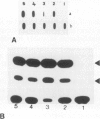
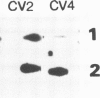
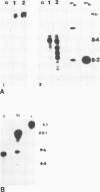
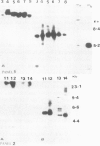
Abstract
The introduction of copia-based vectors in Drosophila hydei cells results in their high-level transient expression and the subsequent establishment of stably transformed cell lines containing multiple copies of vector integrated into host genomic DNA. Using transformation frequency and transient expression analysis as assays of promoter strength, we have defined the regions of copia essential for expression. We find that the essential sequences reside within the long terminal repeat, but 3' to the site of initiation of copia RNA. Deletion of the consensus enhancer-like sequences from copia appears to have no effect on vector expression.
Keywords: transposable element, copia, transformation, deletion analysis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg P. E., Anderson W. F. Correlation of gene expression and transformation frequency with the presence of an enhancing sequence in the transforming DNA. Mol Cell Biol. 1984 Feb;4(2):368–370. doi: 10.1128/mcb.4.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouis M., Jarry B. Vectors containing a prokaryotic dihydrofolate reductase gene transform Drosophila cells to methotrexate-resistance. EMBO J. 1983;2(7):1099–1104. doi: 10.1002/j.1460-2075.1983.tb01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F., Sinclair J. H., Sang J. H., Ish-Horowicz D. An assay for transient gene expression in transfected Drosophila cells, using [3H]guanine incorporation. EMBO J. 1984 Nov;3(11):2549–2554. doi: 10.1002/j.1460-2075.1984.tb02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Raymond K., Ju G. Functional analysis of the transcription control region located within the avian retroviral long terminal repeat. Mol Cell Biol. 1985 Mar;5(3):438–447. doi: 10.1128/mcb.5.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emori Y., Shiba T., Kanaya S., Inouye S., Yuki S., Saigo K. The nucleotide sequences of copia and copia-related RNA in Drosophila virus-like particles. 1985 Jun 27-Jul 3Nature. 315(6022):773–776. doi: 10.1038/315773a0. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Ish-Horowicz D. Extrachromosomal circular copies of the eukaryotic transposable element copia in cultured Drosophila cells. Nature. 1981 Aug 13;292(5824):591–595. doi: 10.1038/292591a0. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Levis R., Simon M. A., Rubin G. M. The 5' termini of RNAs encoded by the transposable element copia. Nucleic Acids Res. 1981 Dec 11;9(23):6279–6291. doi: 10.1093/nar/9.23.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. H., Ringler J. Ribonucleic acid synthesis in isolated Drosophila nuclei. Biochemistry. 1979 Oct 30;18(22):4923–4927. doi: 10.1021/bi00589a021. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Tsichlis P., Khoury G. Multiple enhancer domains in the 3' terminus of the Prague strain of Rous sarcoma virus. Nucleic Acids Res. 1984 Aug 24;12(16):6427–6442. doi: 10.1093/nar/12.16.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Rubin G. M. Complete nucleotide sequence of the Drosophila transposable element copia: homology between copia and retroviral proteins. Mol Cell Biol. 1985 Jul;5(7):1630–1638. doi: 10.1128/mcb.5.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S. S., Brorein W. J., Jr, Dunsmuir P., Rubin G. M. Transposition of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979 Jun;17(2):415–427. doi: 10.1016/0092-8674(79)90168-5. [DOI] [PubMed] [Google Scholar]
- Richardson K. K., Fostel J., Skopek T. R. Nucleotide sequence of the xanthine guanine phosphoribosyl transferase gene of E. coli. Nucleic Acids Res. 1983 Dec 20;11(24):8809–8816. doi: 10.1093/nar/11.24.8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S., Rose A. B., Pearlman R. E. Transposable element sequences involved in the enhancement of yeast gene expression. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5428–5432. doi: 10.1073/pnas.82.16.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz H. E., Lockett T. J., Young M. W. Analysis of transcripts from two families of nomadic DNA. J Mol Biol. 1982 May 5;157(1):49–68. doi: 10.1016/0022-2836(82)90512-5. [DOI] [PubMed] [Google Scholar]
- Shiba T., Saigo K. Retrovirus-like particles containing RNA homologous to the transposable element copia in Drosophila melanogaster. Nature. 1983 Mar 10;302(5904):119–124. doi: 10.1038/302119a0. [DOI] [PubMed] [Google Scholar]
- Sinclair J. H., Saunders S. E., Burke J. F., Sang J. H. Regulated expression of a Drosophila melanogaster heat shock locus after stable integration in a Drosophila hydei cell line. Mol Cell Biol. 1985 Nov;5(11):3208–3213. doi: 10.1128/mcb.5.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermeijer P. J., Derksen J. W., Lubsen N. H. New cell line: established cell lines of Drosophila hydei. In Vitro. 1980 Nov;16(11):913–914. doi: 10.1007/BF02619327. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]