ABSTRACT
This article highlights the critical importance of identifying the classic triad of hemoptysis, anemia, and diffuse pulmonary infiltrates, offering clinicians a structured approach for the timely diagnosis and management of the diffuse alveolar hemorrhage in setting of GPA. Post‐intubation HRCT findings revealed diffuse patchy ground glass opacities in both lungs, along with right lobar consolidation showing liquefaction and an air‐fluid level.
Keywords: diffuse alveolar hemorrhage (DAH), granulomatosis, hemoptysis, pneumonia, polyangiitis, respiratory distress
1. Introduction
Diffuse alveolar hemorrhage (DAH) represents a rare and critical clinical entity characterized by hemorrhagic involvement of the pulmonary vasculature, specifically the pulmonary arteries, pulmonary venules, and alveolar capillaries [1]. This pathological process culminates in the accumulation of erythrocytes within the alveolar space, posing a significant and potentially life‐threatening challenge to patient health [2, 3]. The clinical spectrum of DAH varies from acute respiratory distress syndrome to more subtle presentations, such as a persistent cough. This article emphasizes the pivotal role of recognizing the classic triad of symptoms—hemoptysis, anemia, and diffuse pulmonary infiltrates—as a diagnostic framework, providing clinicians with a strategic approach to timely identification and management of this complex and high‐stakes condition [4, 5].
Given the nuanced and diverse clinical presentations associated with DAH, healthcare professionals are urged to maintain a heightened clinical suspicion, particularly when confronted with patients displaying unexplained respiratory distress or subtle constitutional symptoms. This article underscores the critical need for an advanced understanding of the classic triad, serving as a practical tool for clinicians to navigate the diagnostic challenges inherent in DAH [4]. The timely application of this knowledge is imperative for facilitating accurate diagnoses and implementing appropriate therapeutic interventions in order to mitigate the potentially dire consequences of this condition [6].
The report highlights the diagnostic challenges and emphasizes the need for a comprehensive approach in managing such complex clinical presentations.
2. Case History and Examination
A 15‐year‐old female patient referred from multiple healthcare facilities, presenting with a 1‐week history of fever, progressive dyspnea, chest pain, and hemoptysis. Despite the nuanced absence of classical symptoms such as joint pain, weight loss, rash, photosensitivity, or hematuria, her clinical picture evolved from initial exertional dyspnea to a distressing state even at rest. Physical examination at presentation revealed pallor and mild with vital signs indicating a temperature of 100.2°F, oxygen saturation of 87% in room air and 92% at 4 L of oxygen, respiratory rate of 22/min, pulse of 92 beats per minute, and blood pressure measuring 100/60 mmHg. Auscultation unveiled bilateral diffuse crepitation, predominantly on the right side of the chest. In contrast, per abdominal, cardiovascular, and neurological examinations yielded unremarkable findings.
3. Investigations
The initial laboratory profile of the patient is shown in Table 1.
TABLE 1.
Laboratory results of the patient.
| Test | Result | Reference range | Unit |
|---|---|---|---|
| Hemoglobin | 6.5 | 12.0–15.5 (Female), 13.5–17.5 (Male) | gm/dL |
| Total leukocyte count (TLC) | 6300 | 4000—11,000 | /cumm |
| Platelet count | 166,000 | 150,000—450,000 | /cumm |
| Urea | 31 | 7–20 | mg/dL |
| Creatinine | 0.9 | 0.6–1.2 | mg/dL |
| Erythrocyte sedimentation rate (ESR) | 120 | < 20 (Male), < 30 (Female) | mm/h |
| C reactive protein (CRP) | 450 | < 10 | mg/L |
| Urine red blood cells (RBC) | Plenty | 0–3 | /hpf |
| Urine albumin | 2+ | Negative | — |
| Prothombin time (PT) | 18 | 10–13 | seconds |
| INR | 1.01 | 0.8–1.2 | ratio |
Serial chest X‐rays shown in Figures 1 and 2, unveiled progressive bilateral opacities in the middle and lower zones over a day. The blue arrows on Figure 1 indicates predominant unilateral opacification that appeared dense with alveolar infiltrates and mostly in the central regions, findings consistent with alveolar filling due to hemorrhage. The rapid progression is clearly visible in Figure 2, with the red arrows highlighting bilateral pleural effusion due to concurrent pneumonia.
FIGURE 1.
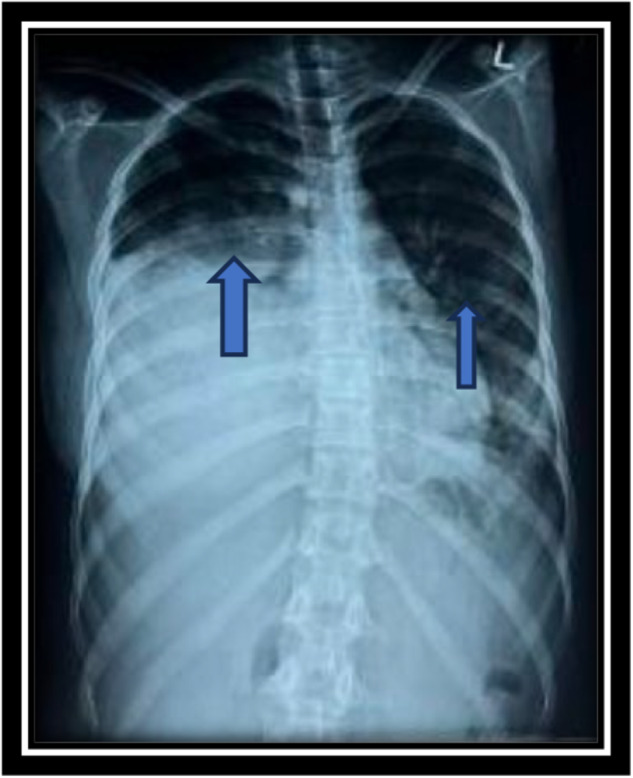
Chest X ray showing bilateral infiltrate more on right.
FIGURE 2.
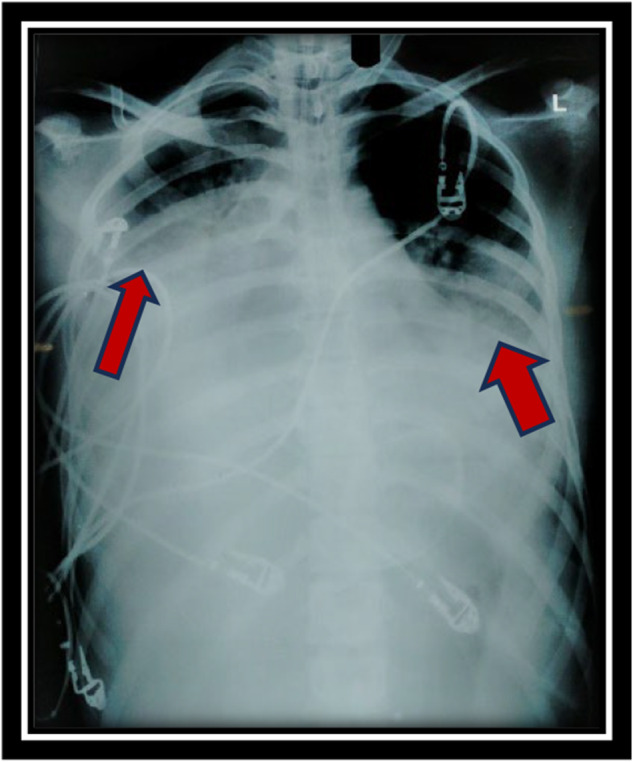
Chest X ray showing progression of hemorrhage side.
Notably, a bed side‐ USG guided diagnostic pleural tapping showed no evidence of an infective effusion. However, as the patient's respiratory distress worsened, with an increased respiratory rate of 28, subcostal retractions, low oxygen saturation of 82% on 10 L/min of supplemental oxygen, tachycardia, and altered mental status, intervention became necessary. Consequently, elective endotracheal intubation was performed.
Subsequent high‐resolution computed tomography (HRCT) post‐intubation revealed diffuse patchy ground glass opacities in bilateral lungs, with right lobar consolidation exhibiting liquefaction and an air‐fluid level. Further diagnostic endeavors included serological testing, which revealed an elevated myeloperoxidase (MPO) titer with a positive antineutrophil cytoplasmic antibody (ANCA) report. Conversely, tests for proteinase 3 (PR3), anti‐glomerular basement membrane (Anti‐GBM) antibody, double‐stranded DNA (ds‐DNA), and anti‐histone antibody returned negative results. Notably, given the suspicion of pneumonia superimposed on DAH, a sputum culture was sent, unraveling a multidrug‐resistant Acinetobacter infection, sensitive to polymyxin B and colistin.
4. Treatment and Outcome
The patient's therapeutic regimen commenced with intravenous immunoglobulin (IVIg) and intravenous methylprednisolone pulse therapy (1 g daily) for 5 days to address the DAH. Colistin, at a dosage of 4.5 million units twice daily for 10 days, was initiated for the concurrent pneumonia. Over the course of her hospital stay, the patient received six units of blood transfusion. As her consciousness level gradually improved and chest findings ameliorated, she underwent successful extubation after 9 days. Post‐extubation, the patient was administered rituximab at dosage of 500 mg escalating per hour and was subsequently discharged on an oral prednisolone regimen (40 mg daily for 2 weeks). Follow‐up care involved weekly visits for rituximab infusion over an additional 3‐week period.
5. Discussion
DAH is a rare and potentially life‐threatening condition characterized by bleeding within the pulmonary vasculature, resulting in the accumulation of red blood cells in the alveolar spaces [2]. This case report highlights the complex presentation of a 15‐year‐old female who initially presented with symptoms mimicking severe pneumonia but was later diagnosed with DAH, unveiling an underlying association with Granulomatosis with Polyangiitis (GPA) [7, 8].
The initial challenge in this case was the absence of classical symptoms typically associated with GPA, such as joint pain, weight loss, rash, myalgia, or hematuria [9]. This highlights the varied clinical presentations of GPA, as noted in the case report by Vanoli et al. [10], underscoring the importance of recognizing atypical manifestations, particularly in pediatric patients. The progressive evolution of dyspnea, chest pain, and hemoptysis from exertion to rest further added to the diagnostic complexity, necessitating a thorough investigation [8, 9, 10].
The rapid clinical deterioration and progression of pulmonary infiltrates raised concerns that the patient's condition was more than a simple pneumonia. Further inquiry with the parents revealed a history of frequent joint pain, which heightened suspicion of an underlying autoimmune etiology. A multidisciplinary approach, including input from the rheumatology team, supported the possibility of an autoimmune disorder, prompting further investigation for vasculitis and a comprehensive autoimmune panel.
Laboratory findings played a crucial role in establishing the diagnosis. The elevated MPO titer and positive ANCA result were indicative of an autoimmune etiology, prompting further exploration into GPA [11]. Negative results for other autoantibodies like PR3, Anti‐GBM antibody, ds‐DNA, and Anti‐histone antibody ruled out alternative autoimmune conditions, reinforcing the specificity of the association between DAH and GPA in this case [11, 12].
Imaging studies, including serial chest X‐rays and HRCT, revealed the extent of pulmonary involvement with bilateral opacities and pleural effusion. The notable post‐intubation finding of right lobar consolidation with liquefaction and an air‐fluid level, marked by the orange arrow on HRCT in Figure 3, accompanied by ground‐glass opacification, provided significant insight into the characterization of the disease course. These imaging findings, coupled with the clinical progression, supported the diagnosis of DAH associated with GPA. In addition to imaging and ANCA titers, a similar case reported by Kaya et al. suggests that bronchoalveolar lavage can be valuable for establishing a definitive diagnosis [13, 14].
FIGURE 3.
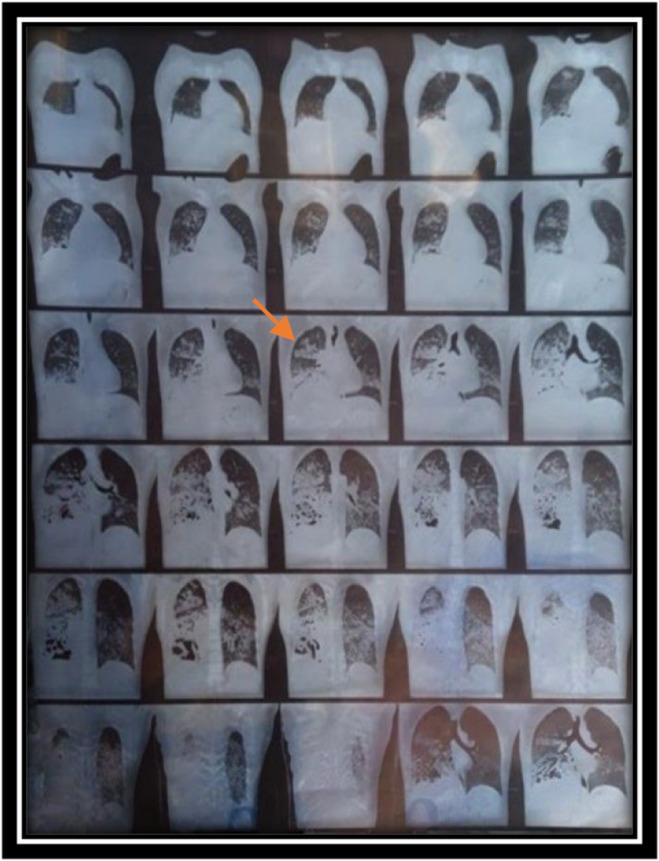
CT chest showing progression of inflitration.
The subsequent detection of multidrug‐resistant Acinetobacter infection further complicated the clinical situation, underscoring the potential difficulties in managing infectious complications in patients with autoimmune conditions. Consequently, antibiotics were prolonged for an extended duration. However, a multinational study conducted in the ICUs of Singapore, Thailand, and Nepal by Yin Mo et al. [15] demonstrated that short‐course treatment (lasting ≤ 7 days) was as effective as the conventional longer treatment (lasting ≥ 8 days) in terms of mortality or pneumonia recurrence within 60 days. This study proposes that tailoring antibiotic regimens to individual needs and shortening their duration could alleviate side effects and reduce the risk of antibiotic resistance, benefiting both well‐resourced and resource‐limited healthcare settings.
The multidisciplinary approach to treatment, involving intravenous immunoglobulin (IVIg) and intravenous methylprednisolone pulse therapy for DAH, colistin for pneumonia, and blood transfusions for severe anemia, exemplified the intricate balance required in addressing both the autoimmune and infectious aspects of the patient's condition.
Author Contributions
Shrijan Shrestha: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, visualization, writing – original draft, writing – review and editing. Pragya Rai: formal analysis, investigation, methodology, project administration, visualization, writing – original draft, writing – review and editing. Gyan Kayastha: investigation, methodology, project administration, supervision, visualization, writing – review and editing.
Consent
A written consent was taken from the patient and patient's parents, adhering to the journal's policy on patient consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding: The authors received no specific funding for this work.
Data Availability Statement
The data underlying this case report are available from the corresponding author upon reasonable request.
References
- 1. Specks U., “Diffuse Alveolar Hemorrhage Syndromes,” Current Opinion in Rheumatology 13, no. 1 (2001): 12–17. [DOI] [PubMed] [Google Scholar]
- 2. Susarla S. C. and Fan L. L., “Diffuse Alveolar Hemorrhage Syndromes in Children,” Current Opinion in Pediatrics 19, no. 3 (2007): 314–320. [DOI] [PubMed] [Google Scholar]
- 3. Gkogkou E., Broux I., Kempeneers C., et al., “Diffuse Alveolar Hemorrhage in Infants: Report of Five Cases,” Respiratory Medicine Case Reports 31 (2020): 101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taytard J., Nathan N., De Blic J., et al., “New Insights Into Pediatric Idiopathic Pulmonary Hemosiderosis: The French RespiRare® Cohort,” Orphanet Journal of Rare Diseases 8, no. 1 (2013): 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wells J. and Frankel S. K., “Alveolar Hemorrhage,” in Orphan Lung Diseases, eds. Cottin V., Cordier J. F., and Richeldi L. (London: Springer, 2015), 155–175, 10.1007/978-1-4471-2401-6_10. [DOI] [Google Scholar]
- 6. Saha B. K., “Idiopathic Pulmonary Hemosiderosis: A State of the Art Review,” Respiratory Medicine 176 (2021): 106234. [DOI] [PubMed] [Google Scholar]
- 7. Mahajan V., Whig J., Kashyap A., and Gupta S., “Diffuse Alveolar Hemorrhage in Wegener's Granulomatosis,” Lung India 28, no. 1 (2011): 52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Da Silva R. C. and Adhikari P., “Granulomatosis With Polyangiitis Presenting With Diffuse Alveolar Hemorrhage: A Systematic Review,” Cureus 14, no. 10 (2022): e29909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greco A., Marinelli C., Fusconi M., et al., “Clinic Manifestations in Granulomatosis With Polyangiitis,” International Journal of Immunopathology and Pharmacology 29, no. 2 (2016): 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vanoli J., Riva M., Vergnano B., et al., “Granulomatosis With Polyangiitis Presenting With Diffuse Alveolar Hemorrhage Requiring Extracorporeal Membrane Oxygenation With Rapid Multiorgan Relapse: A Case Report,” Medicine 96, no. 13 (2017): e6024, 10.1097/MD.0000000000006024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Emmi G., Bettiol A., Gelain E., et al., “Evidence‐Based Guideline for the Diagnosis and Management of Eosinophilic Granulomatosis With Polyangiitis,” Nature Reviews Rheumatology 19, no. 6 (2023): 378–393. [DOI] [PubMed] [Google Scholar]
- 12. Grayson P. C., Monach P. A., Pagnoux C., et al., “Value of Commonly Measured Laboratory Tests as Biomarkers of Disease Activity and Predictors of Relapse in Eosinophilic Granulomatosis With Polyangiitis,” Rheumatology 54, no. 8 (2015): 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reisman S., Chung M., and Bernheim A., “A Review of Clinical and Imaging Features of Diffuse Pulmonary Hemorrhage,” American Journal of Roentgenology 216, no. 6 (2021): 1500–1509. [DOI] [PubMed] [Google Scholar]
- 14. Kaya H., Yilmaz S., Sezgi C., et al., “Two Cases of Extrapulmonary Onset Granulomatosis With Polyangiitis Which Caused Diffuse Alveolar Haemorrhage,” Respiratory Medicine Case Reports 13 (2014): 32–36, 10.1016/j.rmcr.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mo Y., Booraphun S., Li A. Y., et al., “Individualised, Short‐Course Antibiotic Treatment Versus Usual Long‐Course Treatment for Ventilator‐Associated Pneumonia (REGARD‐VAP): A Multicentre, Individually Randomised, Open‐Label, Non‐Inferiority Trial,” Lancet Respiratory Medicine 12 (2024): 399–408, 10.1016/S2213-2600(23)00418-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this case report are available from the corresponding author upon reasonable request.


