Abstract
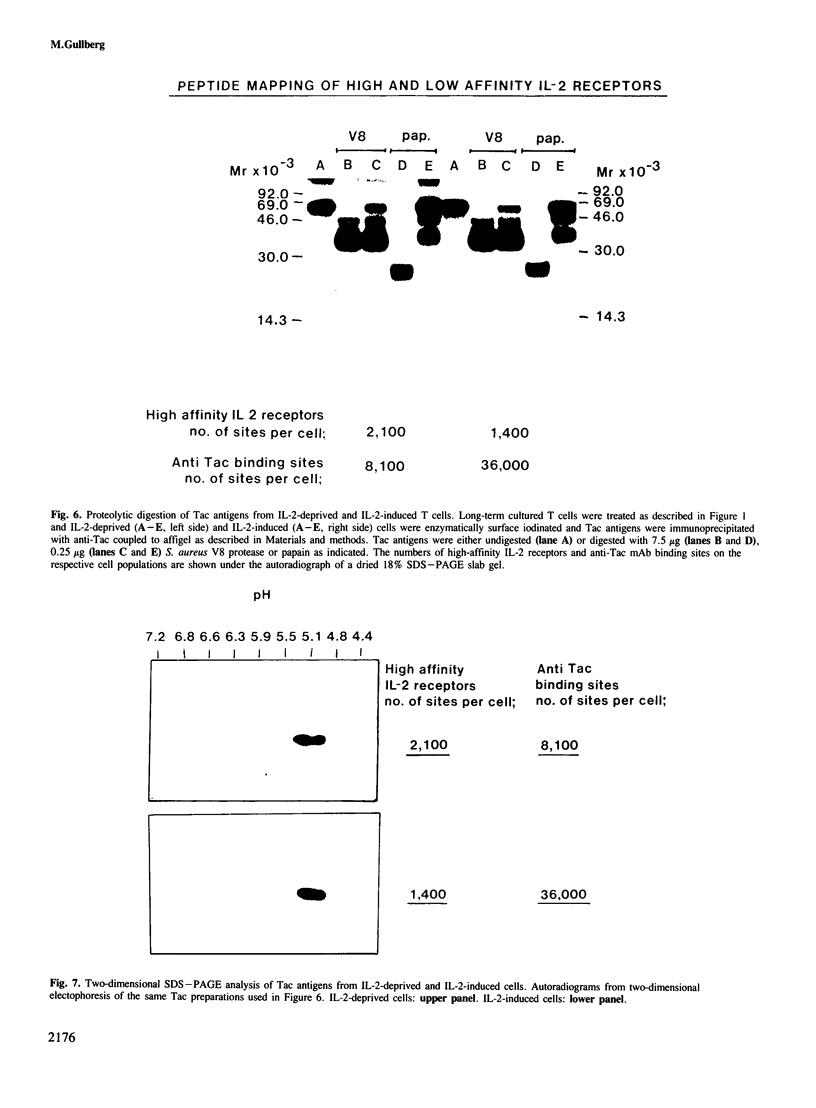
Activated T cells express at least two distinct affinity classes of interleukin-2 (IL-2) receptors. The number of low-affinity receptors per cell is normally 10-30 times greater than that of the high-affinity receptors, and the difference in the dissociation constant between the two classes of receptors is in the order of 1,000-fold. In this report normal human T cells are used in a cellular system in which the number of low-affinity receptors can be manipulated. It is demonstrated that a cell population could be achieved with such low levels of low-affinity IL-2 receptors that almost half of the surface pool of anti-IL-2 receptor antibody (anti-Tac) binding sites represented high-affinity receptors. By using this cellular system it was possible to show that anti-Tac recognizes both receptor classes with similar affinity and that IL-2 inhibits Tac binding to both receptor classes in a competitive fashion. Tac antigens were purified from surface 125I-labeled cells expressing high levels of high-affinity IL-2 receptors, but low levels of the low-affinity receptor class, and this preparation was compared with another pool of Tac antigens obtained from cells expressing the normal 10- to 20-fold excess of low-affinity IL-2 binding sites over high-affinity IL-2 receptors. Biochemical characterization by peptide mapping by limited proteolysis and two-dimensional gel analysis revealed that these distinct preparations of Tac antigens were indistinguishable.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buxser S. E., Kelleher D. J., Watson L., Puma P., Johnson G. L. Change in state of nerve growth factor receptor. Modulation of receptor affinity by wheat germ agglutinin. J Biol Chem. 1983 Mar 25;258(6):3741–3749. [PubMed] [Google Scholar]
- Buxser S. E., Watson L., Johnson G. L. A comparison of binding properties and structure of NGF receptor on PC12 pheochromocytoma and A875 melanoma cells. J Cell Biochem. 1983;22(4):219–233. doi: 10.1002/jcb.240220404. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Drogula C., Krönke M., Waldmann T. A., Greene W. C. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Greene W. C., Robb R. J., Svetlik P. B., Rusk C. M., Depper J. M., Leonard W. J. Stable expression of cDNA encoding the human interleukin 2 receptor in eukaryotic cells. J Exp Med. 1985 Jul 1;162(1):363–368. doi: 10.1084/jem.162.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob P. M., Bothwell M. A. Modification of nerve growth factor receptor properties by wheat germ agglutinin. J Biol Chem. 1983 Dec 10;258(23):14136–14143. [PubMed] [Google Scholar]
- Hatakeyama M., Minamoto S., Uchiyama T., Hardy R. R., Yamada G., Taniguchi T. Reconstitution of functional receptor for human interleukin-2 in mouse cells. Nature. 1985 Dec 5;318(6045):467–470. doi: 10.1038/318467a0. [DOI] [PubMed] [Google Scholar]
- Kondo S., Shimizu A., Maeda M., Tagaya Y., Yodoi J., Honjo T. Expression of functional human interleukin-2 receptor in mouse T cells by cDNA transfection. Nature. 1986 Mar 6;320(6057):75–77. doi: 10.1038/320075a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Robb R. J., Waldmann T. A., Greene W. C. Characterization of the human receptor for T-cell growth factor. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6957–6961. doi: 10.1073/pnas.80.22.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Corthésy P., Tougne C., Lees R., MacDonald H. R., Nabholz M. High and low affinity IL 2 receptors: analysis by IL 2 dissociation rate and reactivity with monoclonal anti-receptor antibody PC61. J Immunol. 1985 Dec;135(6):3988–3994. [PubMed] [Google Scholar]
- Mason J. C., Tager H. S. Identification of distinct receptor complexes that account for high-and low-affinity glucagon binding to hepatic plasma membranes. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6835–6839. doi: 10.1073/pnas.82.20.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- Reeves W. G., Zamkoff K. W., Poiesz B. J., Paolozzi F. P., Tomar R. H., Moore J. L., Ruscetti F. W. T cell growth factor required for optimal induction of T cell growth factor receptor expression in phytohemagglutinin-stimulated T cells. J Biol Response Mod. 1985 Feb;4(1):83–95. [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Favata M. F., Oroszlan S. Production and characterization of monoclonal antibodies to human interleukin 2: strategy and tactics. J Immunol. 1983 Oct;131(4):1808–1815. [PubMed] [Google Scholar]
- Wano Y., Uchiyama T., Fukui K., Maeda M., Uchino H., Yodoi J. Characterization of human interleukin 2 receptor (Tac antigen) in normal and leukemic T cells: co-expression of normal and aberrant receptors on Hut-102 cells. J Immunol. 1984 Jun;132(6):3005–3010. [PubMed] [Google Scholar]
- Welte K., Andreeff M., Platzer E., Holloway K., Rubin B. Y., Moore M. A., Mertelsmann R. Interleukin 2 regulates the expression of Tac antigen on peripheral blood T lymphocytes. J Exp Med. 1984 Nov 1;160(5):1390–1403. doi: 10.1084/jem.160.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]




