Abstract
INTRODUCTION:
United States Multi-Society Task Force colonoscopy surveillance intervals are based solely on adenoma characteristics, without accounting for other risk factors. We investigated whether a risk model including demographic, environmental, and genetic risk factors could individualize surveillance intervals under an “equal management of equal risks” framework.
METHODS:
Using 14,069 individuals from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial who had a diagnostic colonoscopy following an abnormal flexible sigmoidoscopy, we modeled the risk of colorectal cancer, considering the diagnostic colonoscopy finding, baseline risk factors (e.g., age and sex), 19 lifestyle and environmental risk factors, and a polygenic risk score for colorectal cancer. Ten-year absolute cancer risks for each diagnostic colonoscopy finding (advanced adenomas [N = 2,446], ≥3 non-advanced adenomas [N = 483], 1–2 non-advanced adenomas [N = 4,400], and no adenoma [N = 7,183]) were used as implicit risk thresholds for recommended surveillance intervals.
RESULTS:
The area under the curve for the model including colonoscopy findings, baseline characteristics, and polygenic risk score was 0.658. Applying the equal management of equal risks framework, 28.2% of individuals with no adenoma and 42.7% of those with 1–2 non-advanced adenomas would be considered high risk and assigned a significantly shorter surveillance interval than currently recommended. Among individuals who developed cancer within 10 years, 52.4% with no adenoma and 48.3% with 1–2 non-advanced adenomas would have been considered high risk and assigned a shorter surveillance interval.
DISCUSSION:
Using a personalized risk-based model has the potential to identify individuals with no adenoma or 1–2 non-advanced adenomas, who are higher risk and may benefit from shorter surveillance intervals.
KEYWORDS: colorectal cancer, PLCO, surveillance, adenoma, guidelines
INTRODUCTION
Colorectal cancer (CRC) screening in asymptomatic individuals reduces CRC incidence and mortality through the identification and removal of precancerous adenomatous polyps and through early detection of cancer, leading to better prognosis (1,2). Current guidelines recommend starting screening between ages 45–50 years (3–5). For surveillance following detection of an adenoma, the 2020 United States Multi-Society Task Force (USMSTF) (6) recommend individuals with an adenoma ≥ 10 mm in diameter, tubulovillous or villous histology, or high-grade dysplasia (advanced adenoma) undergo repeat colonoscopy at 3 years, whereas individuals with tubular adenomas < 10 mm in diameter (non-advanced adenoma) are recommended to be re-examined in 3–10 years, depending on the number of adenomas. However, adherence to these guidelines is poor (7), in part due to the presence of other suspected risk factors (e.g., family history), the fear of missed polyps or interval cancers, and uncertainty in the strength of evidence for the recommendations.
The inclusion of other risk factors, including genetic and environmental factors, into surveillance recommendations could provide a more individualized risk assessment, with the potential to improve outcomes, efficiency, and cost-effectiveness. Known lifestyle, environmental, and genetic risk factors for CRC have been combined into models for cancer risk (8–11), but it is unclear how to apply these risk models to recommend surveillance intervals following CRC screening. To be consistent with existing practice, one approach is to utilize implicit risk thresholds to inform surveillance intervals; this approach has been successfully implemented for cervical cancer screening (12,13). The 10-year absolute CRC risk for colonoscopy findings (i.e., advanced adenoma, ≥ 3 non-advanced adenomas, 1–2 non-advanced adenomas, and no adenoma) can serve as implicit risk thresholds for recommended surveillance intervals. Using the risk model, one can calculate each person's CRC risk and assign them to an interval consistent with their risk. This allows equal management of equal risks (14), such that the surveillance interval assigned based on the risk computed by a risk model that includes environmental and lifestyle and/or genetic factors is consistent with the surveillance interval that would be assigned to someone with the same estimated risk from adenoma characteristics alone.
We therefore aimed to determine whether lifestyle and environmental and/or genetic risk factors predicted future CRC risk among individuals with an abnormal flexible sigmoidoscopy screen who completed a diagnostic colonoscopy examination. We used a previously validated lifestyle and environmental score (e-score) based on known CRC risk factors (11) and a polygenic risk score (PRS) derived from genome-wide association studies to predict risk. Our goal was to evaluate the potential utility of using these environmental and genetic risk scores to inform surveillance intervals.
METHODS
This study included individuals from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial (15,16), which enrolled approximately 155,000 individuals aged 55–74 years from 10 centers in the United States between 1993 and 2001. The National Cancer Institute and each center's Institutional Review Board approved the protocol, and all study participants provided written informed consent. Information on demographic, lifestyle, environmental, and dietary risk factors was collected at baseline using questionnaires. Individuals randomized to the intervention arm (N = 77,465) were offered a flexible sigmoidoscopy at baseline and at either 3 years (before April 1995) or 5 years after randomization. Individuals with an abnormal flexible sigmoidoscopy were referred to their physician for follow-up, and their medical records were abstracted for subsequent diagnostic workup. Individuals with an inadequate flexible sigmoidoscopy (<50-cm depth of insertion or visual inspection limited to < 90% of the mucosal surface due to inadequate bowel preparation, with no detection of a polyp or mass) were invited for a repeat flexible sigmoidoscopy (17); approximately 8.1% of PLCO participants had an inadequate screen (18). This study was limited to participants with an abnormal flexible sigmoidoscopy, defined as a screen with visible or palpable evidence of mucosal abnormality, rectal nodules, rectal or colon masses, and rectal or colon polyp(s). Individuals with screen-detected abnormalities were referred to their physician for follow-up (with colonoscopy referral optional for diverticulosis and other noncancer-related abnormalities); their medical records were abstracted for subsequent diagnostic workup (15,17). In total, 15,512 participants in the intervention arm completed a baseline questionnaire, had an abnormal flexible sigmoidoscopy at either the baseline or follow-up screen, and underwent a follow-up diagnostic colonoscopy. Adenomas were classified as non-advanced (tubular adenomas < 10 mm in size) or advanced (tubulovillous or villous histology, high-grade dysplasia, or ≥ 10 mm in size). Participants were classified according to their worst finding on the colonoscopy.
Follow-up
Cancer incidence was ascertained from annual study questionnaires and confirmed by medical record abstraction for cancer diagnoses through 2009, after which information on cancer incidence was collected via passive linkage to cancer registries through December 31, 2016 (19). Mortality was assessed through linkage to the National Death Index through December 31, 2018.
Genotyping and PRS
As described previously (20), PLCO participants were genotyped on a high density single nucleotide polymorphism (SNP) array and imputed to the TOPMed reference panel. Because of limited statistical power to evaluate risk in other ancestries, this analysis was restricted to individuals of European ancestry with genotyping available (N = 14,313).
A genome-wide PRS for CRC was generated using GCTB SBayesR (v2.03beta) (21,22) and genome-wide association study (GWAS) summary statistics from the GECCO Consortium (23), excluding individuals from PLCO to generate unbiased weights. This PRS, which included 455,995 SNPs, was applied to the PLCO cohort. As a sensitivity analysis, we additionally considered a PRS based only on published loci (23) (including 194 of 205 reported SNPs). PRS quartiles were based on the distribution in the full cohort.
Lifestyle and environmental risk score
Using data collected from baseline risk factor and dietary questionnaires, a lifestyle and environmental risk score (e-score) was calculated for each individual based on the model developed by Jeon et al (11). The Supplement contains details on the variables included and how they were combined to form the e-score (Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/B219). Sex-specific quartile cut points for the e-score were generated using the distribution of the entire cohort.
Statistical methodology
Kaplan-Meier survival analyses for CRC risk were performed to examine crude associations with screening colonoscopy result, PRS, and e-score. Cox proportional hazard models were used to model CRC risk, adjusting for age, sex, previous self-reported CRC screening in the past 3 years, family history of CRC, and genotyping platform (baseline characteristics). Individuals were censored at the earliest of CRC diagnosis, death, or end of follow-up. Five models were developed for CRC risk based on (i) colonoscopy findings, (ii) colonoscopy findings + baseline characteristics, (iii) colonoscopy findings + baseline characteristics + e-score, (iv) colonoscopy findings + baseline characteristics + PRS, and (v) colonoscopy findings + baseline characteristics + e-score + PRS. The area under the curve (AUC) was calculated for each model to assess the models' ability to discriminate between individuals who did and did not develop CRC within 10 years. A two-sided P value < 0.05 was considered statistically significant.
To inform whether a different surveillance interval would be assigned based on the modeled risk compared with their colonoscopy result, we first determined each participant's recommended surveillance interval based on their colonoscopy results under the USMSTF recommendations. Because of small sample size, participants with ≥ 3 non-advanced adenomas were combined. We used Kaplan-Meier models to estimate the 10-year CRC risk for individuals with each colonoscopy finding (i.e., no adenoma, 1–2 non-advanced adenomas, ≥ 3 non-advanced adenomas, or advanced adenoma). These 10-year risks were then used as thresholds to assign surveillance intervals to each individual based on the USMSTF recommendations (Supplementary Table 2, Supplementary Digital Content 1, http://links.lww.com/CTG/B219). Individuals whose modeled risk was below the estimated risk of individuals with no adenoma (very low risk) were assigned a 10-year surveillance interval; individuals with a risk between the risk of individuals with no adenoma and individuals with 1–2 non-advanced adenomas (low risk) were assigned a 7-10-year surveillance interval; individuals with a risk between the risk of individuals with 1–2 non-advanced adenomas and ≥ 3 non-advanced adenomas (moderate risk) were assigned a 3-7-year surveillance interval; individuals with a risk between the risk of individuals with ≥ 3 non-advanced adenomas and an advanced adenoma (high risk) were assigned a 3-yearly interval, and individuals with a risk above individuals with an advanced adenoma (very high risk) were assigned a 1-3-year surveillance interval. The proportion of individuals assigned a different surveillance interval was calculated for all individuals in the study and for individuals who developed CRC within 10 years.
Levels of missing data were low (< 7%), so median and mean imputation was performed for categorical and continuous variables, respectively (see Supplement for more details, including levels of missing data for each variable, Supplementary Table 3, Supplementary Digital Content 1, http://links.lww.com/CTG/B219). A complete case analysis was performed as a sensitivity analysis.
RESULTS
Of the 14,313 individuals with an abnormal flexible sigmoidoscopy result who attended colonoscopy, 244 were excluded because they were diagnosed with CRC within 60 days of their sigmoidoscopy (N = 28) or had > 12 months between their sigmoidoscopy and diagnostic colonoscopy (N = 216), leaving 14,069 individuals for analysis (Figure 1, Table 1). Approximately 17% (N = 2,446) had an advanced adenoma detected on their colonoscopy, 32% (N = 4,440) had a non-advanced adenoma (N = 3,957 [28%] with 1–2 adenomas and N = 483 [3%] with ≥ 3 adenomas), and 51% had no adenoma (N = 7,183).
Figure 1.
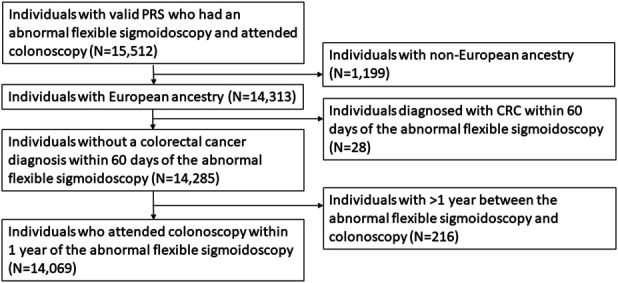
Consolidated Standards of Reporting Trials flowchart for inclusion in the study population. CRC, colorectal cancer; PLCO, Prostate, Lung, Colorectal, and Ovarian; PRS, polygenic risk score.
Table 1.
Descriptive characteristics of the 14,069 individuals from the PLCO Cancer Screening Trial, who had a diagnostic colonoscopy and met the inclusion criteria for this study
| Advanced adenoma | 3+ non-advanced adenoma | 1–2 non-advanced adenoma | No adenoma | Total | ||||||
| N | % | N | % | N | % | N | % | N | % | |
| Total | 2,446 | 17 | 483 | 3 | 3,957 | 28 | 7,183 | 51 | 14,069 | 100 |
| Sex | ||||||||||
| Male | 1,652 | 68 | 360 | 75 | 2,514 | 64 | 3,927 | 55 | 8,453 | 60 |
| Female | 794 | 32 | 123 | 25 | 1,443 | 36 | 3,256 | 45 | 5,616 | 40 |
| Age at colonoscopy (yr) | ||||||||||
| 55–59 | 518 | 21 | 81 | 17 | 791 | 20 | 1,410 | 20 | 2,800 | 20 |
| 60–64 | 796 | 33 | 191 | 40 | 1,373 | 35 | 2,602 | 36 | 4,962 | 35 |
| 65–69 | 668 | 27 | 128 | 27 | 1,069 | 27 | 1,918 | 27 | 3,783 | 27 |
| 70–74 | 396 | 16 | 70 | 14 | 591 | 15 | 1,016 | 14 | 2073 | 15 |
| ≥75 | 68 | 3 | 13 | 3 | 133 | 3 | 237 | 3 | 451 | 3 |
| Family history | ||||||||||
| Yes | 310 | 13 | 59 | 12 | 438 | 11 | 785 | 11 | 1,592 | 11 |
| No | 2,059 | 84 | 406 | 84 | 3,376 | 85 | 6,146 | 86 | 11,987 | 85 |
| Unknown | 77 | 3 | 18 | 4 | 143 | 4 | 252 | 4 | 490 | 3 |
| Smoking status | ||||||||||
| Never | 903 | 37 | 163 | 34 | 1,575 | 40 | 2,799 | 39 | 5,440 | 39 |
| Current | 366 | 15 | 77 | 16 | 548 | 14 | 1,015 | 14 | 2,006 | 14 |
| Former | 1,177 | 48 | 243 | 50 | 1,834 | 46 | 3,369 | 47 | 6,623 | 47 |
| BMI, median (IQR) | 27.2 (24.6, 30.3) | 27.3 (24.7, 30.3) | 27.6 (25.1, 30.7) | 27.1 (24.4, 30.1) | 27.1 (24.6, 30.2) | |||||
| Aspirin and/or ibuprofen use in past 12 mo | ||||||||||
| Yes | 1,376 | 56 | 283 | 59 | 2,376 | 60 | 4,354 | 61 | 8,389 | 60 |
| No | 1,070 | 44 | 200 | 41 | 1,581 | 40 | 2,829 | 39 | 5,680 | 40 |
| Prior self-reported endoscopya | ||||||||||
| Yes | 746 | 30 | 184 | 38 | 1,695 | 43 | 3,447 | 48 | 6,072 | 43 |
| No | 1,700 | 70 | 299 | 62 | 2,262 | 57 | 3,736 | 52 | 7,997 | 57 |
| First positive FSG | ||||||||||
| Baseline | 1,814 | 74 | 338 | 70 | 2,509 | 63 | 4,171 | 58 | 8,832 | 63 |
| Year 3 | 128 | 5 | 25 | 5 | 267 | 7 | 468 | 7 | 888 | 6 |
| Year 5 | 504 | 21 | 120 | 25 | 1,181 | 30 | 2,544 | 35 | 4,349 | 31 |
| e-score | ||||||||||
| Quartile 1 (lowest) | 556 | 23 | 117 | 24 | 967 | 24 | 1,877 | 26 | 3,517 | 25 |
| Quartile 2 | 586 | 24 | 106 | 22 | 1,034 | 26 | 1,791 | 25 | 3,517 | 25 |
| Quartile 3 | 627 | 26 | 121 | 25 | 990 | 25 | 1,779 | 25 | 3,517 | 25 |
| Quartile 4 (highest) | 677 | 28 | 139 | 29 | 966 | 24 | 1,736 | 24 | 3,518 | 25 |
| PRS | ||||||||||
| Quartile 1 (lowest) | 454 | 19 | 99 | 20 | 969 | 24 | 1,972 | 27 | 3,494 | 25 |
| Quartile 2 | 509 | 21 | 124 | 26 | 1,060 | 27 | 1,932 | 27 | 3,625 | 26 |
| Quartile 3 | 590 | 24 | 149 | 31 | 1,110 | 28 | 1,959 | 27 | 3,808 | 27 |
| Quartile 4 (highest) | 893 | 37 | 111 | 23 | 818 | 21 | 1,320 | 18 | 3,142 | 22 |
| Length of follow-up, median (IQR) (yr) | 15.9 (12.1–19.0) | 15.5 (12.2–18.7) | 15.4 (12.5–18.9) | 15.1 (12.2–18.5) | 15.3 (12.2–18.7) | |||||
BMI, body mass index; e-score, environmental risk score; FSG, flexible sigmoidoscopy; IQR, interquartile range; PLCO, Prostate, Lung, Colorectal, and Ovarian; PRS, polygenic risk score.
Individuals whose first positive flexible sigmoidoscopy was at year 3 or 5 and attended the baseline screen in PLCO were considered to have a prior endoscopy.
The median follow-up for CRC incidence after colonoscopy was 15.3 years (IQR: 12.2–18.7 years). A total of 116 individuals (1.6%) were diagnosed with CRC within 10 years, with 40% (N = 46) diagnosed within 5 years of their initial diagnostic colonoscopy following an abnormal flexible sigmoidoscopy. Among those who developed CRC within 10 years, 35% (N = 41) had an advanced adenoma, 28% (N = 33) had a non-advanced adenoma (including 25% [N = 29] with 1–2 non-advanced adenomas), and 36% (N = 42) had no adenoma on their initial colonoscopy.
CRC risk varied by diagnostic colonoscopy finding (log-rank test P < 0.001), with a higher risk observed for individuals with advanced adenomas (Figure 2a); individuals with a higher PRS were also at increased risk (P = 0.002, Figure 2b); however, no significant difference in CRC risk was observed by e-score quartile (P = 0.7, Figure 2c).
Figure 2.
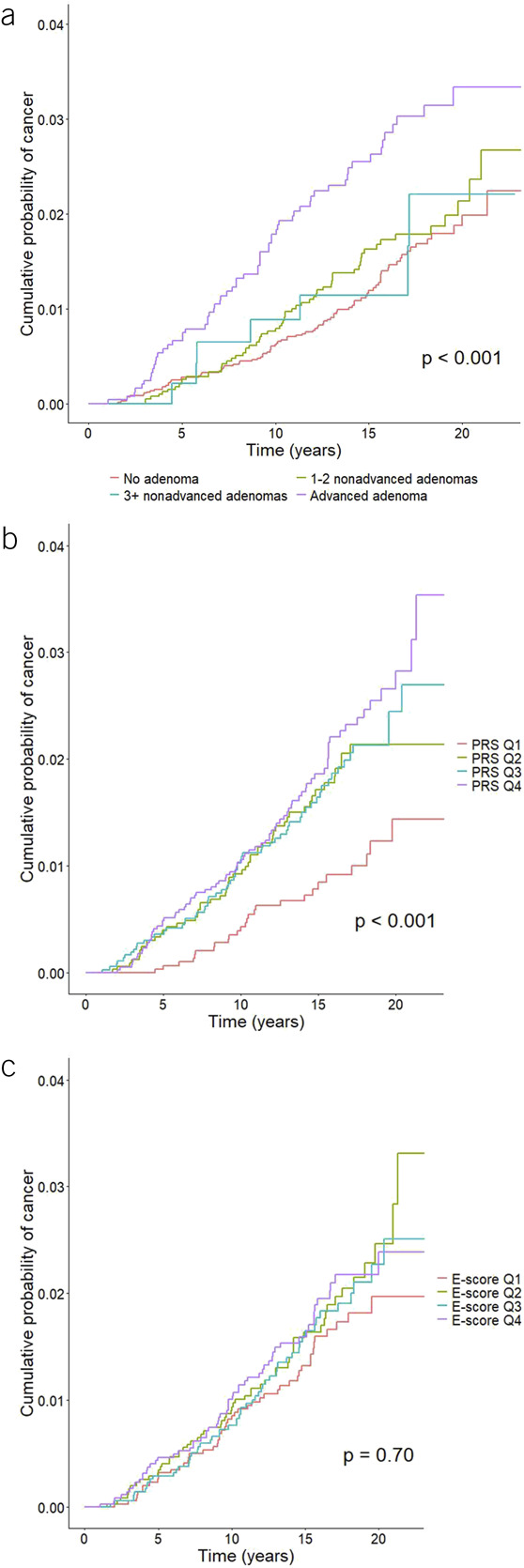
Cumulative probability of colorectal cancer by (a) colonoscopy result, (b) PRS quintile, and (c) lifestyle and environmental risk score (e-score) quartile. PRS, polygenic risk score.
In multivariate models including baseline characteristics and baseline colonoscopy result, there was no significant association between e-score and risk of CRC; however, higher PRS was associated with an increased risk of CRC (HRper quartile increase = 1.22, 95% CI: 1.08–1.38, Table 2), resulting in an hazard ratio (HR) of 1.81 for individuals in the highest PRS quartile compared with the lowest quartile. When PRS was considered as a continuous variable, standardized to have a mean of 0 and SD of 1, each SD increase in PRS was associated with an HR of 1.29 (95% CI: 1.11–1.50). The 10-year AUC for the model including only colonoscopy findings was 0.606; adding baseline characteristics increased the AUC to 0.648. The AUC was slightly higher for the models including PRS (0.658). There was no difference between the AUCs of the models including and excluding the e-score (0.649 vs 0.648). Complete case results were very similar, with AUCs approximately 0.02 higher (Supplementary Table 4, Supplementary Digital Content 1, http://links.lww.com/CTG/B219).
Table 2.
Hazard ratios (95% CI) of risk factors associated with colorectal cancer under different modelsa
| Model 1: Colonoscopy result | Model 2: Model 1 + baseline characteristics | Model 3: Model 2 + e-score | Model 4: Model 2 + PRS | Model 5: Model 2 + e-score + PRS | |||||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| PRS quartile | 1.22 | 1.08, 1.38 | 0.002 | 1.22 | 1.08, 1.38 | 0.002 | |||||||||
| e-score quartile | 1.06 | 0.95, 1.20 | 0.308 | 1.06 | 0.94, 1.20 | 0.314 | |||||||||
| Age | 1.06 | 1.03, 1.08 | <0.001 | 1.06 | 1.03, 1.09 | <0.001 | 1.06 | 1.03, 1.09 | <0.001 | 1.06 | 1.03, 1.09 | <0.001 | |||
| Sex | |||||||||||||||
| Male | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||||||
| Female | 0.98 | 0.74, 1.29 | 0.870 | 0.98 | 0.74, 1.29 | 0.859 | 0.97 | 0.73, 1.28 | 0.818 | 0.97 | 0.73, 1.28 | 0.810 | |||
| Prior self-reported endoscopyb | |||||||||||||||
| No | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||||||
| Yes | 0.97 | 0.72, 1.30 | 0.829 | 0.97 | 0.73, 1.31 | 0.858 | 0.98 | 0.73, 1.31 | 0.879 | 0.98 | 0.73, 1.32 | 0.906 | |||
| Family history of colorectal cancer | |||||||||||||||
| No | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||||||
| Yes | 1.17 | 0.79, 1.72 | 0.436 | 1.16 | 0.79, 1.71 | 0.448 | 1.13 | 0.77, 1.66 | 0.547 | 1.12 | 0.76, 1.65 | 0.558 | |||
| Unsure | 0.82 | 0.36, 1.86 | 0.638 | 0.81 | 0.36, 1.84 | 0.621 | 0.81 | 0.36, 1.84 | 0.619 | 0.81 | 0.36, 1.82 | 0.601 | |||
| Diagnostic colonoscopy result | |||||||||||||||
| Advanced adenoma | 2.56 | 1.70, 3.85 | <0.001 | 2.45 | 1.62, 3.69 | <0.001 | 2.43 | 1.61, 3.67 | <0.001 | 2.32 | 1.53, 3.50 | <0.001 | 2.30 | 1.52, 3.48 | <0.001 |
| Adenoma: 3+ | 1.13 | 0.52, 2.44 | 0.752 | 1.12 | 0.52, 2.41 | 0.781 | 1.11 | 0.51, 2.40 | 0.789 | 1.07 | 0.49, 2.31 | 0.872 | 1.06 | 0.49, 2.30 | 0.881 |
| Adenoma: 1-2 | 1.20 | 0.87, 1.66 | 0.272 | 1.19 | 0.86, 1.65 | 0.301 | 1.19 | 0.86, 1.65 | 0.297 | 1.16 | 0.84, 1.61 | 0.368 | 1.16 | 0.84, 1.61 | 0.365 |
| No adenoma | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||||
| AUC at 10 yrc | 0.606 | 0.648 | 0.649 | 0.658 | 0.658 | ||||||||||
AUC, area under the curve; HR, hazard ratio; PLCO, Prostate, Lung, Colorectal, and Ovarian; PRS, polygenic risk score.
All models were additionally adjusted for genotyping platform.
Individuals whose first positive flexible sigmoidoscopy was at year 3 or 5 and attended the baseline screen in PLCO were considered to have a prior endoscopy.
The AUCs at 10 yr exclude genotyping platform.
In our study, the 10-year CRC risk for individuals who had no adenoma, 1–2 non-advanced adenomas, ≥ 3 non-advanced adenomas, and an advanced adenoma were 0.62% (95% CI: 0.43%–0.81%), 0.79% (95% CI: 0.50%–1.08%), 0.89% (95% CI: 0.02%–1.75%), and 1.79% (95% CI: 1.12%–2.23%), respectively. Under an equal management of equal risks framework and using the model with PRS (Model 4), over a quarter (28.2%) of individuals with no adenoma would be considered high or very high risk and assigned a shorter surveillance interval of ≤ 3 years, since their 10-year estimated absolute CRC risk exceeded those with ≥ 3 non-advanced adenomas (i.e., ≥ 0.89%), compared with 10 years under the USMSTF Recommendations (Figure 3, Supplementary Table 5, Figure S1, Supplementary Digital Content 1, http://links.lww.com/CTG/B219). Similarly, 42.7% of individuals with 1–2 non-advanced adenomas would be considered high or very high risk and assigned a shorter surveillance interval of ≤ 3 years, compared with 7–10 years under the USMSTF Recommendations. Over half the individuals with ≥ 3 non-advanced adenomas (51.8%) had a 10-year estimated absolute risk < 0.79% and would therefore be considered low or very low risk and assigned a longer surveillance interval of 7–10 years. One in 8 individuals (12.6%) with an advanced adenoma would be considered low or very low risk (10-year estimated absolute risk < 0.79%) and assigned a longer surveillance interval of 7–10 years. The results were similar for the model with baseline characteristics (Model 2, Supplementary Tables 4 and 5, Supplementary Digital Content 1, http://links.lww.com/CTG/B219), as well as for the complete case analyses (Supplementary Tables 4 and 6, Supplementary Digital Content 1, http://links.lww.com/CTG/B219).
Figure 3.
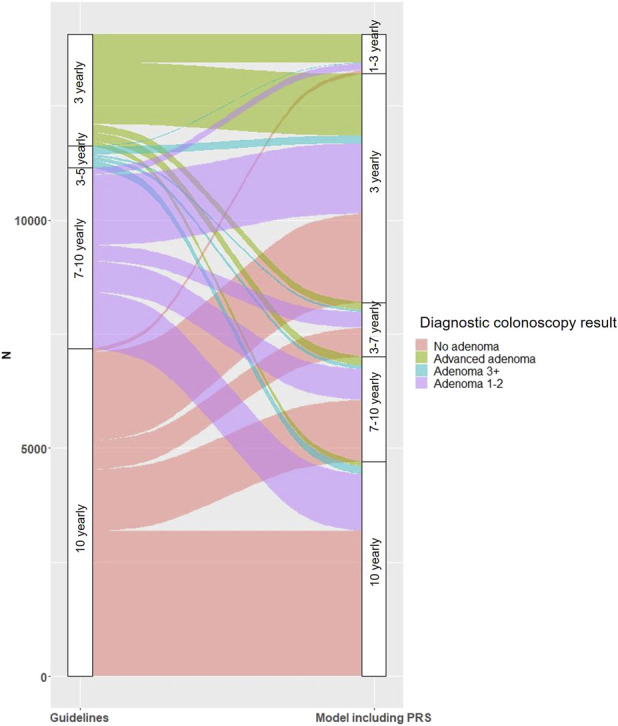
Screening intervals for the full population under the US Multi-Society 2020 Task Force Recommendations on the left and the model containing colonoscopy findings, baseline characteristics, and PRS on the right. PRS, polygenic risk score.
To understand how these changes in surveillance intervals might affect CRC, we restricted our analysis to the 116 individuals who developed CRC within 10 years (Figure S3, Supplementary Digital Content 1, http://links.lww.com/CTG/B219). Overall, 33.6% of these individuals would have been considered higher risk than their colonoscopy result alone suggested (Supplementary Table 7, Supplementary Digital Content 1, http://links.lww.com/CTG/B219) and would therefore have been assigned a shorter surveillance interval under the risk model including PRS (Model 4) as well as the model with only baseline characteristics (Model 2). Approximately 13.8% would have been assigned a longer surveillance interval under the risk model with PRS (Model 4), with slightly fewer (11.2%) for the baseline risk model (Model 2) (Supplementary Table 7, Supplementary Digital Content 1, http://links.lww.com/CTG/B219).
Under the model with PRS (Model 4), 52.4% of CRC cases with no adenoma on colonoscopy and 48.3% of CRC cases with 1–2 non-advanced adenomas would have been considered high or very high risk (10-year estimated absolute risk ≥ 0.89%) and assigned a shorter surveillance interval of ≤ 3 years (Figure S2, Supplementary Table 5, Supplementary Digital Content 1, http://links.lww.com/CTG/B219). In addition, 90.2% of individuals with advanced adenomas who subsequently developed cancer within 10 years would have been considered high or very high risk and assigned a surveillance interval of ≤ 3 years under the model with PRS (Model 4). No individuals with advanced adenomas assigned to 10-year surveillance interval developed CRC within 10 years. Similar results were observed for the model containing only baseline characteristics (Model 2).
Among all individuals in this study (Supplementary Table 7, Supplementary Digital Content 1, http://links.lww.com/CTG/B219), both those who did and did not develop CRC within 10 years, 31.0% would be assigned a shorter surveillance interval when PRS was included in the risk model (Model 4), with similar results for the baseline risk model (Model 2). Using the midpoint of each surveillance interval, the mean time until the first surveillance visit among all individuals in this study would be 6.32 years when PRS was included in the risk model (Model 4) compared with 8.13 years under the USMSTF Recommendations, implying more surveillance colonoscopies would be carried out using risk-based surveillance intervals.
We additionally constructed a model using a PRS based only the published loci (23) (Model 6); results were very similar (Supplementary Table 8, Supplementary Digital Content 1, http://links.lww.com/CTG/B219). Among individuals who developed CRC within 10 years, 34.5% would be assigned a shorter surveillance interval under the model using PRS with published loci (Supplementary Table 7, Supplementary Digital Content 1, http://links.lww.com/CTG/B219), compared with 33.6% for the original genome-wide PRS model (Model 4).
DISCUSSION
Our study explored the potential benefit of including genetic and lifestyle/environmental risk factors as well as other baseline characteristics into a risk assessment model to recommend colorectal surveillance intervals following colonoscopy. PRS was a statistically significant predictor of CRC risk after accounting for colonoscopy result, with a hazard ratio of 1.81 for individuals in the highest quartile compared with the lowest, whereas the lifestyle and environmental risk score was not significant. Although the discrimination was moderate for all models evaluated, including baseline characteristics (e.g., age) and, to a lesser extent, PRS, improved the model's ability to discriminate between individuals who did and did not develop CRC compared with the model only containing colonoscopy findings. Using either the PRS or baseline risk model, a substantial proportion of individuals would be moved to more frequent surveillance because of an estimated increase in risk (e.g., 28.2% of individuals with no adenoma would be assigned a surveillance interval of ≤3 years using the PRS model). A small portion of individuals under the PRS model had substantially lower estimated risk than their colonoscopy result would imply (e.g., 4.4% of individuals with advanced adenomas were classified as very low risk), and a long surveillance interval would be recommended under this model.
Risk-stratified screening and/or surveillance using genetic or lifestyle/environmental factors has the potential to improve efficiency and cost-effectiveness, as well as reduce harms among low-risk individuals and allow limited resource settings to make the best use of their resources (24,25). In this study, if individuals were assigned surveillance intervals based on their estimated cancer risk, many more people would be assigned a shorter surveillance interval, which may not always be feasible; however, different risk thresholds could be used for determining surveillance intervals based on available resources. We used implicit risk thresholds from current surveillance guidelines to demonstrate the principle of risk-based surveillance, but these may not be the optimal thresholds for guiding clinical management. Previous work considered what proportion of colorectal cancers in the PLCO intervention arm which were not screen-detected could have been detected at screening; with a mean follow-up of 11.5 years, they estimated that almost half the cancers could not have been detected at screening, with another 27% considered prevalent but not detected (26).
Current surveillance recommendations in the United States depend only on characteristics of adenomas identified at colonoscopy (6); however, CRC risks have been shown to differ by PRS among individuals with low-risk and high-risk adenomas (27). Although cohort studies have suggested that individuals with low-risk adenomas do not have an increased risk of CRC compared with individuals with no adenoma (28–30), these studies cannot account for surveillance colonoscopy and adenoma removal, which reduces CRC incidence (31,32). Randomized trials of surveillance colonoscopy at 5 and 10 vs 10 years in individuals with 1–2 non-advanced adenomas are underway (33,34) which should help clarify CRC risk in the absence of surveillance. We found that 3.8% of individuals with 1–2 non-advanced adenomas have an estimated 10-year absolute risk that exceeds the average risk for individuals with advanced adenoma, suggesting substantial heterogeneity in risk among those with non-advanced adenoma. We note that family history was not a significant predictor in this model, despite being associated with CRC incidence and mortality in the PLCO in an earlier study (35). This was also true when family history was the only variable in the model (HR = 1.18, 0.80–1.74), which suggests that among individuals with an abnormal flexible sigmoidoscopy, family history is not a strong predictor of cancer risk. There may be benefits of incorporating other risk factors into the risk assessment to identify those at high risk; however, the benefits of shortening surveillance intervals for individuals traditionally considered to be low risk must be balanced against harms, as the majority will not develop cancer.
In practice, any advantage of including PRS in models may be outweighed by the logistical and financial challenges involved with generating genetic scores in the population eligible for CRC surveillance; however, genotyping costs have substantially decreased over the last decade and the same genetic data can be used to predict many common complex diseases and inform preventive interventions, such as statin treatment, diabetes prevention, or screening for other cancers (36). As such, PRS may be broadly used in health care in the next decade.
Our study has some limitations. For most participants, we do not know what screening or polypectomies took place following the trial. These practices can reduce the risk of CRC; previous work showed a 44% reduction in cancer incidence from a single surveillance visit (31). However, our data represent real-life clinical practice in the United States. In addition, individuals who had no adenoma detected on colonoscopy in this study are not necessarily representative of all individuals without adenoma since they all had an abnormal flexible sigmoidoscopy prior to colonoscopy. However, the 10-year CRC risk among PLCO intervention arm participants who had a negative flexible sigmoidoscopy at baseline was 0.81%, comparable with the 0.62% observed among individuals with no adenoma with abnormal sigmoidoscopy in this analysis. Because of small numbers of racial and ethnic minority individuals in the PLCO, we were unable to accurately assess risk in these populations and had to limit our analysis to individuals with European ancestry. As a result, the generalizability of our study to other populations is limited; future studies involving more diverse populations are warranted.
Our study had many strengths. We prospectively followed > 14,000 individuals who underwent colonoscopy for a median of 15.3 years, allowing us to compare the predictive ability of a lifestyle and environmental risk score and PRS together with baseline questionnaire data. The detailed information on adenomas allowed us to accurately categorize individuals according to risk, and the high-quality long-term follow-up for CRC incidence and death allowed us to assess 10-year risks. PLCO participants were healthy individuals recruited across the United States and randomly assigned to screening, making the PLCO more representative of the general population than a clinical-based study. We provided absolute risk estimates, in addition to relative risks, allowing the comparison of surveillance intervals based on adenoma characteristics with those based on risk estimates. Finally, we were able to show, using an equal management of equal risks framework, how an individualized risk assessment using baseline characteristics and PRS may be beneficial in identifying those at high risk for more frequent surveillance.
In conclusion, we have demonstrated that the use of a risk model containing additional information beyond colonoscopy findings has the potential to change surveillance recommendations for a substantial proportion of individuals. Our study is one of the first to demonstrate the potential utility of using baseline characteristics as well as PRS to guide colorectal surveillance intervals after CRC screening. More prospective studies and randomized trials are required before clinical implementation of personalized risk-based surveillance decisions, but this study suggests that the incorporation of individual risk factors may be beneficial.
CONFLICTS OF INTEREST
Guarantor of the article: Sonja I. Berndt, PhD.
Specific author contributions: Conception and design: H.A.K., W.Y.H., N.D.F., U.P., L.H., R.E.S., S.I.B. Acquisition of data: W.Y.H., N.D.F. Statistical analysis: R.L., H.A.K., D.W., M.T., F.Q., J.S. Writing—original draft: R.L. Writing—review and editing: H.A.K., W.Y.H., D.W., M.T., F.Q., N.D.F., E.L., J.S., U.P., L.H., R.E.S., S.I.B. Study supervision: H.A.K., S.I.B.
Financial support: Intramural Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute.
Potential competing interests: R.E.S.: research support from Immunovia, Freenome, Exact Sciences. All other authors declare no conflicts of interest.
Data availability: PLCO genotype data are available through dbGaP (accession number phs001286.v3.p2) and by request via https://cdas.cancer.gov/learn/plco/instructions/?type=data. Summary-level data for the European GWAS is available through the GWAS catalog (accession no. GCST90129505).
Study Highlights.
WHAT IS KNOWN
✓ Current surveillance guidelines for individuals with colorectal adenomas depend only on adenoma characteristics such as number and size.
WHAT IS NEW HERE
✓ Adding age, sex, colonoscopy history, and family history improves colorectal cancer risk prediction beyond colonoscopy findings.
✓ This could be useful for assigning surveillance intervals.
✓ Adding a genetic risk score slightly further improves colorectal cancer risk prediction, whereas the lifestyle and environmental risk score does not contribute additional information.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Patrick Wright and Mike Furr at the Information Management Services, Inc. for their support with this study. PLCO Cancer incidence data have been provided by the Colorado Central Cancer Registry, District of Columbia Cancer Registry, Georgia Cancer Registry, Hawaii Cancer Registry, Cancer Data Registry of Idaho, Minnesota Cancer Surveillance System, Missouri Cancer Registry, Nevada Central Cancer Registry, Pennsylvania Cancer Registry, Texas Cancer Registry, Virginia Cancer Registry, and Wisconsin Cancer Reporting System. All are supported in part by funds from the Center for Disease Control and Prevention, National Program for Central Registries, local states, or by the National Cancer Institute, Surveillance, Epidemiology, and End Results program. The results reported here and the conclusions derived are the sole responsibility of the authors.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/B219
REFERENCES
- 1.Kahi CJ, Imperiale TF, Juliar BE, et al. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol 2009;7(7):770–11. [DOI] [PubMed] [Google Scholar]
- 2.Lauby-Secretan B, Vilahur N, Bianchini F, et al. The IARC perspective on colorectal cancer screening. N Engl J Med 2018;378(18):1734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaukat A, Kahi CJ, Burke CA, et al. ACG clinical guidelines: Colorectal cancer screening 2021. Am J Gastroenterol 2021;116(3):458–79. [DOI] [PubMed] [Google Scholar]
- 4.Wolf AM, Fontham ET, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American cancer Society. CA Cancer J Clin 2018;68(4):250–81. [DOI] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 2021;325(19):1965–77. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc 2020;91(3):463–85.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong J, Wang LF, Ardolino E, et al. Real-world compliance with the 2020 US Multi-Society Task Force on Colorectal Cancer polypectomy surveillance guidelines: An observational study. Gastrointest Endosc 2023;97(2):350–6.e3. [DOI] [PubMed] [Google Scholar]
- 8.Jantzen R, Payette Y, de Malliard T, et al. Five-year absolute risk estimates of colorectal cancer based on CCRAT model and polygenic risk scores: A validation study using the Quebec population-based cohort CARTaGENE. Prev Med Rep 2022;25:101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Liu L, Lu M, et al. Implications of lifestyle factors and polygenic risk score for absolute risk prediction of colorectal neoplasm and risk-adapted screening. Front Mol Biosci 2021;8:685410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGeoch L, Saunders CL, Griffin SJ, et al. Risk prediction models for colorectal cancer incorporating common genetic variants: A systematic review. Cancer Epidemiol Biomarkers Prev 2019;28(10):1580–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon J, Du M, Schoen RE, et al. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental, and genetic factors. Gastroenterology 2018;154(8):2152–64.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Lower Genital Tract Dis 2020;24(2):132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung LC, Egemen D, Chen X, et al. 2019 ASCCP risk-based management consensus guidelines: Methods for risk estimation, recommended management, and validation. J Lower Genital Tract Dis 2020;24(2):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castle PE, Katki HA. Screening: A risk-based framework to decide who benefits from screening. Nat Rev Clin Oncol 2016;13(9):531–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366(25):2345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Controlled Clin Trials 2000;21(6 Suppl):273S–309S. [DOI] [PubMed] [Google Scholar]
- 17.Weissfeld JL, Schoen RE, Pinsky PF, et al. Flexible sigmoidoscopy in the PLCO cancer screening trial: Results from the baseline screening examination of a randomized trial. J Natl Cancer Inst 2005;97(13):989–97. [DOI] [PubMed] [Google Scholar]
- 18.Weissfeld JL, Schoen RE, Pinsky PF, et al. Flexible sigmoidoscopy in the randomized prostate, lung, colorectal, and ovarian (PLCO) cancer screening trial: Added yield from a second screening examination. J Natl Cancer Inst 2012;104(4):280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinsky PF, Miller E, Prorok P, et al. Extended follow-up for prostate cancer incidence and mortality among participants in the Prostate, Lung, Colorectal and Ovarian randomized cancer screening trial. BJU Int 2019;123(5):854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machiela MJ, Huang W-Y, Wong W, et al. GWAS explorer: An open-source tool to explore, visualize, and access GWAS summary statistics in the PLCO Atlas. Scientific Data 2023;10(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Jones LR, Zeng J, Sidorenko J, et al. Improved polygenic prediction by Bayesian multiple regression on summary statistics. Nat Commun 2019;10(1):5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng J. GCTB Accessed July 1, 2023. (https://cnsgenomics.com/software/gctb/#Download).
- 23.Fernandez-Rozadilla C, Timofeeva M, Chen Z, et al. Deciphering colorectal cancer genetics through multi-omic analysis of 100,204 cases and 154,587 controls of European and east Asian ancestries. Nat Genet 2023;55(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naber SK, Kundu S, Kuntz KM, et al. Cost-effectiveness of risk-stratified colorectal cancer screening based on polygenic risk: Current status and future potential. JNCI Cancer Spectr 2020;4(1):pkz086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas C, Mandrik O, Saunders CL, et al. The costs and benefits of risk stratification for colorectal cancer screening based on phenotypic and genetic risk: A health economic analysis. Cancer Prev Res 2021;14(8):811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal cancers not detected by screening flexible sigmoidoscopy in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Gastrointest Endosc 2012;75(3):612–20. [DOI] [PubMed] [Google Scholar]
- 27.Guo F, Edelmann D, Cardoso R, et al. Polygenic risk score for defining personalized surveillance intervals after adenoma detection and removal at colonoscopy. Clin Gastroenterol Hepatol 2023;21(1):210–9.e11. [DOI] [PubMed] [Google Scholar]
- 28.Lee JK, Jensen CD, Levin TR, et al. Long-term risk of colorectal cancer and related death after adenoma removal in a large, community-based population. Gastroenterology 2020;158(4):884–94. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Click B, Pinsky PF, Hickey T, et al. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. Jama 2018;319(19):2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He X, Hang D, Wu K, et al. Long-term risk of colorectal cancer after removal of conventional adenomas and serrated polyps. Gastroenterology 2020;158(4):852–61.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cross AJ, Robbins EC, Pack K, et al. Long-term colorectal cancer incidence after adenoma removal and the effects of surveillance on incidence: A multicentre, retrospective, cohort study. Gut 2020;69(9):1645–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinsky PF, Schoen RE. Contribution of surveillance colonoscopy to colorectal cancer prevention. Clin Gastroenterol Hepatol 2020;18(13):2937–44.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jover R, Bretthauer M, Dekker E, et al. Rationale and design of the European Polyp Surveillance (EPoS) trials. Endoscopy 2016;48(6):571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg DS, Schoen RE. Preneoplastic colorectal polyps:“I found them and removed them—now what?”. Ann Intern Med 2019;171(9):667–8. [DOI] [PubMed] [Google Scholar]
- 35.Schoen RE, Razzak A, Yu KJ, et al. Incidence and mortality of colorectal cancer in individuals with a family history of colorectal cancer. Gastroenterology 2015;149(6):1438–45.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennon NJ, Kottyan LC, Kachulis C, et al. Selection, optimization and validation of ten chronic disease polygenic risk scores for clinical implementation in diverse US populations. Nat Med 2024;30(2):480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


