focused on microRNA (miRNA, miR) assessment in blood serum or granulosa cells, suggesting the high potential clinical utility of such management. The greatest number of studies focused on the association between miRNAs and IR, obesity, or lipid disorders, and some miRNAs were characteristics of all these processes concomitantly. The altered expression of miR-222, miR-223, miR-320, and miR-122 has been most commonly mentioned as the regulator of these metabolic distortions and seems to result from common regulation pathways of metabolic disturbances. In turn, the current literature lacked the miRNA which could be identified as a reliable marker of type 2 diabetes mellitus or NAFLD accompanying PCOS. Therefore, the main objective of future studies should be determining miRNA markers of these most serious metabolic complications. This article aims to review the role of microRNAs as biomarkers for metabolic disorders in PCOS.
Keywords: Insulin Resistance, Metabolic Diseases, MicroRNAs, Obesity, Polycystic Ovary Syndrome
Introduction
Polycystic ovary syndrome (PCOS) is a complex disorder characterized by hormonal and metabolic imbalances, which affects approximately 6% to 20% of women of reproductive age [1–3]. Mounting evidence suggests that environmental and genetic factors together with inflammation and microbiome alterations are of fundamental importance in the development of PCOS [4–7].
Among the wide range of dysfunctions and symptoms, affected women most frequently display hirsutism, acne, hyperandrogenemia, oligo- or amenorrhea, infertility, polycystic morphology of the ovaries, and insulin resistance (IR) [8,9]. Some of the above-mentioned symptoms were included in the revised Rotterdam criteria, which allow for diagnosing PCOS with a high degree of certainty after excluding other possible causes. According to the criteria, for PCOS diagnosis the patients must present at least 2 of the following symptoms: clinical or biochemical hyperandrogenism, chronic oligo- or an-ovulation, and polycystic ovarian morphology, or elevated levels of anti-Müllerin hormone (AMH) [10]. Nevertheless, despite diagnostic guidelines, years of research on the etiology of PCOS have revealed that even the diagnostic criteria insufficiently cover the complexity of biological alterations accompanying the disease [11].
These endocrine and metabolic disorders underlying PCOS form a network of interacting connections that further entail metabolic complications of this condition [12]. Hyperandrogenemia, which is the flagship biochemical disorder characteristic for patients suffering from PCOS, predisposes to disruption in insulin metabolism, leading to IR and hyperinsulinemia [13]. Further, all these disturbances substantially contribute to the occurrence of metabolic syndrome (MetS) components, including impaired glucose metabolism, as well as obesity, hypertension, and lipid metabolism disruption [14,15]. Accumulating data suggest that MetS fosters the development of type 2 diabetes mellitus, non-alcoholic fatty liver disease (NAFLD), and other related metabolic disorders [16–18]. Such links between metabolic complications and PCOS are directly reflected in statistics reporting a significantly higher incidence of metabolic disorders in patients with PCOS in comparison to healthy women [19].
In response to this phenomenon, guidelines elaborated by the European Society of Human Reproduction and Embryology (ESHRE) [10] and the American College of Obstetricians and Gynecologists (ACOG) [20] are consistent with the urgency of screening for cardiovascular disease, glucose metabolism disorders, and type 2 diabetes mellitus in patients with PCOS to avoid the development of more severe forms of these complications. Although the recommendations are generally not entirely in agreement, the screening schemes regarding metabolic risk share many similarities. Firstly, the screening tools, aimed to predict these disorders, are limited to the clinical features, including body mass index (BMI) and blood pressure, and the utility of some laboratory tests, including fasting glucose, cholesterol, lipoproteins, and triglycerides. Secondly, the recommended screening methods do not allow for identifying the cohort at risk of developing IR, the individual components of MetS, or NAFLD. Thus, the recommendations assume that the occurrence of some metabolic discrepancies will be a predictor of the occurrence of others more complex. Since it implies the initial presence of some pathological process, risk cannot be estimated in patients who have not yet developed any metabolic pathologies [10,20].
Many recent studies have investigated clinical features or metabolic markers for estimation of metabolic risk [21–23]. In addition, some authors have also proposed other indicators of metabolic abnormalities in patients with PCOS, such as oxidative stress [24–26] or inflammatory biomarkers [27,28]. Nevertheless, as the studies were most often the only ones to investigate the effectiveness of these markers, these results are difficult to interpret and should be further validated [24–28].
During the last decade, the role of microRNA (miRNA, miR) in PCOS has been the subject of extensive scientific research [29]. One of the most novel reviews concerning this issue has emphasized the crucial role of miRNAs in regulating pivotal pathways involved in the pathogenesis of PCOS, and proposed the utility of miRNAs as therapeutic targets [30]. According to the current literature, miRNAs are also molecules associated with metabolic disorders, including IR, MetS, and NAFLD in the normal population [31–33]. Therefore, this article aims to review the role of microRNAs as biomarkers for metabolic disorders in PCOS.
miRNAs in Insulin Resistance (IR)
IR is a condition involving decreased sensitivity of an organism’s tissues to insulin [34]. In addition to being one of the central features of PCOS, IR has been also identified as a risk factor predisposing to numerous PCOS complications such as MetS, NAFLD, or type 2 diabetes mellitus [35]. Moreover, the most recent research suggests that IR is a risk factor for poor in vitro fertilization (IVF) outcomes [36]. Although have tools to diagnose IR, including Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), or insulin resistance index (IRI), it has been shown that available markers have limitations and novel ones are needed [37–39].
Among all metabolic disorders associated with PCOS for which links with miRNA expression have been evaluated, IR was most extensively analyzed (Table 1). Numerous studies have observed a substantial role of miR-223 in the pathogenesis of IR associated with PCOS, and almost all were in line with miR-223 overexpression both in serum and adipose tissue of IR-affected patients with PCOS compared to patients with PCOS without IR [40–42]. Besides these encouraging results, some discrepancies regarding the usefulness of miR-223 were also noted. Huo et al observed reduced expression of miR-223 in patients with PCOS and IR [43]. Although they did not indicate the possible causes, we hypothesize that this effect could have resulted from the ability of miR-223 to reduce IR by inhibiting macrophages with pro-inflammatory properties [44].
Table 1.
Associations between dysregulated microRNAs (miRNAs) and insulin resistance (IR) in patients with polycystic ovary syndrome (PCOS).
| Authors | Year | Collected material | Upregulated miRNA | Downregulated miRNA |
|---|---|---|---|---|
| Chen et al [40] | 2013 | Adipose tissue | miR-223; miR-133 | – |
| Murri et al [41] | 2018 | Serum | miR-197-3p; miR-378a-5p; miR-223-5p; miR-122-5p; miR-203a; miR-16-1-3p; miR-193a-5p; miR-378a-3p; miR-24-3p; miR-361-5p; miR-365a-3p; miR-192-5p; miR-1468; miR-425-3p | miR-151a-5p/3p; miR-199a-5p/3p; miR-98-5p; miR-103a-3p; miR-107; miR-30c-5p; miR-18a-5p; miR-326; miR-30b-5p; let-7f-5p |
| Huang et al [42] | 2023 | Serum | miR-21; miR-27a; miR-93; miR-221; miR-222; miR-223 | – |
| Huo et al [43] | 2022 | Serum | miR-486-5p; miR-6088; miR-122-5p | miR-223-3p |
| Chuang et al [45] | 2015 | Adipose tissue | miR-223 | – |
| Wang et al [48] | 2022 | Serum | miR-222-3p | – |
| Jiang et al [50] | 2016 | Serum | miR-122; miR-193b; miR-194 | – |
| Hu et al [51] | 2020 | Granulosa cells | hsa-miR-612; hsa-miR-4449; hsa-miR-4488; hsa-miR-6510-5p; hsa-miR-548ar-3p; hsa-miR-3656; hsa-miR-7110-5p; hsa-miR-4516; hsa-miR-4492; hsa-miR-3162-3p; hsa-miR-3141; hsa-miR-3195; hsa-miR-6087; hsa-miR-1273g-3; hsa-miR-3196 | hsa-miR-548aq-3p; hsa-miR-122-5p; hsa-miR-548t-5p; hsa-miR-3591-3p; hsa-miR-204-3p; hsa-miR-767-5p; hsa-miR-107; hsa-miR-33a-5p; hsa-miR-802; hsa-miR-548aj-5p; hsa-miR-190b; hsa-miR-103a-2-5p; hsa-let-7g-3p; hsa-miR-548g-5p; hsa-miR-590-5p; hsa-miR-105-5p; hsa-miR-585-3p; hsa-miR-144-3p; hsa-miR-199b-3p; hsa-miR-1298-5p; hsa-miR-20b-5p; hsa-miR-181c-5p; hsa-miR-6511b-3p; hsa-miR-126-5p; hsa-miR-378d; hsa-miR-29b-2-5p; hsa-miR-18b-5p; hsa-miR-3144-3p; hsa-miR-1255b-5p; hsa-miR-144-5p; hsa-miR-16-1-3p; hsa-miR-9-3p; hsa-miR-196b-5p; hsa-miR-34a-3p; hsa-miR-7114-5p; hsa-miR-3135b; hsa-miR-592 |
| Krentowska et al [55] | 2024 | Serum | miR-27a | miR-320 |
| Liu et al [56] | 2023 | Serum | miR-4488 | – |
| Rashad et al [58] | 2019 | Serum | – | miR-320 |
| Wander et al [60] | 2022 | Serum | miR-132-3p | miR-486-5p; miR-503-5p; miR-16-5p; miR-4732-5p/3p; miR-363-3p; miR-25-3p; miR-451a; miR-20b-5p; miR-15a-5p; miR-92-3p; miR-144-3p; miR-7-5p |
| Sang et al [61] | 2023 | Serum | – | miR-363-3p |
| Díaz et al [62] | 2020 | Serum | – | miR-451a; miR-652-3p; miR-106b-5p; miR-206 |
| Wu et al [65] | 2014 | Adipose tissue | miR-25; miR-106b | – |
| Xu et al [66] | 2023 | Granulosa cells | miR-1298-5p | – |
| Gao et al [67] | 2022 | Serum | miR-184; miR-326 | – |
| Nanda et al [68] | 2020 | Serum | – | miR-24 |
| Udesen et al [69] | 2023 | Serum | – | miR-342-3p |
| Zhang et al [70] | 2023 | Serum | – | miR-335-5p |
| Mu et al [71] | 2021 | Ovarian tissues of rats | miR-103 | – |
| Soyman et al [72] | 2022 | Serum | – | miR-132 |
| Sørensen et al [73] | 2019 | Serum | – | miR-1225-5p |
| Yang et al [74] | 2018 | Ovarian tissues of rats | miR-33b-5p | – |
miRNA, miR – microRNA; hsa-miR – homo sapiens microRNA.
In contrast to the above-mentioned reports, Chuang et al found that use of this miRNA did not allow for differentiation between patients with and without IR in women with PCOS, despite the presence of a positive association between miR-223 and HOMA-IR values patients with PCOS and in healthy women [45], which might reflect the limited usefulness of this miRNA in the diagnosis of IR in patients with PCOS. Nevertheless, as the available literature has overall widely implicated the role of miR-223 in IR pathogenesis via identifying miR-223 interaction with type 4 glucose transporter (GLUT-4) and insulin substrate receptor-1 (IRS-1), involved in the insulin signaling pathway, miR-223 seems to be a reliable miRNA candidate to predict IR in patients with PCOS [46,47]. Further, elevated levels of the miRNAs of the 221 family, including miR-221 and miR-222, were linked to enhanced risk of IR in patients with PCOS [42,48]. Their multi-faceted role in pathways controlling insulin metabolism has long been debated and the regulation of GLUT-4 and the receptors for adiponectin has been described [49]. Several studies have also highlighted the importance of higher miR-122 expression in the pathogenesis of IR associated with PCOS [41,43,50]. Although most researchers agreed that elevated levels of this miRNA corresponded with an enhanced risk of IR [41,43,50], Hu et al found that in patients with PCOS who were diagnosed with IR, miR-122 was downregulated in comparison to the PCOS group without IR [51]. Analysis of studies describing the pathways in which this miR-122 is involved does not let us draw clear conclusions regarding a more relevant pattern of miRNA action. Thus, on the one hand, it was postulated that in the non-PCOS model, enhanced expression of miR-122 corresponded with intensified IR [52,53]. On the other hand, it was found that inhibition of this miRNA can modulate the expression of GLUT-4, IRS-1, and Forkhead Box A2 (FOXA2), which subsequently resulted in IR enhancement [54]. Also, some other miRNAs, whose increased expression was associated with the presence of IR in patients with PCOS, have been described by independent research teams, including miR-27a [42,55] and miR-4488 [51,56]. All these miRNAs have also previously been to some extent investigated in terms of association with the pathogenesis of insulin metabolism disturbances. The role of miR-27 has been well characterized and was observed to affect the PIK3/AKT pathway [57]. In contrast, although miR-4488 was found to be a regulator of genes participating in insulin metabolism, the details of the crosstalk are poorly understood [56].
A great number of studies have also pointed to the existence of miRNAs, whose reduced expression corresponded with IR. Krentowska et al, consistent with Rashad et al, found lower miR-320 expression to be linked with higher IR risk [55,58]. Nevertheless, these results should be treated cautiously, as in non-PCOS cohorts miR-320 has displayed different patterns of associations with IR [59]. Other miRNAs mentioned by at least 2 researchers as negatively correlated with impaired insulin metabolism include miR-199-3p [41,51], miR-20b-5p [51,60], miR-363-3p [60,61], miR-107 [41,51], miR-144-3p [51,60], and miR-451a [60,62]. The role of these miRNAs seems to result from different mechanisms of action; eg, miR-363-3p regulates the PI3K-Akt pathway [63], miR-107 affects insulin secretion and pancreatic beta cell development [46], and miR-144-3p acts via the regulation of IRS-1 [64]. There were also several miRNAs, including miR-106b-5p [62,65], miR-486-5p [43,60], miR-1298-5p [51,66], miR-326 [41,67], miR-16-1-3p [41,51], and miR-378 [41,51], in which altered expression was noticed by different research teams. Nevertheless, as the obtained results were contradictory, the role of these miRNAs should be further thoroughly analyzed.
Hu et al found correlations between IR and both increased and decreased expression of different miRNAs, but most of their findings have yet to be confirmed by other researchers, and exploring these observations is required to draw any firm conclusions [51], and this is also needed for many other analyzed miRNAs for which the links with IR have been described only once [40–43,50,55,60–62,65–74] including dysfunctional glucose metabolism in adipose tissue (AT) (Table 1). Taking together the results from our research and data from the literature, it now appears that further efforts should rather focus on the investigation of miRNAs for which correlations with IR were found in multiple studies and for which pathways of action are relatively well known.
miRNAs in Obesity
Excess body weight is another metabolic disorder specific to PCOS that has been extensively discussed in terms of its association with miRNA expression. Obesity is a crucial factor in the development of a broad range of PCOS-related symptoms. Moreover, it has been postulated that the relationship between PCOS and obesity is reciprocal [9]. Research has shown many correlations between the level of specific miRNAs and obesity in patients with PCOS (Table 2).
Table 2.
Associations between dysregulated microRNAs (miRNAs) and various indicators of excessive body weight including body mass index (BMI), waist-hip ratio (WHR), and waist circumference in patients with polycystic ovary syndrome (PCOS).
| Authors | Year | Collected material | Assessed parameter of obesity | Upregulated miRNA | Downregulated miRNA |
|---|---|---|---|---|---|
| Murri et al [41] | 2018 | Serum | BMI | miR-24-3p; miR-122-5p; miR-1537; miR-140-5p; miR-148a-3p; miR-223-5p; miR-23a-3p; miR-29c-3p; miR-30e-5p; miR-338-3p; miR-361-5p; miR-365a-3p; miR-378a-5p; miR-424-5p; miR-425-3p; miR-425-5p; miR-877-5p; miR-197-3p; miR-223-3p | miR-151a-3p; miR-151a-5p; let-7d-5p; miR-199a-3p; miR-199a-5p; miR-199b-5p; miR-379-3p |
| WHR | miR-192-5p; miR-193a-5p; miR-203a; miR-122-5p; miR-378a-5p; miR-877-5p | miR-151a-3p; miR-151a-5p; miR-199a-3p; miR-199a-5p; miR-181a-2-3p; miR-30d-3p; miR-331-3p; miR-98-5p; miR-30b-5p; miR-326; miR-126-5p | |||
| Wander et al [60] | 2022 | Serum | Waist circumference | miR-326; miR-339-5p | miR-1294; miR-4732-5p; miR-451a; miR-486-5p; miR-16-5p; miR-15a-3p; miR-451b; miR-4732-3p; miR-1180-3p |
| Xu et al [66] | 2023 | Granulosa cells | BMI | miR-1298-5p | – |
| WHR | |||||
| Gao et al [67] | 2022 | Serum | BMI | miR-184; miR-326 | – |
| Nanda et al [68] | 2020 | Serum | BMI | – | miR-24 |
| Udesen et al [69] | 2023 | Serum | WHR | miR-376-3p | - |
| Zhang et al [70] | 2023 | Serum | BMI | – | miR-335-5p |
| Udesen et al [75] | 2020 | Serum | BMI | miR-122; miR-223 | – |
| Zhao et al [76][71] | 2015 | Serum | BMI | miR-223 | – |
| Murri et al [78] | 2013 | Serum | BMI | miR-21; miR-27b; miR-103; miR-155 | – |
| Xiong et al [79] | 2017 | Serum | BMI | miR-23a | – |
| Lin et al [80] | 2020 | Serum | BMI | miR-23a | – |
| Romero-Ruiz et al [86] | 2021 | Serum | BMI | miR-33a-5p; miR-143-3p | – |
| Yang et al [87] | 2022 | Granulosa cells | BMI | miR-133a-3p | – |
| Cirillo et al [88] | 2019 | Granulosa cells | BMI | – | miR-486 |
miRNA, miR – microRNA; hsa-miR – homo sapiens microRNA; BMI – body mass index; WHR –waist-hip ratio.
Similarly, as in the case of IR, miRNA-223 was the most often mentioned obesity predictor and it has been revealed to positively correlate with higher BMI values [41,75,76]. These observations are inconsistent with reports regarding links between miR-223 and obesity in the general population, as a recent meta-analysis conducted by Veie et al has proven that elevated miR-223 levels correlated with weight reduction [77]. Thus, we hypothesize that patients with PCOS may display different regulatory patterns of obesity-associated miRNAs. A potential example of this phenomenon was described in the study by Murri et al, in which other miRNAs such as miR-21, miR-27b, miR-103, and miR-155 tended to present opposing links with BMI values between patients with PCOS and control groups [78]. Besides miR-223, the association between heightened expression of several other miRNAs, including miR-326 [60,67], miR-23a [79,80], and miR-122 [41,75], and greater obesity risk was confirmed by several researchers. The involvement of miR-122 in obesity development seems well-established in the literature, as several studies conducted on non-PCOS groups have shown obesity-related overexpression of this miRNA [81–83]. Nevertheless, there are many discrepancies regarding the action of other above-described miRNAs. A study conducted on non-PCOS cohorts indicated that lower miR-23a levels were associated with obesity [84]. Therefore, based on this observation, different mechanisms of miR-23a action in the pathogenesis of obesity cannot be excluded. Further, although Vega-Cardenas et al found that the role of miR-326 in obesity development results from this microRNA’s ability to enhance inflammation in adipose tissue [85], Murri et al found that lower miR-326 concentrations corresponded with higher waist-hip ratio (WHR) in patients with PCOS [41]; therefore, details of the activity of this molecule should be further examined.
In turn, many other miRNAs mentioned in Table 2 displaying positive associations with obesity were only once described in this context [25,41,60,66,67,69,78,86,87], which requires further research.
On the contrary, the evaluation of associations between downregulated miRNAs and greater risk of excess body weight in patients with PCOS has not led to clear conclusions. Only Wander et al [60] and Cirillo et al [88] consistently noticed the lowering levels of miR-486, probably contributing to obesity development through PI3K/Akt pathway targeting [89,90]. Some other miRNAs, including miR-151a-3p/5p, miR-199a-3p/5p [41], miR-4732-3p/5p, miR-451a, miR-486-5p, miR-16-5p [60], miR-24 [68], and miR-335-5p [70], for which lower regulation was proven to be associated with obesity development, have also been mentioned above to be underregulated in individuals with IR, showing potential common pathways underlying obesity and IR. Unfortunately, these links have only been described by single research teams; therefore, without additional confirmation of their role in the pathogenesis of obesity, these possible associations are unclear.
Lipid Metabolism Disorders Characteristic of Polycystic Ovary Syndrome (PCOS)
The characteristic picture of lipid metabolism disorders in patients with PCOS includes increased triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and non-high-density lipoprotein cholesterol (non-HDL-C), as well as an accompanying tendency for decreased high-density lipoprotein cholesterol (HDL-C) serum concentrations [91]. Lipid disorders in PCOS have a genetic basis and are determined by the presence of IR and excess androgens. It is estimated that lipid disorders affect approximately 70% of PCOS patients, which indicates a significant population-level concern [92]. Among individuals affected by PCOS, such unfavorable serum lipid profiles can be observed throughout their whole lifetime [91] and contribute to deteriorated outcomes of assisted reproductive technology (ART) procedures and a higher risk of future cardiovascular complications [36,93].
microRNAs in Triglycerides (TG) Metabolism Disorders
The overwhelming majority of correlations between miRNAs and TG concentrations agree with the associations identified for IR. Thus, the reduced expression of miR-24 [68], miR-320 [58], and miR-361-3p [73], as well as enhanced expression of miR-184 [67], miR-326 [67], and miR-4488 [56], were noted to be predictors of higher TG concentrations. This strongly supports the hypothesis that the pathomechanisms of metabolic disorders in PCOS overlap. Moreover, according to Wang et al, miR-222-3p is inversely linked to TG concentrations but only in the group of obese women with PCOS, which further confirms that metabolic disorders in PCOS are inextricably linked [48]. The most correlations between the expression of various miRNAs and TG were found by Murri et al [41], most of them revealed for the first time. However, thorough analysis allowed us to observe some discrepancies between the results of this study and those obtained by other authors; the contradictory results regarded the concentrations of miR-24 [41,68] and miR-151a-5p [41,56]. While Murri et al noted a positive correlation between miR-24 and TG concentrations [41], Nanda et al obtained contradictory results [68]. In the same study by Murri et al, a negative correlation between miR-151a-5p and TG was found [41], but Liu et al found that elevated miR-151a-5p levels corresponded with higher TG concentrations [56].
microRNAs in Total Cholesterol (TC) Metabolism Disorders
Overall, the association between TC and miRNA expression in patients with PCOS has been less frequently described compared to other lipid fractions. Some of the miRNAs for which negative correlations with TC have been found, including miR-361-3p [41], miR-222-3p [48], and miR-363-3p [61], were also mentioned to be associated with IR in the same studies. In addition, some novel miRNAs were investigated and while miR-598 and miR-433-3p presented a positive link with TC, enhanced miR-429 expression was a predictor of lower TC concentrations [41,73]. The action of all of these 3 miRNAs on cholesterol metabolism regulation could be hypothetically explained by their influence on the PI3k/Akt pathway [94–97]. Interestingly, these miRNAs have not been proven in other studies to be the markers of IR in PCOS patients, the occurrence of which is also related to this pathway [98]. Hence, on the one hand, these miRNAs may be particular specific TC biomarkers. On the other, taking into consideration the aforementioned common regulation of both processes, they may have negligible diagnostic value. Therefore, additional research is needed.
microRNAs in Low-Density Lipoprotein Cholesterol (LDL-C) Disorders
Overall, the current literature draws attention to the similar predictive capabilities of TC and LDL-C in indicating patients are at high risk of CVD development. Following this relationship, some miRNAs, including miR-598 and miR-361-3p, displayed associations with LDL-C, which is also referred to as TC [41]. However the analysis of miRNA’s usefulness in LDL-C level prediction conducted by Wang et al brought some inconsistencies in this regard and revealed that enhanced miR-222-3p expression was simultaneously linked to higher LDL-C and lower TC levels [48]. There is also a group of miRNAs that showed correlations with both LDL-C and IR. Some of them, including miR-107 or miR-320, were associated with LDL-C in the same manner as with IR, but others, including miR-223-3p or miR-151a-5p, displayed opposite relationships [41,56,99].
In addition, a new approach to the prediction of lipids’ fraction via miRNA concentrations was proposed by Yu et al, who found a correlation between miR-4644 and LDL-receptor (LDLR) participating in LDL metabolism and enhanced expression of this miRNA corresponded with lower expression of LDLR. Although this study evaluated LDLR concentrations in follicular fluid and the authors emphasized the potential importance of these results in folliculogenesis, the described regulation should also be studied in the context of its role in development of systemic dyslipidemias [100].
microRNAs in High-Density Lipoprotein Cholesterol (HDL-C) Disorders
The available literature also shows numerous links between miRNAs and HDL-C concentrations [41,48,67,68,73]. The most prominent of these associations was again pointed out by Murri et al [41]. Nevertheless, it remains challenging to demonstrate any consistent results, as none of the other authors has reached the same conclusions. Only miR-361-5p was mentioned twice as a potential biomarker of HDL-C levels, but researchers disagreed on the type of this regulation [41,73]. While Murri et al have observed under-expression of this miRNA associated with higher HDL-C values [41], Sørensen et al found high miR-361-5p values predicted high HDL-C levels [73]. Considering the associations between IR and the development of HDL-C metabolism disturbance [101], the fact that in PCOS individuals’ HDL-C concentrations were negatively correlated with the expression of miR-222-3p, miR-223-5p, and miR-326 seems important [41,48,67]. However, to consider these miRNAs as significant biomarkers of the levels of this lipid fraction, further studies demonstrating the association of these miRNAs with HDL-C are required.
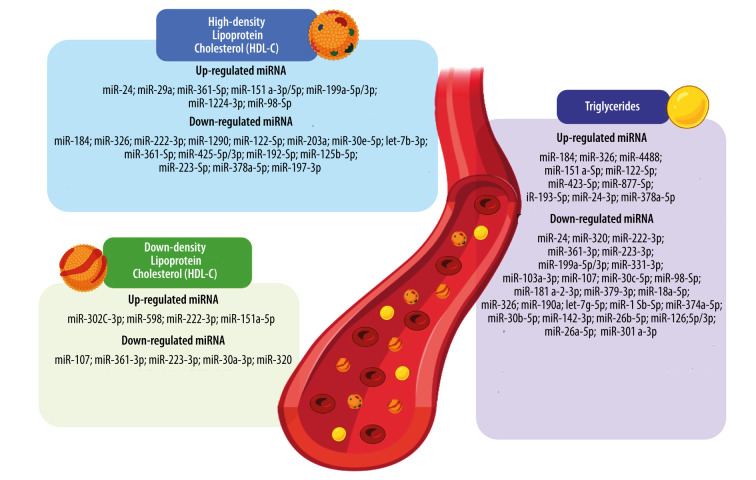
A schematic summary of the relationships between various miRNAs and concentrations of TG, LDL-C, and HDL-C is depicted in Figure 1.
Figure 1.
The graphic summary of alterations of microRNAs (miRNAs) concentrations associated with lipid profile parameters in patients with polycystic ovary syndrome (PCOS). Lipid metabolism disorders characteristics for PCOS include altered high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglyceride serum concentrations. Each alteration of lipid molecule serum level is regulated by a down- or overexpression of a range of different miRNAs. Based on: [41,48,56,58,67,68,73,99]. Created with BioRender software version 04. HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; miRNA, miR – microRNA.
microRNAs in Hypertension
The risk of hypertension poses another important challenge in PCOS patients. The meta-analysis by Wekker et al revealed approximately twice as much hypertension risk among patients with PCOS, in comparison to the population of healthy women [102]. Persistently elevated blood pressure in women of reproductive age may lead to development of other cardiovascular disturbances; therefore, early prediction of this disorder seems crucial in prevention of more severe PCOS complications [103,104].
Despite such a high prevalence of hypertension in patients with PCOS, only a few studies have searched for candidate miRNAs for the prediction of this metabolic dysfunction. Wang et al attempted to find correlations between miR-222-3p and cardiovascular disease (CVD), to which the diagnostic criterion hypertension was included. They discovered that higher miR-222-3p serum levels corresponded with enhanced CVD risk. Overall, it is the only study that revealed miRNA expression can directly reflect the occurrence of the condition, in which higher blood pressure can occur [48].
Zhang et al assessed the impact of miR-339 on functioning of endothelial progenitor cells (EPC), showing that impaired action was involved in the initiation and development of hypertension and other CVD components. They found that PCOS was associated with diminished migration and proliferation of EPC and increased miR-339 serum expression. The researchers observed that higher miR-339 expression contributed to reduced migration and proliferation of EPC and hypothesized that this effect occurred through PI3K/AKT and SIRT1/PGC-1-alpha pathways [105].
In summary, it is surprising that PCOS-related hypertension has received little research attention, especially considering that the authors have often focused on the relationship between miRNA expression and hypertension of various etiologies unrelated to PCOS in the literature [106].
microRNAs in type 2 Diabetes Mellitus
Patients with PCOS who have IR display a significantly greater risk of type 2 diabetes mellitus development [107,108]. Similarly, in patients previously diagnosed with type 2 diabetes mellitus, the occurrence of PCOS and even its more severe forms is also more prevalent [109,110]. Considering such reciprocal influences, these disorders seem to share a common causal background. In clinical practice, multiple clinical signs are useful in predicting the onset of type 2 diabetes mellitus in patients with PCOS. To be able to predict the risk of diabetes at an early stage before any clinical symptoms appear, a single marker is desirable [111].
Using reverse transcription-quantitative polymerase chain reaction (RT-qPCR), Wang et al evaluated the association between serum expression of miR-222-3p and type 2 diabetes mellitus in PCOS patients. Their study included 111 patients with PCOS, 57 of whom were diagnosed with type 2 diabetes mellitus, and 94 women who served as healthy controls. Increased expression of miR-222-3p was a predictor of the complication of PCOS with type 2 diabetes mellitus. As this relationship concerned both overweight and normal-weight PCOS patients, miR-222-3p can be considered a highly useful marker for patients with different body weights [48].
Wu et al also focused on the role of microRNA in type 2 diabetes mellitus in patients with PCOS. Based on the assessment of the microRNA profile of 44 patients with PCOS and type 2 diabetes mellitus, miR-32-3p appears to be involved in the pathogenesis of this complication of PCOS through the PLA2G4A pathway. Unfortunately, the authors did not include patients with PCOS unaffected by type 2 diabetes mellitus; therefore, comparison of miR-32-3p expression between these groups was not possible [112].
An attempt to establish a common genetic background of PCOS and type 2 diabetes mellitus based on the bioinformatic analysis was also performed by Zhang et al. As a result of the research, 4 genes contributing to the pathophysiology of these diseases were identified. The subsequent evaluation of gene regulators revealed 28 miRNAs involved in post-transcriptional modifications. Nevertheless, as the authors did not determine whether upregulation or downregulation of miRNA expression is linked to enhanced risk of type 2 diabetes mellitus development in patients with PCOS, further investigation is needed [113].
Another interesting study, conducted by Hocaoglu et al, focused on assessment of miRNA expression in pregnant women with PCOS who had gestational diabetes mellitus (GDM). The expression of miR-16-5p and miR-155-5p was evaluated in leukocytes from the peripheral blood. The authors observed higher miR-16-5p expression in pregnant patients with PCOS and GDM in comparison to pregnant women with PCOS without this metabolic complication [114]. Understanding the significantly higher risk of GDM development in patients with PCOS [115] and increased prevalence of many GDM complications affecting women and their offspring, including type 2 diabetes mellitus [116], may improve clinical utility [114].
microRNAs in Non-Alcoholic Fatty Liver Disease (NAFLD)
Among all the metabolic symptoms and early complications of PCOS, NAFLD is one of the most frequently disregarded complications of the disease. NAFLD is caused by metabolic disorders characteristic of PCOS and is also triggered by some microbiome alterations or genetic disturbances [117,118]. Although NAFLD can result in severe complications, currently, due to the lack of optimal screening methods, routine tests in patients with PCOS are usually not recommended [117].
To the best of our knowledge, only 1 published study has evaluated the role of miRNA in NAFLD and PCOS. Chen et al used microarray analysis to find differentially expressed genes (DEGs) in PCOS and NAFLD samples. First, 61 genes were characterized as differentially expressed and the next step of the study included the identification of miRNAs that become targets for DEGs. They observed that miR-20a-5p, miR-101-3p, miR-129-2-3p, and miR-124-3p participated in the regulation of DEGs, but no detailed data on the nature of this regulation was mentioned. Thus, these results should be considered a foundation for further research [119].
Future Directions
The growing number of research studies that focus on miRNAs as markers for various diseases creates new prospects for these non-coding RNAs to predict the risk of metabolic complications of PCOS. The prominent question that results from our review concerns the desired character of metabolic disorder markers in patients with PCOS. Hence, it is crucial to determine whether a single marker for all metabolic disturbances or rather separate one for each will be more useful in everyday clinical practice. The analysis of the links between miRNAs and all metabolic disturbances revealed several miRNAs (eg, miR-222, miR-223, miR-320, or miR-122) which were found to be potential markers of various metabolic disturbances in PCOS. The physiological roles of these miRNAs partially explain the wide range of metabolic pathologies with which miRNAs have been described to be associated. miR-222 and miR-223, through their influence on immune cells, regulate the inflammatory response [120,121]. Thus, the fact that metabolic disorders are associated with inflammation confirms the role of these miRNAs in metabolic state regulation [122]. Another non-coding RNA – miR-320 – alleviates oxidative stress [123], which is another process involved in metabolic disturbances [124,125].
Moreover, the involvement of aforementioned miRNAs in various metabolic pathologies indicates common biological pathways underlying their background. Overall, it can be concluded that selection of miRNA characterizing a multitude of metabolic parameters is relatively easy to conduct. On the other hand, these miRNAs linked to a wide range of metabolic disturbances accompanying PCOS were also reported to be simply the markers of PCOS occurrence [55,56,72]. Therefore, there is a risk of the lack of marker sensitivity, which may manifest as the elevated levels of these miRNAs in most women with PCOS. The opposite research strategy involves the establishment of separate markers for each metabolic disorder. Although it requires more extensive scientific investigation, it seems more valuable, especially for the prediction of more severe PCOS complications, including type 2 diabetes mellitus or NAFLD.
When debating the above-mentioned issues, it also seems important in this context that future studies should evaluate the pathways in which selected miRNAs are involved, as well as identification of the genes targeted by the selected miRNAs. We hypothesized that such a research strategy could elucidate the mechanisms leading to involvement of selected miRNA in several different metabolic processes. Furthermore, this would allow an understanding of the discrepancies in the levels of the same miRNA found for the same metabolic disorder.
Another crucial issue is selection of the most optimal tissues or fluids for miRNA detection. The greatest diversity of biological materials was particularly evident in studies performed to search for correlations between miRNAs and IR. In addition to using serum samples, researchers collected granulosa cells, as well as adipose tissue fragments. The heterogeneity of various tissues could also be observed while investigating the relationships between miRNA and other metabolic impairments. Among all these biomaterials, the measurements performed in serum seem to have the highest diagnostic potential due to the simplicity of material collection. Similarly, granulosa cells derived from follicular fluid can also be easily obtained during follicular puncture before ART procedures; however, this method seems to be limited to patients displaying PCOS-related infertility. On the contrary, obtaining adipose tissue involves invasive techniques; therefore, the utility of this tissue in miRNA detection is lower.
Conclusions
The occurrence of even mild forms of metabolic disorders in patients with PCOS has negative health consequences and is a harbinger of more serious metabolic disorders in the future. Therefore, it is crucial to identify any early exponents of metabolic disorders.
Despite the wide range of scientific reports suggesting the existence of links between metabolic disorders in PCOS and alterations in miRNA expression, it is difficult to identify miRNAs that clearly could be used as markers of these disorders. Currently, more studies have focused on miRNA involvement in pathological processes underlying PCOS, such as IR, obesity, or dyslipidemias. Although the substantial role of several miRNAs, including miR-222, miR-223, miR-320, and miR-122, in such mild PCOS complications has been postulated by several research teams, there is a risk of low specificity of these miRNAs as markers. Therefore, to increase their clinical usefulness, there is a need for in-depth investigation and determining the pathways regulated. On the other hand, few studies have focused on miRNAs characteristic for more severe metabolic complications, including type 2 diabetes mellitus or NAFLD; hence, this issue is poorly studied. In summary, further research should strive to predict more severe complications of PCOS, including type 2 diabetes mellitus or NAFLD, especially in young patients. Moreover, emphasis should be placed on detection of miRNAs with minimally invasive methods to enhance the clinical utility.
Footnotes
Conflict of interest: None declared
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity: All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Siddiqui S, Mateen S, Ahmad R, Moin S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS) J Assist Reprod Genet. 2022;39(11):2439–73. doi: 10.1007/s10815-022-02625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escobar-Morreale HF. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–84. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 3.Lizneva D, Suturina L, Walker W, Brakta S, et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. doi: 10.1016/j.fertnstert.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Ajmal N, Khan SZ, Shaikh R. Polycystic ovary syndrome (PCOS) and genetic predisposition: A review article. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100060. doi: 10.1016/j.eurox.2019.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudnicka E, Suchta K, Grymowicz M, et al. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci. 2021;22(7):3789. doi: 10.3390/ijms22073789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batra M, Bhatnager R, Kumar A, et al. Interplay between PCOS and microbiome: The road less travelled. Am J Reprod Immunol. 2022;88(2):e13580. doi: 10.1111/aji.13580. [DOI] [PubMed] [Google Scholar]
- 7.Eiras MC, Pinheiro DP, Romcy KAM, et al. Polycystic ovary syndrome: the epigenetics behind the disease. Reprod Sci. 2022;29(3):680–94. doi: 10.1007/s43032-021-00516-3. [DOI] [PubMed] [Google Scholar]
- 8.Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132(2):321–36. doi: 10.1097/AOG.0000000000002698. [DOI] [PubMed] [Google Scholar]
- 9.Joham AE, Norman RJ, Stener-Victorin E, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10(9):668–80. doi: 10.1016/S2213-8587(22)00163-2. [DOI] [PubMed] [Google Scholar]
- 10.Teede HJ, Tay CT, Laven JJE, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J Clin Endocrinol Metab. 2023;108(10):2447–69. doi: 10.1210/clinem/dgad463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dapas M, Dunaif A. Deconstructing a syndrome: Genomic insights into PCOS causal mechanisms and classification. Endocr Rev. 2022;43(6):927–65. doi: 10.1210/endrev/bnac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Chen C, Ma Y, et al. Multi-system reproductive metabolic disorder: Significance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS) Life Sci. 2019;228:167–75. doi: 10.1016/j.lfs.2019.04.046. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. 2020;35:100937. doi: 10.1016/j.molmet.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang R, Yang S, Li R, et al. Effects of hyperandrogenism on metabolic abnormalities in patients with polycystic ovary syndrome: A meta-analysis. Reprod Biol Endocrinol. 2016;14(1):67. doi: 10.1186/s12958-016-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim SS, Kakoly NS, Tan JWJ, et al. Metabolic syndrome in polycystic ovary syndrome: A systematic review, meta-analysis and meta-regression. Obes Rev. 2019;20(2):339–52. doi: 10.1111/obr.12762. [DOI] [PubMed] [Google Scholar]
- 16.Diabetes Canada Clinical Practice Guidelines Expert Committee. Punthakee Z, Goldenberg R, Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2018;42(Suppl 1):S10–S15. doi: 10.1016/j.jcjd.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Yki-Järvinen H. Liver fat in the pathogenesis of insulin resistance and type 2 diabetes. Dig Dis. 2010;28(1):203–9. doi: 10.1159/000282087. [DOI] [PubMed] [Google Scholar]
- 18.Mendrick DL, Diehl AM, Topor LS, et al. Metabolic syndrome and associated diseases: From the bench to the clinic. Toxicol Sci. 2018;162(1):36–42. doi: 10.1093/toxsci/kfx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins – Gynecology. ACOG Practice Bulletin No. 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131(6):e157–e71. doi: 10.1097/AOG.0000000000002656. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Wang H, Ma Q, et al. Value of the triglyceride-glucose index and non-traditional blood lipid parameters in predicting metabolic syndrome in women with polycystic ovary syndrome. Hormones (Athens) 2023;22(2):263–71. doi: 10.1007/s42000-023-00438-6. [DOI] [PubMed] [Google Scholar]
- 22.Blum MR, Popat RA, Nagy A, et al. Using metabolic markers to identify insulin resistance in premenopausal women with and without polycystic ovary syndrome. J Endocrinol Invest. 2021;44(10):2123–30. doi: 10.1007/s40618-020-01430-2. [DOI] [PubMed] [Google Scholar]
- 23.Kałużna M, Czlapka-Matyasik M, Kompf P, et al. Lipid ratios and obesity indices are effective predictors of metabolic syndrome in women with polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2022;13:20420188211066699. doi: 10.1177/20420188211066699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nawrocka-Rutkowska J, Szydłowska I, Jakubowska K, et al. Assessment of the parameters of oxidative stress depending on the metabolic and anthropometric status indicators in women with PCOS. Life (Basel) 2022;12(2):225. doi: 10.3390/life12020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Ruan X, Li Y, Cheng J, Mueck AO. Oxidative stress indicators in Chinese women with PCOS and correlation with features of metabolic syndrome and dependency on lipid patterns. Arch Gynecol Obstet. 2019;300(5):1413–21. doi: 10.1007/s00404-019-05305-7. [DOI] [PubMed] [Google Scholar]
- 26.Bannigida DM, Nayak BS, Vijayaraghavan R. Insulin resistance and oxidative marker in women with PCOS. Arch Physiol Biochem. 2020;126(2):183–86. doi: 10.1080/13813455.2018.1499120. [DOI] [PubMed] [Google Scholar]
- 27.Hancerliogullari N, Tokmak A, Guney G, et al. Serum calprotectin levels as markers of inflammation, insulin resistance and hyperandrogenism in women with polycystic ovary syndrome. Medicine (Baltimore) 2022;101(51):e32326. doi: 10.1097/MD.0000000000032326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banaszewska B, Siakowska M, Chudzicka-Strugala I, et al. Elevation of markers of endotoxemia in women with polycystic ovary syndrome. Hum Reprod. 2020;35(10):2303–11. doi: 10.1093/humrep/deaa194. [DOI] [PubMed] [Google Scholar]
- 29.Chen B, Xu P, Wang J, Zhang C. The role of MiRNA in polycystic ovary syndrome (PCOS) Gene. 2019;706:91–96. doi: 10.1016/j.gene.2019.04.082. [DOI] [PubMed] [Google Scholar]
- 30.Rashid G, Khan NA, Elsori D, et al. miRNA expression in PCOS: unveiling a paradigm shift toward biomarker discovery. Arch Gynecol Obstet. 2024;309(5):1707–23. doi: 10.1007/s00404-024-07379-4. [DOI] [PubMed] [Google Scholar]
- 31.Nigi L, Grieco GE, Ventriglia G, et al. MicroRNAs as regulators of insulin signaling: Research updates and potential therapeutic perspectives in type 2 diabetes. Int J Mol Sci. 2018;19(12):3705. doi: 10.3390/ijms19123705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solís-Toro D, Mosquera Escudero M, García-Perdomo HA. Association between circulating microRNAs and the metabolic syndrome in adult populations: A systematic review. Diabetes Metab Syndr. 2022;16(1):102376. doi: 10.1016/j.dsx.2021.102376. [DOI] [PubMed] [Google Scholar]
- 33.Hochreuter MY, Dall M, Treebak JT, Barrès R. MicroRNAs in non-alcoholic fatty liver disease: Progress and perspectives. Mol Metab. 2022;65:101581. doi: 10.1016/j.molmet.2022.101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SH, Park SY, Choi CS. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab J. 2022;46(1):15–37. doi: 10.4093/dmj.2021.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao H, Zhang J, Cheng X, Nie X, He B. Insulin resistance in polycystic ovary syndrome across various tissues: An updated review of pathogenesis, evaluation, and treatment. J Ovarian Res. 2023;16(1):9. doi: 10.1186/s13048-022-01091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang H, Si M, Tian T, et al. Adiposity and lipid metabolism indicators mediate the adverse effect of glucose metabolism indicators on oogenesis and embryogenesis in PCOS women undergoing IVF/ICSI cycles. Eur J Med Res. 2023;28(1):216. doi: 10.1186/s40001-023-01174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewandowski KC, Płusajska J, Horzelski W, et al. Limitations of insulin resistance assessment in polycystic ovary syndrome. Endocr Connect. 2018;7(3):403–12. doi: 10.1530/EC-18-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewandowski KC, Skowrońska-Jóźwiak E, Łukasiak K, et al. How much insulin resistance in polycystic ovary syndrome? Comparison of HOMA-IR and insulin resistance (Belfiore) index models. Arch Med Sci. 2019;15(3):613–18. doi: 10.5114/aoms.2019.82672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amisi CA. Markers of insulin resistance in Polycystic ovary syndrome women: An update. World J Diabetes. 2022;13(3):129–49. doi: 10.4239/wjd.v13.i3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YH, Heneidi S, Lee JM, et al. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes. 2013;62(7):2278–86. doi: 10.2337/db12-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murri M, Insenser M, Fernández-Durán E, et al. Non-targeted profiling of circulating microRNAs in women with polycystic ovary syndrome (PCOS): Effects of obesity and sex hormones. Metabolism. 2018;86:49–60. doi: 10.1016/j.metabol.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Huang CC, Yang PK, Huang YS, et al. The role of circulating miRNAs in mechanism of action and prediction of therapeutic responses of metformin in polycystic ovarian syndrome. Fertil Steril. 2023;119(5):858–68. doi: 10.1016/j.fertnstert.2022.12.045. [DOI] [PubMed] [Google Scholar]
- 43.Huo Y, Ji S, Yang H, et al. Differential expression of microRNA in the serum of patients with polycystic ovary syndrome with insulin resistance. Ann Transl Med. 2022;10(14):762. doi: 10.21037/atm-22-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhuang G, Meng C, Guo X, et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125(23):2892–903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 45.Chuang TY, Wu HL, Chen CC, et al. MicroRNA-223 expression is upregulated in insulin resistant human adipose tissue. J Diabetes Res. 2015;2015:943659. doi: 10.1155/2015/943659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakraborty C, Doss CG, Bandyopadhyay S, Agoramoorthy G. Influence of miRNA in insulin signaling pathway and insulin resistance: Micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip Rev RNA. 2014;5(5):697–712. doi: 10.1002/wrna.1240. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Ceinos J, Rangel-Zuñiga OA, Clemente-Postigo M, et al. miR-223-3p as a potential biomarker and player for adipose tissue dysfunction preceding type 2 diabetes onset. Mol Ther Nucleic Acids. 2021;23:1035–52. doi: 10.1016/j.omtn.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q, Fang C, Zhao Y, Liu Z. Correlation study on serum miR-222-3p and glucose and lipid metabolism in patients with polycystic ovary syndrome. BMC Womens Health. 2022;22(1):398. doi: 10.1186/s12905-022-01912-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Estrella Ibarra P, García-Solís P, Solís-Sáinz JC, Cruz-Hernández A. Expression of miRNA in obesity and insulin resistance: A review. Endokrynol Pol. 2021;72(1):73–80. doi: 10.5603/EP.a2021.0002. [DOI] [PubMed] [Google Scholar]
- 50.Jiang L, Huang J, Chen Y, et al. Identification of several circulating microRNAs from a genome-wide circulating microRNA expression profile as potential biomarkers for impaired glucose metabolism in polycystic ovarian syndrome. Endocrine. 2016;53(1):280–90. doi: 10.1007/s12020-016-0878-9. [DOI] [PubMed] [Google Scholar]
- 51.Hu MH, Zheng SX, Yin H, et al. Identification of microRNAs that regulate the MAPK pathway in human cumulus cells from PCOS women with insulin resistance. Reprod Sci. 2020;27(3):833–44. doi: 10.1007/s43032-019-00086-5. [DOI] [PubMed] [Google Scholar]
- 52.Mokhtari Ardekani A, Mohammadzadehsaliani S, Behrouj H, et al. miR-122 dysregulation is associated with type 2 diabetes mellitus-induced dyslipidemia and hyperglycemia independently of its rs17669 variant. Mol Biol Rep. 2023;50(5):4217–24. doi: 10.1007/s11033-023-08344-1. [DOI] [PubMed] [Google Scholar]
- 53.Dong L, Hou X, Liu F, et al. Regulation of insulin resistance by targeting the insulin-like growth factor 1 receptor with microRNA-122-5p in hepatic cells. Cell Biol Int. 2019;43(5):553–64. doi: 10.1002/cbin.11129. [DOI] [PubMed] [Google Scholar]
- 54.Sendi H, Mead I, Wan M, et al. miR-122 inhibition in a human liver organoid model leads to liver inflammation, necrosis, steatofibrosis and dysregulated insulin signaling. PLoS One. 2018;13(7):e0200847. doi: 10.1371/journal.pone.0200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krentowska A, Ponikwicka-Tyszko D, Łebkowska A, et al. Serum expression levels of selected microRNAs and their association with glucose metabolism in young women with polycystic ovary syndrome. Pol Arch Intern Med. 2024;134(1):16637. doi: 10.20452/pamw.16637. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y, Shi X, Xu B, et al. Differential expression of plasma-derived exosomal miRNAs in polycystic ovary syndrome as a circulating biomarker. Biomed Rep. 2023;19(6):92. doi: 10.3892/br.2023.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen T, Zhang Y, Liu Y, et al. MiR-27a promotes insulin resistance and mediates glucose metabolism by targeting PPAR-γ-mediated PI3K/AKT signaling. Aging (Albany NY) 2019;11(18):7510–24. doi: 10.18632/aging.102263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rashad NM, Ateya MA, Saraya YS, et al. Association of miRNA-320 expression level and its target gene endothelin-1 with the susceptibility and clinical features of polycystic ovary syndrome. J Ovarian Res. 2019;12(1):39. doi: 10.1186/s13048-019-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du H, Zhao Y, Yin Z, et al. The role of miR-320 in glucose and lipid metabolism disorder-associated diseases. Int J Biol Sci. 2021;17(2):402–16. doi: 10.7150/ijbs.53419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wander PL, Enquobahrie DA, Bammler TK, et al. Associations of plasma miRNAs with waist circumference and insulin resistance among women with polycystic ovary syndrome – pilot study. Mol Cell Endocrinol. 2022;554:111723. doi: 10.1016/j.mce.2022.111723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sang M, Yu Y, Zhou Z, et al. Differential expression of serum mir-363-3p in patients with polycystic ovary syndrome and its predictive value for their pregnancy. BMC Womens Health. 2023;23(1):264. doi: 10.1186/s12905-023-02337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Díaz M, Bassols J, López-Bermejo A, de Zegher F, Ibáńez L. Low circulating levels of miR-451a in girls with polycystic ovary syndrome: different effects of randomized treatments. J Clin Endocrinol Metab. 2020;105(3):dgz204. doi: 10.1210/clinem/dgz204. [DOI] [PubMed] [Google Scholar]
- 63.Shu L, Zhao H, Huang W, et al. Resveratrol upregulates mmu-miR-363-3p via the PI3K-Akt pathway to improve insulin resistance induced by a high-fat diet in mice. Diabetes Metab Syndr Obes. 2020;13:391–403. doi: 10.2147/DMSO.S240956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karolina DS, Armugam A, Tavintharan S, et al. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PLoS One. 2011;6(8):e22839. doi: 10.1371/journal.pone.0022839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu HL, Heneidi S, Chuang TY, et al. The expression of the miR-25/93/106b family of micro-RNAs in the adipose tissue of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99(12):E2754–E61. doi: 10.1210/jc.2013-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu C, Luo M, Liu X, et al. MicroRNA-1298-5p in granulosa cells facilitates cell autophagy in polycystic ovary syndrome by suppressing glutathione-disulfide reductase. Cell Tissue Res. 2023;392(3):763–78. doi: 10.1007/s00441-023-03747-9. [DOI] [PubMed] [Google Scholar]
- 67.Gao C, Guo L, Jiang Z, Cao L. Evaluation of serum MiR-184 and MiR-326 expression in PCOS subjects: Correlation with PCOS related parameters. Clin Lab. 2022;68(7):210823. doi: 10.7754/Clin.Lab.2021.210823. [DOI] [PubMed] [Google Scholar]
- 68.Nanda D, Chandrasekaran SP, Ramachandran V, et al. Evaluation of serum miRNA-24, miRNA-29a and miRNA-502-3p expression in PCOS subjects: Correlation with biochemical parameters related to PCOS and insulin resistance. Indian J Clin Biochem. 2020;35(2):169–78. doi: 10.1007/s12291-018-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Udesen PB, Sørensen AE, Svendsen R, et al. Circulating miRNAs in women with polycystic ovary syndrome: A longitudinal cohort study. Cells. 2023;12(7):983. doi: 10.3390/cells12070983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang S, Liu Y, Wang M, et al. Role and mechanism of miR-335-5p in the pathogenesis and treatment of polycystic ovary syndrome. Transl Res. 2023;252:64–78. doi: 10.1016/j.trsl.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 71.Mu J, Yu P, Li Q. microRNA-103 contributes to progression of polycystic ovary syndrome through modulating the IRS1/PI3K/AKT signal axis. Arch Med Res. 2021;52(5):494–504. doi: 10.1016/j.arcmed.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Soyman Z, Durmus S, Ates S, et al. Circulating MIR-132, MIR-146A, MIR-222, and MIR-320 expression in differential diagnosis of women with polycystic ovary syndrome. Acta Endocrinol (Buchar) 2022;18(1):13–19. doi: 10.4183/aeb.2022.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sørensen AE, Udesen PB, Maciag G, et al. Hyperandrogenism and metabolic syndrome are associated with changes in serum-derived microRNAs in women with polycystic ovary syndrome. Front Med (Lausanne) 2019;6:242. doi: 10.3389/fmed.2019.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Y, Jiang H, Xiao L, Yang X. MicroRNA-33b-5p is overexpressed and inhibits GLUT4 by targeting HMGA2 in polycystic ovarian syndrome: An in vivo and in vitro study. Oncol Rep. 2018;39(6):3073–85. doi: 10.3892/or.2018.6375. [DOI] [PubMed] [Google Scholar]
- 75.Udesen PB, Glintborg D, Sørensen AE, et al. Metformin decreases miR-122, miR-223 and miR-29a in women with polycystic ovary syndrome. Endocr Connect. 2020;9(11):1075–84. doi: 10.1530/EC-20-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao C, Liu X, Shi Z, et al. Role of serum miRNAs in the prediction of ovarian hyperstimulation syndrome in polycystic ovarian syndrome patients. Cell Physiol Biochem. 2015;35(3):1086–94. doi: 10.1159/000373934. [DOI] [PubMed] [Google Scholar]
- 77.Veie CHB, Nielsen IMT, Frisk NLS, Dalgaard LT. Extracellular microRNAs in relation to weight loss-a systematic review and meta-analysis. Noncoding RNA. 2023;9(5):53. doi: 10.3390/ncrna9050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murri M, Insenser M, Fernández-Durán E, et al. Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. J Clin Endocrinol Metab. 2013;98(11):E1835–E44. doi: 10.1210/jc.2013-2218. [DOI] [PubMed] [Google Scholar]
- 79.Xiong W, Lin Y, Xu L, et al. Circulatory microRNA 23a and microRNA 23b and polycystic ovary syndrome (PCOS): the effects of body mass index and sex hormones in an Eastern Han Chinese population. J Ovarian Res. 2017;10(1):10. doi: 10.1186/s13048-016-0298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin J, Huang H, Lin L, Li W, Huang J. MiR-23a induced the activation of CDC42/PAK1 pathway and cell cycle arrest in human cov434 cells by targeting FGD4. J Ovarian Res. 2020;13(1):90. doi: 10.1186/s13048-020-00686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang R, Hong J, Cao Y, et al. Elevated circulating microRNA-122 is associated with obesity and insulin resistance in young adults. Eur J Endocrinol. 2015;172(3):291–300. doi: 10.1530/EJE-14-0867. [DOI] [PubMed] [Google Scholar]
- 82.Hess AL, Larsen LH, Udesen PB, et al. Levels of circulating miR-122 are associated with weight loss and metabolic syndrome. Obesity (Silver Spring) 2020;28(3):493–501. doi: 10.1002/oby.22704. [DOI] [PubMed] [Google Scholar]
- 83.González-Arce LM, Lara-Riegos JC, Pérez-Mendoza GJ, et al. High expression levels of circulating microRNA-122 and microRNA-222 are associated with obesity in children with Mayan ethnicity. Am J Hum Biol. 2021;33(6):e23540. doi: 10.1002/ajhb.23540. [DOI] [PubMed] [Google Scholar]
- 84.Lozano-Bartolomé J, Llauradó G, Portero-Otin M, et al. Altered expression of miR-181a-5p and miR-23a-3p is associated with obesity and TNFα-induced insulin resistance. J Clin Endocrinol Metab. 2018;103(4):1447–58. doi: 10.1210/jc.2017-01909. [DOI] [PubMed] [Google Scholar]
- 85.Vega-Cárdenas M, Uresti-Rivera EE, Cortés-García JD, et al. Increased levels of adipose tissue-resident Th17 cells in obesity associated with miR-326. Immunol Lett. 2019;211:60–67. doi: 10.1016/j.imlet.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Romero-Ruiz A, Pineda B, Ovelleiro D, et al. Molecular diagnosis of polycystic ovary syndrome in obese and non-obese women by targeted plasma miRNA profiling. Eur J Endocrinol. 2021;185(5):637–52. doi: 10.1530/EJE-21-0552. [DOI] [PubMed] [Google Scholar]
- 87.Yang X, Wang K, Lang J, et al. Up-regulation of miR-133a-3p promotes ovary insulin resistance on granulosa cells of obese PCOS patients via inhibiting PI3K/AKT signaling. BMC Womens Health. 2022;22(1):412. doi: 10.1186/s12905-022-01994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cirillo F, Catellani C, Lazzeroni P, et al. MiRNAs regulating insulin sensitivity are dysregulated in polycystic ovary syndrome (PCOS) ovaries and are associated with markers of inflammation and insulin sensitivity. Front Endocrinol (Lausanne) 2019;10:879. doi: 10.3389/fendo.2019.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang Y, Ji C, Guo S, et al. The miR-486-5p plays a causative role in prostate cancer through negative regulation of multiple tumor suppressor pathways. Oncotarget. 2017;8(42):72835–46. doi: 10.18632/oncotarget.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Savova MS, Mihaylova LV, Tews D, et al. Targeting PI3K/AKT signaling pathway in obesity. Biomed Pharmacother. 2023;159:114244. doi: 10.1016/j.biopha.2023.114244. [DOI] [PubMed] [Google Scholar]
- 91.Cooney LG, Dokras A. Beyond fertility: Polycystic ovary syndrome and long-term health. Fertil Steril. 2018;110(5):794–809. doi: 10.1016/j.fertnstert.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 92.Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos GP. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab. 2007;18(7):280–85. doi: 10.1016/j.tem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 93.Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: A retrospective study in Chinese population. Front Endocrinol (Lausanne) 2022;13:892125. doi: 10.3389/fendo.2022.892125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Su X, Gu D, Xu L, et al. PI3K/Akt pathway expression in children with different obesity degrees and its relationship with glucolipid metabolism and insulin resistance. Am J Transl Res. 2021;13(6):6592–98. [PMC free article] [PubMed] [Google Scholar]
- 95.Lin X, Huang X, Wang L, Liu W. The long noncoding RNA MALAT1/microRNA-598-3p axis regulates the proliferation and apoptosis of retinoblastoma cells through the PI3K/AKT pathway. Mol Vis. 2022;28:269–79. [PMC free article] [PubMed] [Google Scholar]
- 96.Cheng H, Yan W. miR-433 regulates myocardial ischemia reperfusion injury by targeting NDRG4 via the PI3K/Akt pathway. Shock. 2020;54(6):802–9. doi: 10.1097/SHK.0000000000001532. [DOI] [PubMed] [Google Scholar]
- 97.Li W, Tang T, Yao S, et al. Low-dose lipopolysaccharide alleviates spinal cord injury-induced neuronal inflammation by inhibiting microRNA-429-mediated suppression of PI3K/AKT/Nrf2 signaling. Mol Neurobiol. 2024;61(1):294–307. doi: 10.1007/s12035-023-03483-9. [DOI] [PubMed] [Google Scholar]
- 98.Tong C, Wu Y, Zhang L, Yu Y. Insulin resistance, autophagy and apoptosis in patients with polycystic ovary syndrome: Association with PI3K signaling pathway. Front Endocrinol (Lausanne) 2022;13:1091147. doi: 10.3389/fendo.2022.1091147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arancio W, Calogero Amato M, et al. Serum miRNAs in women affected by hyperandrogenic polycystic ovary syndrome: the potential role of miR-155 as a biomarker for monitoring the estroprogestinic treatment. Gynecol Endocrinol. 2018;34(8):704–8. doi: 10.1080/09513590.2018.1428299. [DOI] [PubMed] [Google Scholar]
- 100.Yu L, Wang C, Zhang D, et al. Exosomal circ_0008285 in follicle fluid regulates the lipid metabolism through the miR-4644/LDLR axis in polycystic ovary syndrome. J Ovarian Res. 2023;16(1):113. doi: 10.1186/s13048-023-01199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baneu P, Văcărescu C, Drăgan SR, et al. The triglyceride/HDL ratio as a surrogate biomarker for insulin resistance. Biomedicines. 2024;12(7):1493. doi: 10.3390/biomedicines12071493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wekker V, van Dammen L, Koning A, et al. Long-term cardiometabolic disease risk in women with PCOS: A systematic review and meta-analysis. Hum Reprod Update. 2020;26(6):942–60. doi: 10.1093/humupd/dmaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kovell LC, Juraschek SP, Michos ED. Hypertension in young women: Implications of the polycystic ovary syndrome and opportunities for prevention and further research. J Clin Endocrinol Metab. 2021;106(9):e3775–e77. doi: 10.1210/clinem/dgab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gomez JMD, VanHise K, Stachenfeld N, et al. Subclinical cardiovascular disease and polycystic ovary syndrome. Fertil Steril. 2022;117(5):912–23. doi: 10.1016/j.fertnstert.2022.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang J, Xu W, Li S, et al. The role of miRNA-339-5p in the function of vascular endothelial progenitor cells in patients with PCOS. Reprod Biomed Online. 2022;44(3):423–33. doi: 10.1016/j.rbmo.2021.09.017. [DOI] [PubMed] [Google Scholar]
- 106.Jusic A, Devaux Y. EU-CardioRNA COST Action (CA17129). Noncoding RNAs in hypertension. Hypertension. 2019;74(3):477–92. doi: 10.1161/HYPERTENSIONAHA.119.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Orio F, Muscogiuri G, Nese C, et al. Obesity, type 2 diabetes mellitus and cardiovascular disease risk: An uptodate in the management of polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2016;207:214–19. doi: 10.1016/j.ejogrb.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 108.Toulis KA, Goulis DG, Kolibianakis EM, et al. Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: A systematic review and a meta-analysis. Fertil Steril. 2009;92(2):667–77. doi: 10.1016/j.fertnstert.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 109.Long C, Feng H, Duan W, et al. Prevalence of polycystic ovary syndrome in patients with type 2 diabetes: A systematic review and meta-analysis. Front Endocrinol (Lausanne) 2022;13:980405. doi: 10.3389/fendo.2022.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sim SY, Chin SL, Tan JL, et al. Polycystic ovary syndrome in type 2 diabetes: Does it predict a more severe phenotype? Fertil Steril. 2016;106(5):1258–63. doi: 10.1016/j.fertnstert.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 111.Glintborg D, Kolster ND, Ravn P, Andersen MS. Prospective risk of type 2 diabetes in normal weight women with polycystic ovary syndrome. Biomedicines. 2022;10(6):1455. doi: 10.3390/biomedicines10061455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu J, Chen X. Acupuncture therapy protects PCOS patients with diabetes by regulating miR-32-3p/PLA2G4A pathway. Am J Transl Res. 2021;13(8):8819–32. [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang J, Zhang FJ, Zhang L, et al. Identification of key genes and molecular pathways in type 2 diabetes mellitus and polycystic ovary syndrome via bioinformatics analyses. Eur Rev Med Pharmacol Sci. 2023;27(8):3255–69. doi: 10.26355/eurrev_202304_32097. [DOI] [PubMed] [Google Scholar]
- 114.Hocaoglu M, Demirer S, Loclar Karaalp I, et al. Identification of miR-16-5p and miR-155-5p microRNAs differentially expressed in circulating leukocytes of pregnant women with polycystic ovary syndrome and gestational diabetes. Gynecol Endocrinol. 2021;37(3):216–20. doi: 10.1080/09513590.2020.1843620. [DOI] [PubMed] [Google Scholar]
- 115.Yu HF, Chen HS, Rao DP, Gong J. Association between polycystic ovary syndrome and the risk of pregnancy complications: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016;95(51):e4863. doi: 10.1097/MD.0000000000004863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moon JH, Jang HC. Gestational diabetes mellitus: Diagnostic approaches and maternal-offspring complications. Diabetes Metab J. 2022;46(1):3–14. doi: 10.4093/dmj.2021.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Doycheva I, Ehrmann DA. Nonalcoholic fatty liver disease and obstructive sleep apnea in women with polycystic ovary syndrome. Fertil Steril. 2022;117(5):897–911. doi: 10.1016/j.fertnstert.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 118.Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–24. doi: 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 119.Chen Y, Ma L, Ge Z, et al. Key genes associated with non-alcoholic fatty liver disease and polycystic ovary syndrome. Front Mol Biosci. 2022;9:888194. doi: 10.3389/fmolb.2022.888194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Y, Yang J, Zhou X, et al. Knockdown of miR-222 inhibits inflammation and the apoptosis of LPS-stimulated human intervertebral disc nucleus pulposus cells. Int J Mol Med. 2019;44(4):1357–65. doi: 10.3892/ijmm.2019.4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiao P, Wang XP, Luoreng ZM, et al. miR-223: An effective regulator of immune cell differentiation and inflammation. Int J Biol Sci. 2021;17(9):2308–22. doi: 10.7150/ijbs.59876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen J, Yang Y, Kong W. Cross Talk between inflammation and metabolic disorders. Mediators Inflamm. 2022;2022:9821506. doi: 10.1155/2022/9821506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ding C, Qian C, Hou S, et al. Exosomal miRNA-320a is released from hAMSCs and regulates SIRT4 to prevent reactive oxygen species generation in POI. Mol Ther Nucleic Acids. 2020;21:37–50. doi: 10.1016/j.omtn.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kruczkowska W, Gałęziewska J, Kciuk M, et al. Senescent adipocytes and type 2 diabetes - current knowledge and perspective concepts. Biomol Concepts. 2024;15(1):bmc-2022-0046. doi: 10.1515/bmc-2022-0046. [DOI] [PubMed] [Google Scholar]
- 125.Panic A, Stanimirovic J, Sudar-Milovanovic E, Isenovic ER. Oxidative stress in obesity and insulin resistance. Explor Med. 2022;3:58–70. [Google Scholar]