Abstract
PURPOSE
Nivolumab plus ipilimumab (NIVO + IPI) is a first-in-class combination immunotherapy for the treatment of intermediate- or poor (I/P)-risk advanced or metastatic renal cell carcinoma (mRCC). Currently, there are limited real-world data regarding clinical effectiveness beyond 12-24 months from treatment initiation. In this real-world study, treatment patterns and clinical outcomes were evaluated for NIVO + IPI in a community oncology setting.
METHODS
A retrospective analysis using electronic medical record data from The US Oncology Network examined patients with I/P-risk clear cell mRCC who initiated first-line (1L) NIVO + IPI between January 4, 2018, and December 31, 2019, with follow-up until June 30, 2022. Baseline demographics, clinical characteristics, treatment patterns, clinical effectiveness, and safety outcomes were assessed descriptively. Overall survival (OS) and real-world progression-free survival (rwPFS) were analyzed using Kaplan-Meier methods.
RESULTS
Among 187 patients identified (median follow-up, 22.4 months), with median age 63 (range, 30-89) years, 74 (39.6%) patients had poor risk and 37 (19.8%) patients had Eastern Cooperative Oncology Group performance status score ≥2. Of 86 patients who received second-line therapy, 54.7% received cabozantinib and 10.5% received pazopanib. The median (95% CI) OS and rwPFS were 38.4 (24.7-46.1) months and 11.1 (7.5-15.0) months, respectively. Treatment-related adverse events (TRAEs) were reported in 89 (47.6%) patients, including fatigue (n = 25, 13.4%) and rash (n = 19, 10.2%).
CONCLUSION
This study provides data to support the understanding of the real-world utilization and long-term effectiveness of 1L NIVO + IPI in patients with I/P-risk mRCC. TRAE rates were low relative to clinical trials.
INTRODUCTION
Kidney cancer is the seventh most common cancer in the United States with an estimated 81,800 new cases and 14,890 deaths in 2023.1 Approximately 85% of kidney tumors are renal cell carcinomas, and among them, approximately 75% has clear cell histology.2 Overall, the incidence rate has been rising slightly, likely because of changing prevalence of leading risk factors (tobacco use, obesity, and hypertension) within certain subpopulations.3 While mortality rates have leveled off as patients are diagnosed in earlier stages of disease, the 5-year relative survival among patients with metastatic kidney cancer is 17%.1
CONTEXT
Key Objective
In this real-world study, treatment patterns and clinical outcomes were evaluated among patients with intermediate- or poor-risk metastatic renal cell carcinoma (mRCC) treated with first-line (1L) nivolumab plus ipilimumab (NIVO + IPI) in a community oncology setting.
Knowledge Generated
In 187 patients (median follow-up, 22.4 months), the median (95% CI) overall survival and real-world progression-free survival were 38.4 (24.7 to 46.1) months and 11.1 (7.5 to 15.0) months, respectively. Treatment-related adverse events were reported in 89 (47.6%) patients, including fatigue (n = 25, 13.4%) and rash (n = 19, 10.2%).
Relevance (Z. Bakouny)
This study evaluates the use of NIVO + IPI in the 1L treatment of mRCC in a large community oncology population. It adds significant data to the literature for a regimen that is widely adopted in this setting across the world.*
*Relevance section written by JCO Clinical Cancer Informatics Associate Editor Ziad Bakouny, MD, MSc.
The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) prognostic model was developed to identify factors associated with survival among patients with metastatic renal cell carcinoma (mRCC).4 The IMDC model considers six risk factors: anemia, thrombocytosis, neutrophilia, hypercalcemia, Karnofsky performance status <80%, and <1 year from diagnosis to initiation of systemic therapy. Patients with no risk factors are classified as favorable, whereas the presence of one to two risk factors is considered intermediate risk and the presence of ≥3 risk factors is considered poor risk. At initiation of frontline targeted therapy for mRCC, IMDC intermediate- or poor (I/P)-risk was observed in 81.5% of patients, and an increasing number of risk factors were associated with a higher risk of death.4
The first-line (1L) treatment paradigm in advanced renal cell carcinoma (aRCC) continues to evolve with the approvals of combinations of immunotherapies and targeted agents.5-11 Combination therapy with nivolumab plus ipilimumab (NIVO + IPI) received US Food and Drug Administration approval in April 2018 for the treatment of patients with untreated I/P-risk aRCC on the basis of results from the phase III CheckMate 214 trial.7,9 In this study, NIVO + IPI was associated with superior efficacy versus sunitinib in the 1L treatment of I/P-risk aRCC.7 At the primary disclosure with a median follow-up of 25.2 months, the 18-month overall survival (OS) rate was 75% (95% CI, 70-78) versus 60% (95% CI, 55-65), with an objective response rate (ORR) of 42% versus 27%, respectively.7 The clinical efficacy of NIVO + IPI versus sunitinib was maintained at 8 years (median follow-up, 99.1 months), with a median OS of 47.7 versus 26.0 months, a median progression-free survival (PFS) of 12.4 versus 8.5 months, and an ORR of 42% versus 27%.12
While randomized controlled trials (RCTs) are considered the gold standard, they often do not reflect real-world patient populations. Real-world evidence (RWE) collected from routine clinical practice plays an important role in complementing results observed in RCTs. A few RWE studies have previously reported outcomes among patients with I/P-risk mRCC who received 1L NIVO + IPI.13-17 Given the unmet need in this patient population, this study aims to use real-world data to examine the demographics, clinical characteristics, and outcomes of patients who receive 1L NIVO + IPI for mRCC outside of the clinical trial setting. This study adds to the existing literature by examining outcomes in patients with a follow-up of up to 51 months.
METHODS
Study Design and Population
This was a real-world retrospective observational study using electronic health record (EHR) data from The US Oncology Network to evaluate patients with I/P-risk mRCC receiving 1L NIVO + IPI in US community oncology practices. Patients were included in this analysis if they were 18 years or older at diagnosis of mRCC with IMDC I/P-risk disease, had ≥2 visits to a US Oncology Network clinic, and had initiated 1L NIVO + IPI, defined as the first systemic therapy for mRCC during the study identification period (April 1, 2018, to December 31, 2019). Patients were excluded from the study if they were enrolled in a clinical trial or had other documented primary cancer diagnoses during the study observation period (April 1, 2018, to June 30, 2022).
The index date was the date of initiation of 1L NIVO + IPI. Patients were followed for at least 3 weeks and up to 51 months from the index date through the last visit date during the study observation period or date of death, whichever occurred first.
Data Source
Study data were sourced from The US Oncology Network's EHR system, iKnowMed (iKM), which includes over 1.2 million patients treated annually within community-based outpatient practices receiving treatment across the network.18 iKM data from structured fields were supplemented with unstructured data abstracted from medical charts to capture other information of interest, including patients' treatment history, date of diagnosis, and physician-assessed response. The Social Security Administration's Death Master File was used to supplement the data available in iKM on vital status and death dates.
Outcomes
Clinical effectiveness was assessed using OS, real-world progression-free survival (rwPFS), real-world response rate (rwRR), real-world time to treatment response (rwTTR), real-world time to treatment discontinuation or death (rwTTD), real-world treatment-free interval (rwTFI), and real-world time to next treatment or death (rwTTNT).
OS was defined as the interval between the index date and the date of death. rwPFS was the interval between the index date and the date of clinician-documented disease progression or death. Patients who did not die (for OS) or did not progress or die (for rwPFS) were censored on the study end date or the last visit date available in the database, whichever occurred first. rwRR was calculated as the proportion of patients who achieved a complete response (CR) or partial response (PR) on the basis of providers' assessments as documented in the charts, which may use different criteria compared with response assessments in clinical trials (eg, RECIST19). rwTTR was defined as the interval between the index date and the first documented response among patients who experienced a CR or PR. rwTTD was the interval between the index date and the end date of the dosing regimen. Patients without treatment discontinuation were censored at the last visit or death. rwTFI was defined as the interval between the end date of the dosing regimen and the initiation date of second-line (2L) therapy, and rwTTNT was defined as the interval between the index date and 2L initiation. Patients were censored if they continued treatment through the study end date.
Safety outcomes included treatment-related adverse events (TRAEs), immune-related adverse events (irAEs), and clinically significant TRAEs (csTRAEs). TRAEs were defined as adverse events of any grade that were explicitly attributed to the treatment regimen as documented by the physicians in patients' charts. irAEs were defined as a subset of TRAEs that induced inflammatory side effects. csTRAEs were defined as TRAEs that led to dose modification, treatment holds or discontinuation, oral or intravenous steroid use (prednisone, prednisolone, dexamethasone, methylprednisolone), hospitalization, or emergency department (ED) visits.
Statistical Analysis
Descriptive analyses were performed on demographic and clinical characteristics and treatment patterns. Kaplan-Meier methods were used to assess rwPFS and OS along with an unadjusted, univariate analysis of demographics and clinical characteristics associated with rwPFS and OS. Analyses were conducted using SAS v.9.4 (SAS Institute Inc., Cary, NC).
Ethical Approval
The Institutional Review Board and Compliance/Privacy approval was gained before initiation of the retrospective research. Since this project involved the analysis of existing data and records, study information was analyzed in such a manner that research participants could not be directly identified. Patient informed consent was not required because of the nature of the study design. Thus, exemption status and a waiver of informed consent were approved by The US Oncology, Inc Institutional Review Board. Data were handled in compliance with Health Insurance Portability and Accountability Act and the Health Information Technology for Economic and Clinical Health (HITECH) Act.
RESULTS
Patient Characteristics
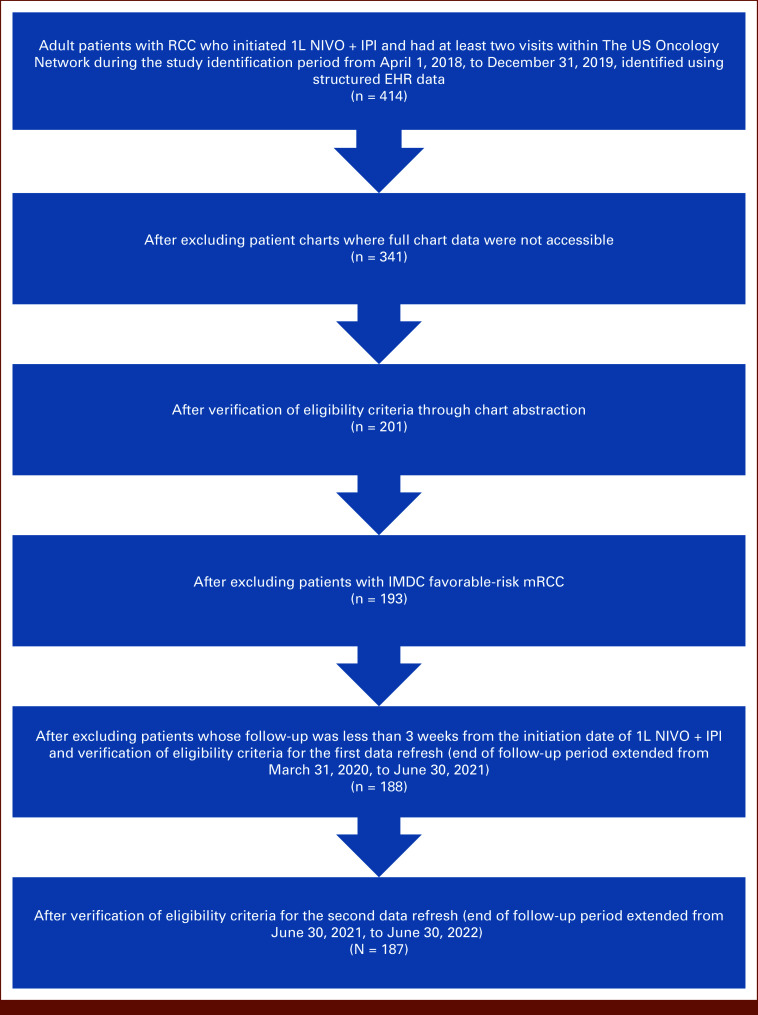
The study included 187 patients with I/P-risk mRCC treated with 1L NIVO + IPI (Fig 1). The median (range) follow-up was 22.4 (0.7-50.2) months. The median (range) age was 63 (30-89) years, and most patients were White (70.1%) and male (74.3%); about half (50.8%) were current or former smokers (Table 1).
FIG 1.
Patient selection and attrition. 1L, first-line; EHR, electronic health record; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IPI, ipilimumab; mRCC, metastatic renal cell carcinoma; NIVO, nivolumab; RCC, renal cell carcinoma.
TABLE 1.
Demographic and Clinical Characteristics of Patients With IMDC I/P-Risk mRCC Receiving 1L NIVO + IPI Combination Therapy
| Characteristica | NIVO + IPI (N = 187) |
|---|---|
| Age at index, years, median (min-max) | 63 (30-89) |
| Age category at index, years, No. (%) | |
| <65 | 100 (53.5) |
| ≥65 | 87 (46.5) |
| Race, No. (%) | |
| White | 131 (70.1) |
| Black/African American | 15 (8.0) |
| Other | 10 (5.3) |
| Not documented | 31 (16.6) |
| Sex, No. (%) | |
| Female | 48 (25.7) |
| Male | 139 (74.3) |
| US practice location, No. (%) | |
| South | 81 (43.3) |
| West | 63 (33.7) |
| Midwest | 39 (20.9) |
| Northeast | 4 (2.1) |
| BMI category, kg/m2, No. (%) | |
| Underweight or normal (<25.0) | 46 (24.5) |
| Overweight (≥25.0 and <30.0) | 62 (33.2) |
| Obese (≥30.0) | 69 (36.9) |
| Not documented | 10 (5.4) |
| Smoking status, No. (%) | |
| Current | 24 (12.8) |
| Former | 71 (38.0) |
| Never | 76 (40.6) |
| Not documented | 16 (8.6) |
| IMDC risk category, No. (%) | |
| Intermediate | 113 (60.4) |
| Poor | 74 (39.6) |
| ECOG PS, No. (%) | |
| 0-1 | 114 (61.0) |
| 2+ | 37 (19.8) |
| Not documented | 36 (19.3) |
| Charlson comorbidity index,b No. (%) | |
| 0 | 116 (62.0) |
| 1-2 | 59 (31.6) |
| 3+ | 12 (6.4) |
| Comorbidities,c No. (%) | |
| Hypertension | 99 (52.9) |
| Diabetes mellitus | 51 (27.3) |
| Chronic obstructive pulmonary disease | 17 (9.1) |
| Renal disease | 15 (8.0) |
| Depression | 10 (5.3) |
| Creatinine clearance, mL/min, No. (%) | |
| <60 | 30 (16.0) |
| ≥60 | 89 (47.6) |
| Not documented | 68 (36.4) |
| PD-L1 testing, No. (%) | |
| Yes | 13 (7.0) |
| Not documented | 174 (93.0) |
| Stage at initial RCC diagnosis,a No. (%) | |
| I | 13 (7.0) |
| II | 17 (9.1) |
| III | 21 (11.2) |
| IV | 125 (66.8) |
| Not documented | 11 (5.9) |
| No. of organs with metastases, No. (%) | |
| 0 or 1 | 101 (54.0) |
| 2 or more | 86 (46.0) |
| Site of metastasis, No. (%) | |
| Lung | 108 (57.8) |
| Bone | 59 (31.6) |
| Lymph node | 57 (30.5) |
| Liver | 33 (17.6) |
| Brain | 12 (6.4) |
| Other | 44 (23.5) |
| Not recorded | 4 (2.1) |
Abbreviations: 1L, first-line; ECOG PS, Eastern Cooperative Oncology Group performance status; I/P, intermediate or poor; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IPI, ipilimumab; mRCC, metastatic renal cell carcinoma; NIVO, nivolumab; RCC, renal cell carcinoma.
Baseline characteristics were captured within 30 d before or after index unless otherwise specified.
The Charlson comorbidity index predicts risk of death within 1 year of hospitalization for patients with specific comorbid conditions.
Comorbid conditions were assessed within 6 months before and including the index date, and a patient can report more than one condition. Comorbidities with a prevalence >5% are reported.
Among the study cohort, 60.4% and 39.6% had an IMDC prognostic risk of intermediate and poor, respectively (Table 1). The majority (61.0%) had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-1. The most common sites of metastasis were lung (57.8% of patients), bone (31.6%), and lymph node (30.5%), and 46.0% had two or more metastatic sites. Metastases were also observed in liver and brain (17.6% and 6.4%, respectively). The most common comorbidities, observed in at least 5% of study patients, included hypertension (52.9%), diabetes mellitus (27.3%), chronic obstructive pulmonary disease (9.1%), renal disease (8.0%), and depression (5.3%).
Clinical Effectiveness
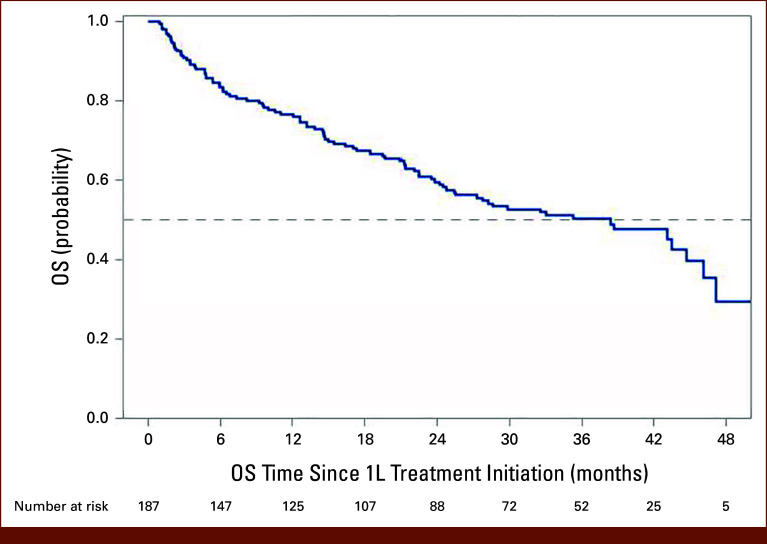
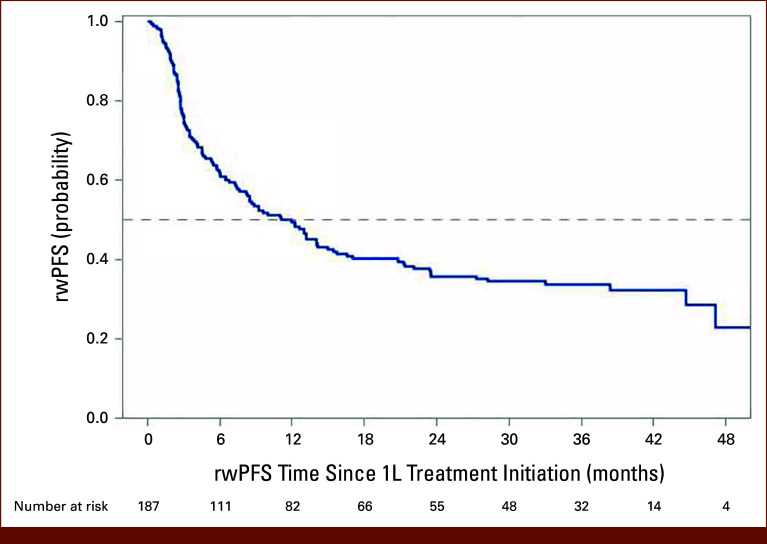
Overall, 89 (47.6%) patients died and 120 (64.2%) patients died or developed progressive disease during the study period (Table 2). The median OS (95% CI) was 38.4 months (24.7 to 46.1 months). The landmark OS (95% CI) was 76.5% (69.5 to 82.0) at 12 months and 59.5% (51.6 to 66.5) at 24 months (Table 2; Fig 2). The median rwPFS (95% CI) was 11.1 (7.5 to 15.0) months. The landmark rwPFS rate at 12 months after initiation of 1L NIVO + IPI was 49.3% (95% CI, 41.8 to 56.4), and that at 24 months after initiation of 1L NIVO + IPI was 35.9% (95% CI, 28.8 to 43.0; Table 2; Fig 3). Statistically significant predictors of OS and rwPFS included ECOG PS 2 or greater (OS: hazard ratio [HR], 1.77 [95% CI, 1.08 to 2.92]; P = .0246; rwPFS: HR, 2.383 [95% CI, 1.54 to 3.69]; P = .0001), stage IV disease at initial diagnosis (OS: HR, 1.786 [95% CI, 1.06 to 3.02]; P = .0305; rwPFS: HR, 1.757 [95% CI, 1.13 to 2.73]; P = .0124), and IMDC poor-risk mRCC (OS: HR, 2.39 [95% CI, 1.57 to 3.64]; P < .0001; rwPFS: HR, 1.74 [95% CI, 1.21 to 2.49]; P = .0026).
TABLE 2.
Landmark OS and rwPFS of Patients With IMDC I/P-Risk mRCC Receiving 1L NIVO + IPI Combination Therapy
| Variable | NIVO + IPI (n = 187) | |
|---|---|---|
| OS | rwPFS | |
| Events, No. (%) | 89 (47.6) | 120 (64.2) |
| Median, months (95% CI) | 38.4 (24.7 to 46.1) | 11.1 (7.5 to 15.0) |
| Q1-Q3 | 12.6-NR | 3.0-47.1 |
| Probability, % (95% CI) | ||
| Month 6 | 83.5 (77.2 to 88.2) | 62.0 (54.6 to 68.6) |
| Month 12 | 76.5 (69.5 to 82.0) | 49.3 (41.8 to 56.4) |
| Month 18 | 67.2 (59.7 to 73.7) | 40.3 (33.0 to 47.5) |
| Month 24 | 59.5 (51.6 to 66.5) | 35.9 (28.8 to 43.0) |
| Month 30 | 52.6 (44.6 to 66.0) | 34.6 (27.5 to 41.7) |
| Month 36 | 50.0 (41.9 to 57.6) | 33.8 (26.7 to 40.9) |
Abbreviations: 1L, first-line; I/P, intermediate or poor; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IPI, ipilimumab; mRCC, metastatic renal cell carcinoma; NIVO, nivolumab; NR, not reached; OS, overall survival; Q, quartile; rwPFS, real-world progression-free survival.
FIG 2.
Real-world OS among patients with IMDC I/P-risk mRCC receiving 1L NIVO + IPI combination therapy. 1L, first-line; I/P, intermediate or poor; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IPI, ipilimumab; mRCC, metastatic renal cell carcinoma; NIVO, nivolumab; OS, overall survival.
FIG 3.
Real-world PFS among patients with IMDC I/P-risk mRCC receiving 1L NIVO + IPI combination therapy. 1L, first-line; I/P, intermediate or poor; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IPI, ipilimumab; mRCC, metastatic renal cell carcinoma; NIVO, nivolumab; PFS, progression-free survival; rw, real-world.
Among patients with a response assessment (n = 133), the rwRR (95% CI) was 42.9% (34.3 to 51.7), which mostly consisted of PR (n = 55, 41.4%). Among patients who experienced a CR or PR (n = 57), the median rwTTR (min-max) was 2.8 months (0.3-4.6).
Safety
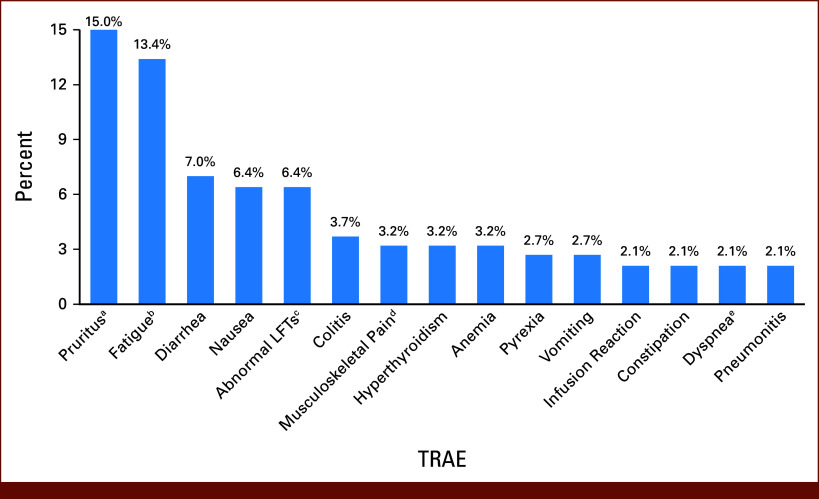
Overall, TRAEs of any grade were reported in 89 (47.6%) patients, among which 42.7% (n = 38) experienced irAEs. The most common TRAEs included fatigue (13.4%), rash (10.2%), diarrhea (7.0%), nausea (6.4%), and colitis (3.7%; Fig 4). Among patients with a TRAE (n = 89), 51.7% experienced a TRAE within 1 month of initiation of 1L NIVO + IPI and 95.5% experienced a TRAE within 3 months of initiation of 1L NIVO + IPI.
FIG 4.
TRAEs occurring in 2% or more of patients with IMDC I/P-risk mRCC receiving 1L NIVO + IPI combination therapy. aIncludes rash and inflamed and irritated nodules along skull. bIncludes tiredness. cIncludes increased ALT and increased AST; dIncludes myalgia, body aches, chest and back discomfort, muscle aches, neck pain, hip pain, and leg pain. eIncludes dyspnea and shortness of breath. 1L, first-line; I/P, intermediate or poor; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IPI, ipilimumab; LFTs, liver function tests; mRCC, metastatic renal cell carcinoma; NIVO, nivolumab; TRAE, treatment-related adverse event.
csTRAEs were reported in 49 (26.2%) patients, among whom outcomes for csTRAEs included steroid use (79.6%), treatment holds (38.8%), treatment discontinuation (36.7%), hospitalization (22.4%), or ED visit (12.2%). Overall, 177 (94.7%) patients discontinued 1L NIVO + IPI. The most common reasons for discontinuation were progressive disease (25.1%), toxicity (21.4%), completed planned treatment (11.8%), hospice (4.8%), and death (4.8%).
Treatment Patterns
The median rwTTD (95% CI) was 5.8 (4.2 to 7.5) months. Most patients (54.0%) did not proceed to 2L therapy during the study period. The median rwTFI (95% CI) was 1.2 (1.0 to 2.0) months, and the probability of being treatment-free at 3, 6, and 12 months after discontinuation was 35.6% (95% CI, 28.2 to 43.0), 27.7% (95% CI, 20.8 to 35.0), and 20.6% (95% CI, 14.3 to 27.7), respectively. The median rwTTNT (95% CI) was 12.0 (8.4 to 13.9) months, and among patients who did initiate 2L during the study period (n = 86), the most common 2L regimens were cabozantinib monotherapy (54.7%), pazopanib (10.5%), and cabozantinib plus NIVO (9.3%).
DISCUSSION
This real-world study reports patient characteristics, treatment utilization, clinical effectiveness, and safety outcomes of patients who received a 1L NIVO + IPI combination therapy for mRCC in a large community oncology network in the United States. Most patients in this study were younger than 65 years, White, male, and overweight or obese and had a good ECOG PS (0-1). With a median follow-up of 22.4 months, the median (95% CI) OS was 38.4 (24.7 to 46.1) months, the median (95% CI) rwPFS was 11.1 (7.5 to 15.0) months, the rwRR (95% CI) was 42.9% (34.3 to 51.7), and 46.0% of patients proceeded to 2L with a median (95% CI) TTNT of 12.0 (8.4 to 13.9) months.
Relative to patients with I/P-risk aRCC who received 1L NIVO + IPI in the CheckMate 214 trial, this real-world study included a greater proportion of patients with ECOG 2+ (24.5% in this RWE study v 0% in CheckMate 214 trial), poor IMDC risk (39.6% in this RWE study v 21.4% in the CheckMate 214 trial), and brain metastasis (6.4% in this RWE study v 0% in the CheckMate 214 trial). Despite these differences in patient characteristics, median rwPFS (11.1 months) and rwRR (43.3%) from this study were consistent with the median PFS (12.4 months) and ORR (42.4%) from the CheckMate 214 trial.12 However, it should be noted that physician-documented assessments of response and progression in this real-world study may not necessarily mirror RECIST criteria used in clinical trials. The median OS of 38.4 months in our study was slightly lower than the median OS observed in the CheckMate 214 trial (47.7 months) although this may be due to the lower average duration of follow-up (median, 99.1 months v our study 22.4 [0.7-50.2] months).12 Given the relative consistency of real-world clinical outcomes with those observed in the CheckMate 214 trial, results from this study support the effectiveness of NIVO + IPI in the real-world community oncology setting.
Other real-world (RW) studies have examined similar patient populations, most of which demonstrated similar effectiveness of 1L NIVO + IPI. Lai et al14 performed a retrospective analysis of patients from the US Collaborative Network who were receiving 1L systemic treatment for mRCC, including a cohort receiving 1L NIVO + IPI, within the period of January 2009 to May 2023. While this study included data from 57 health care organizations across the United States, some factors associated with outcomes such as IMDC risk stratification and reasons for treatment discontinuation were not available. At a follow-up of 19.7 months, Lai et al14 found a median OS of 39.7 months and a 12-month survival rate of 74.2%,14 whereas our median OS and 12-month survival rate was 38.4 months and 76.5%, respectively. The Lai study population was similar to our study with respect to age, sex, race, types of comorbidities, and sites of metastasis.14 Ernst et al13 performed a retrospective analysis of patients from the IMDC database treated with 1L NIVO + IPI for mRCC between 2002 and 2021 across 40 international centers. At a follow-up of 14.3 months, for the patients who survived the entire study period, the 12-month survival rates were 85% and 61% for the IMDC intermediate- and poor-risk cohorts, respectively, and the ORR was 40.6% and 33.0% for the intermediate- and poor-risk cohorts, respectively. These survival outcomes were similar to our study. While age and sex of patients in their study were similar to those in this study, they observed higher rates of lung metastases in the intermediate- and poor-risk cohorts than those found in this study population. Comparison is limited because this study did not perform separate analyses for poor-risk and intermediate-risk patients, and their study had a shorter follow-up time. Zarrabi et al17 performed a retrospective analysis of patients with clear cell mRCC from the Flatiron Health database with a study period from April 2018 through April 2022. The median age in their study was 66 years compared with 63 years in this study, and 64% was White compared with 70% in this study; a similar proportion was male in both studies. The median (range) follow-up time was 20.0 (0.2-47.6 months). Among patients with IMDC I/P-risk mRCC, they found a median OS of 23.3 months, a 24-month survival rate of 48.5%, and a median PFS of 6.4 months. However, comparison with our study is limited because Zarrabi et al17 did not report performance status scores, comorbidities, or adverse event profiles. Collectively, our study in combination with the existing body of RWE provides further support for the clinical effectiveness of NIVO + IPI for I/P mRCC.
As for treatment patterns, the probability of remaining treatment-free 3 months after discontinuation of 1L NIVO + IPI was 35.6% (95% CI, 28.2 to 43.0) in this study, whereas approximately half of patients who experienced a response in the CheckMate 214 trial were reported to be treatment-free.20 In addition, in our study, the median rwTTD was 5.8 months and the median rwTTNT was 12.0 months. Other RW studies have found results approaching ours.13,14 Ernst et al13 observed median treatment durations of 5.7 and 3.7 months and the median TTNT of 14.7 and 8.4 months for the intermediate risk- and poor-risk cohorts, respectively. Lai et al14 found a median time on treatment of 5.4 months and a median TTNT of 16.6 months irrespective of IMDC risk. As for 2L treatments which were initiated in 46.0% of patients in our study, the most common regimens were cabozantinib monotherapy, pazopanib, and cabozantinib plus NIVO. In a retrospective study of the Flatiron database that used the CheckMate 214 trial inclusion criteria, 33.5% of patients who initiated 1L NIVO + IPI initiated 2L therapy, the most common of which included cabozantinib and pazopanib (50.0% and 12.1%, respectively).21 Similar to our study, 44.5% of patients in the study by Lai et al14 received 2L.14 While cabozantinib was the most common 2L regimen in the study by Lai et al14 (57.3%), 10.3% received 2L axitinib.14 However, conclusions regarding our study versus the study by Lai et al14 are limited because Lai et al14 did not report the type of regimen for the remaining third of those who received 2L treatment. Broadly, this study combined with the existing body of RW studies shows a consistent historical trend in the use of subsequent tyrosine kinase inhibitor (TKI) monotherapy. More novel treatments in subsequent lines of therapy including combinations of immuno-oncology and TKI therapies were less commonly observed and might not have been available at the time of the study. This adds confidence that the effectiveness results observed in our study are not biased by novel treatments in subsequent lines of therapy not available at the time of the CheckMate 214 trial.
While 94% of patients experienced a TRAE in the RCT, TRAEs were only documented among 48% of patients in this study, all of which occurred within 6 months of treatment initiation. Because of the nature of TRAEs, documentation may vary among clinical practices. Adverse event rates and severity in RWE studies may differ from RCT,22 and results should be interpreted with caution. Still, overall trends of the most common TRAEs observed in this RW study were consistent with those observed in the CheckMate 214 trial.
This was one of the first studies to describe patient characteristics, treatment patterns, and clinical outcomes among patients with mRCC treated with 1L NIVO + IPI in the community oncology setting, providing further insights into the patterns and tolerability of this regimen outside of RCTs. Despite the shorter follow-up relative to RCTs, this study followed most patients for 18 months, which enhances the current literature and understanding of long-term effectiveness of NIVO + IPI for mRCC in real-world settings. This study evaluated several real-world outcomes including OS, rwPFS, rwTTR, rwTTD, rwTFI, and rwTTNT. In addition, the iKM database captures data on outpatient medical oncology care for patients treated across the United States, providing confidence that results will be generalizable to other community oncology practices that follow evidence-based treatment guidelines.
There are several limitations specific to retrospective, real-world research that should be considered. The iKM system is used for clinical practice purposes and not solely for research purposes. Therefore, certain variables such as response, progression, and adverse events were recorded as documented by physicians in the EHR, which may not be consistent with the definitions used in RCTs. In addition, some variables of interest may not as complete across the entire population (eg, comorbidities), and data entry errors at the point of care cannot be detected or corrected during analysis. While the study periods and eligibility criteria allowed for a sufficient number of patients to describe clinical outcomes in the overall cohort, sample sizes of certain subgroups of interest (eg, high comorbidity burden, brain metastasis) were limited. Although iKM data contain rich, detailed information about the histories of patients with cancer, patients enrolled in this study might have received medical services outside the network, which might not have been captured.
While this study allows for ample follow-up relative to other RWE studies of similar patient populations, additional follow-up may be required to further refine estimates of OS. In addition, because this is a single-arm study, we cannot infer comparative effectiveness of NIVO + IPI relative to other treatments. Furthermore, all patients included in this study initiated 1L NIVO + IPI from April 2018 to December 2019 at which point the best practices and considerations for the treatment of mRCC in use today were not yet established.
In conclusion, this real-world study supports the clinical effectiveness of 1L NIVO + IPI combination therapy for patients with IMDC I/P-risk mRCC in the community oncology setting. Clinical effectiveness outcomes from initiation of 1L NIVO + IPI in this real-world study were consistent with efficacy outcomes reported in the CheckMate 214 trial. These findings also suggest that the 1L NIVO + IPI combination was generally well tolerated in the real-world setting, with low rates of documented TRAEs relative to the CheckMate 214 trial that decreased over time.
PRIOR PRESENTATION
Presented as a poster at the 2023 International Kidney Cancer Symposium: North America, Nashville, TN, November 8-10, 2023.
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/CCI.24.00132.
AUTHOR CONTRIBUTIONS
Conception and design: Andrew J. Osterland, Annette Yim, Sarah B. Guttenplan, Xin Yin, Paul R. Conkling
Financial support: Lisa Rosenblatt
Administrative support: Annette Yim, Lisa Rosenblatt, Paul R. Conkling
Provision of study materials or patients: Gurjyot K. Doshi, Ping Shi, Annette Yim
Collection and assembly of data: Andrew J. Osterland, Paul R. Conkling
Data analysis and interpretation: Gurjyot K. Doshi, Andrew J. Osterland, Ping Shi, Viviana Del Tejo, Sarah B. Guttenplan, Samantha Eiffert, Xin Yin, Lisa Rosenblatt, Paul R. Conkling
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Andrew J. Osterland
Employment: McKesson
Viviana Del Tejo
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Sarah B. Guttenplan
Employment: Bristol Myers Squibb/Medarex
Stock and Other Ownership Interests: Bristol Myers Squibb/Medarex
Samantha Eiffert
Employment: Bristol Myers Squibb, IntegraConnect
Xin Yin
Employment: Bristol Myers Squibb, Johnson & Johnson/Janssen
Lisa Rosenblatt
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb, Merck (I), Amgen Astellas BioPharma (I)
Travel, Accommodations, Expenses: Bristol Myers Squibb
Paul R. Conkling
Employment: Virginia Oncology Associates, Ontada/McKesson
Research Funding: Bristol Myers Squibb (Inst), EMD Serono (Inst), Janssen (Inst), Bayer (Inst), Daiichi Sankyo/Lilly
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Cancer Institute : Surveillance, Epidemiology, and End Results Program: Cancer Stat Facts, Kidney and Renal Pelvis, 2023. https://seer.cancer.gov/statfacts/html/kidrp.html
- 2.Wong MCS, Goggins WB, Yip BHK, et al. : Incidence and mortality of kidney cancer: Temporal patterns and global trends in 39 countries. Sci Rep 7:15698, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow WH, Dong LM, Devesa SS: Epidemiology and risk factors for kidney cancer. Nat Rev Urol 7:245-257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heng DY, Xie W, Regan MM, et al. : External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol 14:141-148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choueiri TK, Powles T, Burotto M, et al. : Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384:829-841, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer R, Alekseev B, Rha SY, et al. : Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384:1289-1300, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Tannir NM, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rini BI, Plimack ER, Stus V, et al. : Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116-1127, 2019 [DOI] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration : FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma. 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-ipilimumab-combination-intermediate-or-poor-risk-advanced-renal-cell
- 10.US Food and Drug Administration : FDA approves pembrolizumab plus axitinib for advanced renal cell carcinoma. 2019. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-plus-axitinib-advanced-renal-cell-carcinoma
- 11.US Food and Drug Administration : FDA approves avelumab plus axitinib for renal cell carcinoma. 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-avelumab-plus-axitinib-renal-cell-carcinoma
- 12.Tannir NM, Escudier B, McDermott DF, et al. : Nivolumab plus ipilimumab (NIVO+IPI) vs sunitinib (SUN) for first-line treatment of advanced renal cell carcinoma (aRCC): Long-term follow-up data from the phase 3 CheckMate 214 trial. J Clin Oncol 42, 2024. (suppl 4; abstr 363) [Google Scholar]
- 13.Ernst MS, Navani V, Wells JC, et al. : Outcomes for International Metastatic Renal Cell Carcinoma Database Consortium Prognostic Groups prognostic groups in contemporary first-line combination therapies for metastatic renal cell carcinoma. Eur Urol 84:109-116, 2023 [DOI] [PubMed] [Google Scholar]
- 14.Lai GS, Li JR, Wang SS, et al. : Real world treatment sequences and outcomes for metastatic renal cell carcinoma. PLoS One 18:e0294039, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah NJ, Sura SD, Shinde R, et al. : Real-world treatment patterns and clinical outcomes for metastatic renal cell carcinoma in the current treatment era. Eur Urol Open Sci 49:110-118, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thana M, Basappa NS, Ghosh S, et al. : Utilization and safety of ipilimumab plus nivolumab in a real-world cohort of metastatic renal cell carcinoma patients. Clin Genitourin Cancer 20:210-218, 2022 [DOI] [PubMed] [Google Scholar]
- 17.Zarrabi KK, Handorf E, Miron B, et al. : Comparative effectiveness of front-line ipilimumab and nivolumab or axitinib and pembrolizumab in metastatic clear cell renal cell carcinoma. Oncologist 28:157-164, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The US Oncology Network: Our Company . https://usoncology.com/our-company/
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, McDermott DF, Escudier B, et al. : Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer 128:2085-2097, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geynisman DM, Faccone J, Zhang Y, et al. : The end of the beginning—Lessons from the first 10 years as an oncologist. J Clin Oncol 39:2516-2517, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Hirsch BR, Harrison MR, George DJ, et al. : Use of “Real-World” data to describe adverse events during the treatment of metastatic renal cell carcinoma in routine clinical practice. Med Oncol 31:156, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/CCI.24.00132.