ABSTRACT
Background and Aim
The study ‘Periodontitis and Its Relation to Coronary Artery Disease’ (PAROKRANK) reported an association between periodontitis (PD) and the first myocardial infarction (MI). This follow‐up study aims to test the hypothesis that those with PD—compared to periodontally healthy individuals—are at increased risk for cardiovascular (CV) events and death.
Methods
A total of 1587 participants (age <75 years; females 19%) had a dental examination including panoramic radiographs between 2010 and 2014. PD was categorized as healthy (≥80% alveolar bone height), mild/moderate (79%–66%) or severe (<66%). A composite CV event (first of all‐cause death, non‐fatal MI or stroke and hospitalization following to heart failure) was investigated during a mean follow‐up period of 9.9 years (range 0.2–12.5 years). Participants were divided into two groups: those with and without PD. The primary event rate, stratified by periodontal status at baseline, was calculated using the Kaplan–Meier method and Cox regression.
Results
The number of events was 187 in the 985 periodontally healthy participants (19%) and 174 in the 602 participants with PD (29%; p < 0.0001). Those with PD had a higher likelihood for a future event (hazard ratio [HR] = 1.26; 95% CI: 1.01–1.57; p = 0.038), following adjustment for age, smoking and diabetes.
Conclusion
The PAROKRANK follow‐up revealed that CV events were more common among participants with PD, which supports the assumption that there might be a direct relation between PD and CV disease.
Keywords: cardiovascular disease, long‐term follow‐up, myocardial infarction, periodontitis, prognosis
1. Introduction
Cardiovascular disease (CVD) remains a leading cause of morbidity and mortality (WHO 2022a, 2022b). Traditional risk factors should be prevented (Visseren et al. 2021) but they cannot fully explain all cardiovascular (CV) events. Among other possible risk factors, inflammatory activation has been linked to accelerated atherosclerosis and an enhanced risk for plaque rupture provoking acute coronary syndromes (Libby and Aikawa 2002; Hansson 2005). Pharmacological agents counteracting such activation have been shown to reduce coronary events (Ridker et al. 2017; Tardif et al. 2019).
Periodontal disease, ranging from gingivitis to severe periodontitis (PD), is a chronic tissue‐destructive inflammatory condition. It is an often‐neglected health burden, with a global prevalence of the severe form of around 19% and in Sweden of around 11%, and estimated to be the sixth chronic health burden globally (WHO 2022a, 2022b; Wahlin et al. 2018; 1999 International Workshop 1999).
Even though the reason is debated, there is a link between PD and CVD (Pihlstrom, Michalowicz, and Johnson 2005; Blaizot et al. 2009). One possibility is shared risk factors, while another is that PD may promote CVD via inflammatory activation, that is, a causal relationship. That periodontal treatment decreases C‐reactive protein and low‐density lipoproteins and improves endothelial function has been advocated as support for the latter relationship (Lockhart et al. 2012; Teeuw et al. 2014; Moura Foz et al. 2010; Buhlin et al. 2009; Tonetti et al. 2007). Such surrogate endpoints must, however, be interpreted with caution, leaving an uncertainty regarding the true nature of the association. The 2020 consensus report from the European Federation of Periodontology and the World Heart Federation, written by global experts on both periodontal and cardiovascular diseases, concluded that there is evidence supporting an independent relationship between severe PD and several diseases including CVD (Sanz et al. 2020). Special attention was given to a meta‐analysis which reported an increased risk of a first coronary heart event in people with PD (Dietrich et al. 2013). A more recent systematic review and meta‐analysis showed a modest but consistent increase of CVD in populations with PD (Larvin et al. 2021). Further, very extensive bibliometric analyses of the links between PD and CVD have concluded that inflammation may constitute a shared aetiological factor. It was, however, underlined that further research was required to establish a definitive cause–effect relationship (Tang et al. 2023).
The case–control study PAROKRANK (‘Periodontitis and Its Relation to Coronary Artery Disease’), which carefully considered potential confounders, found an independent association between PD and myocardial infarction (MI), highlighting the possibility of an independent relationship (Rydén et al. 2016).
To further explore this possibility, we present data from a pre‐planned, long‐term follow‐up of the PAROKRANK participants.
2. Materials and Methods
2.1. Study Population
Extensive descriptions of the PAROKRANK protocol have been published elsewhere (Rydén et al. 2016; Norhammar et al. 2019). Briefly, the study included 805 patients of <75 years with a first MI, prospectively recruited at 17 Swedish hospitals during the years 2010–2014. Controls (n = 805) of the same gender and age (±3 months), free from previous MI and heart valve replacement and who were living in the same postal code areas as the patients, were randomly selected from the national population registry. The present study consists of the 1587 participants for whom follow‐up data were available, while information on 23 of them (patients = 9; controls = 14) were lost to follow‐up due to erroneous identification numbers.
Outpatient visits for study enrolment were scheduled at the local departments of cardiology and hospital dentistry approximately 6–10 weeks after the acute event in accordance with national routine care. To ascertain that the controls would undergo their investigations during the same season, they were usually contacted within 10 days after their matched patient had his or her visit. All study participants had fasted and abstained from smoking during at least 12 h prior to their venous blood sampling and physical examination.
2.2. Laboratory Analyses
The following analyses were performed on blood samples at the local laboratory: complete blood count, total and high‐density lipoprotein (HDL) cholesterol and triglycerides, P‐creatinine, P‐fibrinogen, P‐glucose and glycated haemoglobin A1c (HbA1c). Plasma (6 mL) and whole blood (4 mL) were collected and stored in a central bio bank at the Karolinska Institutet at −70°C. High‐sensitivity C‐reactive protein was analysed at a central laboratory (Redhot Diagnostics, Södertälje, Sweden) through ELISA (MP Biomedicals, New York, USA), a method with a functional sensitivity of 0.1 mg/L.
2.3. Study Protocol
Information on the patients at the time of their initial hospitalization and at the follow‐up 6–10 weeks after the MI was collected via a PAROKRANK case‐record form linked to the National Quality Registry SWEDEHEART (Jernberg et al. 2010). Patients were recruited at hospitals participating in this registry and were followed during at least 12 months at the cardiology departments with access to standardized secondary preventive and physical training programs.
Similar information was collected in a separate database for the control population.
2.4. Definitions
MI was diagnosed according to international criteria as issued during the study period (Thygesen et al. 2007, 2012). The medical history was based on self‐reported information in standardized questionnaires, while the diagnoses of hypertension, diabetes and stroke were obtained by the study personnel.
Smoking was registered as ‘yes’ (previous or ongoing) or ‘no’ (never smoked) for the patients presented as recorded at the follow‐up visit, as was ongoing pharmacological treatment.
2.5. Dental Examinations
A dental examination, including a panoramic dental radiograph, was performed according to a standardized protocol (20). Analogue or digital panoramic radiographs were taken from both dentate and edentulous subjects at the local centres for central analysis at the Department of Dental Medicine, Karolinska Institutet Huddinge (Image Tool 3.0 software program, Department of Dental Diagnostics Science, University of Texas Health Science Center, TX, USA). Measurements were carried out with a high‐resolution computer monitor in a dimly lit room. Each tooth was measured at the site with the most pronounced bone loss. Measurements were made from the marginal bone crest to the tooth apex (total bone height) and from the cemento‐enamel junction (CEJ) to the tooth apex (total root length) mesially and distally. The arithmetic mean, calculated from the total root length and bone height, was used as a measure of the proportion of remaining bone height supporting each tooth. Measurements were made of all teeth with visible CEJs and visible apexes. Participants were subsequently—based on the mean value of all teeth—allocated to the following groups: healthy (≥80% remaining bone); mild to moderate periodontitis (79%–66%); and severe periodontitis (<66%). The radiographic examinations were carried out by three dentists blinded to whether the panoramic radiograph came from a patient or control and trained in the use of the equipment. For inter‐individual calibration purposes, 42 randomly selected panoramic radiographs were examined. These dental x‐rays were graded by the three dentists individually, that is, in 126 separate observations. The three graders were in complete agreement in 121 of these observations (96%). The correlation between dentists 1 and 2 was 0.95; between 1 and 3 it was 0.90; and between 2 and 3 it was 0.90 (κ = 0.82).
2.6. Follow‐Up and Endpoints
The starting date for the present follow‐up was at the time of the dental examination (i.e., 6–10 weeks after the acute MI for the patients). The study participants were followed until the time of death or 31 December 2022. The primary endpoint was a composite of the first of all‐cause mortality, non‐fatal MI, non‐fatal stroke and hospitalization following heart failure. The date and cause of death were obtained from the Swedish Cause of Death Register, and information on morbidity was obtained from the National Patient Register according to the International Classification of Diseases (ICD‐10) codes (Table S1).
2.7. Ethical Approval
The PAROKRANK study, including the follow‐up, was approved by the Regional Ethics Committee at Stockholm (Dnr: 2008/152‐31/2; 2017/1803‐32; 2018/2210‐32; 2019‐02871; 2023‐03203‐02). All patients provided written informed consent. PAROKRANK was conducted according to principles outlined in the Helsinki Declaration.
2.8. Statistical Considerations
Calculations based on an assumed prevalence of severe PD in the Swedish population (Hugoson, Sjödin, and Norderyd 2008), supported by an analysis of the first 120 patients and 120 controls in PAROKRANK, revealed that to detect an increased risk of MI (odds ratio [OR] = 1.4) among subjects with PD with a power of 80%, there was a need for 800 patients and a similar number of matched controls. No further power calculation was performed for the follow‐up part, in which patients and controls were merged into one cohort.
Descriptive statistics was used to characterize the data. Quantitative variables are expressed as mean and standard deviation and categorical variables as frequencies and/or percentages. Survival rates in the total cohort were estimated by the Kaplan–Meier method and compared by the Cox regression analysis. Hazard ratio (HR) and corresponding 95% confidence intervals (CIs) adjusted for factors of importance for cardiovascular prognosis (age, smoking and diabetes) were calculated. All analyses were carried out using the SAS system (The SAS system 9.4 for Windows, SAS Institute Inc., Cary, NC, USA), and the level of significance was set to 5%.
3. Results
3.1. Comparison Between Participants With and Without Periodontitis
At baseline, the periodontal status among the 1587 study participants was healthy in 985 (62%) and PD in 602 (38%). Pertinent clinical characteristics of the participants by periodontal status are presented in Table 1. The mean age was 60 ± 8 years in periodontically healthy participants and 65 ± 6 years in those with PD. The most apparent differences between the two groups were that a first MI was more common (56% vs. 47%) and smoking (previous and ongoing) more frequent (73% vs. 51%) among participants with PD. Blood pressure, cholesterol levels and HbA1c were well controlled at the start of follow‐up in both groups.
TABLE 1.
Clinical characteristics by the presence of periodontal disease (mild/moderate or severe) or not (healthy).
| Variables | Healthy | Periodontitis |
|---|---|---|
| n = 985 | n = 602 | |
| Age (years) | 60 ± 8 | 65 ± 6 |
| Male gender (%) | 822 (84) | 468 (78) |
| Family history cardiovascular disease | 305 (31) | 169 (28) |
| Medical history | ||
| Myocardial infarction | 458 (47) | 338 (56) |
| Stroke | 19 (2) | 20 (3) |
| Hypertension | 315 (32) | 233 (39) |
| Diabetes mellitus | 71 (7) | 73 (12) |
| Kidney disease | 34 (4) | 30 (5) |
| Cancer | ||
| Oral cavity‐throat | 9 (1) | 5 (1) |
| Elsewhere | 68 (7) | 41 (7) |
| Smoker | ||
| Yes (past or present) | 499 (51) | 436 (73) |
| No | 477 (49) | 160 (27) |
| Body mass index (kg/m2) | 26.9 ± 3.9 | 27.1 ± 4.2 |
| Blood pressure (mm Hg) | ||
| Systolic | 132 ± 17 | 135 ± 18 |
| Diastolic | 80 ± 11 | 80 ± 11 |
| Laboratory data | ||
| Haemoglobin (g/L) | 145 ± 11 | 142 ± 12 |
| White blood cell count (×109/L) | 5.8 ± 1.6 | 6.2 ± 1.7 |
| Cholesterol (mmol/L) | 4.8 ± 1.3 | 4.6 ± 1.3 |
| Triglycerides (mmol/L) | 1.4 ± 1.2 | 1.3 ± 0.8 |
| HbA1c (mmol/mol) | 39 ± 7 | 41 ± 9 |
| High‐sensitivity CRP (mg/L) | 2.0 ± 2.4 | 2.5 ± 2.8 |
| Pharmacological treatment | ||
| Renin‐angiotensin system blockers | 524 (53) | 365 (61) |
| Acetylsalicylic acid | 485 (49) | 363 (61) |
| Beta‐blockers | 480 (49) | 352 (59) |
| Statins | 515 (52) | 382 (64) |
| NSAID | 26 (2.7) | 19 (3.2) |
| Corticosteroids | 31 (3.2) | 23 (3.9) |
Note: Data are presented as mean ± SD or number (%). If not otherwise stated, patient data were retrieved at the visit 6–10 weeks after the myocardial infarction for the patients and at the outpatient visit for the controls.
3.2. Outcome
The mean follow‐up was 9.9 ± 1.7 years (range 0.2–12.5). The total number of primary events during follow‐up was 361 (death = 117; non‐fatal MI = 123; non‐fatal stroke = 85; heart failure leading to hospitalization = 36; Table 2). Fifty‐four of the 117 participants who died were periodontally healthy (group mortality: 54/985 [5%]), while 63 had periodontitis (mortality 63/602 [10%]). Of the 361 events, 187 occurred among the 985 periodontally healthy participants (19%) and 174 among the 602 participants with PD (29%; p < 0.0001). Following adjustment (age, smoking and diabetes), participants with PD had a higher likelihood to develop the primary event (HR 1.26; 95% CI: 1.01–1.57). Kaplan–Meier curves for time to the primary outcome are presented in Figure 1. Corresponding curves for patients with MI and controls are presented in Figure 2A,B. There seems to be a graded increased risk to develop the primary endpoint by the severity of the periodontal disease, as revealed by Figure S1.
TABLE 2.
First of future cardiovascular events or deaths during follow‐up by periodontal status.
| Variable | Healthy | Periodontitis |
|---|---|---|
| n = 985 | n = 602 | |
| Death | 54 (5) | 63 (10) |
| Cardiovascular events | 8 (1) | 9 (1.5) |
| Non‐fatal myocardial infarction | 74 (7) | 49 (8) |
| Non‐fatal stroke | 43 (4) | 42 (7) |
| Hospitalization for heart failure | 16 (2) | 20 (3) |
| Total | 187 (19) | 174 (29) |
Note: Data are presented as numbers and percent within brackets.
FIGURE 1.
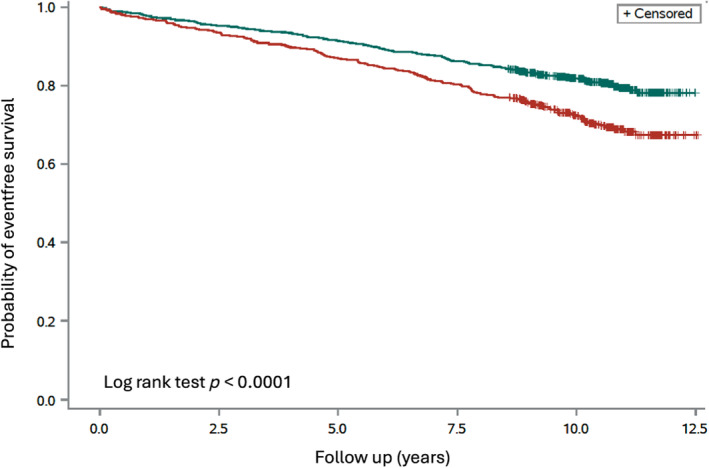
Time to the primary event, a composite of all‐cause mortality, non‐fatal myocardial infarction, non‐fatal stroke and heart failure hospitalizations by periodontal status at baseline in the total cohort (n = 1587) (log‐rank test, p < 0.0001). Green line, participants without periodontitis (n = 985); red line, participants with periodontitis (n = 602).
FIGURE 2.
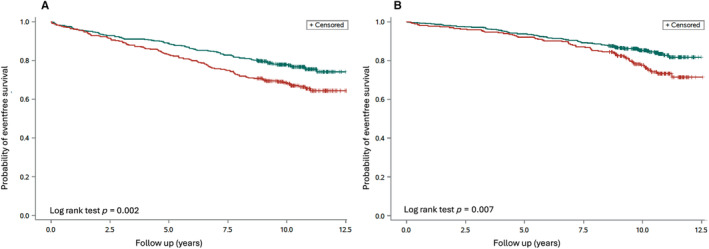
(A) Time to the primary event, a composite of the first of all‐cause mortality, non‐fatal myocardial infarction, non‐fatal stroke and heart failure hospitalization, by periodontal status at baseline in patients (n = 796; log‐rank test p = 0.002). Green line, participants without periodontitis (n = 458); red line, participants with periodontitis (n = 338). (B) Time to the primary event, a composite of the first of all‐cause mortality, non‐fatal myocardial infarction, non‐fatal stroke and heart failure hospitalization, by periodontal status at baseline in controls (n = 791; log‐rank test p = 0.007). Green line, participants without periodontitis (n = 527); red line, participants with periodontitis (n = 264).
4. Discussion
This follow‐up of the PAROKRANK cohort revealed that the primary endpoint was more common in the presence of PD.
The association between PD and CVD is well established (Pihlstrom, Michalowicz, and Johnson 2005; Blaizot et al. 2009; Lockhart et al. 2012; Teeuw et al. 2014; Moura Foz et al. 2010; Buhlin et al. 2009; Tonetti et al. 2007; Tang et al. 2023; Orlandi, Graziani, and D'Aiuto 2020). In 2012, the American Heart Association assessed whether available evidence supported that PD is an independent risk factor for CVD and whether treatment modified the risk. It was concluded that there was support for an association independent of known confounders but not for a causative relationship. Moreover, whereas periodontal treatment reduced systemic inflammation and endothelial dysfunction in short‐term studies, there was no evidence that such interventions reduced cardiovascular events (Lockhart et al. 2012).
The risk of cardiovascular events or death according to self‐reported oral hygiene was investigated in a cross‐sectional Scottish Health Survey recruiting 11,869 participants followed during an average of 8.1 years. Participants with self‐reported poor oral hygiene were at increased risk (de Oliveira, Watt, and Hamer 2010). Recently, a large retrospective study from primary care in the United Kingdom reported on 64,379 adult patients with a diagnosis of periodontal disease matched to controls without periodontitis. The exposed cohort had a 43% increased likelihood of CVD at the start of the observation and an 18% enhanced risk of developing CVD during a median follow‐up of 3.4 years (Zemedikun et al. 2021).
The strongest evidence for a causal relation between PD and CVD would be provided by the outcome of a randomized, prospective trial allocating individuals with PD and without CVD to dental treatment or no periodontal intervention with a composite of cardiovascular mortality and morbidity as endpoint. To the best of our knowledge, the outcome of a such trial has not been reported apart from a feasibility study, the Periodontitis and Vascular Events (PAVE) Study (Beck et al. 2008). In this trial, 303 patients with coronary artery disease and PD were randomized to either community care or protocol providing scaling and root planing of the periodontal disease. During a time of up to 25 months, only 12 patients had experienced a cardiovascular event, without any difference between the treatment groups. A reasonably powered trial would indeed meet several obstacles. Among them are the requirement of a very large sample size of the order of 1000s and a substantial follow‐up time of at least a decade (Cullinan and Seymour 2013). Further, it may be deemed unethical to deprive people with diagnosed PD available treatment.
PAROKRANK, a large case–control study, recruited patients and controls across Sweden during 2010–2014. Patients with a first MI were all <75 years old to avoid too many concomitant disorders, which might confound the results. Control populations, individually matched for age, sex and living location, were also investigated. All participants were meticulously investigated for cardiovascular risk factors and compared with age, sex and geographically matched controls. The study revealed that periodontal disease was associated with an almost 30% increased risk of MI (adjusted OR = 1.28; 95% CI: 1.03–1.58). This association was somewhat more pronounced in women, in participants <65 years and in those with previously undetected diabetes (Norhammar et al. 2019; Nordendahl et al. 2018). The original protocol included a prospective follow‐up.
The present result, namely an increase of cardiovascular events among those with periodontitis compared to those without, extends previous findings (Rydén et al. 2016). This increase seems more pronounced in the presence of an existing CVD in this population, a first MI. It was less pronounced among the controls. Thus, the findings cannot be seen as representative for a general population. The present results are supported by a recent report from the Atherosclerosis Risk in Communities (ARIC) study, a 13‐year long‐term follow‐up study of over 6700 persons (Molinsky et al. 2022). Heart failure events were reported to be more common among edentulous participants and in those with periodontal disease (adjusted HR = 2.4 and 1.7, respectively). Moreover, the ARIC study has reported on an association between future stroke and periodontitis (Sen et al. 2018).
4.1. Strengths
Major strengths with the PAROKRANK study are the large, carefully characterized population together with the prespecified objective to analyse the relationship between PD and CVD both in a case–control design and, as in the present report, in a prospective follow‐up of all participants. PD was diagnosed with an objective radiographic method, evaluated at a core centre, in contrast to many large observational studies which based their reports only on the number of teeth. Further, recruiting patients <75 years old reduced the potential problem with age‐related multi‐morbidity, while the recruitment of participants across Sweden ensured the selection of a representative population. Moreover, the follow‐up was complete and based on the collection of events from reliable and validated Swedish registries (Ludvigsson et al. 2011). Another strength is the use of historical bone loss on panoramic radiographs, which provided insights into the patient's overall periodontal health over time. It helped identify historical disease patterns that may not be apparent from current clinical measurements.
By considering historical bone loss, we can assess the patient's risk for future complications. A history of significant bone loss may indicate a higher risk of disease recurrence.
4.2. Limitations
First, the event rate in both groups was lower than expected, mirroring the contemporary rather benign prognosis among survivors after an MI (Ferrannini et al. 2022). The high access to CVD prevention and dental care in Sweden, as well as the enrollment in the secondary prevention registries of SWEDEHEART for the patients, may explain the low rate of periodontal disease and low cardiovascular risk, hampering the possibility to show a stronger association between PD and CVD. This is also mirrored by the distribution of deaths with relatively few direct cardiovascular events. It was, however, felt important to account for total mortality, which may have introduced a competing risk. Another important limitation was that information on change in drug treatment and dental hygiene during follow‐up could not be retrieved. As outlined, the patients were exposed to a more substantial pharmacological treatment than controls, already at the start of follow‐up. It should, however, be noted that pharmacological treatment was substantial even among the control group at the time of study start (see Table 1). Unfortunately, the study design did not allow us to collect information on biological variables that may have been influenced during the time of follow‐up. Another problem is the lack of current signs of inflammation. These shortcomings limit the generalizability of our findings. Historical bone loss does not provide a real‐time view of the current periodontal state. As underlined, follow‐up was registry‐based, and there were no opportunities to re‐examine the dental state. Thus, historical bone loss might have led to both over‐ and under‐diagnosis of periodontal disease.
A dilemma when converting a case–control study to a prospective cohort is that the case selection (MI) may be a stronger predictor than the exposure of interest (PD). To overcome this problem, we reported Kaplan–Meier survival curves both for the complete population (Figure 1) and for patients and controls separately (Figure 2A,B). As can be seen, both groups had an increase in events in the presence of PD. Since this split decreased the number of participants in each group, we did not—due to lack of statistical power—perform a formal comparison within these groups.
5. Conclusion
This pre‐planned follow‐up of the PAROKRANK study revealed an increase in the risk for cardiovascular events and death in the presence of PD. Together with the previous case control–based report on an association between PD and a first MI, the present data support the assumption that PD may increase cardiovascular risk, especially in patients with coronary artery disease.
Author Contributions
Lars Rydén: study design, data collection, data analysis, data interpretation, drafting the first manuscript (together with Per Näsman). Per Näsman: study design, data analysis, data interpretation, drafting the first manuscript (together with Lars Rydén). Kåre Buhlin: study design, data collection, manuscript review. Ulf de Faire: study design, data interpretation, manuscript review. Giulia Ferrannini: data interpretation, manuscript review. Anders Gustafsson: study design, data analysis and interpretation, manuscript review. Barbro Kjellström: study design, data collection, data interpretation, manuscript review. Björn Klinge: study design, manuscript review. Thomas Kvist: data interpretation, manuscript review. Eva Levring Jäghagen: data interpretation, manuscript review. Bertil Lindahl: study design, manuscript review. Anna Norhammar: study design, data collection, data interpretation, manuscript review. Åke Nygren: study design, data interpretation, manuscript review. Ulf Näslund: data interpretation, manuscript review. Elisabet Svenungsson: study design, data interpretation, manuscript review. All authors gave their final approval and agree to be accountable for all aspects of the work.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Data S1:
Acknowledgements
We are grateful to the personnel in the 17 participating hospitals and to all study participants. A special thanks to Merja Heinonen RN for the devoted work at the coordinating centre and for primary contacts with all controls. In addition, we are grateful to the BBMRI.se and KI Biobank at Karolinska Institutet for professional service and to all locally participating dentists and cardiologist in Sweden.
Funding: The PAROKRANK study was supported by generous grants from AFA Insurance, Swedish Heart‐Lung Foundation, Swedish Research Council, Swedish Society of Medicine, Region Stockholm (ALF project), Steering committee KI/SLL for odontological research, Eklund Foundation for Odontological Research, Baltic Child Foundation and the 1.6 Million Club.
Anna Norhammar and Per Näsman shared first authorship.
Björn Klinge and Lars Rydén shared senior authorship.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1999 International Workshop . 1999. “1999 International Workshop for a Classification of Periodontal Diseases and Conditions. Papers. Oak Brook, Ill, October 30–November 2, 1999.” Annals of Periodontology 4: i1–i112. [DOI] [PubMed] [Google Scholar]
- Beck, J. D. , Couper D. J., Falkner K. L., et al. 2008. “The Periodontitis and Vascular Events (PAVE) Pilot Study: Adverse Events.” Journal of Periodontology 79: 90–96. [DOI] [PubMed] [Google Scholar]
- Blaizot, A. , Vergnes J. N., Nuwwareh S., Amara J., and Sixon M.. 2009. “Periodontal Diseases and Cardiovascular Events: Meta‐Analysis of Observational Studies.” International Dental Journal 59: 197–209. [PubMed] [Google Scholar]
- Buhlin, K. , Hultin M., Norderyd O., et al. 2009. “Periodontal Treatment Influences Risk Markers for Atherosclerosis in Patients With Severe Periodontitis.” Atherosclerosis 206: 518–522. [DOI] [PubMed] [Google Scholar]
- Cullinan, M. P. , and Seymour G. J.. 2013. “Periodontal Disease and Systemic Illness: Will the Evidence Ever Be Enough?” Periodontology 2000 62: 271–286. [DOI] [PubMed] [Google Scholar]
- de Oliveira, C. , Watt R., and Hamer M.. 2010. “Toothbrushing, Inflammation, and Risk of Cardiovascular Disease: Results From Scottish Health Survey.” BMJ 340: c2451. 10.1136/bmj.c2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, T. , Sharma P., Walter C., Weston P., and Beck J.. 2013. “The Epidemiological Evidence Behind the Association Between Periodontitis and Incident Atherosclerotic Cardiovascular Disease.” Journal of Clinical Periodontology 40, no. Suppl 14: S70–S84. [DOI] [PubMed] [Google Scholar]
- Ferrannini, G. , Almosawi M., Buhlin K., et al. 2022. “Long‐Term Prognosis After a First Myocardial Infarction: Eight Years Follow‐Up of the Case‐Control Study PAROKRANK.” Scandinavian Cardiovascular Journal 56: 337–342. [DOI] [PubMed] [Google Scholar]
- Hansson, G. K. 2005. “Inflammation, Atherosclerosis, and Coronary Artery Disease.” New England Journal of Medicine 352: 1685–1695. [DOI] [PubMed] [Google Scholar]
- Hugoson, A. , Sjödin B., and Norderyd O.. 2008. “Trends Over 30 Years 1973–2003 in the – Prevalence and Severity of Periodontal Disease.” Journal of Clinical Periodontology 35: 405–414. [DOI] [PubMed] [Google Scholar]
- Jernberg, T. , Attebring M. F., Hambraeus K., et al. 2010. “The Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies.” Heart 96: 1617–1621. [DOI] [PubMed] [Google Scholar]
- Larvin, H. , Kang J., Aggarwal V. R., Pavitt S., and Wu J.. 2021. “Risk of Incident Cardiovascular Disease in People With Periodontal Disease: A Systematic Review and Analysis.” Clinical and Experimental Dental Research 7: 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby, P. , and Aikawa M.. 2002. “Stabilization of Atherosclerotic Plaques: New Mechanisms and Clinical Targets.” Nature Medicine 11: 1257–1262. [DOI] [PubMed] [Google Scholar]
- Lockhart, P. B. , Bolger A. F., Papapanou P. N., et al. 2012. “Periodontal Disease and Atherosclerotic Vascular Disease: Does the Evidence Support an Independent Association? A Scientific Statement From the American Heart Association.” Circulation 125: 2520–2544. [DOI] [PubMed] [Google Scholar]
- Ludvigsson, J. F. , Andersson E., Ekbom A., et al. 2011. “External Review and Validation of the Swedish National Inpatient Register.” BMC Public Health 11, 450. 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinsky, R. L. , Yuzefpolskaya M., Norby F. L., et al. 2022. “Periodontal Status, C‐Reactive Protein, NT‐proBNP, and Incident Heart Failure: The ARIC Study.” JACC Heart Failure 10: 731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura Foz, A. , Alexandre Romito G., Manoel Bispo C., et al. 2010. “Periodontal Therapy and Biomarkers Related to Cardiovascular Risk.” Minerva Stomatologica 59: 271–283. [PubMed] [Google Scholar]
- Nordendahl, E. , Gustafsson A., Norhammar A., et al. 2018. “Severe Periodontitis Is Associated With Myocardial Infarction in Females.” Journal of Dental Research 97: 1114–1121. [DOI] [PubMed] [Google Scholar]
- Norhammar, A. , Kjellström B., Habib N., et al. 2019. “Undetected Dysglycemia Is an Important Risk Factor for Two Common Diseases, Myocardial Infarction and Periodontitis: A Report From the PAROKRANK Study.” Diabetes Care 42: 1504–1511. [DOI] [PubMed] [Google Scholar]
- Orlandi, M. , Graziani F., and D'Aiuto F.. 2020. “Periodontal Therapy and Cardiovascular Risk.” Periodontology 2000 83: 107–124. [DOI] [PubMed] [Google Scholar]
- Pihlstrom, B. L. , Michalowicz B. S., and Johnson N. W.. 2005. “Periodontal Diseases.” Lancet 366: 1809–1820. [DOI] [PubMed] [Google Scholar]
- Ridker, P. M. , Everett B. M., Thuren T., et al. 2017. “Antiinflammatory Therapy With Canakinumab for Atherosclerotic Disease.” New England Journal of Medicine 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- Rydén, L. , Buhlin K., Ekstrand E., et al. 2016. “Periodontitis Increases the Risk of a First Myocardial Infarction. A Report From the PAROKRANK Study.” Circulation 133: 576–583. [DOI] [PubMed] [Google Scholar]
- Sanz, M. , Marco Del Castillo A., Jepsen S., et al. 2020. “Periodontitis and Cardiovascular Diseases: Consensus Report.” Journal of Clinical Periodontology 47: 268–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, S. , Giamberardino L. D., Moss K., et al. 2018. “Periodontal Disease, Regular Dental Care Use, and Incident Ischemic Stroke.” Stroke 49: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, K. , Wu Y., Zheng O., and Chen X.. 2023. “Bibliometric Research on Analysis of Links Between Periodontitis and Cardiovascular Diseases.” Frontiers in Cardiovascular Medicine 10: 1255722. 10.3389/fcvm.2023.1255722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif, J. C. , Kouz S., Waters D. D., et al. 2019. “Efficacy and Safety of Low‐Dose Colchicine After Myocardial Infarction.” New England Journal of Medicine 381: 2497–2505. [DOI] [PubMed] [Google Scholar]
- Teeuw, W. J. , Slot D. E., Susanto H., et al. 2014. “Treatment of Periodontitis Improves the Atherosclerotic Profile: A Systematic Review and Meta‐Analysis.” Journal of Clinical Periodontology 41: 70–79. [DOI] [PubMed] [Google Scholar]
- Thygesen, K. , Alpert J. S., Jaffe A. S., et al. 2012. “Third Universal Definition of Myocardial Infarction.” Circulation 126: 2020–2035. [DOI] [PubMed] [Google Scholar]
- Thygesen, K. , Alpert J. S., White H. D., and Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction . 2007. “Universal Definition of Myocardial Infarction.” Circulation 116: 2634–2653. [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , D'Aiuto F., Nibali L., et al. 2007. “Treatment of Periodontitis and Endothelial Function.” New England Journal of Medicine 356: 911–920. [DOI] [PubMed] [Google Scholar]
- Visseren, F. L. J. , Mach F., Smulders Y. M., et al. 2021. “2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice.” European Heart Journal 42: 3227–3337. [DOI] [PubMed] [Google Scholar]
- Wahlin, Å. , Papias A., Jansson H., and Norderyd O.. 2018. “Secular Trends Over 40 Years of Periodontal Health and Disease in Individuals Aged 20‐80 Years in Jönköping, Sweden: Repeated Cross‐Sectional Studies.” Journal of Clinical Periodontology 45: 1016–1024. [DOI] [PubMed] [Google Scholar]
- WHO . 2022a. “WHO Report on Global Burden of Disease.” Accessed May 2024. https://www.who.int/publications/i/item/9789240061484.
- WHO . 2022b. “WHO: Cardiovascular‐Diseases‐(CVDs).” Accessed September 2022. https://www.who.int/en/news‐room/fact‐sheets/detail/cardiovascular‐diseases‐(cvds).
- Zemedikun, D. T. , Chandan J. S., Raindi D., et al. 2021. “Burden of Chronic Diseases Associated With Periodontal Diseases: A Retrospective Cohort Study Using UK Primary Care Data.” BMJ Open 11, no. 12: e048296. 10.1136/bmjopen-2020-048296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1:
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


