Abstract
INTRODUCTION:
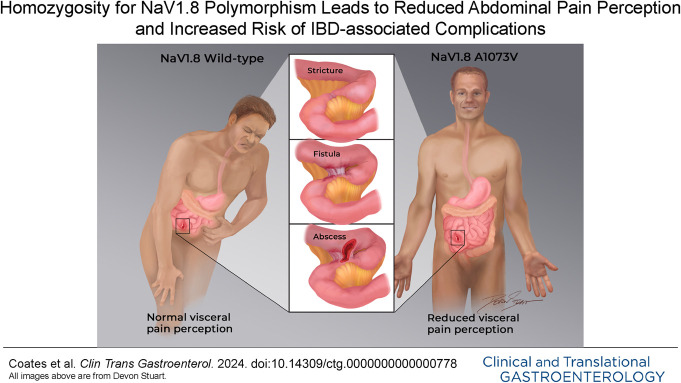
Hypoalgesic inflammatory bowel disease (IBD) may provide critical insights into human abdominal pain. This condition was previously associated with homozygosity for a polymorphism (rs6795970, A1073V; 1073val/val) related to Nav1.8, a voltage-gated sodium channel preferentially expressed on nociceptors. It was unclear whether this relationship existed for both Crohn's disease (CD) and ulcerative colitis (UC). This study evaluated a larger, carefully phenotyped IBD cohort to investigate this question.
METHODS:
Allelic and genotypic frequencies of rs6795970 were compared among study cohorts characterized by concomitant assessment of intestinal inflammatory status and abdominal pain experience. Visceral sensory perception was performed in healthy individuals using rectal balloon distension.
RESULTS:
We analyzed 416 patients with IBD (261CD:155UC) and 142 healthy controls. In the IBD cohort, 84 individuals (43CD:41UC) were determined to have hypoalgesic disease. The allelic frequency of rs6795970 was significantly higher in patients with hypoalgesic IBD when compared with other patients with IBD and healthy controls. Patients with hypoalgesic IBD were also more likely to be homozygous for this polymorphism when compared with other patients with IBD and healthy controls. Hypoalgesic CD (30% vs 12%, P = 0.004) and hypoalgesic UC (32% vs 15%, P = 0.036) were each significantly more likely to be associated with homozygosity for the rs6795970 polymorphism. In a cohort of healthy individuals (n = 50), rs6795970 homozygotes (n = 11) also demonstrated reduced abdominal discomfort to rectal balloon distension.
DISCUSSION:
These findings indicate that Nav1.8 plays a key role in human visceral pain perception, and could serve as a novel diagnostic target in the management of hypoalgesic CD and UC, and potential therapeutic target for conditions associated with chronic abdominal pain.
KEYWORDS: inflammatory bowel disease, voltage-gated sodium channels, NaV1.8, SCN10A, polymorphism
INTRODUCTION
Inflammatory bowel disease (IBD), which includes Crohn's disease (CD) and ulcerative colitis (UC), describes chronic disorders characterized by relapsing and remitting inflammation of the gastrointestinal (GI) tract. IBD affects more than 3 million Americans, most of whom are diagnosed at a relatively early age (1). CD and UC are both life-long conditions that can be treated, but that are currently incurable. Abdominal pain is described in up to 70% of patients at the onset or during exacerbations of the disease (2). It is also one of the primary reasons patients with IBD seek medical attention (3), a key factor influencing their treatment decisions (4), and a major determinant of healthcare cost and resource utilization (5–8).
Importantly, however, the absence of abdominal pain in IBD can also pose significant challenges. Individuals with hypoalgesic IBD (also sometimes known as “silent” IBD) have grossly evident intestinal inflammatory changes that do not result in painful or clinically significant noxious sensations. Various estimates have suggested that a third or more of IBD patients with active disease will be asymptomatic or at least hypoalgesic (9–11). Hypoalgesic IBD is important because this patient population is less likely to seek appropriate medical attention (on a timely basis or ever) and more likely to develop complications (e.g., strictures and fistulae) and ultimately incur major healthcare costs, including hospitalization (10–12).
We previously described a voltage-gated sodium channel (VGSC), Nav1.8, that appears to play an important role in hypoalgesic IBD (12). Specifically, in a cohort carefully characterized using concomitant pain survey data and endoscopically determined disease activity, we revealed that patients with hypoalgesic IBD were significantly more likely to demonstrate homozygosity for the Nav1.8 gene (SCN10A) single nucleotide polymorphism (SNP), rs6795970, compared with other patients with IBD (12). In humans, this SNP produces a nonsynonymous amino acid change (alanine at amino acid 1073 to valine in SCN10A, A1073V) within an intracellular loop of the Nav1.8 channel. This was an intriguing finding, as Nav1.8 is preferentially expressed on sensory neurons associated with pain perception (13). Separate studies have also revealed that homozygosity for the rs6795970 SNP (1073val/val; homozygous minor allele) is associated with somatosensory hypoalgesia (14) and reduced abdominal pain scores in postsigmoidectomy patients (15). In addition, there is evidence that the variant channel is functionally altered (14,15). Thus, there is growing biological plausibility that this Nav1.8 gene variant could play an important role in hypoalgesic IBD and other conditions associated with altered pain perception.
It remains unclear, however, whether this SNP is relevant to both hypoalgesic CD and hypoalgesic UC. These conditions represent pathophysiologically distinct sets of diseases, and the causes of abdominal pain may vary tremendously among patients. Questions also remain from previous studies regarding the potential contribution that other demographic and clinical variables have in this context, including age, sex, comorbid psychiatric conditions, and medication use. No previous study had objectively compared the visceral pain experience of individuals genotyped based on this polymorphism. To further investigate these issues, we undertook a prospective study evaluating large cohorts of patients with CD and UC who had been genotyped based on the presence or absence of rs6795970, carefully characterizing their inflammatory status and concurrent abdominal pain experience. We also evaluated the potential impact of this polymorphism on visceral sensory perception in a pilot cohort of healthy individuals who had undergone SCN10A genotyping. We hypothesized that both hypoalgesic CD and UC would be more likely to exhibit homozygosity for the polymorphism on A1073V. We also anticipated that individuals who were homozygous for this variant would exhibit reduced visceral and somatosensory pain to appropriate standardized stimuli.
METHODS
Study participant selection
We obtained relevant clinical and patient survey data from individuals who had consented to take part in a prospective IBD natural history registry and tissue biorepository associated with the IBD Center at our institution (approved under protocols PRAMSHY98-057 and STUDY00015545) and who had undergone a colonoscopy between October 1, 2015, and September 30, 2022. We prospectively and consecutively enrolled all individuals who met the inclusion criteria and did not have the exclusion criteria listed below. Our previous study demonstrated homozygosity in approximately 45% of patients with hypoalgesic (silent) IBD and 20% of “other” patients with IBD (12). Based on this finding (assuming a normal binomial distribution), we determined that cohort sizes of n = 38 would provide at least 80% power to detect the same incidences of rs6795970 homozygosity if our statistical comparisons were conducted at the 0.05 level. Thus, we continued recruitment until both the hypoalgesic and other subcohorts within the CD and UC patient groups each had at least 38 individuals.
Inclusion criteria
We included patients with established diagnoses of IBD (for at least 12 months) who had (i) undergone a colonoscopy, (ii) completed a pain questionnaire (see below) at the time of endoscopy, and (iii) provided a blood sample as part of their participation in the registry and biorepository. In addition, IBD study patients had to meet the following criteria: (i) age equal to or greater than 18 years; (ii) established diagnosis of IBD (either CD or UC, based on standard clinical criteria incorporating historical, laboratory, endoscopic, and histological evaluation); (iii) no coexisting condition that could explain abdominal pain, including pregnancy, trauma, non-IBD-associated malignancy, infection or non-IBD-associated inflammatory disorder. We also included a cohort of healthy adult control patients (age greater than or equal to 18 years) who had no documented history of chronic GI illness, current or recent abdominal pain, chronic pain disorder, chronic neurological disorder or neuropathy, diabetes, or current or recent (i.e., at the time or within 7 days of the clinical encounter) analgesic usage.
Exclusion criteria
Patients with IBD were excluded if they had an indeterminate form of IBD, microscopic colitis, a form of inflammatory enteritis or colitis that was not associated with IBD or had not provided information about abdominal pain at the time of the ileocolonoscopy. Patients were also excluded if they had undergone a total colectomy or proctocolectomy at any time or any intra-abdominal surgery within the calendar year before the time of the study encounter. Patients with UC who had undergone any intestinal resection were excluded. Healthy controls were excluded if they had a documented history of chronic GI illness, current or recent abdominal pain, chronic pain disorder, chronic neurological disorder or neuropathy, diabetes, or current or recent (i.e., at the time or within 7 days of the clinical encounter) analgesic usage.
Characterization of intestinal inflammatory status and complications
Disease severity and location were recorded for each IBD study participant using contemporary endoscopic, histologic, and radiologic information. CD and UC location and phenotype were classified according to the Montreal Classification system (16). The severity of UC disease activity was determined based on the appearance of the mucosa at the time of endoscopy and characterized using the Mayo Clinic endoscopy subscore (17). The severity of CD was also determined by appearance at endoscopy using the CD Simple Endoscopic Score (SES-CD) (17). Both the Mayo score (ranging from 0 to 3, with 0 = inactive, 1 = mild activity, 2 = moderate activity and 3 = severe activity in UC) and the SES-CD (ranging from 0 to 12 in 5 intestinal segments [the TI and 4 colonic segments], with 0–6 indicating in remission or mild disease, and scores of 7–12 as moderately to severely active disease) are based on Likert-type scales. For the purposes of this study, we defined active UC as a Mayo score of 2–3 and active CD as a SES-CD of 7–12 in at least one intestinal segment. All complications described were intra-abdominal/luminal in nature (e.g., fistulae described were not perianal phenomena).
Abdominal pain assessment
Abdominal pain ratings were based primarily on responses to the Short Inflammatory Bowel Disease Questionnaire (SIBDQ), that asks patients to grade pain on a frequency-based inverse Likert scale (“How often over the past 2 weeks have you been troubled by pain in the abdomen?”), with 1 representing pain all of the time and 7 representing pain none of the time (18). We also asked patients about abdominal pain severity (using a scale adopted from the CD activity index (19)), with potential responses including 0 [no abdominal pain], 1 [mild], 2 [moderate], and 3 [severe]). As we found in previous investigations (12,20), we determined that abdominal pain frequency and intensity scores closely correlated with one another (Pearson correlation, r = −0.73, P < 0.001). For the purposes of this study and to increase the rigor of our analysis, we defined the presence of clinically meaningful abdominal pain as SIBDQ pain ratings of ≤ 5 (with 5 defined as a little of the time) or an abdominal pain severity rating of > 1. In addition, the absence of clinically meaningful abdominal pain was defined as a SIBDQ pain rating of 6 or greater (hardly any or no pain over the prior 2 week period) and a severity rating of 0.
Determination of study cohorts
Three cohorts were evaluated in each of the genetic analyses undertaken in associated with this study: (i) IBD patients (total, CD, or UC) with active disease who had an SIBDQ pain score of >5 (describing hardly any to no abdominal pain) (i.e., active disease with no pain or hypoalgesic IBD), (ii) all other patients with IBD (total, CD, or UC), and (iii) healthy controls. Basic demographic and disease characteristics of each group are presented in Table 1. Of note, all patients identified as having active disease had a Mayo or SES-CD score (in at least one bowel segment) of 2 or greater. In addition, inflammatory activity assessments were based on consensus determinations provided by 3 expert endoscopists using the Mayo and SES-CD criteria described above (MC, MW, KC). The interobserver concordance rate (determining whether an individual had active or inactive disease at the time of endoscopy) was 92%. If there was disagreement in this assessment, the final determination was based on the 2 matching scores. Of note, the experts determining inflammatory activity in each case were blinded to these pain scores.
Table 1.
Demographic and clinical characteristics of the hypoalgesic and other IBD cohorts
| Variable | Hypoalgesic IBD | Other IBD | P value |
| Cohort (% women) | 84 (38.1%) | 332 (58.1%) | <0.001 |
| Age (yr) | 46.3 ± 1.9 | 43.1 ± 0.8 | 0.10 |
| BMI | 25.9 ± 0.6 | 29.0 ± 0.9 | 0.10 |
| Race (AI-AN/Asian/BoAA/White) | 1/4/4/75 | 4/17/25/286 | 0.84 |
| Disease type (CD/UC) | 43/41 | 218/114 | 0.01 |
| Disease duration (yr) | 10.1 ± 1.1 | 12.5 ± 0.6 | 0.06 |
| Any history of EIM | 31 (36.9%) | 187 (56.3%) | 0.002 |
| SIBDQ | 57.3±1.5 | 44.3±0.7 | <0.001 |
| Pain scores (SIBDQ4 = 6/SIBDQ4 = 7) | 32/52 | ||
| Laboratory studies | |||
| WBC (103 cells/mm3) | 8.6 ± 0.4 | 7.9 ± 0.2 | 0.14 |
| ESR (mm/hr) | 19.7 ± 2.6 | 18.4 ± 1.3 | 0.58 |
| CRP (mg/dL) | 1.6 ± 0.3 | 1.3 ± 0.1 | 0.52 |
| Current IBD medications | |||
| Corticosteroid | 6 (7.1%) | 43 (13.0%) | 0.14 |
| Mesalamine | 32 (38.1%) | 66 (19.9%) | <0.001 |
| Immunomodulator | 26 (31.0%) | 77 (23.2%) | 0.16 |
| Biologic | 44 (52.4%) | 208 (62.6%) | 0.10 |
| Active pain medication/substance use | |||
| Tobacco use | 9 (10.7%) | 35 (10.5%) | 1.0 |
| Alcohol use | 27 (32.1%) | 87 (26.2%) | 0.28 |
| Cannabis use | 2 (2.4%) | 22 (6.6%) | 0.19 |
| Illicit drug use | 4 (4.8%) | 20 (6.0%) | 0.80 |
| NSAID use | 14 (16.7%) | 56 (16.9%) | 1.0 |
| Opioid use | 2 (2.4%) | 42 (12.7%) | 0.005 |
| Other pain medication use | 20 (23.8%) | 148 (44.6%) | 0.0001 |
| Symptoms of anxiety/depression | 21 (25.0%) | 178 (53.6%) | <0.0001 |
| Antidepressant/anxiolytic use | 9 (10.7%) | 117 (35.2%) | <0.0001 |
AI-AN, American Indian-Alaska Native; BMI, body mass index; BoAA, Black or African American; CD, Crohn's disease; CRP, C-reactive protein; EIM, extraintestinal manifestation; ESR, sedimentation rate; IBD, inflammatory bowel disease; NSAID, nonsteroidal anti-inflammatory drug; SIBDQ, Short Inflammatory Bowel Disease Questionnaire; SIBDQ4, SIBDQ Pain Score (note: a SIBDQ4 score of 6 indicates abdominal pain hardly any of the time over the prior 2 wk period, while a score of 7 indicates no pain); UC, ulcerative colitis.
Items in bold-italics demonstrated statistically significant differences from one another.
DNA isolation
High-quality genomic DNA was isolated from whole blood using silica-based spin columns (QIAmp DNeasy Blood & Tissue Kit, Qiagen, Hilden, Germany). Spectrophotometry was used to quantify DNA, and the quality of the isolated material was evaluated with an Agilent Bioanalyzer.
TaqMan SNP genotyping
Genotype analysis of all IBD and healthy control samples described above was performed with commercially available TaqMan assays using the OpenArray platform on a QuantStudio 12K Flex instrument (Thermo Fisher Scientific; formerly Life Technologies, Grand Island, NY). This analysis was performed on the SNP rs6795970 (PN4351376; Thermo Fisher Scientific). Twenty nanograms of genomic DNA were amplified per the manufacturer's directions and scaled to a total volume of 5 µL in an Applied Biosystems Veriti 384-well thermal cycler. As a routine quality control, 10% of the patient samples were subjected to independent (and investigator-blinded) DNA sequencing of gene-specific amplicons containing the polymorphic site. There was 100% concordance with the TaqMan SNP genotyping results.
Visceral sensory testing
To further objectively assess the potential impact of the polymorphism above on visceral sensory function, we performed barostatic rectal balloon distension (RBD) in a pilot cohort of 50 consecutively enrolled healthy controls (as defined above). This study was approved by the Institutional Review Board (STUDY00010688). We chose this sample size based on the reported incidence of rs6795970 homozygosity in the general population (eg, approximately 15%–25%) and the likelihood of recruiting at least 10 individuals for each of our homozygous and other cohorts, as this was a threshold which we determined would provide us with at least 65% power for a Student t-test conducted at alpha = 0.05 to detect an effect size similar to that reported in Duan et al. (14) Of note, due to challenges associated with recruitment for this testing, we could not perfectly match the demographic characteristics of these particular study patients to those of the IBD cohort. All subjects underwent a bowel preparation (Fleet phosphate enema, self-administered at least 1 hour before the testing) and an overnight fast. A customized rectal barostat catheter (MUI Scientific) attached to a 600 mL polyethylene bag (MUI Scientific) was inserted into the rectum so that the middle of the balloon was located approximately 10 cm from the anal verge. To decrease the effects of abdominal viscera on the balloon volume, patients were placed in a semi-prone position and the foot of the bed elevated 15°. The bag was then unfolded by inflating it with 75 mL of air and then deflating it completely. After a 10–15-minute recovery period, the catheter was connected to a barostat (G&J Electronics Inc). Baseline operating pressure (BOP) was then determined as previously described (21,22). The bag was then deflated to 0 mm Hg, and subjects were allowed to rest for 10 minutes before proceeding to the following assessments.
Sensory threshold assessment
Sensory thresholds were measured by ramp inflation, starting at 0 mm Hg and increasing in steps of 4 mm Hg for 1 minute per step to a maximum of 60 mm Hg. Thresholds for first sensation, urgency, discomfort, and pain were determined by patients pressing a button at the distension pressure at which sensations were perceived. Ramp inflation was terminated as soon as the subjects reported the first sensation of pain. After this procedure, the bag was deflated to BOP and the subjects allowed to rest.
Phasic distensions and sensory ratings
Phasic distensions of 12, 24, 36, and 48 mm Hg above BOP were each applied once in random order. Each distension was maintained for 60 seconds with an interstimulus interval of 2 minutes during which the balloon will be deflated to BOP. Subjects were asked to mark on a 100 mm visual analog scale 30 seconds after the onset of the distension for the sensation of discomfort (or pain if that was experienced). These scales will be anchored at each end by the descriptions “unnoticeable” and “unbearable”. Study patients were blinded to the distension order. Study personnel were blinded to patient genotype. Of note, during the assessment of sensation, interaction between the subject and the study investigator will be kept to a minimum.
Statistical analysis
Patients' baseline and clinical characteristics were summarized as descriptive statistics. The Fisher exact test was used to compare categorical variables between groups, and a Monte Carlo version of the Fisher exact test was applied when analyzing contingency tables with at least 3 rows or columns. To ensure reproducibility of results, a random seed was set when applying the Monte Carlo version of the Fisher exact test. The Student t-test or Wilcoxon rank-sum and Kruskal-Wallis tests were used to compare continuous variables between groups as appropriate. Odds ratios and corresponding 95% confidence intervals were computed using epitools R package (Tomas J. Aragon [2020] epitools: Epidemiology Tools. R package version 0.5–10.1; https://CRAN.R-project.org/package=epitools). Specifically, the odds ratio() function was applied with the Wald option. Data analyses were performed using GraphPad Prism v.8.0 (La Jolla, CA) or RStudio Team (RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA, http://www.rstudio.com/). All P values of less than 0.05 were considered statistically significant.
RESULTS
IBD and healthy control cohort characteristics
We initially identified 450 individuals with IBD who provided blood samples, completed the surveys and underwent concurrent endoscopic examination. A total of 34 individuals were subsequently excluded (Figure 1). Thus, we eventually evaluated 416 consecutively recruited patients with IBD (261 CD, 155 UC; 223 women, 193 men) who provided blood samples for DNA analysis, while also completing concomitant clinical surveys and received endoscopic evaluation to permit determination of current abdominal pain experience and IBD-associated disease activity (Table 1). We also recruited 142 healthy controls. In the total IBD cohort, 84 patients (20.2%) were identified as having hypoalgesic IBD, while the remaining 332 patients were classified in the other IBD category. The hypoalgesic and other IBD cohorts had statistically similar mean ages and mean disease durations. Participant-reported race was also similar between the cohorts. The hypoalgesic IBD cohort was significantly more likely to be male compared with the other IBD and healthy control cohorts (Table 1). Patients with IBD made up most of each IBD cohort, though they made up a lower proportion of the hypoalgesic IBD cohort. There were no significant differences in disease location (using the Montreal Classification system (16)) in the CD or UC patient cohorts when comparing the hypoalgesic and other IBD cohorts. The mean SIBDQ, Harvey-Bradshaw Index (CD only), and Simple Clinical Colitis Activity Index (UC only) scores were each significantly different between the IBD cohorts (Tables 1, 3 and 5). The mean white blood cell count (WBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were statistically similar between the IBD cohorts. In addition, there was no significant difference in the use of immunomodulator (IMM) or biologic/small molecule medications between the IBD cohorts, but the hypoalgesic IBD cohort was more likely to use mesalamine. Patients with hypoalgesic IBD were significantly less likely to have developed an extraintestinal manifestation (EIM) of IBD (Table 1).
Figure 1.
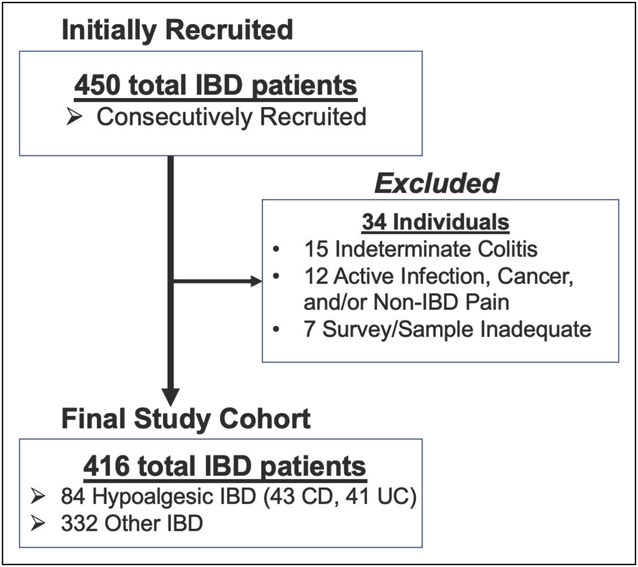
Study recruitment. Hypoalgesic IBD is defined as Mayo > 1 (in ulcerative colitis) or CD Simple Endoscopic Score > 6 (in Crohn’s disease) with concomitant Short Inflammatory Bowel Disease Questionnaire pain score of > 5 or abdominal pain severity score of < 2. CD, Crohn's disease; IBD, inflammatory bowel disease; UC, ulcerative colitis.
Table 3.
Demographic and clinical characteristics of the hypoalgesic and other CD cohorts
| Variable | Hypoalgesic CD | Other CD | P value |
| Cohort (% women) | 43 (37.2%) | 218 (60.6%) | 0.005 |
| Age (yr) | 46.3 ± 1.9 | 43.1 ± 0.8 | 0.10 |
| BMI | 25.6 ± 0.8 | 28.8 ± 1.2 | 0.14 |
| Race (AI-AN/Asian/BoAA/White) | 0/2/3/38 | 3/13/20/182 | 0.80 |
| Disease location (Montreal) | |||
| L1 | 7 (16.3%) | 62 (28.4%) | 0.33 |
| L2 | 11 (25.6%) | 38 (17.4%) | |
| L3 | 25 (58.1%) | 118 (54.2%) | |
| L4 | 2 (4.7%) | 11 (5.0%) | |
| Crohn's disease behavior (Montreal) | |||
| Nonstricturing/nonpenetrating (B1) | 11 (25.6%) | 85 (39.0%) | 0.08 |
| Stricturing (B2) | 14 (32.6%) | 75 (34.4%) | 0.82 |
| Penetrating (B3) | 18 (41.8%) | 58 (26.6%) | 0.04 |
| Disease duration (yr) | 10.7 ± 1.4 | 12.8 ± 0.7 | 0.31 |
| Any history of EIM | 20 (46.5%) | 134 (61.5%) | 0.09 |
| Short Inflammatory Bowel Disease Questionnaire | 57.8±1.5 | 44.7±0.9 | <0.001 |
| Pain scores (SIBDQ4 = 6/SIBDQ4 = 7) | 18/25 | ||
| Harvey-Bradshaw Index | 3.3±0.4 | 6.9±0.3 | <0.001 |
| Prior history of surgery (CD) | 18 (41.8%) | 101 (46.3%) | 0.62 |
| Laboratory studies | |||
| WBC (103 cells/mm3) | 8.4 ± 0.6 | 7.8 ± 0.3 | 0.57 |
| ESR (mm/hr) | 17.8 ± 3.3 | 17.9 ± 1.7 | 0.73 |
| CRP (mg/dL) | 1.6 ± 0.5 | 1.2 ± 0.1 | 0.70 |
| Current IBD medications | |||
| Corticosteroid | 1 (2.3%) | 24 (11.0%) | 0.08 |
| Mesalamine | 7 (16.3%) | 28 (13.1%) | 0.62 |
| Immunomodulator | 17 (39.5%) | 55 (25.2%) | 0.06 |
| Biologic | 29 (67.4%) | 153 (70.2%) | 0.72 |
| Active pain medication/substance use | |||
| Tobacco use | 5 (11.6%) | 25 (11.6%) | 1.0 |
| Alcohol use | 15 (34.9%) | 53 (24.3%) | 0.18 |
| Cannabis use | 1 (2.3%) | 16 (7.3%) | 0.32 |
| Illicit drug use | 3 (7.0%) | 12 (5.5%) | 0.72 |
| NSAID use | 8 (18.6%) | 29 (13.3%) | 0.35 |
| Opioid use | 0 (0.0%) | 26 (11.9%) | 0.01 |
| Other pain medication use | 10 (23.3%) | 91 (41.7%) | 0.03 |
| Symptoms of anxiety/depression | 13 (30.2%) | 120 (55.0%) | 0.004 |
| Antidepressant/anxiolytic use | 4 (9.3%) | 84 (38.5%) | <0.001 |
Individuals with L4 (Crohn’s disease of the upper gastrointestinal tract) could exhibit this disease distribution in addition to disease at other sites in the gut.
AI-AN, American Indian-Alaska Native; BMI, body mass index; BoAA, Black or African American; CD, Crohn's disease; CRP, C-reactive protein; EIM, extraintestinal manifestation; ESR, sedimentation rate; NSAID, nonsteroidal anti-inflammatory drug; IBD, inflammatory bowel disease; SIBDQ, Short Inflammatory Bowel Disease Questionnaire; SIBDQ4, SIBDQ Pain Score (note: a SIBDQ4 score of 6 indicates abdominal pain hardly any of the time over the prior 2 wk period, while a score of 7 indicates no pain).
Items in bold-italics demonstrated statistically significant differences from one another.
Table 5.
Demographic and Clinical Characteristics of the Hypoalgesic and Other UC cohorts
| Variable | Hypoalgesic UC | Other UC | P value |
| Cohort (% women) | 41 (39.0%) | 114 (51.8%) | 0.16 |
| Age (yr) | 48.1 ± 2.6 | 46.1 ± 1.4 | 0.48 |
| BMI | 26.2 ± 0.7 | 29.2 ± 1.2 | 0.15 |
| Race (AI-AN/Asian/BoAA/White) | 1/2/1/37 | 1/4/5/104 | 0.79 |
| Disease location (Montreal) | |||
| E1 | 3 (7.3%) | 7 (6.1%) | 0.68 |
| E2 | 9 (22.0%) | 33 (28.9%) | |
| E3 | 29 (70.7%) | 77 (65.0%) | |
| Disease duration (yr) | 9.4 ± 1.6 | 11.9 ± 0.9 | 0.17 |
| Any history of EIM | 11 (26.8%) | 53 (46.5%) | 0.03 |
| Short Inflammatory Bowel Disease Questionnaire | 56.7±1.9 | 43.6±1.4 | <0.001 |
| Pain scores (SIBDQ4 = 6/SIBDQ4 = 7) | 14/27 | ||
| Short Clinical Colitis Activity Index | 2.7±0.4 | 4.6±0.3 | 0.002 |
| Laboratory studies | |||
| WBC (103 cells/mm3) | 8.8 ± 0.6 | 8.2 ± 0.4 | 0.16 |
| ESR (mm/hr) | 22.0 ± 4.1 | 19.3 ± 2.0 | 0.73 |
| CRP (mg/dL) | 1.5 ± 0.5 | 1.6 ± 0.3 | 0.60 |
| Current IBD medications | |||
| Corticosteroid | 5 (12.2%) | 19 (16.7%) | 0.50 |
| Mesalamine | 25 (61.0%) | 38 (33.3%) | 0.003 |
| Immunomodulator | 9 (22.0%) | 22 (19.3%) | 0.82 |
| Biologic | 16 (39.0%) | 54 (47.4%) | 0.36 |
| Active pain medication/substance use | |||
| Tobacco | 4 (9.8%) | 10 (8.8%) | 0.99 |
| Alcohol | 12 (29.3%) | 34 (29.8%) | 1.0 |
| Cannabis | 1 (2.4%) | 6 (5.3%) | 0.68 |
| NSAID | 6 (14.6%) | 27 (23.7%) | 0.27 |
| Opioid | 2 (4.9%) | 16 (14.0%) | 0.16 |
| Illicit drug | 1 (2.4%) | 8 (7.0%) | 0.45 |
| Other pain medications | 10 (24.4%) | 57 (50.0%) | 0.006 |
| Symptoms of anxiety/depression | 8 (19.5%) | 58 (50.9%) | <0.001 |
| Antidepressant/anxiolytic use | 5 (12.2%) | 33 (28.9%) | 0.04 |
AI-AN, American Indian-Alaska Native; BMI, body mass index; BoAA, Black or African American; CRP, C-reactive protein; EIM, extraintestinal manifestation; ESR, sedimentation rate; NSAID, nonsteroidal anti-inflammatory drug; IBD, inflammatory bowel disease; SIBDQ, Short Inflammatory Bowel Disease Questionnaire; SIBDQ4, SIBDQ Pain Score (note: a SIBDQ4 score of 6 indicates abdominal pain hardly any of the time over the prior 2 wk period, while a score of 7 indicates no pain); UC, ulcerative colitis.
Items in bold-italics demonstrated statistically significant differences from one another.
Patients with hypoalgesic IBD were significantly less likely to exhibit clinically significant anxious or depressive symptoms or to use antidepressant/anxiolytic medication (Table 1). Patients with hypoalgesic IBD and other patients with IBD had similar likelihoods of using tobacco, alcohol, and illicit drugs (e.g., heroin, methamphetamine, cocaine) (Table 1). Patients with hypoalgesic IBD were significantly less likely to use opioids or analgesic medications of any kind (Table 1).
Allelic and genotypic comparison of the IBD and healthy control cohorts
Table 2 presents the homozygotic and allelic frequencies of the SNP rs6795970 in each of the hypoalgesic IBD, other IBD, and healthy control cohorts. Of note, the mean allelic frequency for rs6795970 was 42.2%, and the total evaluated cohort was found to be in Hardy-Weinberg equilibrium when considering this polymorphism (Χ2 = 0.077). The hypoalgesic IBD cohort had significantly higher allelic and homozygotic frequencies for the rs6795970 polymorphism when compared with the other IBD and healthy control cohorts.
Table 2.
Genotypic and allelic frequencies of the SCN10A polymorphism, rs6795970, in the IBD and healthy control cohorts
| Genotype | Hypoalgesic IBD (n = 84) | Other IBD (n = 332) | Healthy controls (n = 142) | P value |
| A/A (1073Val/Val) | 26 (31.0%) | 42 (12.7%) | 25 (17.6%) | 0.0003 |
| A/G (1073Val/Ala) or G/G (1073Ala/Ala) | 58 (69.0%) | 290 (87.3%) | 117 (82.4%) | |
| Allele | ||||
| A (valine) | 85 (50.6%) | 248 (37.3%) | 118 (41.5%) | 0.007 |
| G (alanine) | 83 (49.4%) | 416 (62.7%) | 166 (58.5%) | |
Frequencies are listed as number of subjects (% of subcohort). The homozygous genotype (A/A) for the rs6795970 polymorphism and the frequency of this polymorphic allele were each significantly more common in the hypoalgesic IBD cohort.
A, rs6795970 polymorphism(c.3218G>A); G, wild type; IBD, inflammatory bowel disease.
CD cohort characteristics
We evaluated the same clinical and demographic characteristics described above in both the CD and UC subcohorts. In the CD cohort (148 women, 113 men), 43 individuals (16.5%) were determined to have the hypoalgesic phenotype (Table 3). There was a significantly higher proportion of men in the hypoalgesic CD cohort when compared with the other CD group and the healthy controls. In CD, as with the total IBD cohort, there was no significant difference in age, disease duration, or race between the hypoalgesic and other cohorts. Of note, the hypoalgesic CD cohort was significantly more likely to develop intra-abdominal fistulae when compared with the other CD cohort. As previously indicated, the mean SIBDQ and Harvey-Bradshaw Index scores were significantly different between the hypoalgesic and other CD cohorts (Table 3). However, measures of inflammation, including the WBC, ESR, and CRP, were all also statistically similar between the CD cohorts. Mesalamine, IMM, biologic, and corticosteroid medication use were also similar between the IBD cohorts.
Patients with hypoalgesic CD were significantly less likely to exhibit clinically significant anxious or depressive traits or to use antidepressant/anxiolytic medication (Table 3). Patients with hypoalgesic CD and other patients with CD had similar likelihoods of using tobacco, alcohol, cannabis/cannabis derivatives, and illicit drugs. Patients with hypoalgesic CD were significantly less likely to use opioids or other analgesic medications (Table 3).
Allelic and genotypic comparison of the CD cohorts
Table 4 presents the homozygotic and allelic frequencies of the SNP rs6795970 in each of the hypoalgesic CD and other CD cohorts. The hypoalgesic CD cohort had a significantly higher homozygotic and allelic frequency for the rs6795970 polymorphism when compared with the other IBD cohort (Table 4).
Table 4.
Genotypic and allelic frequencies of the SCN10A polymorphism, rs6795970, in Hypoalgesic and Other CD cohorts
| Genotype | Hypoalgesic CD (n = 43) | Other CD (n = 218) | Odds ratio (confidence interval) | P value |
| A/A (1073Val/Val) | 13 (30.2%) | 25 (11.4%) | 3.35 (1.56,7.05) | 0.004 |
| A/G (1073Val/Ala) or G/G (1073Ala/Ala) | 30 (69.8%) | 193 (88.6%) | ||
| Allele | ||||
| A (valine) | 42 (48.8%) | 157 (36.0%) | 1.70 (1.07, 2.67) | 0.03 |
| G (alanine) | 44 (51.2%) | 279 (64.0%) | ||
Frequencies are listed as number of subjects (% of subcohort). The homozygous genotype (A/A) for the rs6795970 polymorphism was more common in the hypoalgesic CD cohort.
A, rs6795970 polymorphism(c.3218G>A); CD, Crohn's disease; G, wild type.
UC cohort characteristics
In the UC cohort (75 women, 80 men), 41 individuals (26.4%) were determined to have the hypoalgesic phenotype (Table 5). Unlike with the total IBD and CD cohorts, sex distribution was not significantly different among the hypoalgesic UC, the other UC group, and the healthy control cohorts. There was no significant difference in age or race between the UC cohorts. As with the CD cohorts, the mean SIBDQ and Simple Clinical Colitis Activity Index scores were significantly different between the hypoalgesic and other UC cohorts (Table 5). However, the mean values for the inflammatory markers, WBC, ESR, and CRP, were all also statistically similar between the UC cohorts. Of note, the relative proportions of patients with hypoalgesic UC reporting SIBDQ pain scores of 6 and 7 (14 and 27, respectively) were similar to those reported in the hypoalgesic CD cohort (18 and 25, respectively) (P = 0.507) (Tables 3 and 5). Patients with hypoalgesic UC were more likely to use mesalamine but had statistically similar rates of IMM, biologic, and corticosteroid medication use when compared with the other UC cohort. Patients with hypoalgesic UC were less likely to have developed an EIM of IBD (Table 5).
Patients with hypoalgesic UC were also significantly less likely to exhibit clinically significant anxious or depressive traits or to use antidepressant/anxiolytic medication (Table 5). Patients with hypoalgesic UC and other patients with UC had similar likelihoods of using tobacco, alcohol, cannabis, and illicit drugs. Patients with hypoalgesic UC had similar rates of nonsteroidal anti-inflammatory drug and opioid use compared with other patients with UC, but they were significantly less likely to use other pain medications as a whole (Table 5).
Allelic and genotypic comparison of the UC cohorts
Table 6 presents the homozygotic and allelic frequencies of the SNP rs6795970 in each of the hypoalgesic UC and other UC cohorts. The hypoalgesic UC cohort had a significantly higher homozygotic frequency for the rs6795970 polymorphism when compared with the other IBD cohort (Table 6). Allelic frequency of the SNP also trended toward being higher in the hypoalgesic UC cohort (P = 0.052).
Table 6.
Genotypic and allelic frequencies of the SCN10A polymorphism, rs6795970, in the UC cohorts
| Genotype | Hypoalgesic UC (n = 41) | Other UC (n = 114) | Odds ratio (confidence intervals) | P value |
| A/A (1073Val/Val) | 13 (31.7%) | 17 (14.9%) | 2.65 (1.113, 5.977) | 0.036 |
| A/G (1073Val/Ala) or G/G (1073Ala/Ala) | 28 (68.3%) | 97 (85.1%) | ||
| Allele | ||||
| A (valine) | 43 (52.4%) | 91 (39.9%) | 1.66 (0.999, 2.759) | 0.052 |
| G (alanine) | 39 (47.6%) | 137 (60.1%) | ||
Frequencies are listed as number of subjects (% of subcohort). The homozygous genotype (A/A) for the rs6795970 polymorphism was more common in the hypoalgesic UC cohort.
A, rs6795970 polymorphism(c.3218G>A); G, wild type; UC, ulcerative colitis.
Visceral sensory testing
As described above, we performed RBD in a cohort of 50 healthy individuals (20 women, 30 men; mean age 54.2 years). Eleven were subsequently found to be homozygous for rs6795970, while 39 individuals exhibited the heterozygous or wild-type genotype.
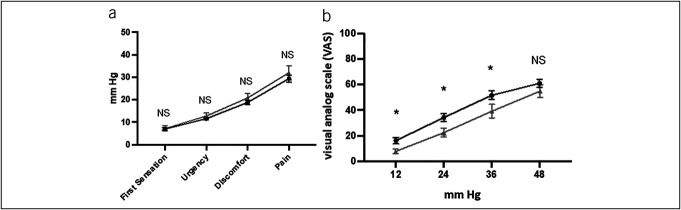
Figure 2a demonstrates the findings associated with sensory threshold testing in the homozygotes and heterozygote/wild-type cohorts. No significant differences were identified in the mean threshold pressure values for first sensation, fecal urgency, abdominal discomfort, or abdominal pain.
Figure 2.
(a) Subject-reported sensory threshold assessment. The gray triangles represent mean values for subjects that are homozygous for the rs6795970 polymorphism (n = 11), and the black circles represent mean values for subjects that have a heterozygous or wild-type genotype for SCN10A (n = 39). (b) Subject-reported severity of discomfort to phasic rectal balloon distension (visual analog scale 0–100). The gray triangles represent mean values for subjects that are homozygous for the rs6795970 polymorphism (n = 11), and the black circles represent mean values for subjects that have a heterozygous or wild-type genotype for SCN10A (n = 39). NS, not significant. *, < 0.05.
Figure 2b demonstrates the results associated with phasic distension and sensory rating testing. The homozygote cohort exhibited a significantly lower mean reported visual analog scale score compared with the heterozygote/wild-type cohort at each pressure except at 48 mm Hg.
DISCUSSION
Hypoalgesic, or silent, IBD is relatively common and uniquely impactful. We demonstrated here, and in previous studies (11,12), that individuals with this condition are at increased risk of serious complications, including the development of strictures and intra-abdominal fistulae. Patients with IBD and their healthcare providers need more refined and effective methods to identify those at risk of this condition, to monitor these individuals more intensively and to reduce the risk of complications like those referenced above. This study addresses this issue in several ways, as it provides further evidence of the critical role that NaV1.8 has in human visceral pain perception, and of the impact that the rs6795970 polymorphism has on that function. Our findings confirmed that homozygosity for this polymorphism is significantly associated with hypoalgesia in IBD. This is the first study to demonstrate that homozygosity for this SNP is also significantly more likely in both hypoalgesic CD and UC when compared with nonhypoalgesic IBD cohorts and healthy controls. Considering these findings, we believe that rs6795970 could be used as a tool to screen for individuals with hypoalgesic IBD.
This study is also important because it represents the largest investigation of hypoalgesic IBD, to date. This is also the largest prospective study of this patient population and the largest genetic analysis performed in this cohort. In addition, this study reinforced previous findings that characterize the hypoalgesic IBD cohort (11,12). For example, we demonstrated that patients with hypoalgesic IBD are less likely to use pain medications (e.g., opioids and other analgesic agents). We again demonstrated that patients with hypoalgesic IBD are older and more likely to be male while being less likely to exhibit EIM of IBD. They are also less likely to exhibit concomitant anxiety and/or depression, or variations in disease type, complications, and severity. As indicated previously, we found that patients with hypoalgesic CD were also more likely to exhibit certain IBD-associated complications (e.g., intra-abdominal fistulae). In addition, in a healthy control cohort, we demonstrated that individuals exhibiting homozygosity for rs6795970 reported reduced perceived abdominal discomfort to RBD.
As indicated above, these results are similar to those of previous investigations in a variety of ways. First, the mean allelic frequency for rs6795970 in our study population was 42.2%. This is similar to other previous reports (e.g., 1000 Genomes Project mean allelic frequency = 38% (23,24)). In addition, these findings reinforce those of our previous study performed in a smaller cohort of individuals with IBD, which demonstrated that homozygosity for rs6795970 was significantly more likely in those demonstrating hypoalgesia (12). In a previous study of hypoalgesic IBD, we also demonstrated a significant association with older age, male sex, and the development of IBD-associated complications (including intra-abdominal fistulae), along with an inverse association with coexistent EIM of IBD, anxiety/depression, use of antidepressants and anxiolytics, and use of opioids or analgesic medications (11). These characteristics are particularly relevant when considering that the major variables assessing IBD disease type, extent, and severity were statistically similar. They also support the idea that patients with hypoalgesic IBD are not hypoalgesic because of a disproportionality of analgesic use and/or because they harbor a less severe disease phenotype.
The findings of this study are novel for several reasons. While other investigations have linked VGSC-associated SNPs and disorders of brain-gut interaction, including irritable bowel syndrome (NaV1.5 (25)) and functional dyspepsia (NaV1.8 (26)), our initial study and the results reported here are the first to describe an association between a VGSC gene polymorphism and an IBD-associated visceral pain phenotype. This is the only genetic factor specifically linked with hypoalgesic IBD or any other visceral hyposensitivity disorder that we are aware of. In addition, this is the only study to have objectively evaluated visceral sensory experience in humans genotyped based on the rs6795970 polymorphism. It is also one of the few studies to have demonstrated diminished visceral sensory perception in any population. Importantly, based on our previous studies, the association only appears to exist for individuals who are homozygotic for this SNP, suggesting that a single nonpolymorphic gene copy is sufficient to abrogate hypoalgesia.
Our investigation reveals important insights about the physiology underlying human visceral pain sensation. Specifically, it adds to the growing evidence that the rs6795970 polymorphism and the associated NaV1.8 channel have significant influence on human visceral pain perception. NaV1.8 is particularly relevant in this context because it is primarily expressed in the peripheral nervous system and appears to play a critical role in the transmission of pain-related signals (13). The rs6795970 polymorphism is notable because it encodes a nonsynonymous amino acid substitution (alanine at amino acid 1073 to valine, A1073V) on an intracellular loop in the NaV1.8 membrane protein. Thus, it is very possible that this polymorphism results in significant changes in biophysical function of the NaV1.8 protein. In support of this hypothesis, we and others have demonstrated that the rs6795970 variant results in an electrophysiologically modified channel associated with altered activation characteristics that lead to reduced firing of dorsal root ganglion neurons (14,15,23). The variant channel has also been associated with diminished somatosensory pain perception (14,15). In this study, both the hypoalgesic CD and UC patient cohorts were significantly more likely to exhibit homozygosity for the rs6795970 polymorphism. Although they are both categorized as forms of IBD, CD, and UC are pathophysiologically distinct conditions that are themselves composed of heterogeneous disorders that manifest through myriad inflammatory pathways which just happen to share phenotypic and symptomatic traits. Some of these shared symptoms, including abdominal pain, can manifest as a result of several factors that do not necessarily overlap between the conditions (e.g., stricturing, viscous stretch, disease location and extent). The fact that abdominal pain in both CD and UC is affected by the rs6795970 polymorphism strongly suggests that NaV1.8 plays a fundamental role in visceral and maybe all pain perception. This concept is bolstered by our demonstration that healthy human beings exhibiting homozygosity for this variant demonstrate diminished discomfort as a result of RBD. In other words, while this SNP is certainly relevant to pain experience in IBD, it is not necessarily specific to this condition. IBD simply appears to serve as a convenient backdrop on which to study NaV1.8 function and the physiological effects of this particular genetic variant.
Potential limitations of this study include the fact that all of the patients were derived from a single tertiary medical center. In addition, our designation of each study participant's IBD phenotype was based on endoscopic assessments and survey responses made from a single clinical encounter. It would be helpful to determine how consistent our clinical determinations were over multiple time points. It would also be useful to evaluate other disorders associated with chronic abdominal pain, including brain-gut disorders, such as irritable bowel syndrome. The visceral sensory testing we performed evaluated a relatively small cohort of homozygous patients and did not include explicit evaluation of fecal urgency. Finally, we did not have the opportunity to perform visceral sensory testing in our IBD cohorts. Although validated surveys were used to provide information about patient abdominal pain experience in our IBD cohort, there was no objective measure of visceral nociceptive perception. It will be important to pair this type of analysis with more objective assessments of abdominal pain experience (e.g., visceromotor response to RBD).
In spite of the limitations described above, the results of this study provide additional compelling evidence that NaV1.8 has a critical role in human visceral nociceptive function. In the future, it will be important to investigate IBD cohorts longitudinally, to determine whether patient pain and symptom experience vary over time. It would also be useful to simultaneously investigate other GI conditions associated with alterations in visceral sensation (e.g., irritable bowel syndrome), to evaluate whether the impact of this polymorphism is limited to inflammatory conditions of the gut. Similarly, it will be interesting to evaluate other conditions associated with hypoalgesia (e.g., silent gastroesophageal reflux disease, silent pancreatitis, etc.) to determine whether they were also associated with this SNP. Evaluating the pathophysiological relationship between this SNP, NaV1.8 function, and visceral nociception through further mechanistic studies will also be critical. Finally, other factors (including age and sex) appear to have a significant role related to visceral pain perception in the setting of IBD. Thus, it will be important to clarify how these, and other variables, specifically influence nociception in this setting.
In summary, the findings of this study further substantiate the significant role that the SCN10A SNP, rs6795970, has in hypoalgesic IBD, including in both hypoalgesic CD and UC, and visceral pain perception in general. This information could be used to develop novel tools, including genetic tests, to identify patients at risk of hypoalgesic or silent IBD. This is particularly important considering the increased risk of complications exhibited in patients with hypoalgesic IBD. These findings also support the potential of NaV1.8, a VGSC that appears to be specifically localized to peripheral nociceptive neurons, as a novel target that could be used to develop more targeted and less toxic visceral analgesic options. In fact, recent studies have demonstrated specific promise in this regard, particularly for the management of somatosensory pain syndromes (27). The successful development of agents targeting NaV1.8 could also help to reduce patient and provider reliance on other medications, including nonsteroidal anti-inflammatory drugs and opioids, which have been repeatedly associated with increased morbidity and mortality. That would be particularly impactful, considering the tens of millions of individuals in the United States alone who suffer from conditions associated with chronic or recurrent abdominal pain.
CONFLICTS OF INTEREST
Guarantor of the article: Matthew D. Coates, MD, PhD.
Specific author contributions: M.D.C.: Conceptualization, investigation, formal analysis, funding acquisition, and writing original draft. V.W.: Formal analysis, data curation, and review and editing of manuscript. A.S.: Investigation, data curation, project administration, and review and editing of manuscript. J.S.: Investigation, data curation, and review and editing of manuscript. S.D.: Review and editing of manuscript. N.C.: Investigation and review and editing of manuscript. A.O.: Investigation and review and editing of manuscript. K.C.: Investigation and review and editing of manuscript. A.T.: Investigation and review and editing of manuscript. E.W.: Investigation and review and editing of manuscript. P.J.: Investigation and review and editing of manuscript. V.R.: Investigation and review and editing of manuscript. K.E.V.: Investigation, funding acquisition, and review and editing of manuscript. All authors approved the final version of the article.
Financial support: This work was supported by NIH NIDDK (R01DK122364), the Peter and Marshia Carlino Career Development Professorship in Medicine (M.D.C.), the Margot E. Walrath Career Development Professorship in Gastroenterology (M.D.C.) and the Elliot S. Vesell Professorship (K.E.V.). Of note, the Genome Sciences Core services and instruments used in this project were funded, in part, by the Pennsylvania State University College of Medicine via the Office of the Vice Dean of Research and Graduate Students and the Pennsylvania Department of Health using Tobacco Settlement Funds (CURE). The content is solely the responsibility of the authors and does not necessarily represent the official views of the University or College of Medicine. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. The study design, data collection and analysis, and interpretation of the findings presented in this manuscript were each performed independent of the funding sources.
Potential competing interests: None of the authors have any known direct or indirect personal, professional, or financial conflict of interest to declare in relation to this publication or the work described herein.
IRB approval statement: All of the work described herein was performed following the guidelines set forth by and with the permission of the Penn State College of Medicine Institutional Review Board. All study participants gave written informed consent in accordance with the Declaration of Helsinki.
Study Highlights.
WHAT IS KNOWN?
✓ Hypoalgesic inflammatory bowel disease is relatively common and associated with increased risk for severe complications, such as intra-abdominal fistula.
✓ A voltage-gated sodium channel gene polymorphism has been associated with hypoalgesic inflammatory bowel disease.
WHAT IS NEW HERE?
✓ Homozygosity for a NaV1.8 polymorphism (rs6795970) is associated with both hypoalgesic Crohn’s disease and ulcerative colitis, and diminished abdominal discomfort to rectal balloon distension.
ACKNOWLEDGEMENTS
The authors wish to thank Leonard Harris for his outstanding technical assistance during the course of this study. We also wish to thank Drs Walter Koltun and Gregory Yochum for provision of blood samples to support this study.
Contributor Information
Vonn Walter, Email: vwalter1@pennstatehealth.psu.edu.
August Stuart, Email: astuart@pennstatehealth.psu.edu.
Jeffrey Small, Email: jsmall1@pennstatehealth.psu.edu.
Shannon Dalessio, Email: sdalessio@pennstatehealth.psu.edu.
Nurgul Carkaci-Salli, Email: nsalli@pennstatehealth.psu.edu.
Ann Ouyang, Email: aouyang@pennstatehealth.psu.edu.
Kofi Clarke, Email: kclarke@pennstatehealth.psu.edu.
Andrew Tinsley, Email: atinsley@pennstatehealth.psu.edu.
Emmanuelle D. Williams, Email: ewilliams3@pennstatehealth.psu.edu.
Piotr Janicki, Email: pjanicki@pennstatehealth.psu.edu.
Victor Ruiz-Velasco, Email: vjr10@psu.edu.
Kent E. Vrana, Email: kvrana@pennstatehealth.psu.edu.
REFERENCES
- 1.Dahlhamer JM, Zammitti EP, Ward BW, et al. Prevalence of inflammatory bowel disease among adults aged ≥18 years - United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65(42):1166–9. [DOI] [PubMed] [Google Scholar]
- 2.Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflamm Bowel Dis 2009;15(5):778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norton C, Czuber-Dochan W, Artom M, et al. Systematic review: Interventions for abdominal pain management in inflammatory bowel disease. Aliment Pharmacol Ther 2017;46(2):115–25. [DOI] [PubMed] [Google Scholar]
- 4.Louis E, Siegel CA, James B, et al. Patients with inflammatory bowel disease have heterogeneous treatment preferences that are largely determined by the avoidance of abdominal pain and side effects [P-POWER IBD study]. J Crohns Colitis 2023;17(2):231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G, Pedarla V, Null KD, et al. Health care costs and resource utilization among patients with Crohn's disease with and without perianal fistula. Inflamm Bowel Dis 2022;28(6):870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen R, Skup M, Ozbay AB, et al. Direct and indirect healthcare resource utilization and costs associated with ulcerative colitis in a privately-insured employed population in the US. J Med Econ 2015;18(6):447–56. [DOI] [PubMed] [Google Scholar]
- 7.Park KT, Ehrlich OG, Allen JI, et al. The cost of inflammatory bowel disease: An initiative from the Crohn's & Colitis Foundation. Inflamm Bowel Dis 2020;26(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogale K, Maheshwari P, Kang M, et al. Symptoms associated with healthcare resource utilization in the setting of inflammatory bowel disease. Sci Rep 2022;12(1):10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut 2014;63(1):88–95. [DOI] [PubMed] [Google Scholar]
- 10.Click B, Vargas EJ, Anderson AM, et al. Silent Crohn's disease: Asymptomatic patients with elevated C-reactive protein are at risk for subsequent hospitalization. Inflamm Bowel Dis 2015;21(10):2254–61. [DOI] [PubMed] [Google Scholar]
- 11.Coates MD, Soriano C, Dalessio S, et al. Gastrointestinal hypoalgesia in inflammatory bowel disease. Ann Gastroenterol 2020;33(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Lopez E, Imamura Kawasawa Y, Walter V, et al. Homozygosity for the SCN10A polymorphism rs6795970 is associated with hypoalgesic inflammatory bowel disease phenotype. Front Med (Lausanne) 2018;5:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coates MD, Vrana KE, Ruiz-Velasco V. The influence of voltage-gated sodium channels on human gastrointestinal nociception. Neurogastroenterol Motil 2019;31(2):e13460. [DOI] [PubMed] [Google Scholar]
- 14.Duan G, Han C, Wang Q, et al. A SCN10A SNP biases human pain sensitivity. Mol Pain 2016;12:1744806916666083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coates MD, Kim JS, Carkaci-Salli N, et al. Impact of the naV1.8 variant, A1073V, on post-sigmoidectomy pain and electrophysiological function in rat sympathetic neurons. J Neurophysiol 2019;122(6):2591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006;55(6):749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peyrin-Biroulet L, Panes J, Sandborn WJ, et al. Defining disease severity in inflammatory bowel diseases: Current and future directions. Clin Gastroenterol Hepatol 2016;14(3):348–54.e17. [DOI] [PubMed] [Google Scholar]
- 18.Irvine EJ, Zhou Q, Thompson AK. The short inflammatory bowel disease questionnaire: A quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT investigators. Canadian Crohn's relapse prevention trial. Am J Gastroenterol 1996;91(8):1571–8. [PubMed] [Google Scholar]
- 19.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National cooperative Crohn's disease study. Gastroenterology 1976;70(3):439–44. [PubMed] [Google Scholar]
- 20.Coates MD, Lahoti M, Binion DG, et al. Abdominal pain in ulcerative colitis. Inflamm Bowel Dis 2013;19(10):2207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cremonini F, Houghton LA, Camilleri M, et al. Barostat testing of rectal sensation and compliance in humans: Comparison of results across two centres and overall reproducibility. Neurogastroenterol Motil 2005;17(6):810–20. [DOI] [PubMed] [Google Scholar]
- 22.Hammer HF, Phillips SF, Camilleri M, et al. Rectal tone, distensibility, and perception: Reproducibility and response to different distensions. Am J Physiol 1998;274(3):G584–90. [DOI] [PubMed] [Google Scholar]
- 23.Chambers JC, Zhao J, Terracciano CM, et al. Genetic variation in SCN10A influences cardiac conduction. Nat Genet 2010;42(2):149–52. [DOI] [PubMed] [Google Scholar]
- 24.Delaney JT, Muhammad R, Shi Y, et al. Common SCN10A variants modulate PR interval and heart rate response during atrial fibrillation. Europace 2014;16(4):485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito YA, Strege PR, Tester DJ, et al. Sodium channel mutation in irritable bowel syndrome: Evidence for an ion channelopathy. Am J Physiol Gastrointest Liver Physiol 2009;296(2):G211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arisawa T, Tahara T, Shiroeda H, et al. Genetic polymorphisms of SCN10A are associated with functional dyspepsia in Japanese subjects. J Gastroenterol 2013;48(1):73–80. [DOI] [PubMed] [Google Scholar]
- 27.Jones J, Correll DJ, Lechner SM, et al. Selective inhibition of Na(v)1.8 with VX-548 for acute pain. N Engl J Med 2023;389(5):393–405. [DOI] [PubMed] [Google Scholar]