Abstract
We have used in situ hybridization to determine the sites of insertion of Agrobacterium rhizogenes Ri T-DNA in the chromosomes of Crepis capillaris (2n = 6) transformed roots. Four transformed root lines were obtained by infecting Crepis stem segments with A. rhizogenes. Southern hybridization analysis indicated that each root line was the result of one or more independent T-DNA insertion events. In two root lines, one copy of T-DNA was present; the other two root lines each contained two copies of T-DNA. To localize these T-DNA inserts on Crepis chromosomes, metaphase spreads were perpared from each root line, and hybridized in situ to a biotinlabeled T-DNA probe. The results indicated that T-DNA was present in a different chromosomal location in each root line, and that each chromosome had been a target for T-DNA insertion at least once. In the root lines containing two T-DNA inserts, two patterns of integration were observed: in one case the T-DNAs were present on separate chromosomes; in the other case the two T-DNAs were close together (but not tandemly arranged) on a single chromosome. A comparison of these results and those obtained previously for a fifth Crepis-transformed root line demostrated that Ri T-DNA does not insert preferentially into a particlar chromosomal location.
Keywords: Agrobacterium rhizogenes, Crepis capillaris, in situ hybridization, plant chromosomes, T-DNA
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. W., Chilton M. D. Multiple transcripts of T-DNA detected in nopaline crown gall tumors. J Mol Appl Genet. 1982;1(6):539–546. [PubMed] [Google Scholar]
- Bevan M. W., Chilton M. D. T-DNA of the Agrobacterium Ti and Ri plasmids. Annu Rev Genet. 1982;16:357–384. doi: 10.1146/annurev.ge.16.120182.002041. [DOI] [PubMed] [Google Scholar]
- Bevan M., Barnes W. M., Chilton M. D. Structure and transcription of the nopaline synthase gene region of T-DNA. Nucleic Acids Res. 1983 Jan 25;11(2):369–385. doi: 10.1093/nar/11.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
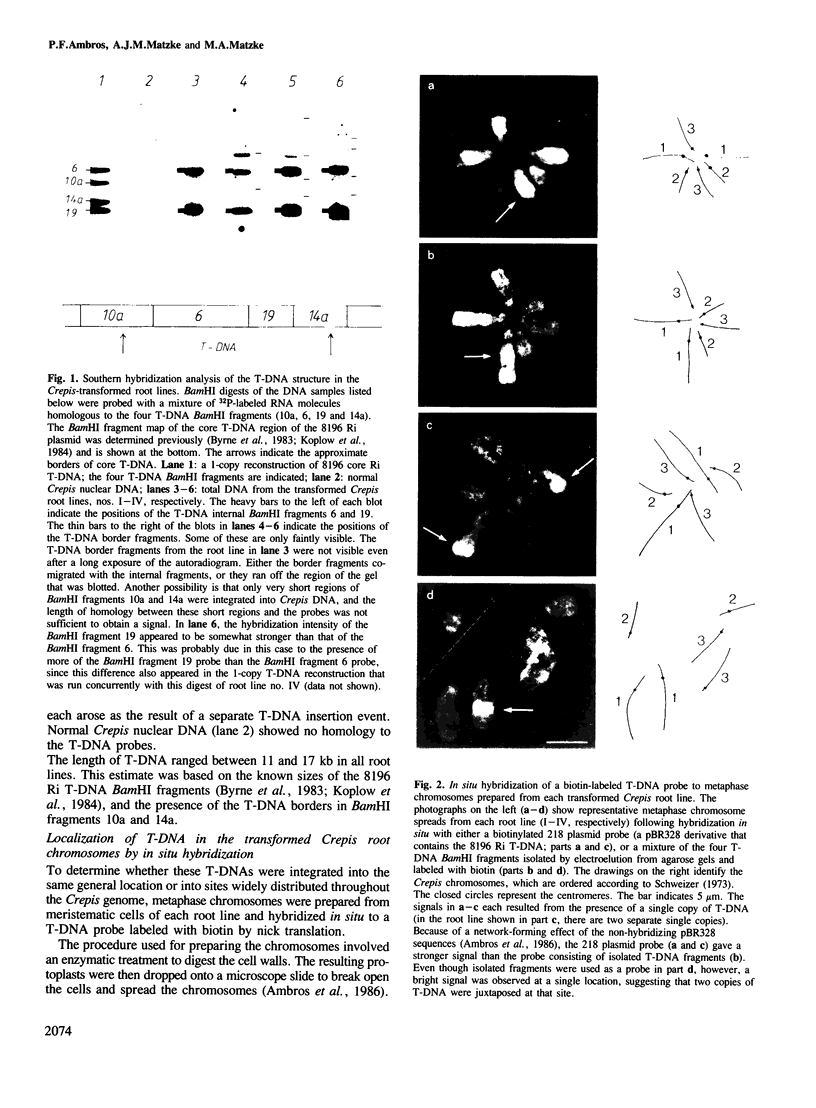
- Byrne M. C., Koplow J., David C., Tempé J., Chilton M. D. Structure of T-DNA in roots transformed by Agrobacterium rhizogenes. J Mol Appl Genet. 1983;2(2):201–209. [PubMed] [Google Scholar]
- Chilton M. D., Drummond M. H., Merio D. J., Sciaky D., Montoya A. L., Gordon M. P., Nester E. W. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977 Jun;11(2):263–271. doi: 10.1016/0092-8674(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Greve H., Dhaese P., Seurinck J., Lemmers M., Van Montagu M., Schell J. Nucleotide sequence and transcript map of the Agrobacterium tumefaciens Ti plasmid-encoded octopine synthase gene. J Mol Appl Genet. 1982;1(6):499–511. [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Durand-Tardif M., Broglie R., Slightom J., Tepfer D. Structure and expression of Ri T-DNA from Agrobacterium rhizogenes in Nicotiana tabacum. Organ and phenotypic specificity. J Mol Biol. 1985 Dec 5;186(3):557–564. doi: 10.1016/0022-2836(85)90130-5. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Simpson R. B., Ream L. W., White F. F., Gordon M. P., Nester E. W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981 Nov;27(1 Pt 2):143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- Joos H., Inzé D., Caplan A., Sormann M., Van Montagu M., Schell J. Genetic analysis of T-DNA transcripts in nopaline crown galls. Cell. 1983 Apr;32(4):1057–1067. doi: 10.1016/0092-8674(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Koplow J., Byrne M. C., Jen G., Tempé J., Chilton M. D. Physical map of the Agrobacterium rhizogenes strain 8196 virulence plasmid. Plasmid. 1984 Jan;11(1):17–27. doi: 10.1016/0147-619x(84)90003-9. [DOI] [PubMed] [Google Scholar]
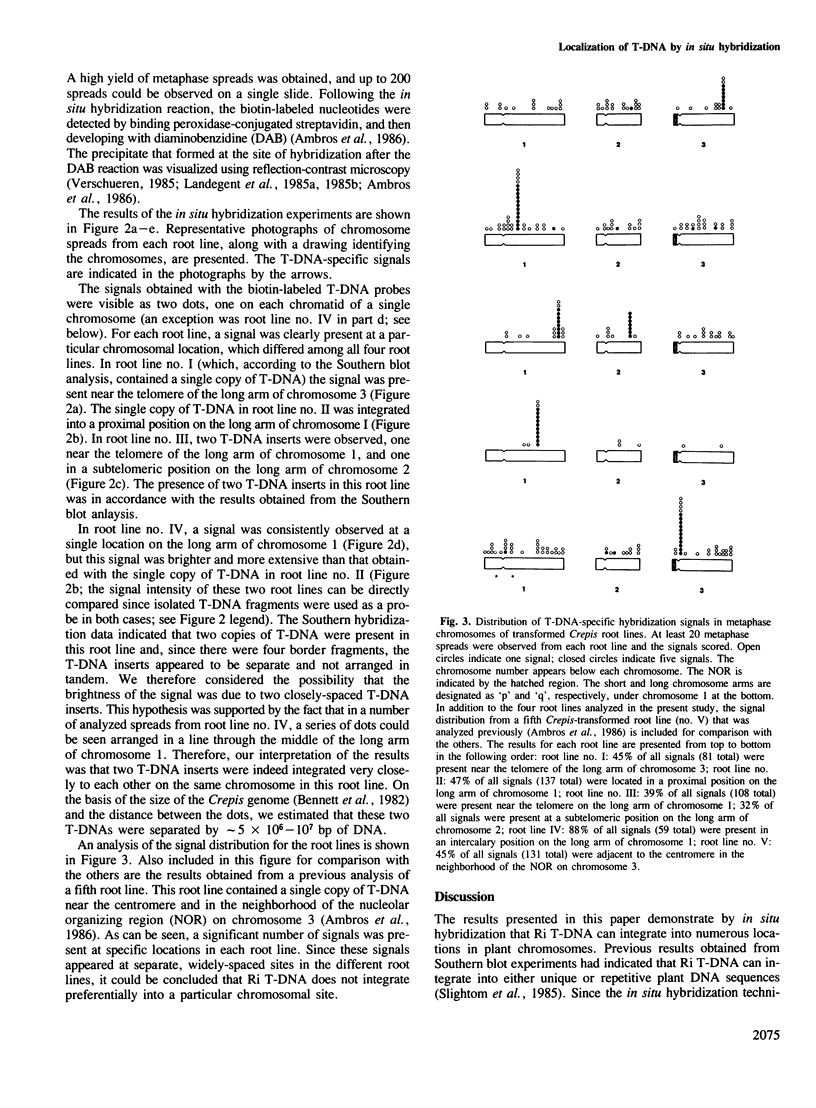
- Landegent J. E., Jansen in de Wal N., Ploem J. S., Van der Ploeg M. Sensitive detection of hybridocytochemical results by means of reflection-contrast microscopy. J Histochem Cytochem. 1985 Dec;33(12):1241–1246. doi: 10.1177/33.12.2415575. [DOI] [PubMed] [Google Scholar]
- Landegent J. E., Jansen in de Wal N., van Ommen G. J., Baas F., de Vijlder J. J., van Duijn P., Van der Ploeg M. Chromosomal localization of a unique gene by non-autoradiographic in situ hybridization. Nature. 1985 Sep 12;317(6033):175–177. doi: 10.1038/317175a0. [DOI] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers M., De Beuckeleer M., Holsters M., Zambryski P., Depicker A., Hernalsteens J. P., Van Montagu M., Schell J. Internal organization, boundaries and integration of Ti-plasmid DNA in nopaline grown gall tumours. J Mol Biol. 1980 Dec 15;144(3):353–376. doi: 10.1016/0022-2836(80)90095-9. [DOI] [PubMed] [Google Scholar]
- Matzke A. J., Chilton M. D. Site-specific insertion of genes into T-DNA of the Agrobacterium tumor-inducing plasmid: an approach to genetic engineering of higher plant cells. J Mol Appl Genet. 1981;1(1):39–49. [PubMed] [Google Scholar]
- Slightom J. L., Jouanin L., Leach F., Drong R. F., Tepfer D. Isolation and identification of TL-DNA/plant junctions in Convolvulus arvensis transformed by Agrobacterium rhizogenes strain A4. EMBO J. 1985 Dec 1;4(12):3069–3077. doi: 10.1002/j.1460-2075.1985.tb04047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepfer D. Transformation of several species of higher plants by Agrobacterium rhizogenes: sexual transmission of the transformed genotype and phenotype. Cell. 1984 Jul;37(3):959–967. doi: 10.1016/0092-8674(84)90430-6. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Verschueren H. Interference reflection microscopy in cell biology: methodology and applications. J Cell Sci. 1985 Apr;75:279–301. doi: 10.1242/jcs.75.1.279. [DOI] [PubMed] [Google Scholar]
- White F. F., Ghidossi G., Gordon M. P., Nester E. W. Tumor induction by Agrobacterium rhizogenes involves the transfer of plasmid DNA to the plant genome. Proc Natl Acad Sci U S A. 1982 May;79(10):3193–3197. doi: 10.1073/pnas.79.10.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. F., Taylor B. H., Huffman G. A., Gordon M. P., Nester E. W. Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of Agrobacterium rhizogenes. J Bacteriol. 1985 Oct;164(1):33–44. doi: 10.1128/jb.164.1.33-44.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Dhaese P., Schreier P. H., Schmalenbach W., Van Montagu M., Schell J. Size, location and polarity of T-DNA-encoded transcripts in nopaline crown gall tumors; common transcripts in octopine and nopaline tumors. Cell. 1983 Apr;32(4):1045–1056. doi: 10.1016/0092-8674(83)90289-1. [DOI] [PubMed] [Google Scholar]
- Zambryski P., Holsters M., Kruger K., Depicker A., Schell J., Van Montagu M., Goodman H. M. Tumor DNA structure in plant cells transformed by A. tumefaciens. Science. 1980 Sep 19;209(4463):1385–1391. doi: 10.1126/science.6251546. [DOI] [PubMed] [Google Scholar]