About 50% of women with fibromyalgia have reduced skin innervation. We discuss the potential relevance of this finding in the context of pathophysiology and phenotype.
Keywords: Fibromyalgia, Small fiber pathology, Small fiber neurology, Evoked potentials, Quantitative sensory testing, Microneurography, Corneal confocal microscopy
Abstract
About 50% of women with fibromyalgia syndrome have reduced skin innervation. This finding is consistent in patient cohorts from different regions of the world. Small fiber function may also be affected, as shown by various studies using different methods, such as quantitative sensory testing or special small fiber neurophysiology such as C-fiber microneurography. Microneurography in particular has shown increased spontaneous activity, mechanosensitivity, and enhanced activity-induced slowing in C fibers of patients with fibromyalgia. Generalized reduction of skin innervation, ie, proximally and distally, was associated with higher symptom severity and more pronounced central nervous system changes as seen in magnetic resonance tomography. The question whether peripheral or central nervous system changes come first, or whether both are signs of an underlying pathology, has not been resolved yet. For clinical practice, it is important to note that reduced skin innervation in fibromyalgia must not be confused with small fiber neuropathy, which is a separate entity with different characteristics and pathophysiology. Further prospective research is warranted to transfer these findings in the peripheral nervous system into clinical fibromyalgia patient management.
1. Introduction
The first description of reduced small nerve fiber function and density in skin of patients with fibromyalgia syndrome (FMS)57 was rapidly confirmed by other groups.9,12,25,37,46 Since then, the discussion has been ongoing, whether there are communalities between FMS and small fiber neuropathy (SFN),10,42,53,56 whether small fiber pathology in FMS is primary or secondary,26,33,42 and whether it has an impact on FMS symptomatology.19,42 We, therefore, did a systematic literature search using the key words “fibromyalgia” AND “small fiber,” where we found 118 articles published between 2013 and 2024. In this narrative review, we summarize data on small fiber pathology in FMS, provide evidence that FMS and SFN are distinct entities, and discuss further implications of malfunction of small nerve fibers for the pathophysiology of FMS.
2. Small fiber pathology in fibromyalgia: data from skin biopsy studies
In our first case–control study, the marked reduction of intraepidermal nerve fiber density (IENFD) in the skin of the lower and upper leg of patients with FMS came by surprise.57 In this study, we had systematically applied 3 small fiber tests in women with FMS and found that indeed IENFD was lowered. This finding was paralleled by elevated thermal perception thresholds in quantitative sensory testing (QST) and findings indicating A-delta fiber malconduction in pain-related evoked potentials (PREP). When regarding the group medians, no reduction in skin innervation was found in healthy persons and in a disease control group consisting of women with major depression and no pain. Initially, these data were received very skeptically by the scientific community because FMS was regarded mainly as a disorder of central pain processing, if a disease at all. However, the finding of reduced skin innervation in FMS was confirmed rapidly by a number of independent groups,9,12,25,37,46 such that there was no more doubt that this was a valid and reproducible finding. Later studies showed that IENFD is reduced in about 50% of women with FMS, as confirmed by a recent meta-analysis including 20 articles involving 903 patients with FMS.23
Going further, we used electron microscopy to analyze skin nerve fibers in FMS in more depth. In a new cohort of patients with FMS, compared with healthy controls and with patients with SFN, we obtained skin biopsies from both distal and proximal areas of the leg, as well as the index finger.15 These biopsies underwent processing for immunofluorescence and electron microscopy. To gauge the diameter of small unmyelinated nerve fibers, we meticulously measured 10 transversely cut axons from each biopsy, the investigator being blinded as to the allocation of samples. Our findings revealed a noteworthy reduction in the mean axon diameter among patients with FMS, as compared to those with SFN and the healthy control group. Of note, this finding was present at all biopsy sites, indicating a general and not length-dependent process. In addition, we corroborated previous observations of disrupted small fiber function by QST and reduced IENFD in women with FMS.
In a later, comprehensive study involving 117 women with FMS, we conducted various assessments, including skin punch biopsy, corneal confocal microscopy (CCM), microneurography, QST, and PREP. Results revealed a significant reduction in IENFD at different biopsy sites in 63% of patients with FMS, compared with 10% in those with major depressive disorder and pain and 18% in healthy controls. Different patterns of skin innervation were identified in patients with FMS, and microneurography indicated distinct abnormalities, emphasizing the extent of small fiber pathology in FMS.19 Of note, those individuals with generalized small fiber loss had a more severe form of FMS. We then investigated potential pathophysiological factors underlying the small fiber pathology. In 128 patients and 26 healthy controls, skin punch biopsies were investigated, revealing elevated expression of transforming growth factor-ß1 (TGF-ß1), hyperpolarization-activated cyclic nucleotide-gated ion channel 2, ephrin-A4, and ephrin receptor-A4 in fibroblasts and keratinocytes. We concluded from these findings that skin cells may contribute to cutaneous nociception by differentially expressing membrane-bound and soluble pain mediators and axon pathfinders and that a disturbance in attractant and repellant ephrins in the epidermis might contribute to reduced IENFD in FMS.20 This could also be an explanation for the reduced number of regenerating fibers in the epidermis as indicated by immunoreactivity for growth-associated protein 43 (GAP43).57
As to dermal and blood-vessel innervation in FMS, data are scarce. In our first cohort with reduced IENFD, we found reduced numbers of dermal unmyelinated fibers and normal numbers of dermal myelinated fibers, indicating a C-fiber-associated pathological process.57 In a second cohort, dermal myelinated and dermal peptidergic fibers were not different between patients with FMS and controls, but patients with FMS had a markedly reduced density of nerve fibers alongside blood vessels.18 In an earlier study, the innervation of arteriole–venule shunts of glabrous hypothenar skin was assessed in patients with FMS and healthy controls revealing that patients with FMS had excessive peptidergic innervation.1 In addition, arteriole–venule shunt profiles from patients with FMS were larger in size compared with those in age-matched control subjects. This finding was not present in thoracic skin, but thoracic skin had a mildly reduced IENFD compared with controls. Interestingly, the authors observed that neurovascular sensory innervation is estrogen-dependent in rats,6 that females normally have twice the sensory innervation to arterioles and arteriole–venule shunts as males, and that this might predispose women to developing the excessive sensory innervation of the arteriole–venule shunts observed in patients with FMS .1 See also Table 1 for a summary of findings.
Table 1.
Overview of selected studies on small fiber pathology in fibromyalgia syndrome.
| Author, y | Reference | IENFD reduced (leg) | QST indicates small fiber dysfunction | EP indicates dysfunction of small fibers or their ascending tracts | CCM pathological | MN pathological | Other tests/comments |
|---|---|---|---|---|---|---|---|
| Üçeyler et al. 2013 | 57 | Yes | Yes | Yes | — | — | |
| Oaklander et al. 2013 | 46 | Yes | — | — | — | — | |
| Albrecht et al. 2013 | 1 | — | — | — | — | — | Excessive peptidergic innervation in dermal arteriovenous shunts |
| de Tommaso et al. 2014 | 12 | Yes | — | Yes | — | — | |
| Caro and Winter 2014 | 9 | Yes | — | — | — | — | |
| Giannoccaro et al. 2014 | 25 | Yes | — | — | — | — | |
| Kosmidis et al. 2014 | 37 | Yes | — | — | — | — | |
| Serra et al. 2014 | 52 | — | — | — | — | Yes | |
| Doppler et al. 2015 | 15 | Yes | Yes | — | — | — | Electron microscopy reveals reduced axonal diameters |
| Evdokimov et al. 2019 | 19 | Yes | Yes | Yes | Yes | Yes | |
| Evdokimov et al. 2020 | 18 | — | — | — | — | — | Dermal blood vessel innervation reduced |
| Fasolino et al. 2020 | 22 | Yes* | Yes* | Yes* | — | — | SFP not related to somatosensory function |
| Vecchio et al. 2020 | 59 | Yes | — | No | — | — | LEPs indicative of central impairment |
| Van Assche et al. 2020 | 58 | — | — | No | — | — | LEPs not pathologic in FMS |
| Pickering et al. 2020 | 47 | — | Yes | — | — | — | Sudomotor function impaired |
| Leone et al. 2023 | 40 | Yes | Yes | — | — | — | |
| Quitadamo et al. 2023 | 50 | Yes | — | — | — | — | IENFD not changed over time (18 mo) |
| Falco et al. 2024 | 21 | Yes | — | — | — | — | Innervation of piloerector muscles and sweat glands reduced |
CCM, corneal confocal microscopy; EPs, evoked potentials; FMS, fibromyalgia syndrome; IENFD, intraepidermal nerve fiber density; LEPs, laser-evoked potentials; MN, microneurography; QST, quantitative sensory testing; SFP, small fiber pathology; —, not performed.
Partially.
3. The difference between small fiber pathology in fibromyalgia and small fiber neuropathy
Although a reduced IENFD can be found in about 50% of female patients with FMS, this does not automatically imply that they suffer from an additional SFN or that SFN is even the cause of fibromyalgia symptoms. First of all, FMS and SFN are clinically distinct31; see Table 2. A recent cross-sectional study on 158 women with FMS and 53 with SFN showed that patients with FMS were younger at symptom onset, had higher pain intensities, and had more generalized pain. In FMS, pain was accompanied by irritable bowel syndrome or sleep disturbances, and in SFN, pain was accompanied by paresthesias, numbness, and impaired glucose metabolism. Family history revealed chronic pain and affective disorders in FMS and other neurological disorders in patients with SFN. Also, the pattern of small fiber loss was different, with patients with FMS mainly showing proximally reduced skin innervation and patients with SFN often having distally accentuated small fiber loss.31 It is plausible that patients with SFN could develop a widespread pain phenotype over time, as suggested in Reference 17. However, as highlighted in Reference 31, there are notable differences in the assessment of symptoms and additional symptoms between the 2 entities when examined in greater detail. Of note, skin denervation as an isolated finding is not sufficient to make the diagnosis of SFN13,17 and that up to 18% of healthy controls have impaired skin innervation.17 The finding of reduced IENFD needs to be evaluated in context of the patient's pain history and the results of the neurological examination.
Table 2.
Differences between fibromyalgia syndrome and small fiber neuropathy.
| Parameter | FMS | SFN | References |
|---|---|---|---|
| Age at onset (median, range) | 35 (4–65) | 47 (12–67) | 31 |
| Pain localization | Proximal, generalized | Distal, feet | 31 |
| Accompanying symptoms | IBS, sleep disturbance | IGT, numbness | 31 |
| Pattern of small fiber loss | Generalized | Length-dependent | 31 |
| QST | Mild deficits in temperature detection | Strong deficits in temperature detection | 40 |
| Activity-induced slowing (MN) | Increased | Regular | 52 |
| Gene expression in keratinocytes | Not different from controls | Inflammatory genes upregulated | 33 |
FMS, fibromyalgia syndrome; IBS, irritable bowel syndrome; IGT, impaired glucose tolerance; MN, microneurography; QST, quantitative sensory testing; SFN, small fiber neuropathy.
Although some patients with FMS have a length-dependent reduction of skin innervation, as in length-dependent SFN,19,55 many have proximally accentuated or even generalized fiber loss.19 In one study, IENFD was reduced at the thigh in 85% of patients with FMS and in only 12% of patients at the lower leg.59 The finding of a proximal reduction of skin innervation is surprising in view of the normally distal-to-proximal spread of a peripheral neuropathy. The underlying pathophysiological mechanism remains to be explored.
Previous studies have shown important physiological and anatomical differences in C fibers between FMS and SFN. Activity-induced slowing, a hallmark of C fibers in microneurography, was markedly increased in FMS, but not in SFN.52 As speculation, this may be related to the finding that dermal C fibers were considerably thinner in FMS, compared with SFN and with healthy controls, irrespective of the biopsy site (lower leg, upper leg, and finger); see Figure 1.8,15
Figure 1.
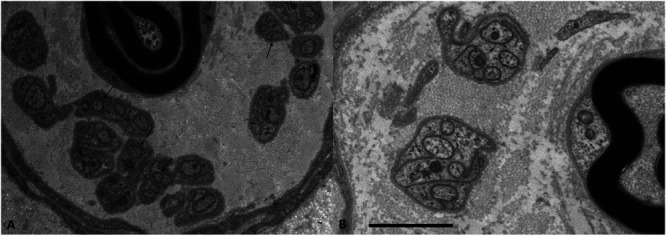
Example of axons with reduced diameters in patients with FMS. Electron micrograph of a Remak bundle. Numerous unmyelinated fibers of small diameter (arrows) are found in a patient with FMS (A), but not in a normal control (B). Bar = 2 mm. FMS, fibromyalgia syndrome. Figure from Reference 15, RightsLink licence number 5817811230620.
A study using QST found more pronounced abnormalities in temperature perception thresholds of patients with SFN than in patients with FMS, regardless of whether the latter had reduced IENFD or not.40 Interestingly, mild deficits in temperature sensation were also present in patients with FMS with normal IENFD, indicating a potential dysfunction that is present even before overt morphological fiber loss. Furthermore, when comparing FMS and SFN, there is always the risk of misdiagnosis, because of overlapping phenotypes, as shown by some patients later detected to harbor sodium channelopathies.22 In addition, QST abnormalities related to thermal thresholds in patients with FMS mostly did not cross the normal ranges (ie, 0 ± 1.96). This may be different from what is usually seen in patients with SFN as was also shown recently.31
RNA sequencing of keratinocytes obtained from patients with SFN, FMS, and healthy controls showed 141 deregulated protein-coding genes between patients with SFN and healthy controls and none between patients with FMS and healthy controls.33 When comparing patients with SFN with patients with FMS, 167 differentially expressed protein-coding genes (129 upregulated and 38 downregulated) were detected. Inflammatory pathways were particularly enriched. Validation in an independent cohort confirmed higher expression of the proinflammatory mediators interleukin-8, C-X-C motif chemokine 3, endothelin receptor type A, and the voltage-gated sodium channel 1.7 in SFN compared with FMS. Thus, a distinct keratinocyte transcriptome signature in SFN, compared with controls, indicates a pathological process in the keratinocytes in this disorder, but not in FMS. Although data interpretation needs caution because of the low number of subjects investigated, these findings support the notion of different pathophysiological mechanisms potentially involving the neurocutaneous unit in SFN and FMS pain.33
A microRNA expression study in patients with FMS identified aberrantly expressed microRNAs (miR) in white blood cells, particularly miR-let-7d, which correlated with reduced small nerve fiber density. The investigation further revealed miR-let-7d and its downstream target, insulin-like growth factor-1 receptor (IGF-1R), as being aberrantly expressed in the skin of patients with FMS with small nerve fiber impairment. Although again caution is needed when interpreting the data obtained in a small patient cohort, these findings may establish a link between systemic miR expression and small fiber pathology in FMS subgroups.39 No data are available for comparison of the miR and IGF-1R data in SFN. Interestingly, serum levels of IGF-1 were reduced in patients with FMS and related to some symptoms,5 supporting the notion of disturbance of the IGF system in FMS.
Interestingly, reduced skin innervation does not seem to be progressive in FMS. A study with 62 patients with FMS observed over 18 months found no further decrease in IENFD over time.50 This is in line with the findings of our cross-sectional study, where IENFD was not age-related in patients with FMS.19 By contrast, IENFD is age-dependently reduced in healthy people38 and in idiopathic and diabetic SFN.35,41,63
4. Small fiber dysfunction in fibromyalgia: neurophysiological studies
A case–control study examined the function and morphology of small nerve fibers in 25 patients with FMS, revealing impaired small fiber function through quantitative sensory testing and PREP. Patients exhibited abnormal N1 latencies when stimulated at the feet, and reduced PREP amplitudes at all stimulation sites compared with healthy and disease controls, indicating abnormalities in small fibers or their central afferents.57 In a later study with also larger control groups, findings on latencies were reversed, although the finding of reduced amplitudes was confirmed.19
Similarly, using laser-evoked potentials (LEPs), mean N2-P2 amplitudes were reduced in FMS, in comparison with healthy controls and disease controls with migraine.12 Intraepidermal nerve fiber density correlated with LEP amplitudes when stimulated at the hand or at the chest tender point, and LEP habituation correlated with pain at tender points. Not all studies confirm this finding. In one cohort, no reduction in LEP amplitudes in FMS was found; however, this was a retrospective analysis.58 These authors found an age-related decrease in LEP N2-P2 amplitudes in both patients with FMS and healthy controls.
Patients with FMS with and without small fiber pathology were compared using multichannel LEPs.12 Amplitudes, and particularly those of the P2 response, were reduced in patients with distal or proximal reduction of skin innervation. Not all the authors' findings were coherent with epidermal nerve fiber density loss, they, therefore, emphasize the complexity of FMS and assume additional CNS mechanisms in FMS.60
Microneurography is a powerful tool to assess the function and characteristics of C fibers.
Silent nociceptors, ie, C fibers that are not active in healthy conditions, exhibited abnormalities in 77% of patients with FMS in one cohort.52 Spontaneous activity was detected in 31% of FMS silent nociceptors in FMS, whereas it was present in only 2% in controls. About 24% of the silent nociceptors in FMS exhibited sensitization to mechanical stimuli. Both these findings were similar to those in a cohort of patients with SFN. However, abnormally high slowing of conduction velocity upon stimulation at 0.25 Hz (“activity-induced slowing”) was markedly enhanced in FMS but not in SFN.52 These findings could later be confirmed in a larger, independent cohort.19
Impaired sudomotor function has been shown in a subgroup of patients with FMS.47 A later study set out to assess a possible association between central sensitization and small nerve fiber impairment in patients with FMS.16 Using measurements of electrochemical skin conductance, 20% of patients with FMS had reduced values. These patients were more severely affected by the FMS and had higher scores in the central sensitization inventory. The authors concluded that in FMS, peripheral and central pathologies coexist and might even promote each other.
In a recent investigation, innervation of piloerector muscles and sweat glands was reduced in patients with FMS that had a reduced IENFD, but not as much as in patients with SFN.21 Measures of the autonomic innervation were not correlated with the severity of autonomic symptoms.
5. Relation of small fiber pathology or dysfunction and fibromyalgia symptoms
Measures of FMS symptom severity and impact show that patients with severely and generally reduced IENFD have higher FMS symptom load than those without.19 The finding that the subgroup of patients with FMS with reduced skin innervation is more severely affected by the disease was also confirmed in another cohort.50 Furthermore, the FMS subgroup with generally reduced IENFD has a higher degree of CNS pathology as shown by different methods of magnetic resonance imaging.3 Specifically, the subgroup with reduced skin innervation showed hyperconnectivity between the inferior frontal gyrus, the angular gyrus, and the posterior parietal gyrus. Cortical thickness was decreased in regions of the frontal, temporal, and parietal of patients with FMS and further decreased in the bilateral pericalcarine cortices in the subgroup with small fiber pathology.3 Although some of these findings were also described by others independent of skin innervation patterns,32 their pathophysiological impact and clinical relevance remain to be elucidated.
Some investigators use CCM parameters to define small fiber pathology in FMS. Applying this method in a small cohort, CCM findings were not associated with neuropathic-like symptoms.42 However, although we also had pathological findings in CCM of our cohorts, not all patients concomitantly have reduced skin innervation and CCM abnormalities,19,36 such that findings derived from each method need to be regarded separately.
It should be mentioned that some studies did not use skin biopsy, but nevertheless looked for and found small fiber dysfunction in FMS, eg, applying screening tools for neuropathic pain14,61 or electrodiagnostic tests of lumbar and sacral nerve root myotomes.30 Also, in a study testing the ratings for pleasant touch, patients with FMS rated this slow brushing less pleasant than controls.7,19 Because the authors found no differences in brain activity during the touch, the underlying issue might be one of peripheral innervation, but this was not tested in the study. It is of note that impairment of small caliber nerve fibers may also lead to autonomic dysfunction such as dyshidrosis, sexual dysfunction, or gastrointestinal symptoms. In a recent study, correlations with small nerve fiber pathology and FMS-associated autonomic symptoms were described.21 Although not specific and lacking a disease control group, these data may shed light on the potentially multifaceted impact of small fiber damage to FMS symptoms.
Fibromyalgia syndrome pain has been labeled as “nociplastic” because of the central sensitization component. In this context, it would be interesting to ask whether small fiber pathology is also present in other disorders with a nociplastic component, such as complex regional pain syndrome (CRPS), chronic primary low back pain, or chronic primary visceral pain. There is little information on this question. In CRPS, some but not all groups found a reduced skin innervation,2,28 and a condition similar to SFN has been suspected in a small number of patients with irritable bowel syndrome, but without biopsy verification.45
6. What comes first, the brain or the peripheral nervous system?
Neuroimaging studies identified increased glutamate levels in the insula of patients with FMS, suggesting a link between fibromyalgia symptoms and heightened central excitatory neurotransmission.49 A study using a glutamate transport inhibitor in rats provided preliminary evidence that insular hyperactivity might be a causal factor in the development of small fiber pathology in FMS.27 Increasing endogenous glutamate in the insula was associated with decreases in mechanical paw withdrawal thresholds and thermal paw withdrawal latency in the rats, as well as increased aversion to noxious mechanical stimuli. In parallel, the treated rats had a decrease in IENFD at the paw. The authors concluded that the reduction in multimodal pain behaviors and lowered density of skin innervation support the notion of insular hyperactivity contributing to small fiber pathology. These findings still need to be confirmed and, in particular, verified in patients.
Although it might be tempting to speculate that abnormalities in the autonomic system, which have been described in FMS to varying degrees,11,62 influence skin innervation, no evidence for this has not been shown yet. If physical activity improves skin innervation, as suggested by a small study,24 physical deconditioning might do the opposite, but this also has not been shown yet.
7. Are fibromyalgia syndrome skin findings relevant for treatment?
Given that about 50% of patients with FMS have small fiber pathology, it is conceivable that there is a “neuropathic” subtype of FMS. Accordingly, we hypothesized that those with and without small fiber pathology would differ in their response to drugs with good efficacy for neuropathic pain, such as pregabalin or duloxetine, which are also approved for FMS treatment by the Food and Drug Administration in the United States. Although no data from prospective studies are available to answer this question, we did a retrospective analysis of our own database and did not find a relation between reduced IENFD and a good response to antineuropathic drugs.4 However, our study was performed in Germany, where none of these drugs is approved for FMS treatment, such that fewer patients may have access to them.
Another question is whether the reduced IENFD in FMS may improve upon adequate treatment. This has occasionally been observed in other disorders, although not in all diseases studied, even under causally oriented treatment.29,34,48,51,54 It is, therefore, surprising to see that in a small study using a home-based multicomponent physical activity intervention that was conducted twice a week, not only measures of disease impact improved, but also IENFD increased after 18 months.24 Changes in IENFD were plus 1 fiber at the proximal and plus 2 fibers at the distal biopsy site in the intervention group, whereas IENFD remained unchanged in the control group. The increase in IENFD correlated with improvement in the Fibromyalgia Impact Questionnaire. Although these data are promising, they need to be replicated in a larger patient group. Also, such studies need to be controlled for test–retest reliability.
A small cohort of patients with FMS and reduced IENFD were treated with intravenous immunoglobulins (IVIg) in a retrospective pilot study.44 Skin punch biopsies were performed, assessing nerve fiber density and symptoms through a fibromyalgia questionnaire. After 6 months of IVIg therapy, patients reported fewer fibromyalgia symptoms, accompanied by improvement in skin biopsy results. This study suggests IVIg as a potential therapy in a subset of fibromyalgia patients with SFN.
8. Discussion
The finding of small fiber pathology in subgroups of women with FMS has opened up a new area in FMS research and has helped recognize FMS as a disease. The fact that findings of small fiber pathology have been confirmed by very many independent research groups worldwide strengthens the notion that the peripheral nervous system is involved in subgroups of patients with FMS and adds to the evidence of a peripheral contribution to FMS pain.
However, 11 years after its first description, there are still substantial blind spots and open questions as for the pathophysiological mechanisms and the clinical relevance of small nerve fiber impairment in patients with FMS. Hence, long-term studies and prospective trials assessing a potential diagnostic relevance of skin punch biopsy assessment in patients with FMS are warranted and also demanded by patients with FMS who have high hopes and expectations regarding the advent of an objective diagnostic tool to be used in clinical practice.
Fibromyalgia syndrome may be rich in its clinical presentation, and it is obvious that peripheral nervous system alterations are not sufficient to explain all aspects of FMS pain, let alone the entire syndrome. To categorize patients with regard to the peripheral nervous system, findings may help not only to improve diagnostics but also to stratify analgesic treatment options. Analgesic treatment attempts often remain insufficient in applying the same algorithms and drugs to all patients despite overt phenotypic heterogeneity spanning a spectrum from focal to generalized pain and pain accompanied by sensory symptoms and without small fiber pathology.43 This is also reflected by heterogeneity in treatment responses, which is a common observation in clinical practice. Integration of small nerve fiber assessment into the clinical management of patients with FMS may help improve treatment stratification when proven efficient in prospective studies. Furthermore, analgesics recommended by national and international FMS guidelines do have strong central effects, and novel developments are warranted to target peripheral pathology.
When assessing current studies on the small-caliber nerve fibers, critical appraisal is necessary as for the size of study cohorts and the statistical analysis performed on each. Here, small patient groups, the lack of appropriate healthy and disease control groups, low statistical power, and the application of inappropriate statistical tests are some aspects to be considered in future studies to obtain more robust data. Particularly, the choice of the control groups is essential because small fiber tests may have divergent results depending on this exact fact as, eg, we reported for QST.19,57 Also, the fact that all findings described to date are based on group effects and not on an individual basis needs to be considered. This hampers transfer of the small fiber tests into clinical practice of FMS management.
The major question about the “why” of small fiber degeneration and sensitization in FMS is still unsolved. Research is advancing rapidly, and it is encouraging to see the increase in state-of-the-art basic science approaches in FMS research including in vivo animal and human in vitro studies adding substantially to the careful clinical work that has been performed over decades. The assessment of potential cellular mechanisms and the impact of immunological processes will hopefully help to link and understand the currently many different, mainly single findings reported in a myriad of articles published on FMS pathophysiology. Particularly, the effect of serum autoantibodies recently raised considerable attention.26 Although the exact mechanisms remain to be elucidated, the induction of peripheral denervation in a murine model is encouraging for future studies.
Eleven years after the first description of small fiber pathology in FMS, there are further crucial questions to be answered. One is, whether the small caliber nerve fibers are also affected in men with FMS. This question has not yet been addressed systematically and data reported from few male study participants in studies mainly investigating women are not adequately informative. Furthermore, the longitudinal development of small nerve fiber degeneration and sensitization needs close attention in sufficiently powered prospective studies including disease and healthy controls. Such studies will shed light on the pathophysiological impact small fiber impairment may have in FMS. Also, the riddle of the different skin innervation patterns ranging from normal to generalized loss needs a solution. Here, the primary proximal loss of intraepidermal nerve fibers is a particularly interesting finding of unknown pathophysiology. Finally, data on the peripheral nervous system need to be linked with the central nervous system because FMS can only be explained and understood when focusing on both.
Still, the finding of small nerve fiber impairment marks a milestone in FMS research and management. Not only has it extended our objective view on a condition otherwise challenging to get a handle on, but even more so: It has helped to destigmatize patients suffering from a condition that was marginalized or even assumed nonexistent for decades.
Disclosures
The authors have no conflicts of interest regarding the content of this manuscript.
Acknowledgements
The authors' own work cited in this review was funded by The Else Kröner-Fresenius-Stiftung (N.Ü., 2014_A129), Evangelisches Studienwerk Villigst (to C.S.), and the Interdisciplinary Center for Clinical Research Würzburg (C.S., F-376). Both authors' work is funded by Deutsche Forschungsgemeinschaft, KFO5001 “ResolvePAIN.” N.Ü. was funded by Deutsche Forschungsgemeinschaft (UE171/15-1).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Albrecht PJ, Hou Q, Argoff CE, Storey JR, Wymer JP, Rice FL. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (AVS) in the palmar glabrous skin of fibromyalgia patients: implications for widespread deep tissue pain and fatigue. Pain Med 2013;14:895–915. [DOI] [PubMed] [Google Scholar]
- [2].Andronic D, Andronic O, Juengel A, Berli MC, Distler O, Brunner F. Skin biomarkers associated with complex regional pain syndrome (CRPS) type I: a systematic review. Rheumatol Int 2022;42:937–47. [DOI] [PubMed] [Google Scholar]
- [3].Aster HC, Evdokimov D, Braun A, Üçeyler N, Kampf T, Pham M, Homola GA, Sommer C. CNS imaging characteristics in fibromyalgia patients with and without peripheral nerve involvement. Sci Rep 2022;12:6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aster HC, Evdokimov D, Braun A, Üçeyler N, Sommer C. Analgesic medication in fibromyalgia patients: a cross-sectional study. Pain Res Manag 2022;2022:1217717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Atamer Y, Şahbaz T, Aşık HK, Saraç S, Atamer A. The relationship between serum leptin, insulin-like growth factor-1, and insulin-like growth factor binding protein-3 levels and clinical parameters in primary fibromyalgia patients. Rev Assoc Med Bras 2023;69:e20230240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Blacklock AD, Cauveren JA, Smith PG. Estrogen selectively increases sensory nociceptor innervation of arterioles in the female rat. Brain Res 2004;1018:55–65. [DOI] [PubMed] [Google Scholar]
- [7].Boehme R, van Ettinger-Veenstra H, Olausson H, Gerdle B, Nagi SS. Anhedonia to gentle touch in fibromyalgia: normal sensory processing but abnormal evaluation. Brain Sci 2020;10:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Campero M, Serra J, Bostock H, Ochoa JL. Slowly conducting afferents activated by innocuous low temperature in human skin. J Physiol 2001;535:855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Caro XJ, Winter EF. Evidence of abnormal epidermal nerve fiber density in fibromyalgia: clinical and immunologic implications. Arthritis Rheumatol 2014;66:1945–54. [DOI] [PubMed] [Google Scholar]
- [10].Clauw DJ. What is the meaning of “small fiber neuropathy” in fibromyalgia? PAIN 2015;156:2115–6. [DOI] [PubMed] [Google Scholar]
- [11].Cohen H, Neumann L, Kotler M, Buskila D. Autonomic nervous system derangement in fibromyalgia syndrome and related disorders. Isr Med Assoc J 2001;3:755–60. [PubMed] [Google Scholar]
- [12].de Tommaso M, Nolano M, Iannone F, Vecchio E, Ricci K, Lorenzo M, Delussi M, Girolamo F, Lavolpe V, Provitera V, Stancanelli A, Lapadula G, Livrea P. Update on laser-evoked potential findings in fibromyalgia patients in light of clinical and skin biopsy features. J Neurol 2014;261:461–72. [DOI] [PubMed] [Google Scholar]
- [13].Devigili G, Rinaldo S, Lombardi R, Cazzato D, Marchi M, Salvi E, Eleopra R, Lauria G. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain 2019;142:3728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Di Carlo M, Cesaroni P, Salaffi F. Neuropathic pain features suggestive of small fibre neuropathy in fibromyalgia syndrome: a clinical and ultrasonographic study on female patients. Clin Exp Rheumatol 2021;9(suppl 10):102–7. [DOI] [PubMed] [Google Scholar]
- [15].Doppler K, Rittner HL, Deckart M, Sommer C. Reduced dermal nerve fiber diameter in skin biopsies of patients with fibromyalgia. PAIN 2015;156:2319–25. [DOI] [PubMed] [Google Scholar]
- [16].Dumolard A, Lefaucheur JP, Hodaj E, Liateni Z, Payen JF, Hodaj H. Central sensitization and small-fiber neuropathy are associated in patients with fibromyalgia. Clin J Pain 2023;39:8–14. [DOI] [PubMed] [Google Scholar]
- [17].Egenolf N, Zu Altenschildesche CM, Kreß L, Eggermann K, Namer B, Gross F, Klitsch A, Malzacher T, Kampik D, Malik RA, Kurth I, Sommer C, Üçeyler N. Diagnosing small fiber neuropathy in clinical practice: a deep phenotyping study. Ther Adv Neurol Disord 2021;14:17562864211004318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Evdokimov D, Dinkel P, Frank J, Sommer C, Üçeyler N. Characterization of dermal skin innervation in fibromyalgia syndrome. PLoS One 2020;15:e0227674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Evdokimov D, Frank J, Klitsch A, Unterecker S, Warrings B, Serra J, Papagianni A, Saffer N, Meyer Zu Altenschildesche C, Kampik D, Malik RA, Sommer C, Üçeyler N. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann Neurol 2019;86:504–16. [DOI] [PubMed] [Google Scholar]
- [20].Evdokimov D, Kreß L, Dinkel P, Frank J, Sommer C, Üçeyler N. Pain-associated mediators and axon pathfinders in fibromyalgia skin cells. J Rheumatol 2020;47:140–8. [DOI] [PubMed] [Google Scholar]
- [21].Falco P, Galosi E, Di Stefano G, Leone C, Di Pietro G, Tramontana L, De Stefano G, Litewczuk D, Esposito N, Truini A. Autonomic small-fiber pathology in patients with fibromyalgia. J Pain 2024;25:64–72. [DOI] [PubMed] [Google Scholar]
- [22].Fasolino A, Di Stefano G, Leone C, Galosi E, Gioia C, Lucchino B, Terracciano A, Di Franco M, Cruccu G, Truini A. Small-fibre pathology has no impact on somatosensory system function in patients with fibromyalgia. PAIN 2020;161:2385–93. [DOI] [PubMed] [Google Scholar]
- [23].Galosi E, Truini A, Di Stefano G. A systematic review and meta-analysis of the prevalence of small fibre impairment in patients with fibromyalgia. Diagnostics (Basel) 2022;12:1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gentile E, Quitadamo SG, Clemente L, Bonavolontà V, Lombardi R, Lauria G, Greco G, Fischetti F, De Tommaso M. A multicomponent physical activity home-based intervention for fibromyalgia patients: effects on clinical and skin biopsy features. Clin Exp Rheumatol 2024;42:1156–63. [DOI] [PubMed] [Google Scholar]
- [25].Giannoccaro MP, Donadio V, Incensi A, Avoni P, Liguori R. Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve 2014;49:757–9. [DOI] [PubMed] [Google Scholar]
- [26].Goebel A, Krock E, Gentry C, Israel MR, Jurczak A, Urbina CM, Sandor K, Vastani N, Maurer M, Cuhadar U, Sensi S, Nomura Y, Menezes J, Baharpoor A, Brieskorn L, Sandström A, Tour J, Kadetoff D, Haglund L, Kosek E, Bevan S, Svensson CI, Andersson DA. Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest 2021;131:e144201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Harte SE, Clauw DJ, Hayes JM, Feldman EL, St Charles IC, Watson CJ. Reduced intraepidermal nerve fiber density after a sustained increase in insular glutamate: a proof-of-concept study examining the pathogenesis of small fiber pathology in fibromyalgia. Pain Rep 2017;2:e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hartmannsberger B, Scriba S, Guidolin C, Becker J, Mehling K, Doppler K, Sommer C, Rittner HL. Transient immune activation without loss of intraepidermal innervation and associated Schwann cells in patients with complex regional pain syndrome. J Neuroinflammation 2024;21:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Havrdova T, Boucek P, Saudek F, Voska L, Lodererova A, Üçeyler N, Vondrova H, Skibova J, Lipar K, Sommer C. Severe epidermal nerve fiber loss in diabetic neuropathy is not reversed by long-term normoglycemia after simultaneous pancreas and kidney transplantation. Am J Transpl 2016;16:2196–201. [DOI] [PubMed] [Google Scholar]
- [30].Hulens M, Bruyninckx F, Rasschaert R, Vansant G, De Mulder P, Stalmans I, Bervoets C, Dankaerts W. Electrodiagnostic abnormalities associated with fibromyalgia. J Pain Res 2020;13:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jänsch S, Evdokimov D, Egenolf N, Meyer Zu Altenschildesche C, Kreß L, Üçeyler N. Distinguishing fibromyalgia syndrome from small fiber neuropathy: a clinical guide. Pain Rep 2024;9:e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Vitton O, Gracely R, Ingvar M, Kong J. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum 2013;65:3293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Karl F, Bischler T, Egenolf N, Evdokimov D, Heckel T, Üçeyler N. Fibromyalgia vs small fiber neuropathy: diverse keratinocyte transcriptome signature. PAIN 2021;162:2569–77. [DOI] [PubMed] [Google Scholar]
- [34].Khoshnoodi M, Truelove S, Polydefkis M. Effect of diabetes type on long-term outcome of epidermal axon regeneration. Ann Clin Transl Neurol 2019;6:2088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Khoshnoodi MA, Truelove S, Burakgazi A, Hoke A, Mammen AL, Polydefkis M. Longitudinal assessment of small fiber neuropathy: evidence of a non-length-dependent distal axonopathy. JAMA Neurol 2016;73:684–90. [DOI] [PubMed] [Google Scholar]
- [36].Klitsch A, Evdokimov D, Frank J, Thomas D, Saffer N, Meyer Zu Altenschildesche C, Sisignano M, Kampik D, Malik R, Sommer C, Üçeyler N. Reduced association between dendritic cells and corneal sub-basal nerve fibers in patients with fibromyalgia syndrome. J Peripher Nerv Syst 2020;25:9–18. [DOI] [PubMed] [Google Scholar]
- [37].Kosmidis ML, Koutsogeorgopoulou L, Alexopoulos H, Mamali I, Vlachoyiannopoulos PG, Voulgarelis M, Moutsopoulos HM, Tzioufas AG, Dalakas MC. Reduction of Intraepidermal Nerve Fiber Density (IENFD) in the skin biopsies of patients with fibromyalgia: a controlled study. J Neurol Sci 2014;347:143–7. [DOI] [PubMed] [Google Scholar]
- [38].Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, Smith AG, Hsieh ST, Mellgren SI, Umapathi T, Ziegler D, Faber CG, Merkies IS. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010;15:202–7. [DOI] [PubMed] [Google Scholar]
- [39].Leinders M, Doppler K, Klein T, Deckart M, Rittner H, Sommer C, Uceyler N. Increased cutaneous miR-let-7d expression correlates with small nerve fiber pathology in patients with fibromyalgia syndrome. PAIN 2016;157:2493–503. [DOI] [PubMed] [Google Scholar]
- [40].Leone C, Galosi E, Esposito N, Falco P, Fasolino A, Di Pietro G, Di Stefano G, Camerota F, Vollert J, Truini A. Small-fibre damage is associated with distinct sensory phenotypes in patients with fibromyalgia and small-fibre neuropathy. Eur J Pain 2023;27:163–73. [DOI] [PubMed] [Google Scholar]
- [41].Løseth S, Stålberg EV, Lindal S, Olsen E, Jorde R, Mellgren SI. Small and large fiber neuropathy in those with type 1 and type 2 diabetes: a 5-year follow-up study. J Peripher Nerv Syst 2016;21:15–21. [DOI] [PubMed] [Google Scholar]
- [42].Marshall A, Rapteas L, Burgess J, Riley D, Anson M, Matsumoto K, Bennett A, Kaye S, Marshall A, Dunham J, Fallon N, Zhao SS, Pritchard A, Goodson N, Malik RA, Goebel A, Frank B, Alam U. Small fibre pathology, small fibre symptoms and pain in fibromyalgia syndrome. Sci Rep 2024;14:3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Martínez MP, Sánchez AI, Prados G, Lami MJ, Villar B, Miró E. Fibromyalgia as a heterogeneous condition: subgroups of patients based on physical symptoms and cognitive-affective variables related to pain. Span J Psychol 2021;24:e33. [DOI] [PubMed] [Google Scholar]
- [44].Metyas S, Chen C, Quismorio A, Abdo N, Kamel K. Improvement of nerve fiber density in fibromyalgia patients treated with IVIg. Curr Rheumatol Rev 2020;16:280–4. [DOI] [PubMed] [Google Scholar]
- [45].Motaghi P, Adibi I, Adibi P, Ghasemi M. Small fiber neuropathy in irritable bowel syndrome. Gastroenterol Hepatol Bed Bench 2024;17:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. PAIN 2013;154:2310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pickering G, Achard A, Corriger A, Sickout-Arondo S, Macian N, Leray V, Lucchini C, Cardot JM, Pereira B. Electrochemical skin conductance and quantitative sensory testing on fibromyalgia. Pain Pract 2020;20:348–56. [DOI] [PubMed] [Google Scholar]
- [48].Ponirakis G, Abdul-Ghani MA, Jayyousi A, Zirie MA, Qazi M, Almuhannadi H, Petropoulos IN, Khan A, Gad H, Migahid O, Megahed A, Al-Mohannadi S, AlMarri F, Al-Khayat F, Mahfoud Z, Al Hamad H, Ramadan M, DeFronzo R, Malik RA. Painful diabetic neuropathy is associated with increased nerve regeneration in patients with type 2 diabetes undergoing intensive glycemic control. J Diabetes Investig 2021;12:1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pyke TL, Osmotherly PG, Baines S. Measuring glutamate levels in the brains of fibromyalgia patients and a potential role for glutamate in the pathophysiology of fibromyalgia symptoms: a systematic review. Clin J Pain 2017;33:944–54. [DOI] [PubMed] [Google Scholar]
- [50].Quitadamo SG, Vecchio E, Delussi M, Libro G, Clemente L, Lombardi R, Modena D, Giannotta M, Iannone F, de Tommaso M. Outcome of small fibre pathology in fibromyalgia: a real life longitudinal observational study. Clin Exp Rheumatol 2023;41:1216–24. [DOI] [PubMed] [Google Scholar]
- [51].Saudek F, Cahová M, Havrdová T, Zacharovová K, Daňková H, Voska L, Lánská V, Üçeyler N, Sommer C. Preserved expression of skin neurotrophic factors in advanced diabetic neuropathy does not lead to neural regeneration despite pancreas and kidney transplantation. J Diabetes Res 2018;2018:2309108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Serra J, Collado A, Solà R, Antonelli F, Torres X, Salgueiro M, Quiles C, Bostock H. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol 2014;75:196–208. [DOI] [PubMed] [Google Scholar]
- [53].Toda K. What is the clinical value of differentiating fibromyalgia from small-fiber neuropathy in clinical practice? Comment on: “The challenge of differentiating fibromyalgia from small-fiber neuropathy in clinical practice” by Bailly F. Joint Bone Spine 2021;88:105232. Joint Bone Spine 2021;88:105265. [DOI] [PubMed] [Google Scholar]
- [54].Üçeyler N, He L, Schönfeld D, Kahn AK, Reiners K, Hilz MJ, Breunig F, Sommer C. Small fibers in Fabry disease: baseline and follow-up data under enzyme replacement therapy. J Peripher Nerv Syst 2011;16:304–14. [DOI] [PubMed] [Google Scholar]
- [55].Üçeyler N, Kafke W, Riediger N, He L, Necula G, Toyka KV, Sommer C. Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology 2010;74:1806–13. [DOI] [PubMed] [Google Scholar]
- [56].Üçeyler N, Sommer C. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. PAIN 2013;154:2569. [DOI] [PubMed] [Google Scholar]
- [57].Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013;136:1857–67. [DOI] [PubMed] [Google Scholar]
- [58].Van Assche DCF, Plaghki L, Masquelier E, Hatem SM. Fibromyalgia syndrome-A laser-evoked potentials study unsupportive of small nerve fibre involvement. Eur J Pain 2020;24:448–56. [DOI] [PubMed] [Google Scholar]
- [59].Vecchio E, Lombardi R, Paolini M, Libro G, Delussi M, Ricci K, Quitadamo SG, Gentile E, Girolamo F, Iannone F, Lauria G, de Tommaso M. Peripheral and central nervous system correlates in fibromyalgia. Eur J Pain 2020;24:1537–47. [DOI] [PubMed] [Google Scholar]
- [60].Vecchio E, Quitadamo SG, Ricci K, Libro G, Delussi M, Lombardi R, Lauria G, de Tommaso M. Laser evoked potentials in fibromyalgia with peripheral small fiber involvement. Clin Neurophysiol 2022;135:96–106. [DOI] [PubMed] [Google Scholar]
- [61].Viceconti A, Geri T, De Luca S, Maselli F, Rossettini G, Sulli A, Schenone A, Testa M. Neuropathic pain and symptoms of potential small-fiber neuropathy in fibromyalgic patients: a national on-line survey. Joint Bone Spine 2021;88:105153. [DOI] [PubMed] [Google Scholar]
- [62].Vincent A, Whipple MO, Low PA, Joyner M, Hoskin TL. Patients with fibromyalgia have significant autonomic symptoms but modest autonomic dysfunction. PM R 2016;8:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ziegler D, Bönhof GJ, Strom A, Straßburger K, Karusheva Y, Szendroedi J, Roden M. Progression and regression of nerve fibre pathology and dysfunction early in diabetes over 5 years. Brain 2021;144:3251–63. [DOI] [PubMed] [Google Scholar]


