Abstract
Background:
Many studies assess aesthetic effectiveness of calcium hydroxylapatite (CaHA), with single-group designs as the most frequently applied designs in practice. This study systematically reviewed CaHA’s effectiveness for aesthetic purposes among these studies.
Methods:
A comprehensive search was conducted across 5 bibliographic databases. Single-group studies with at least 10 human adults were included. Summary measures of patients satisfaction and global aesthetic improvement scores were combined using the generalized linear mixed model. This systematic review adhered to the PRISMA reporting standards.
Results:
Of 3131 records, 46 single-group studies, majority focused on facial areas (n = 32), were included for final qualitative analysis. A total number of 27 studies were included in the meta-analysis. Findings of the meta-analysis showed that 98% (95% confidence interval [CI], 91%–99%; I2, 0.0%) of patients were satisfied with the injection results in the facial area and 90% (95% CI, 67%–97%, I2, 35%) in other treated body areas. Also, patients reported 89% (95% CI, 76%–96%; I2, 65%) improvement on the global aesthetic improvement scale in facial areas and 94% (95% CI, 75%–99%; I2, 0.0%) in other treated regions. Similarly, investigators reported global aesthetic improvement in 92% of patients (95% CI, 33%–100%; I2, 92%) in facial areas and 95% (95% CI, 1%–100%; I2, 89%) in other treated areas.
Conclusions:
Our findings showed aesthetic improvements and satisfaction following CaHA injections in both facial and nonfacial areas. However, studies focusing on nonfacial regions are limited. We recommend more rigorously designed trials to better understand CaHA’s clinical effects.
Takeaways
Question: What is the aesthetic effectiveness of calcium hydroxylapatite (CaHA) reported by single-group studies as the most used study design in real-world practice?
Findings: Forty-six single-group studies were included in the systematic review. The analyses show that over 90% of patients were satisfied with CaHA application results in both facial and nonfacial areas, matching the 90% of patients and investigators who reported improvements on the global aesthetic scale.
Meaning: CaHA injections generally show good results in facial areas, with most patients seeing improvement. Early evidence suggests similar benefits for nonfacial areas, but more rigorous studies are needed to fully understand its clinical effects.
INTRODUCTION
The use of nonsurgical, minimally invasive aesthetic treatments for soft-tissue augmentation is gaining popularity as a choice for aesthetic enhancement to mitigate the effects of facial aging. Dermal filler treatments constitute one of the most prominent noninvasive aesthetic treatment options.1 Calcium hydroxylapatite (CaHA) is an injectable dermal filler consisting of uniform CaHA microspheres suspended in an aqueous carboxymethylcellulose gel carrier that is highly biocompatible with human tissue and is increasingly popular among dermal fillers.2 Currently, Radiesse (Merz North America, Inc., Raleigh, NC) is the only CaHA filler that has obtained US Food and Drug Administration approval for the correction of moderate-to-severe facial wrinkles and folds, HIV lipoatrophy, hand, and jawline augmentation.
The aesthetic effectiveness of CaHA has been investigated in several clinical trials and observational studies, showing varying degrees of enhancement in aesthetic measurements and patient satisfaction levels within the facial and hand regions.3–24 Among these studies, single-group studies including single-arm clinical and observational studies are the most frequently applied designs. Although it is acknowledged that single-group studies have inherent limitations, they are often used for pragmatic and feasibility reasons in real-world practice. Furthermore, whether CaHA maintains consistent effectiveness when used in other body areas other than the face and hands is an area of active exploration.
The objective of this study is to systematically review the characteristics of and findings from the most commonly encountered study designs in real-world practice, single-group studies, focusing on the impact of CaHA on aesthetic outcomes, including aesthetic improvement scores, wrinkle reduction, changes in skin thickness, and patient/investigator satisfaction.
MATERIALS AND METHODS
This systematic review was conducted based on recent systematic review guidelines and reported following the PRISMA reporting standards.25–27 The current systematic review is a part of a lager project. The study protocol of this project was registered in the OSF Registries on December 22, 2022 (Registration doi: https://doi.org/10.17605/OSF.IO/WY49V).
Data Sources, Search Strategy, and Eligibility Criteria
Databases such as Embase, Medline ALL (Ovid), Web of Science Core Collection, and Cochrane Central were searched, up to March 26, 2024. Additionally, the initial 200 results from Google Scholar were imported. The search strategy was developed by an expert research librarian, and included terms related to exposure such as calcium hydroxyapatite and Radiesse. Given that we had multiple outcomes to consider, we conducted a broad search without including any terms specifically related to our outcomes. Detailed information regarding the search strategy and keywords can be found in Supplemental Digital Content 1. (See table, Supplemental Digital Content 1, which displays search strategies, http://links.lww.com/PRSGO/D702.)
To identify additional studies, we reviewed all published reviews for relevant references. Furthermore, the reference lists of the final included studies were manually reviewed.
We included publications involving adults (age ≥ 18 years), regardless of health status, which investigated the impact of CaHA on outcomes related to aesthetics and skin aging, as well as patient satisfaction. We included studies originally designed as single-group studies, defined as single-arm clinical studies or prospective/retrospective observational studies, with at least 10 participants. We excluded case reports with fewer than 10 participants, reviews, letters to editors, conference abstracts, and research conducted on animals, children, or adolescents. Controlled clinical trials were not within the scope of the current review; these findings are summarized in another work from our team elsewhere.28 Non-English publications were excluded.
Study Selection, Data Extraction, and Quality Assessment
Two independent researchers conducted duplicate screenings of all titles and abstracts in accordance with the eligibility criteria. Subsequently, duplicate reviews were carried out for all provided full-text articles. Data from the included studies were extracted using a predefined Excel form. The primary data extracted included the first author’s name, study design, publication year, location, participant number, sex distribution within the population, participants’ health status at the beginning of the study, age, duration of follow-up, ethnicity, skin type, brand of dermal filler, injection site, dilution and dosage, injection depth and method, assessment methods for outcomes, adjustments, and any measures of frequency or association.
The risk of bias in nonrandomized studies of interventions (ROBINS-I) tool was used to assess the quality of the included studies. This tool evaluates the quality of studies based on biases that may arise at different study phases including preintervention (confounding and selection of participants), at intervention (classification of intervention), and postintervention (deviation from intended intervention, missing data, measurements of the outcomes, and selection of the reported outcomes).29 In ROBINS-I, the risk of bias judgments for each domain are no information, critical risk of bias, serious risk of bias, moderate risk of bias, or low risk of bias.
Statistical Analysis
Summary measures were pooled using the generalized linear mixed model with logit-transformed proportions. The Hartung-Knapp method was used to estimate the 95% confidence intervals (95% CIs) for the random-effects models.30 Fixed-effects models were also reported for sensitivity analysis. To ensure consistency among studies regarding the assessment tools for quantifying aesthetic improvement, we included only those studies that used the global aesthetic improvement scale and reported findings as frequencies or proportions. For this outcome, we combined results across all improvement categories, including mildly improved, improved, moderately/much/markedly improved, and very much improved. Similarly, for the meta-analysis of patient satisfaction, we combined findings for all levels of satisfaction, including both very satisfied and satisfied responses following treatment.
Publication bias was assessed when at least 10 studies were available with the Egger test and by visually exploring funnel plots for asymmetry.31,32 Heterogeneity between studies was assessed using I2.33 To assess the influence of individual studies on the overall results (sensitivity analysis), a leave-one-out analysis was conducted, which involves systematically leaving out each study one at a time and re-computing the meta-analysis summary measures. All analyses were performed using R version 4.1.3 with “meta” package.
We present the size, direction of change, and statistical significance of the observed changes in all included studies in the systematic review, and we created tables to outline the study characteristics (Supplemental Digital Content 2), findings (Supplemental Digital Content 3), evaluation of study methodologies (Supplemental Digital Content 4), and assessment tools (Supplemental Digital Content 5). (See table, Supplemental Digital Content 2, which displays characteristics of the included studies, http://links.lww.com/PRSGO/D703.) (See table, Supplemental Digital Content 3, which displays summary of findings of the included studies, http://links.lww.com/PRSGO/D704.) (See table, Supplemental Digital Content 4, which displays risk of bias according to ROBINS-I, http://links.lww.com/PRSGO/D705.) (See table, Supplemental Digital Content 5, which displays assessment methods of the most reported outcomes, http://links.lww.com/PRSGO/D706.)
RESULTS
Eligible Studies
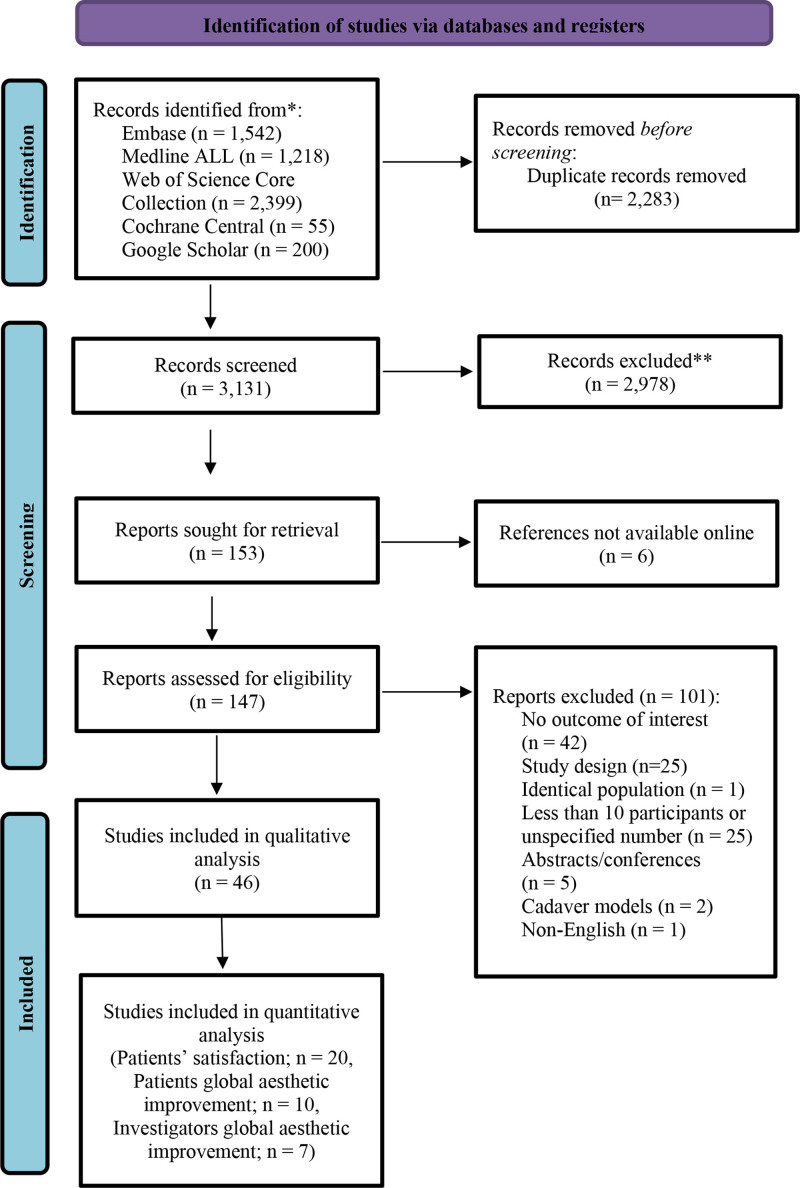
Of 3131 references, 46 studies met the eligibility criteria to be included in the systematic review. Based on the eligibility criteria, 27 studies were included in the meta-analysis (Fig. 1).
Fig. 1.
Flowchart of identification, screening, eligibility, inclusion, and exclusion of retrieved studies.
Study Characteristics and Quality Assessments
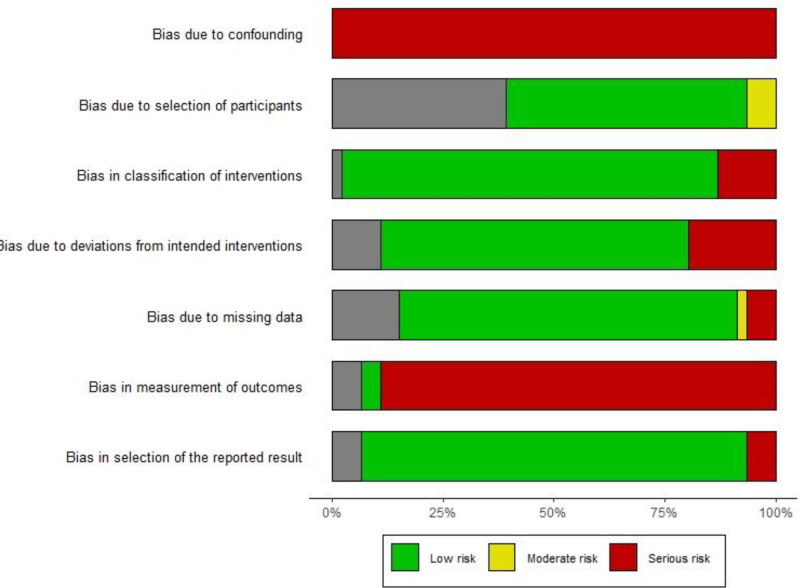
Included studies in the systematic review were published between 2004 to 2024. Of the included studies, 18 (39.1%) were conducted in the United States, 12 (26.1%) in European countries, and 5 (10.9%) in Asian countries. The remaining studies (17.4%) were conducted in South America, Canada, Australia, Russia, and Africa. Also the location of 3 (6.5%) studies were unclear. Facial areas were the major injected regions (n = 32). The majority of studies were designed as pre-post interventional studies (n = 34) and the remaining as observational studies, which majority was retrospective studies (n = 9). The median number of participants was 24, with an interquartile range of 18–41. The median study duration was 21.72 weeks, with an interquartile range of 13.04–52.03 weeks. Among the studies reporting the distribution of sex within the population, the median number of women was 22, with an interquartile range of 15–40, whereas the median number of men was 2, with an interquartile range of 0–5. All studies used Radiesse as the CaHA filler and two studies did not mention the brand name (Supplemental Digital Content 3, http://links.lww.com/PRSGO/D704). Among the included studies, there was low risk of bias in domains related to missing values and reporting of results, whereas the domain of bias due to confounding and measurement of outcomes were judged as a major reason for bias (Fig. 2; Supplemental Digital Content 4, http://links.lww.com/PRSGO/D705).
Fig. 2.
Risk of bias summary of the included studies using risk of bias in nonrandomized studies (ROBINS-I).
Facial Area
Among 46 included studies, 32 investigated the role of CaHA injection in facial areas, the majority of which focused on the mid and/or lower face, such as nasolabial folds, nasal surface, cheeks, lip, marionette lines and jawline. In facial areas, patient satisfaction and global aesthetic improvement were the most reported outcomes (Supplemental Digital Content 3, http://links.lww.com/PRSGO/D704).
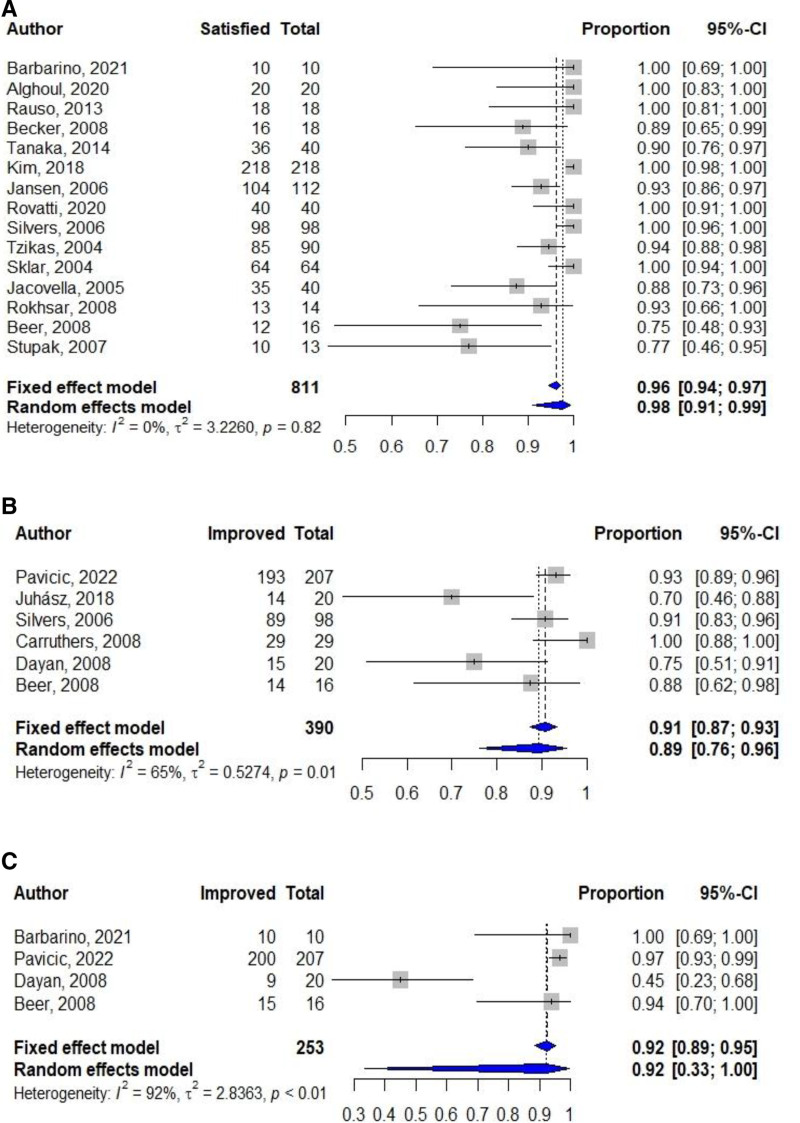
Satisfaction following injection was reported in 20 studies.5,7,8,34–50 Overall, most studies reported moderate-to-high patient satisfaction, with rates varying from 69% to 100% of patients being satisfied to extremely satisfied. Furthermore, combining the findings of 15 eligible studies included in the meta-analysis showed that 98% of the patients (95% CI, 91%–99%; I², 0.0%; n = 811) were satisfied with the injection results (Fig. 3A). Three studies also reported physician satisfaction, showing similar trends with patient satisfaction.44,45,47
Fig. 3.
Summary proportions and pooled estimates of (A) patients’ satisfaction, (B) patients’ aesthetic improvement, and (C) investigators’ aesthetic improvement in facial areas.
A total of 17 studies reported findings on global/overall aesthetic improvement scales in the facial area.4,5,8,11–13,34,35,37,39,46,51–56 Most studies observed some degree of overall aesthetic improvement following CaHA injection as evaluated by investigators or patients. The results of meta-analysis of 6 studies using the global aesthetic improvement scale indicated that 89% of the patients (95% CI, 76%–96%; I², 65%; n = 390) reported some level of improvement on the global aesthetic improvement scale following injection. Additionally, pooled results of 4 studies showed that 92% of the patients (95% CI, 33%–100%; I², 92%; n = 253) were evaluated as improved by the investigators (Figs. 3B, C). The role of CaHA on wrinkle and curve/fold/line correction in this area was investigated in 5 studies.5,7,8,51,55 All showed some level of improvement following injection compared with baseline values as assessed by investigators. The evaluation of skin thickness was reported in 3 studies,46,53,54 and findings were consistent in showing an increase in skin thickness following CaHA injection. Other outcomes such as cheek fullness,5,7 jawline volume loss,56 jawline contour,51 relative enophthalmos measurement,4 temple hollowing scale,35 sulcus deformity,57 temple volume scale,12 and orbital volume58 were reported by a limited number of studies; findings are summarized in Supplemental Digital Content 3 (http://links.lww.com/PRSGO/D704).
Other Treated Body Areas
The role of CaHA on other areas of the body was evaluated in a limited number of studies. Four studies investigated the role of CaHA injection in hands.19,21,22,59 They reported satisfaction rates more than 60% after injection as evaluated by either patients or investigators,19,22 some degree of improvement in global aesthetic improvement scale,19 hand grading scale,21 and severity of wrinkles.22
Of the included studies, three targeted the abdomen and upper arm.60–62 These studies showed improvements in skin thickness,61,62 skin flaccidity,60,62 skin volume,60 skin elasticity,61 and density62 following CaHA injection compared with the baseline values. In both regions, more than 70% of patients were scored as much improved on the global aesthetics improvement scale61 and were satisfied with the results after the injection.60
Four studies examined the role of CaHA on the neck, neck/décolletage, and chest/décolletage regions,63–66 showing improvements in skin laxity,64,66 wrinkles,63,66 and elasticity/viscoelasticity/thickness,65,66 as well as in patient satisfaction63,64,66 and global aesthetics improvement scale.65,66
Additionally, 1 study assessed the role of CaHA on the knee area67 showing improvements in cellulite severity and patient satisfaction; a single study assessed the role of CaHA injection on the dorsum of the foot,68 showing improvements in the global aesthetics improvement scale; and another study assessed the role of CaHA injection in the buttocks,69 showing improvements in cellulite severity, number and depth of dimples as well as improvements in the global aesthetics improvement scale (Supplemental Digital Content 3, http://links.lww.com/PRSGO/D704).
Overall, the pooled results of 5 eligible studies in the meta-analysis of other treated body areas indicated that 90% of the patients (95% CI, 67%–97%; I², 35%; n = 134) were satisfied with the treatment results. Furthermore, meta-analysis of 4 eligible studies based on global aesthetic improvement scales findings indicated that 94% of patients (95% CI, 75%–99%; I², 0.0%, n = 66) reported some level of improvement in the treated areas. Additionally, findings from 3 eligible studies showed that 95% of patients (95% CI, 1%–100%; I², 89%; n = 96) were evaluated as improved by the investigators. (See figure, Supplemental Digital Content 6, which displays summary proportions and pooled estimates of [A] patients’ satisfaction; [B] patients’ aesthetic improvement; and [C] investigators’ aesthetic improvement in other treated body areas, http://links.lww.com/PRSGO/D707.)
Additional Analysis
There were some indications for publication bias based on the Egger test (P value 0.03) for the meta-analysis of patient’s satisfaction in the facial area. In the same analysis, the leave-one-out analysis, showed that no single study had any significant impact. (See figure, Supplemental Digital Content 7, which displays Funnel plots and Egger test P values of the included studies in the meta-analysis of patients satisfaction in facial area, http://links.lww.com/PRSGO/D708.) (See figure, Supplemental Digital Content 8, which displays leave-one-out analysis of the included studies in the meta-analysis of patients satisfaction in facial area, http://links.lww.com/PRSGO/D709.)
DISCUSSION
According to the single-group studies, patients generally reported satisfaction with the results following CaHA injection. Our meta-analysis revealed that more than 90% of patients were satisfied with the treatment. Additionally, improvement in global aesthetic assessment scales for facial areas were observed. Specifically, the meta-analysis indicated an 89% improvement in global aesthetics as evaluated by patients, and a 92% improvement as assessed by investigators. However, it is important to acknowledge that the wide CI for the global aesthetic improvement scale evaluated by investigators reflects a high level of uncertainty in the evidence. Furthermore, studies indicated improvements in wrinkle severity and skin thickness in this region. Findings regarding the role of CaHA in body areas other than the face were based on a very limited number of studies but aligned with those observed in facial areas, particularly in terms of global aesthetic improvement and satisfaction.
CaHA, the primary mineral constituent in bones and teeth, is a naturally occurring substance present in the human body, which lends to its biocompatibility. CaHA has found extensive application in various aesthetic procedures, including volume restoration, contouring, and skin tightening. It has been utilized to improve aesthetic outcomes in a range of body sites, encompassing the jawline, nasolabial folds, orbital area, the back of the hand and foot, neck, chest, décolletage, and abdominal regions. Radiesse consists of synthetic calcium hydroxylapatite microspheres, making up 35% of its composition, with particle sizes ranging from 25 to 45 μm. These microspheres are suspended within an aqueous gel comprising 65% of the product and containing ingredients such as water, glycerin, and carboxymethylcellulose.70 In 2006, the US Food And Drug Administration approved injectable CaHA for treating HIV facial lipoatrophy and moderate to severe facial lines.71 Several reported mechanisms support the utilization of CaHA for aesthetic purposes, including promotion of cell proliferation, collagen production, angiogenesis, and the formation of elastic fibers and elastin.72 Although the effectiveness of CaHA has been examined in previous reviews, these reviews have typically been constrained in scope. They have either focused on specific facial regions, been part of broader assessments encompassing various nonhyaluronic acid fillers, concentrated on safety considerations and potential side effects, or assessed CaHA efficacy for nondermatological applications.38,73–80 The current review, adhering to the PRISMA and evidence-based medicine guidelines, summarizes the literature, focusing solely on CaHA, regardless of treatment area or indication, while taking into account quantitative results from both patients and physicians and providing a thorough systematic overview of the available evidence. It should be highlighted that effectiveness of CaHA in areas other than the face and hand has not been subjected to rigorous controlled investigation and remains an area of active exploration.
The current review provides insights from alternative study designs beyond clinical trials. In this review, we focused on single-group studies as the most frequent study designs in clinical practice. Single-group studies are practical and feasible and are frequently carried out in real-world clinical or practical settings, reflecting the real-life conditions in which treatments or interventions are administered; nonetheless, they come with inherent design limitations. A major limitation of the included studies is the difficulty in attributing the results solely to the CaHA injection as a causal factor. Lack of randomization and the influence of confounders on the results cannot be ruled out, which is also evident from the results of quality assessments. Alternatively, the results may be interpreted as the expected changes in participants’ aesthetic status during follow-up, regardless of the specific underlying causes for these changes. Thus, incorporating a control group allows the researcher to attribute any observed changes in the treatment group to the treatment being examined, rather than external factors. The studies often had small sample sizes, did not assess/report the preinterventional status, did not perform appropriate statistical tests to determine the extent of differences/changes, or did not report the statistical change significance. Additionally, many studies relied on subjective methods, such as questionnaires, raising concerns about bias and the reliability of the results. If an objective approach is not possible, we recommend using validated tools and ensuring both patients and investigators are blinded. Additionally, studies focused predominately on the use of CaHA in facial regions; however, it is worth noting that CaHA is utilized in practice for various other body areas. Although there is some promising evidence suggesting applications of CaHA in areas beyond the face, there is a limited number of studies examining its aesthetic effectiveness in these nonfacial areas, as also recommended by other studies.81 Thus, to gain a more comprehensive understanding of the broad spectrum of potential applications and effectiveness of CaHA, we suggest well-designed randomized controlled clinical trials considering appropriate statistical approaches to minimize biases and enhance the validity of findings, enabling replicability and applicability of study findings. In our study, the heterogeneity in participant characteristics, outcomes, reported estimates, and measurement methods, as well as the limited number of studies, precluded the feasibility of conducting a methodological sound meta-analysis for all outcomes. We limited our inclusion criteria to studies published in English, and thus, potential selection bias could be present. Although safety aspects related to the use of CaHA were not part of the predefined objectives of this systematic review, we observed that the included studies generally reported adverse events that were mild to moderate in severity and resolved without treatment during the study period. In facial areas, the most reported adverse events included hematoma, swelling, bruising, erythema, ecchymosis, edema, and pain/discomfort. Several studies reported the occurrence of nodules following injection,5,11,12,41,44,45 which, in 1 case on the lips, it was surgically removed without complications.40 Migration of the product was reported in 3 cases by 2 studies.4,52 One study reported a case of vascular compression leading to necrosis.5 Ptosis was reported in 2 studies, totaling 4 cases.57,58 Extrusion of the filler with skin discoloration was reported in 1 case.57 When treating the orbital area in patients with postenucleation socket syndrome, 2 cases of internal prosthesis extrusion were reported.58 In other treated body areas, no serious adverse events were reported, with bruising and swelling being the most common adverse events. We acknowledge that the safety profile is an area that needs to be further explored in future research to provide a comprehensive overview of both efficacy and safety of CaHA treatment.
The current studies suggest CaHA improves aesthetic outcomes such as global aesthetic scores, and wrinkles and curves with relatively high patient satisfaction, and most derived from the facial region. Given methodological limitations of single-group studies, caution is required when drawing conclusions about causality or generalizing findings to broader populations. Well-designed controlled clinical trials are recommended to further investigate and confirm the effects of CaHA as a regenerative aesthetic treatment in other body regions outside of the face and hand.
DISCLOSURES
Dr. Phillips is an employee of Merz North America, Inc. Dr. Daughtry was employed in Merz North America, Inc., during the conduct of the study. Dr. Kolb is an employee of Merz Aesthetics GmbH. Dr. Goldie is a consultant for Merz Aesthetics. Dr. Muka is the co-founder and CEO of Epistudia GmbH, which received funding from Merz Aesthetics Inc. to conduct the study. The other authors have no financial interest to declare in relation to the content of this article. This study received funding from Merz North America, Inc.
Supplementary Material
Footnotes
Published online 26 December 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
Drs. Muka and Daughtry are shared last authors to all academic and professional effects, and their names can be legitimately swapped in their respective publication list.
REFERENCES
- 1.Aesthetic plastic surgery national databank statistics 2020-2021. Aesthet Surg J. 2022;42:1–18. [DOI] [PubMed] [Google Scholar]
- 2.AsfAp S. The aesthetic society’s cosmetic surgery national data bank: statistics 2019. Aesthet Surg J. 2020;40:1–26. [DOI] [PubMed] [Google Scholar]
- 3.Moers-Carpi MM, Tufet JO. Calcium hydroxylapatite versus nonanimal stabilized hyaluronic acid for the correction of nasolabial folds: a 12-month, multicenter, prospective, randomized, controlled, split-face trial. Dermatol Surg. 2008;34:210–215. [DOI] [PubMed] [Google Scholar]
- 4.Vagefi MR, McMullan TFW, Burroughs JR, et al. Orbital augmentation with injectable calcium hydroxylapatite for correction of postenucleation/evisceration socket syndrome. Ophthalmic Plast Reconstr Surg. 2011;27:90–94. [DOI] [PubMed] [Google Scholar]
- 5.Pavicic T, Sattler G, Fischer T, et al. Calcium hydroxyapatite filler with integral lidocaine CaHA (+) for soft tissue augmentation: results from an open-label multicenter clinical study. J Drugs Dermatol. 2022;21:481–487. [DOI] [PubMed] [Google Scholar]
- 6.Boen M, Alhaddad M, Goldman MP, et al. A Randomized, evaluator-blind, split-face study evaluating the safety and efficacy of calcium hydroxylapatite for Jawline augmentation. Dermatol Surg. 2022;48:76–81. [DOI] [PubMed] [Google Scholar]
- 7.Rovatti PP, Pellacani G, Guida S. Hyperdiluted calcium hydroxylapatite 1:2 for mid and lower facial skin rejuvenation: efficacy and safety. Dermatol Surg. 2020;46:e112–e117. [DOI] [PubMed] [Google Scholar]
- 8.Kim J. Novel forehead augmentation strategy: forehead depression categorization and calcium-hydroxyapatite filler delivery after tumescent injection. Plast Reconstr Surg, Glob Open. 2018;6:e1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grunebaum LD, Elsaie ML, Kaufman J. Six-month, double-blind, randomized, split-face study to compare the efficacy and safety of calcium hydroxylapatite (CaHA) mixed with lidocaine and CaHA alone for correction of nasolabial fold wrinkles. Dermatol Surg. 2010;36:760–765. [Google Scholar]
- 10.Moers-Carpi M, Storck R, Howell DJ, et al. Physician and patient satisfaction after use of calcium hydroxylapatite for cheek augmentation. Dermatol Surg. 2012;38:1217–1222. [DOI] [PubMed] [Google Scholar]
- 11.Wollina U, Goldman A. Long lasting facial rejuvenation by repeated placement of calcium hydroxylapatite in elderly women. Dermatol Ther. 2020;33:e14183. [DOI] [PubMed] [Google Scholar]
- 12.Juhász MLW, Levin MK, Marmur ES. Pilot study examining the safety and efficacy of calcium hydroxylapatite filler with integral lidocaine over a 12-month period to correct temporal fossa volume loss. Dermatol Surg. 2018;44:93–100. [DOI] [PubMed] [Google Scholar]
- 13.Muti GF. Open-label, post-marketing study to evaluate the performance and safety of calcium hydroxylapatite with integral lidocaine to correct facial volume loss. J Drugs Dermatol. 2019;18:86–91. [PubMed] [Google Scholar]
- 14.Bertucci V, Solish N, Wong M, et al. Evaluation of the Merz hand grading scale after calcium hydroxylapatite hand treatment. Dermatol Surg. 2015;41:S389–S396. [DOI] [PubMed] [Google Scholar]
- 15.Busso M, Moers-Carpi M, Storck R, et al. Multicenter, randomized trial assessing the effectiveness and safety of calcium hydroxylapatite for hand rejuvenation. Dermatol Surg. 2010;36:790–797. [Google Scholar]
- 16.Figueredo VO, Miot HA, Soares Dias J, et al. Efficacy and safety of 2 injection techniques for hand biostimulatory treatment with diluted calcium hydroxylapatite. Dermatol Surg. 2020;46:S54–S61. [DOI] [PubMed] [Google Scholar]
- 17.Goldman MP, Moradi A, Gold MH, et al. Calcium hydroxylapatite dermal filler for treatment of dorsal hand volume loss: results from a 12-month, multicenter, randomized, blinded trial. Dermatol Surg. 2018;44:75–83. [DOI] [PubMed] [Google Scholar]
- 18.Gubanova EI, Starovatova PA. A prospective, comparative, evaluator-blind clinical study investigating efficacy and safety of two injection techniques with Radiesse(R) for the correction of skin changes in aging hands. J Cutan Aesthet Surg. 2015;8:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haq S, Storck R, Baspeyras M, et al. Multinational, multipatient study of calcium hydroxylapatite for treatment of the aging hand: European cosmetic physician group on hand augmentation. Dermatol Surg. 2010;36:782–789. [Google Scholar]
- 20.Kim JS. Detailed sonographic anatomy of dorsal hand augmentation with hyaluronic acid and calcium hydroxyapatite fillers. Aesthet Surg J. 2019;39:1096–1106. [DOI] [PubMed] [Google Scholar]
- 21.Kim JS, Lee W, Oh W, et al. Identification of a suitable layer for injecting calcium hydroxylapatite fillers in the hands. J Plast Reconstr Aesthetic Surg. 2021;74:866–873. [DOI] [PubMed] [Google Scholar]
- 22.Sadick NS. A 52-week study of safety and efficacy of calcium hydroxylapatite for rejuvenation of the aging hand. J Drugs Dermatol. 2011;10:47–51. [PubMed] [Google Scholar]
- 23.Sattler G, Walker T, Buxmeyer B, et al. Efficacy of calcium hydroxylapatite filler versus hyaluronic acid filler in hand augmentation. Aktuel Dermatol. 2014;40:445–451. [Google Scholar]
- 24.Wu DC, Goldman MP. Randomized, double-blinded, sham-controlled, split-hand trial evaluating the safety and efficacy of triamcinolone acetate injection after calcium hydroxylapatite volume restoration of the dorsal hand. Dermatol Surg. 2018;44:534–541. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clin Res Ed). 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muka T, Glisic M, Milic J, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. 2020;35:49–60. [DOI] [PubMed] [Google Scholar]
- 27.Glisic M, Raguindin PF, Gemperli A, et al. A 7-step guideline for qualitative synthesis and meta-analysis of observational studies in health sciences. Public Health Rev. 2023;44:1605454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amiri M, Meçani R, Llanaj E, et al. Calcium hydroxylapatite (CaHA) and aesthetic outcomes: a systematic review of controlled clinical trials. J Clin Med. 2024;13:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 32.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alghoul MS, Vaca EE, Bricker JT, et al. Enhancing the lateral orbital “C-Angle” with calcium hydroxylapatite: an anatomic and clinical study. Aesthet Surg J. 2021;41:952–966. [DOI] [PubMed] [Google Scholar]
- 35.Barbarino SC. Correction of temporal wasting using calcium hydroxylapatite with integral lidocaine: an underused procedure for enhancing overall facial appearance. J Cosmet Dermatol. 2021;20:62–66. [DOI] [PubMed] [Google Scholar]
- 36.Becker H. Nasal augmentation with calcium hydroxylapatite in a carrier-based gel. Plast Reconstr Surg. 2008;121:2142–2147. [DOI] [PubMed] [Google Scholar]
- 37.Beer K, Yohn M, Cohen JL. Evaluation of injectable CaHA for the treatment of mid-face volume loss. J Drugs Dermatol. 2008;7:359–366. [PubMed] [Google Scholar]
- 38.Fakhre GP, Perdikis G, Shaddix KK, et al. An evaluation of calcium hydroxylapatite (Radiesse) for cosmetic nasolabial fold correction: a meta-analysis and patient centric outcomes study. Ann Plast Surg. 2009;63:486–489. [DOI] [PubMed] [Google Scholar]
- 39.Hevia O. A retrospective review of calcium hydroxylapatite for correction of volume loss in the infraorbital region. Dermatol Surg. 2009;35:1487–1494. [DOI] [PubMed] [Google Scholar]
- 40.Jacovella PF, Peiretti CB, Cunille D, et al. Long-lasting results with hydroxylapatite (Radiesse) facial filler. Plast Reconstr Surg. 2006;118:15S–21S. [DOI] [PubMed] [Google Scholar]
- 41.Jansen DA, Graivier MH. Evaluation of a calcium hydroxylapatite-based implant (Radiesse) for facial soft-tissue augmentation. Plast Reconstr Surg. 2006;118:22S–30S, discussion 31S. [DOI] [PubMed] [Google Scholar]
- 42.Rauso R, Curinga G, Rusciani A, et al. Safety and efficacy of one-step rehabilitation of human immunodeficiency virus-related facial lipoatrophy using an injectable calcium hydroxylapatite dermal filler. Dermatol Surg. 2013;39:1887–1894. [DOI] [PubMed] [Google Scholar]
- 43.Rokhsar C, Ciocon DH. Nonsurgical rhinoplasty: an evaluation of injectable calcium hydroxylapatite filler for nasal contouring. Dermatol Surg. 2008;34:944–946. [DOI] [PubMed] [Google Scholar]
- 44.Roy D, Sadick N, Mangat D. Clinical trial of a novel filler material for soft tissue augmentation of the face containing synthetic calcium hydroxylapatite microspheres. Dermatol Surg. 2006;32:1134–1139. [DOI] [PubMed] [Google Scholar]
- 45.Sadick NS, Katz BE, Roy D. A multicenter, 47-month study of safety and efficacy of calcium hydroxylapatite for soft tissue augmentation of nasolabial folds and other areas of the face. Dermatol Surg. 2007;33:S122–6; discussion S126. [DOI] [PubMed] [Google Scholar]
- 46.Silvers SL, Eviatar JA, Echavez MI, et al. Prospective, open-label, 18-month trial of calcium hydroxylapatite (Radiesse) for facial soft-tissue augmentation in patients with human immunodeficiency virus-associated lipoatrophy: one-year durability. Plast Reconstr Surg. 2006;118:34S–45S. [DOI] [PubMed] [Google Scholar]
- 47.Sklar JA, White SM, Klein A. Radiance FN: a new soft tissue filler. Dermatol Surg. 2004;30:764–768. [DOI] [PubMed] [Google Scholar]
- 48.Stupak HD, Moulthrop THM, Wheatley P, et al. Calcium hydroxylapatite gel (Radiesse) injection for the correction of postrhinoplasty contour deficiencies and asymmetries. Arch Facial Plast Surg. 2007;9:130–136. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka Y. Oriental nose occidentalization and perinasal shaping by augmentation of the underdeveloped anterior nasal spine. Plast Reconstr Surg Glob Open. 2014;2:e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tzikas TL. Evaluation of the radiance FN soft tissue filler for facial soft tissue augmentation. Arch Facial Plast Surg. 2004;6:234–239. [DOI] [PubMed] [Google Scholar]
- 51.Baspeyras M, Dallara JM, Cartier H, et al. Restoring jawline contour with calcium hydroxylapatite: a prospective, observational study. J Cosmet Dermatol. 2017;16:342–347. [DOI] [PubMed] [Google Scholar]
- 52.Bernardini FP, Cetinkaya A, Devoto MH, et al. Calcium hydroxyl-apatite (Radiesse) for the correction of periorbital hollows, dark circles, and lower eyelid bags. Ophthalmic Plast Reconstr Surg. 2014;30:34–39. [DOI] [PubMed] [Google Scholar]
- 53.Carruthers A, Carruthers J. Evaluation of injectable calcium hydroxylapatite for the treatment of facial lipoatrophy associated with human immunodeficiency virus. Dermatol Surg. 2008;34:1486–1499. [DOI] [PubMed] [Google Scholar]
- 54.Corduff N. An alternative periorbital treatment option using calcium hydroxyapatite for hyperpigmentation associated with the tear trough deformity. Plast Reconstr Surg Glob Open. 2020;8:e2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dayan SH, Lieberman E, Larimer K. High-volume calcium hydroxylapatite filler to the lower one-third of the face. Arch Facial Plast Surg. 2009;11:145–147. [DOI] [PubMed] [Google Scholar]
- 56.Juhász MLW, Marmur ES. Examining the efficacy of calcium hydroxylapatite filler with integral lidocaine in correcting volume loss of the Jawline—a pilot study. Dermatol Surg. 2018;44:1084–1093. [DOI] [PubMed] [Google Scholar]
- 57.Aletaha M, Salour H, Yadegary S, et al. Orbital volume augmentation with calcium hydroxyapatite filler in anophthalmic enophthalmos. J Ophthalmic Vis Res. 2017;12:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Maria A, Ferraro V, Trenti N, et al. Ten-year follow-up of orbital volume augmentation with calcium hydroxyapatite filler in postenucleation socket syndrome. Ophthal Plast Reconstr Surg. 2024;40:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adel N. Volumization and global biostimulation using calcium hydroxyapatite filler: a dual approach for hand rejuvenation. Plast Reconstr Surg Glob Open. 2023;11:e5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amselem M. Radiesse®: A novel rejuvenation treatment for the upper arms. Clin Cosmet Invest Dermatol. 2015;9:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lapatina NG, Pavlenko T. Diluted calcium hydroxylapatite for skin tightening of the upper arms and abdomen. J Drugs Dermatol. 2017;16:900–906. [PubMed] [Google Scholar]
- 62.Wasylkowski VC. Body vectoring technique with Radiesse® for tightening of the abdomen, thighs, and brachial zone. Clin Cosmet Invest Dermatol. 2015;8:267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fabi SG, Alhaddad M, Boen M, et al. Prospective clinical trial evaluating the long-term safety and efficacy of calcium hydroxylapatite for chest rejuvenation. J Drugs Dermatol. 2021;20:534–537. [DOI] [PubMed] [Google Scholar]
- 64.Guida S, Longhitano S, Spadafora M, et al. Hyperdiluted calcium hydroxylapatite for the treatment of skin laxity of the neck. Dermatol Ther. 2021;34:e15090. [DOI] [PubMed] [Google Scholar]
- 65.Yutskovskaya YA, Kogan EA. Improved neocollagenesis and skin mechanical properties after injection of diluted calcium hydroxylapatite in the neck and déćolletage: a pilot study. J Drugs Dermatol. 2017;16:68–74. [PubMed] [Google Scholar]
- 66.Trindade de Almeida AR, Marques E, Contin LA, et al. Efficacy and tolerability of hyperdiluted calcium hydroxylapatite (Radiesse) for neck rejuvenation: clinical and ultrasonographic assessment. Clin Cosmet Investig Dermatol. 2023;16:1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guida S, Longhitano S, Shaniko K, et al. Hyperdiluted calcium hydroxylapatite for skin laxity and cellulite of the skin above the knee: a pilot study. Dermatol Ther. 2020;33:e14076. [DOI] [PubMed] [Google Scholar]
- 68.Custozzo A, Frank K, Schenck TL, et al. Anatomy of the dorsum of the foot and its relevance for nonsurgical cosmetic procedures. Plast Reconstr Surg. 2020;146:64–72. [DOI] [PubMed] [Google Scholar]
- 69.Durairaj K, Baker O, Yambao M, et al. Safety and efficacy of diluted calcium hydroxylapatite for the treatment of cellulite dimpling on the Buttocks: results from an open-label, investigator-initiated, single-center, prospective clinical study. Aesthetic Plast Surg. 2024;48:1797–1806. [DOI] [PubMed] [Google Scholar]
- 70.Tansavatdi K, Mangat DS. Calcium hydroxyapatite fillers. Facial Plast Surg. 2011;27:510–516. [DOI] [PubMed] [Google Scholar]
- 71.Ridenour B, Kontis TC. Injectable calcium hydroxylapatite microspheres (Radiesse). Facial Plast Surg. 2009;25:100–105. [DOI] [PubMed] [Google Scholar]
- 72.Amiri M, Mecani R, Christa N, et al. Skin regeneration-related mechanisms of calcium hydroxylapatite (CaHA): a systematic review. Front Med. 2023;10:1195934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alsharif SH, Alghamdi AS, Alhumaidi WA, et al. Treatment of striae distensae with filler injection: a systematic review. Clin Cosmet Investig Dermatol. 2023;16:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Henriques DP, Martins RHG, Cataneo AJM. Efficacy of Injectable laryngoplasty with hyaluronic acid and/or calcium hydroxyapatite in the treatment of glottic incompetence. systematic review and meta-analysis. J Voice. [Published online ahead of print February 17, 2023.] [DOI] [PubMed] [Google Scholar]
- 75.Jagdeo J, Ho D, Lo A, et al. A systematic review of filler agents for aesthetic treatment of HIV facial lipoatrophy (FLA). J Am Acad Dermatol. 2015;73:1040–1054. [DOI] [PubMed] [Google Scholar]
- 76.McGuire C, Boudreau C, Tang D. Hand rejuvenation: a systematic review of techniques, outcomes, and complications. Aesthetic Plast Surg. 2022;46:437–449. [DOI] [PubMed] [Google Scholar]
- 77.Ovadia SA, Efimenko IV, Lessard AS. Dorsal hand rejuvenation: a systematic review of the literature. Aesthetic Plast Surg. 2021;45:1804–1825. [DOI] [PubMed] [Google Scholar]
- 78.Shi X-H, Zhou X, Zhang Y-M, et al. Complications from nasolabial fold injection of calcium hydroxylapatite for facial soft-tissue augmentation: a systematic review and meta-analysis. Aesthet Surg J. 2016;36:712–717. [DOI] [PubMed] [Google Scholar]
- 79.Sturm LP, Cooter RD, Mutimer KL, et al. A systematic review of dermal fillers for age-related lines and wrinkles. ANZ J Surg. 2011;81:9–17. [DOI] [PubMed] [Google Scholar]
- 80.Trinh LN, Gupta A. Non-hyaluronic acid fillers for midface augmentation: a systematic review. Facial Plast Surg. 2021;37:536–542. [DOI] [PubMed] [Google Scholar]
- 81.Galadari H, Guida S. A systematic review of Radiesse® (calcium hydroxylapatite): evidence and recommendations for the body. Int J Dermatol. 2024;63:881–889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.