Abstract
Introduction
China is rich in straw resources. The utilization of straw in the cultivation of edible fungi partially resolves the resource conflicts between mushroom cultivation and forest industry and also contributes to environmental protection.
Methods
In this study, based on the technology of replacing wood by grass, the straw formula for mycelial culture of Hericium erinaceus was optimized with Simplex-lattice method commonly used in mixture design. By measuring the growth rate and the activity of lignocellulose degrading enzymes of mycelia in different formulations, and further combining with model optimization, the optimal formulation was screened and validated for mushroom cultivation.
Results
In the experiments, different kinds of straw used as the main material showed interaction effects, further affecting the growth rate of mycelia and the activities of laccase, cellulase, and neutral xylanase. The screened optimal formula was composed of 16.3% rice straw, 59.7% cob, 20.0% wheat bran, 2.0% gypsum, 1.0% sucrose, and 1.0% calcium superphosphate. In the mushroom cultivation, 445.69 g of fresh mushroom were obtained and the biological efficiency reached 89.14%. The growth period of the first mushroom was shortened by 7-9 days. Some nutritional components of fruiting bodies, such as crude fats (6.10%), crude proteins (152.02 g/kg), K (19.71 g/kg), P (2.48 g/kg), and Se (6.06 g/kg), were significantly higher than those of the control formula.
Discussion
These above indicators indicated that the screened formula could be applied in the high-yield and high-quality cultivation of H. erinaceus. Our study lays the foundation for expanding cultivation and strains improvement of H. erinaceus, and is conducive in promoting the rapid development of H. erinaceus industry.
Keywords: Hericium erinaceus, substrate formulation, replacing wood by grass, simplex-lattice method, enzyme activity
1. Introduction
H. erinaceus is a precious edible and medicinal mushroom with delicious taste and high nutritional value (Friedman, 2015; Sangtitanu et al., 2020). Its fruiting bodies are rich in polysaccharide polypeptides, essential amino acids, and trace elements (Gong et al., 2004; Zhu et al., 2014). H. erinaceus has a variety of pharmacological properties, such as relieving gastric diseases (Yuan et al., 2021), anti-inflammatory (Yao et al., 2015; Tada et al., 2022), anti-cancer (Li et al., 2015; Liu et al., 2020), and liver protection (Cui et al., 2016; Jiang et al., 2016). The wide range of medicinal values of H. erinaceus has been increasingly concerned and its demand and cultivation scale have been increasing. As a typical wood-decay fungus, H. erinaceus utilizes wood as the main raw material. However, with the rapid development of the mushroom industry in recent years, the resource conflict between mushroom cultivation and forestry has become increasingly prominent, and the expansion of the cultivation industry of edible fungi are facing difficulties. Therefore, it is necessary to search for new raw materials for H. erinaceus cultivation with the reduced cost.
China is one of the countries with the richest straw resources in the world and the average annual output of straw in China reaches 700 million tons (Yang et al., 2023). Traditional straw treatment methods include open burning and landfilling, which caused waste and serious environmental pollution (He et al., 2016; Montero et al., 2016; He et al., 2018). Replacing wood by grass is a new technology developed in recent years in the edible fungus industry. With the new technology, herbaceous plants (mainly gramineous plants) and waste agricultural residues were used in mushrooms cultivation. This technology can resolve the resource conflicts between mushroom cultivation and forest industry, reduce the cultivation cost of edible fungi, utilize biological resources (Dedousi et al., 2024), decrease environmental pollution, and realize the sustainable development of edible fungus industry. At present, this technology has been widely applied in the cultivation of Auricularia auricula (Zhang et al., 2016), Pleurotus pulmonarius (Wang et al., 2022), Pholiota microspora (Meng et al., 2019), Pleurotus eryngii (Moonmoon et al., 2010), and Lentinula edodes (Pedri et al., 2015). In addition, Simplex-lattice method in mixing design has been applied by mushroom cultivators in the optimization of edible fungus straw cultivation formulas due to its advantages of less test points, simple statistical methods, and high fitting accuracy and has significantly increased mushroom production (Song et al., 2018; Wu et al., 2019a).
During the growth process of edible fungi, they decompose lignin, cellulose, and other biomacromolecules in the substrate. In the growth process, extracellular enzymes play an indispensable role and the activities of extracellular enzymes are related to physicochemical properties of culture media, species of edible fungi, and growth stage (Ball and Jackson, 1995; Kabel et al., 2017; Cesur et al., 2022). At present, the extracellular enzymes of edible fungi detected in production mainly include cellulase (Sun et al., 2021), xylanase (Agustinho et al., 2021), laccase, protease (Majumder et al., 2016) and SOD enzyme, which largely improve the utilization rate of the substrate and the growth rate of mycelium. The measured activities of these extracellular enzymes can be used to guide the high-yield cultivation of edible fungi.
In this study, we screened suitable cultivation substrates from domestic major crop straws and designed the substrate formulas with Simplex-lattice method. The relationships between mycelial growth rate and extracellular enzyme activities in different formulas were analyzed to determine suitable formulas for the cultivation of H. erinaceus. Our study lays the foundation for the large-scale cultivation of H. erinaceus with the technology of replacing wood by grass.
2. Materials and methods
2.1. Test strain
H. erinaceus 20190111, one strain with fast growth rate (4.92mm/d), high yield (biological efficiency 91.85%), and good quality screened by previous germplasm resource evaluation, was provided by the Center of Edible Fungus Research of Jilin Province Vegetable and Flower Research Institute, China.
2.2. Screening straw for the cultivation formula with wood replaced with grass
Based on the basic substrate formula (76.0% wood chips, 20.0% wheat bran, 2.0% gypsum, 1.0% sucrose, 1.0% superphosphate, and 62.0% water), all wood chips (76.0%) were completely replaced by corn straw, cob, rice straw, wheat straw, soybean straw, peanut straw and rapeseed straw, respectively. After the materials were completely mixed, spread in petri dishes (9 cm in diameter), sterilized, and then cooled for use. Round pieces (6 mm in diameter) of mycelia (cultured for 7-10 d on PDA medium at 25 °C, with darkness) were inoculated in the middle of petri dish, and the culture conditions (25 °C, dark) were invariable. When the mycelia were fully grown, draw “+” mark on the back of the petri dishes with the inoculation point as the center, and use the cross-hatch method to measure the colony diameter (Shrestha et al., 2006; Dedousi et al., 2024). Use SPSS software to calculate the daily growth rate of mycelia and perform analysis of variance, with 10 biological replicates per treatment.
2.3. Design and optimization of straw formulas with wood replaced with grass
The Simplex-lattice method in the software Design-Expert 8.0.6.1, which limit the upper and lower boundaries of various components in mixture design (Ghorbani et al., 2021; Jeswani et al., 2021; Ghislain et al., 2022), was used to design and optimize straw formulas for the growth of H. erinaceus mycelia. Based on the preliminary screening results, suitable kinds of straw were selected as the main ingredient (X) to replace wood chips, and the replacement ratio of each straw were set as the level of the investigation factor. All replacement ratios need to meet Xn ≥ 0, and X1+X2+…+Xn=1. After obtaining the replacement ratio of each straw, convert it according to the addition ratio of wood chips (76%) in the formula, and finally obtain the actual addition ratio of each straw. The mycelial growth rate and the activities of laccase (Ren et al., 2023), cellulase (Zhao et al., 2020), and neutral xylanase (Agustinho et al., 2021) of each formula were further determined and the correlation analysis was performed to establish quadratic regression models for each main material. The effects of the changes and interactions of the components in the ratios of the substrates on mycelial growth were analyzed. Further validate the authenticity of the model and optimize its parameters to maximize the response value, and finally obtain the optimal formula.
2.4. Verification test
The optimized formula and control formula were simultaneously verified in mycelial growth test and cultivation test at the Jilin Province Vegetable and Flower Research Institute. The growth conditions (25 °C, dark) in mycelial growth test were consistent with those in straw screening (section 2.2), and the mycelial growth rates were measured using cross-hatch method, and the activities of laccase, cellulase, and neutral xylanase were detected with microcalorimetry enzyme activity assay kits (COMIN, Suzhou, China). The cultivation conditions for the cultivation test were set according to Zhu et al. (2019), and make appropriate modifications. During the mycelial stage, the temperature was controlled at 25 °C, the relative humidity of the air was 60%, and avoid light. After 30 days, the cultivation temperature should be adjusted to 17-22 °C for post ripening (10 d), while keeping other conditions unchanged. Then transferred the mushroom bags to the mushroom room for fruiting, and provided weak scattered light. The temperature should be controlled at 15-18 °C, the air humidity was 90-95%, and the CO2 concentration was 0.03% (no special treatment was required for bud pressing). The cultivation period from inoculation to mushroom emergence was about 50-60 days, and the agronomic traits such as primordia formation time, fresh mass, biological efficiency, color, shape, firmness, fungal spines, and anti-bacteria capacity were recorded. In the above tests, 20 replicates were arranged. Furthermore, the contents of the nutrients in the fruiting bodies of H. erinaceus cultivated in two formulas were detected, including crude fat (Soxhlet method), crude protein (Kjeldahl method), crude fiber (acid-base hydrolysis method), ash (burning method, GB 5009.4-2016), and polysaccharides (phenol sulfuric acid method). Meanwhile, the trace elements (K, Ca, Fe, Zn, Mn, Cu, P, and Se) were also detected using the ICP-MS method in three replicates. The above results were comprehensively analyzed to verify the feasibility of the optimized formula. The results of mycelial growth rate, enzyme activity, nutrient contents and trace elements were analyzed by SPSS software and plotted using Origin software. The Design Expert 8.0.6.1 software was used for formula design, correlation analysis and contour drawing.
2.5. Statistical analysis
The variance analysis of mycelial growth rate, enzyme activity, nutrient contents and trace elements were conducted using SPSS software, and the corresponding figures were drawn using Origin software. The correlation analysis and linear regression analysis between mycelial growth rate/enzyme activity and various straws, as well as the corresponding contour drawing, were conducted in the Design Expert 8.0.6.1 software.
3. Results
3.1. Straw screening test
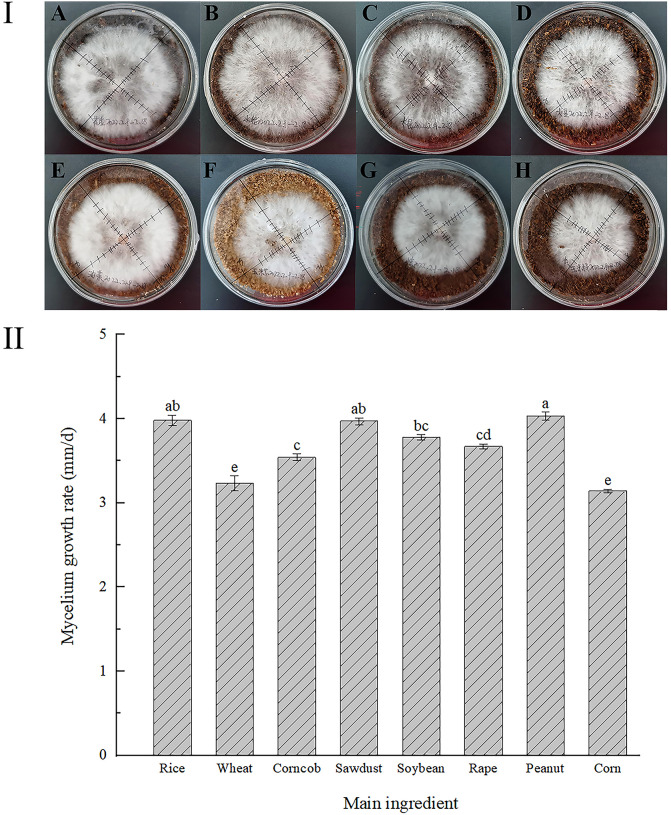
As shown in Figure 1 , the mycelia growth rate was the fastest on peanut straw (4.03 mm/d) and rice straw (3.98 mm/d), which were not significantly different from that on wood chips. Next were soybean straw (3.78 mm/d), rapeseed straw (3.67 mm/d), and corn cob (3.54mm/d), with the slowest growth rate on wheat straw and corn straw. Therefore, a total of five kinds of straws (rice, soybean, peanut, rapeseed straw, and cob) were initially selected as the main substrates for mycelial culture of H. erinaceus.
Figure 1.
Growth states of H. erinaceus mycelium on different straw medium plates. (I-A): Peanut straw; (I-B): Rice straw; (I-C): Wood chips; (I-D): Soybean straw; (I-E): Rapeseed straw; (I-F): Cob; (I-G): Wheat straw; (I-H): Corn straw; II: Mycelial growth rate. Significance, different lowercase letters represent significant differences, and the same letter represents insignificant differences).
3.2. Mixture formula and measurement results
A total of 21 formulas were designed by constraining the proportion of each ingredient in the main material with the design software ( Table 1 ). The mycelial growth rate in formulas 1, 6, 12, and 17 was significantly faster than that in the control formula (CK). The laccase activity in formula 2 was the highest and the laccase activity in most formulas was higher than that in CK. The cellulase activity in formula 4 was the highest and much higher than that in CK. The neutral xylanase activity in Formula 8 was the highest and much higher than that in other formulas and CK. In short, different straw formulas had significant effects on the mycelial growth rate and enzyme activities of H. erinaceus, and some straws were more conducive to the production of extracellular enzymes in H. erinaceus mycelia than wood chips.
Table 1.
Formula design and measurement results of culture materials.
| Formula | Straw (%) | Average growth rate(cm/d) | Laccase activity (nmol/min/g) |
Cellulase activity (μg/min/g) | Neutral xylanase (nmol/min/g) | ||||
|---|---|---|---|---|---|---|---|---|---|
| X1
Rice straw |
X2
Soybean straw |
X3
Cob straw |
X4
Peanut straw |
X5
rapeseed straw |
|||||
| 1 | 100 | 0 | 0 | 0 | 0 | 4.52 ± 0.01a | 77.85 ± 2.38gh | 749.47 ± 7.92de | 3658.81 ± 111.76gh |
| 2 | 0 | 100 | 0 | 0 | 0 | 4.19 ± 0.07fg | 346.44 ± 79.87a | 808.19 ± 1.22bcd | 3607.70 ± 88.17gh |
| 3 | 0 | 0 | 100 | 0 | 0 | 4.17 ± 0.06fg | 189.93 ± 5.81c | 778.12 ± 1.98cde | 5563.85 ± 434.66de |
| 4 | 0 | 0 | 0 | 100 | 0 | 4.22 ± 0.1efg | 170.64 ± 8.52cd | 1028.87 ± 146.05a | 4046.91 ± 112.39gh |
| 5 | 0 | 0 | 0 | 0 | 100 | 3.96 ± 0.13h | 135.35 ± 10.22ef | 809.69 ± 0.92bcd | 6672.49 ± 361.39c |
| 6 | 50 | 50 | 0 | 0 | 0 | 4.54 ± 0.01a | 145.53 ± 11.37de | 832.29 ± 16.71bcd | 7673.40 ± 583.29b |
| 7 | 50 | 0 | 50 | 0 | 0 | 4.27 ± 0.11def | 43.70 ± 5.63i | 861.90 ± 67.67bc | 6768.57 ± 573.17c |
| 8 | 50 | 0 | 0 | 50 | 0 | 4.21 ± 0.02efg | 136.98 ± 11.47def | 797.00 ± 61.21bcd | 8859.79 ± 377.91a |
| 9 | 50 | 0 | 0 | 0 | 50 | 3.8 ± 0.08i | 143.02 ± 4.14de | 572.05 ± 53.61f | 4790.94 ± 388.66f |
| 10 | 0 | 50 | 50 | 0 | 0 | 3.96 ± 0.09h | 156.45 ± 7.80de | 871.98 ± 58.28b | 5014.28 ± 216.97ef |
| 11 | 0 | 50 | 0 | 50 | 0 | 4.1 ± 0.01g | 99.02 ± 4.20gh | 833.88 ± 26.76bcd | 1302.72 ± 77.69j |
| 12 | 0 | 50 | 0 | 0 | 50 | 4.54 ± 0.02a | 284.62 ± 8.65b | 887.90 ± 6.50b | 1884.65 ± 107.93i |
| 13 | 0 | 0 | 50 | 50 | 0 | 4.39 ± 0.02bcd | 256.62 ± 10.33b | 885.74 ± 9.18b | 6023.20 ± 215.44de |
| 14 | 0 | 0 | 50 | 0 | 50 | 4.18 ± 0.03fg | 146.69 ± 1.00de | 869.44 ± 43.69bc | 4908.71 ± 177.69f |
| 15 | 0 | 0 | 0 | 50 | 50 | 4.32 ± 0.03de | 162.74 ± 7.32cde | 699.29 ± 19.55e | 3444.51 ± 156.56h |
| 16 | 60 | 10 | 10 | 10 | 10 | 4.21 ± 0.09efg | 74.94 ± 4.48ghi | 848.21 ± 35.94bc | 6067.39 ± 375.66de |
| 17 | 10 | 60 | 10 | 10 | 10 | 4.46 ± 0.07ab | 107.06 ± 3.15fg | 842.51 ± 33.22bc | 1994.17 ± 12.30i |
| 18 | 10 | 10 | 60 | 10 | 10 | 4.34 ± 0.04cde | 139.88 ± 2.49de | 858.58 ± 22.85bc | 5293.96 ± 360.79ef |
| 19 | 10 | 10 | 10 | 60 | 10 | 4.17 ± 0.09fg | 93.17 ± 3.19gh | 857.60 ± 30.43bc | 5050.34 ± 502.71ef |
| 20 | 10 | 10 | 10 | 10 | 60 | 4.21 ± 0.08efg | 99.61 ± 4.30gh | 841.19 ± 14.86bc | 6064.74 ± 499.49de |
| 21 | 20 | 20 | 20 | 20 | 20 | 4.27 ± 0.08def | 88.56 ± 6.11gh | 800.50 ± 14.66bcd | 5238.31 ± 325.40ef |
| Ck | Wood chips 100 | 4.44 ± 0.04abc | 68.29 ± 1.38hi | 816.05 ± 61.58bcd | 2357.59 ± 165.70i | ||||
Different lowercase letters in the table represent significant differences, whereas the same letter represents insignificant differences.
3.3. Correlation analysis
3.3.1. Correlations between mycelial growth rate and various straws of H. erinaceus
The regression equation between mycelial growth rate and each main material is expressed as: Y=4.52X1 + 4.19X2 + 4.17X3 + 4.22X4 + 3.96X5 + 0.74X1X2 - 0.30X1X3 - 0.65X1X4 - 1.76X1X5 - 0.86X2X3 - 0.41X2X4 + 1.88X2X5 + 0.76X3X4 + 0.44X3X5 + 0.90X4X5 + 103.37X1 2X2X3 - 280.97X1 2X2X4 + 67.85X1 2X2X5 + 78.16X1 2X3X4 + 64.99X1X2 2X3, correlation coefficient of R2 = 0.9178. (1)
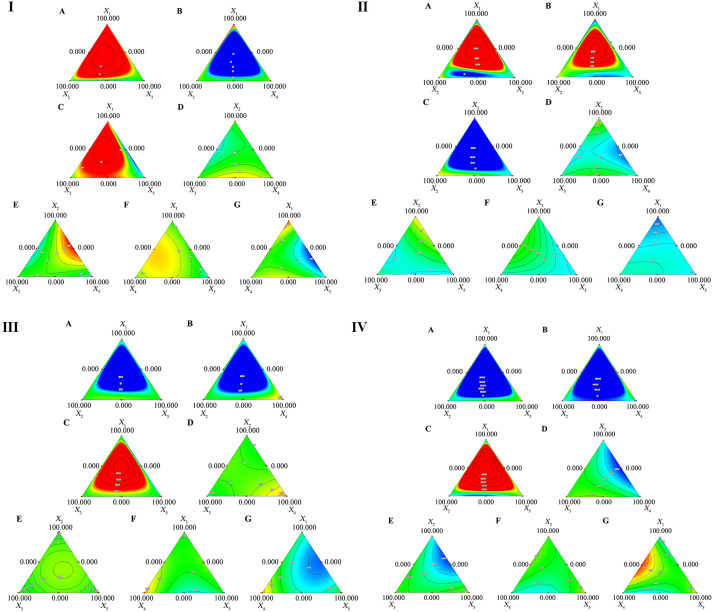
Based on the variance analysis of the quadratic multiple regression model for fitting mycelial growth rate ( Table 2 ), the P-values of the linear mixed model and the quadratic regression model were both less than 0.0001, suggesting both models fitted the relationship between the main ingredients and mycelial growth rate well, and the data could be used in the subsequent analysis. From the regression coefficients (K value) of the regression equation, it can be inferred that the effects degree of various straws on mycelial growth rate was in the following order: rice straw (KX1 = 4.52) > peanut straw (KX4 = 4.22) > soybean straw (KX2 = 4.19) > cob (KX3 = 4.17) > rapeseed straw (KX5 = 3.96). According to the analysis of variance ( Table 2 ), the interaction terms of X1X2, X1X4, X1X5, X2X3, X2X5, X3X4, X4X5 and X1X2 2X3 were extremely significant (p ≤ 0.01), indicating that their interaction can significantly affect the mycelial growth of H. erinaceus. Based on the regression equation and contour plot analysis ( Figure 2I ): for X1X2(k=0.74, red), X1X4(k=-0.65, blue) and X1X5(k=-1.76, blue), the effect of X1 on mycelia growth was associated with the addition of X2, X4, and X5 in the formula; for X2X3 (k=-0.86, blue), X2X5(k=1.88, red) and X1X2 2X3(k=64.99), the effect of X2 on mycelia growth was associated with the addition of X3, X5 and X1-X3 (simultaneously add X1 and X3) in the formula; for X3X4(k=0.76, red), adding X4 to the formula can enhance the effect of X3 on mycelial growth rate, indicating that their interaction was beneficial for mycelia growth; for X4, the interaction effect (k=0.90, red) of adding X5 to the formula was beneficial for improving the mycelial growth rate.
Table 2.
ANOVA for the fitted quadratic polynomial model of mycelium growth rate.
| Sources | Sun of squares | df | Mean square | F | P |
|---|---|---|---|---|---|
| Model | 2.20 | 19 | 0.12 | 25.26 | <0.0001 |
| Linear mixed model | 0.29 | 4 | 0.073 | 15.92 | <0.0001 |
| X1X2 | 0.069 | 1 | 0.069 | 15.10 | 0.0003 |
| X1X3 | 0.011 | 1 | 0.011 | 2.5 | 0.1215 |
| X1X4 | 0.053 | 1 | 0.053 | 11.49 | 0.0015 |
| X1X5 | 0.39 | 1 | 0.39 | 84.73 | <0.0001 |
| X2X3 | 0.093 | 1 | 0.093 | 20.29 | <0.0001 |
| X2X4 | 0.021 | 1 | 0.021 | 4.57 | 0.0383 |
| X2X5 | 0.44 | 1 | 0.44 | 96.14 | <0.0001 |
| X3X4 | 0.072 | 1 | 0.072 | 15.65 | 0.0003 |
| X3X5 | 0.025 | 1 | 0.025 | 5.38 | 0.0252 |
| X4X5 | 0.10 | 1 | 0.10 | 21.97 | <0.0001 |
| X1 2X2X3 | 3.347E-003 | 1 | 3.347E-003 | 0.73 | 0.3976 |
| X1 2X2X4 | 0.032 | 1 | 0.032 | 6.96 | 0.0115 |
| X1 2X2X5 | 3.004E-003 | 1 | 3.004E-003 | 0.66 | 0.4227 |
| X1 2X3X4 | 1.687E-003 | 1 | 1.687E-003 | 0.37 | 0.5473 |
| X1X2 2X3 | 0.041 | 1 | 0.041 | 8.96 | 0.0046 |
| Residual | 0.20 | 1 | 0.20 | ||
| Lack of fit | 3.19E-003 | 1 | 3.19E-003 | 0.69 | 0.4104 |
| Pure error | 0.19 | 42 | 4.617E-003 | ||
| Cor total | 2.40 | 62 |
F, Evaluate whether the influence of inter group factors is significant, and larger F-value represents more significant difference between groups compared to within group differences. P, Evaluate whether the impact is statistically significant, and smaller P-value indicating the higher statistical significance of the result.
Figure 2.
Contour map. I: Contour Figure (I-A) shows the effect of the interaction between various main ingredients on mycelial growth rate: the interaction among rice straw, soybean straw, and cob; (I-B): The interaction between rice straw, soybean straw, and peanut straw; (I-C): The interaction between rice straw, peanut straw, and rapeseed straw; (I-D): The interaction between soybean straw, cob, and peanut straw; (I-E): The interaction between soybean straw, cob straw, and rapeseed straw; (I-F): The interaction between cob, peanut straw, and rapeseed straw; (I-G): The interaction between rice straw, peanut straw, and rapeseed straw. II: Contour Figure (II-A): The interaction between rice straw, soybean straw, and cob; (II-B): The interaction between rice straw, soybean straw, and peanut straw; (II-C): The interaction between rice straw, peanut straw, and rapeseed straw; (II-D): Interaction between soybean straw, cob, and peanut straw; (II-E): The interaction between soybean straw, cob straw, and rapeseed straw; (II-F): The interaction between cob, peanut straw, and rapeseed straw; (II-G): The interaction between rice straw, peanut straw, and rapeseed straw. III: Contour Figure (III-A): The interaction between rice straw, soybean straw, and cob; (III-B): Interaction between rice straw, soybean straw, and peanut straw; (III-C): Interaction among rice straw, peanut straw, and rapeseed straw; (III-D): Interaction among soybean straw, cob, and peanut straw; (III-E): Interaction among soybean straw, cob straw, and rapeseed straw; (III-F): Interaction among cob, peanut straw, and rapeseed straw; (III-G): The interaction between rice straw, peanut straw, and rapeseed straw. IV: Contour (IV-A): The interaction between rice straw, soybean straw, and cob; (IV-B): The interaction between rice straw, soybean straw, and peanut straw; (IV-C): The interaction between rice straw, peanut straw, and rapeseed straw; (IV-D): Interaction between soybean straw, cob, and peanut straw; (IV-E): The interaction between soybean straw, cob straw, and rapeseed straw; (IV-F): The interaction between cob, peanut straw, and rapeseed straw; (IV-G): The interaction between rice straw, peanut straw, and rapeseed straw. The closer the color of the contour map is to red, the higher the numerical value and the contribution rate; the closer it is to blue, the lower the numerical value and the contribution rate).
3.3.2. Correlation of laccase activity and various straws of H. erinaceus
The regression equation between laccase activity and each main material is expressed as: Y = 76.91X1 + 345.50X2 + 188.99X3 + 169.70X4 + 134.41X5 - 264.85X1X2 - 359.14X1X3 + 52.54X1X4 + 147.29X1X5 - 445.31X2X3 - 636.46X2X4 + 176.52X2X5 + 306.94X3X4 - 62.18X3X5 + 40.58X4X5 + 2.134E + 0.05X1 2X2X3 + 60765.33X1 2X2X4 - 1.082E + 005X1 2X2X5 - 1.633E + 005X1 2X3X4 - 46157.78X1X2 2X3, correlation coefficient of R2 = 0.9230. (2)
Based on the variance analysis of the quadratic multiple regression model for laccase activity ( Table 3 ), the P-values of the linear mixed model and the quadratic regression model were both less than 0.0001, suggesting both models fitted the relationship between the main ingredients and laccase activity well, and the data could be used in the subsequent analysis. From the regression coefficients (K value) of the regression equation, it can be inferred that the effects degree of five kinds of straw on laccase activity ranked in the following decreasing order: soybean straw (KX2 = 345.50) > cob (KX3 = 188.99) > peanut straw (KX4 = 169.70) > rapeseed straw (KX5 = 134.41) > rice straw (KX1 = 76.91). According to the analysis of variance ( Table 3 ), the p-values corresponding to the interaction terms of X1X2, X1X3, X1X5, X2X3, X2X4, X2X5, X3X4, X1 2X2X3, X1 2X2X5, X1 2X3X4, and X1X2 2X3 were all less than 0.001, indicating that their interaction can significantly affect the laccase activity of H. erinaceus. Based on the regression equation and contour plot analysis ( Figure 2II ): for X1X2 (k=-264.5, blue), X1X3 (k=-359.14, blue), X1X5 (k=147.25, red), X1 2X2X3 (k=0.05, red), X1 2X2X5 (k=0.05, red) and X1 2X3X4(k=0.05, red), the effect of X1 on laccase activity was associated with the amount of X2, X3, X5, X2-X3 and X2-X5 in the formula; for X2X3 (k=-445.31, blue), X2X4(k=-636.46, blue), X2X5(k=176.52, red) and X1X2 2X3(k=-46157.78), the effect of X2 on laccase activity was associated with the addition of X3, X4, X5 and X1-X3 in the formula; for X3X4(k=306.94, red), adding X4 to the formula can enhance the effect of X3 on laccase activity, indicating that their interaction was beneficial for lignin degradation.
Table 3.
ANOVA for the fitted quadratic polynomial model of laccase activity.
| Source | Sun of squares | df | Mean square | F | P |
|---|---|---|---|---|---|
| Model | 3.19E+05 | 19 | 16805.74 | 45.32 | < 0.0001 |
| Linear mixed model | 1.16E+05 | 4 | 29011.96 | 78.24 | < 0.0001 |
| X1X2 | 8770.3 | 1 | 8770.3 | 23.65 | < 0.0001 |
| X1X3 | 16126.91 | 1 | 16126.91 | 43.49 | < 0.0001 |
| X1X4 | 345.11 | 1 | 345.11 | 0.93 | 0.3401 |
| X1X5 | 2712.52 | 1 | 2712.52 | 7.32 | 0.0098 |
| X2X3 | 24793.68 | 1 | 24793.68 | 66.87 | < 0.0001 |
| X2X4 | 50647.09 | 1 | 50647.09 | 136.59 | < 0.0001 |
| X2X5 | 3896.09 | 1 | 3896.09 | 10.51 | 0.0023 |
| X3X4 | 11779.81 | 1 | 11779.81 | 31.77 | < 0.0001 |
| X3X5 | 483.45 | 1 | 483.45 | 1.3 | 0.2598 |
| X4X5 | 205.87 | 1 | 205.87 | 0.56 | 0.4602 |
| X1 2X2X3 | 14262.73 | 1 | 14262.73 | 38.47 | < 0.0001 |
| X1 2X2X4 | 1493.37 | 1 | 1493.37 | 4.03 | 0.0511 |
| X1 2X2X5 | 7637.26 | 1 | 7637.26 | 20.6 | < 0.0001 |
| X1 2X3X4 | 7361.1 | 1 | 7361.1 | 19.85 | < 0.0001 |
| X1X2 2X3 | 20709.83 | 20709.83 | 55.85 | < 0.0001 | |
| Residual | 15944.16 | 43 | 370.79 | ||
| Lack of fit | 1299.64 | 1 | 1299.64 | 3.73 | 0.0603 |
| Pure error | 14644.52 | 42 | 348.68 | ||
| Cor total | 3.35E+05 | 62 |
3.3.3. Correlations between cellulase activity and various straws of H. erinaceus
The regression equation between cellulase activity and each main material is expressed as: Y=751.86X1 + 810.57X2 + 780.51X3 + 1031.26X4 + 812.07X5 + 209.77X1X2 + 388.33X1X3 - 372.77X1X4 - 834.22X1X5 + 311.21X2X3 - 342.71X2X4 + 311.75X2X5 - 75.14X3X4 + 298.05X3X5 - 884.07X4X5 -1.106E + 005X12X2X3 - 96289.73X1 2X2X4 + 1.307E + 005X1 2X2X5 + 97272.77X1 2X3X4 + 7313.02X1X2 2X3, correlation coefficient of R2 = 0.8195. (3)
Based on the variance analysis of the quadratic multiple regression model for cellulase activity ( Table 4 ), the P-values of the linear mixed model and the quadratic regression model were both less than 0.0001, suggesting both models fitted the relationship between the main ingredients and cellulase activity well, and the data could be used in the subsequent analysis. From the regression coefficients (K value) of the regression equation, it can be inferred that the effects degree of five kinds of straw on cellulase activity ranked in the following decreasing order: peanut straw (KX4 = 1031.26) > rapeseed straw (KX5 = 812.07) > soybean straw (KX2 = 810.57) > cob (KX3 = 780.51) > rice straw (KX1 = 751.86). As shown in Table 4 , The p-values corresponding to the interaction terms of X1X3, X1X4, X1X5 and X4X5 were all less than 0.01, indicating that their interaction can significantly affected the cellulase activity of H. erinaceus. Based on the regression equation and contour plot analysis ( Figure 2III ), for X1X3(k=388.33, red), X1X4(k=-372.77, blue) and X1X5(k=-834.22, blue), the effect of X1 on cellulase activity was associated with the amount of X3, X4 and X5 in the formula; for X4, the interaction effect (k=-884.07, blue) of adding X5 to the formula was not conducive to cellulose degradation.
Table 4.
ANOVA for fitted quadratic polynomial model of cellulase activity.
| Source | Sun of squares | df | Mean square | F | P |
|---|---|---|---|---|---|
| Model | 4.35E+05 | 19 | 22891.07 | 10.28 | < 0.0001 |
| Linear mixed model | 1.39E+05 | 4 | 34649.88 | 15.56 | < 0.0001 |
| X1X2 | 5501.69 | 1 | 5501.69 | 2.47 | 0.1234 |
| X1X3 | 18855.16 | 1 | 18855.16 | 8.47 | 0.0057 |
| X1X4 | 17373.61 | 1 | 17373.61 | 7.8 | 0.0078 |
| X1X5 | 87011.88 | 1 | 87011.88 | 39.07 | < 0.0001 |
| X2X3 | 12109.31 | 1 | 12109.31 | 5.44 | 0.0245 |
| X2X4 | 14684.56 | 1 | 14684.56 | 6.59 | 0.0138 |
| X2X5 | 12151.37 | 1 | 12151.37 | 5.46 | 0.0242 |
| X3X4 | 705.91 | 1 | 705.91 | 0.32 | 0.5764 |
| X3X5 | 11106.83 | 1 | 11106.83 | 4.99 | 0.0308 |
| X4X5 | 97720.89 | 1 | 97720.89 | 43.87 | < 0.0001 |
| X1 2X2X3 | 3831.75 | 1 | 3831.75 | 1.72 | 0.1966 |
| X1 2X2X4 | 3749.86 | 1 | 3749.86 | 1.68 | 0.2014 |
| X1 2X2X5 | 11148.51 | 1 | 11148.51 | 5.01 | 0.0305 |
| X1 2X3X4 | 2612.56 | 1 | 2612.56 | 1.17 | 0.2848 |
| X1X2 2X3 | 519.85 | 1 | 519.85 | 0.23 | 0.6315 |
| Residual | 95772.73 | 43 | 2227.27 | ||
| Lack of fit | 8383.73 | 1 | 8383.73 | 4.03 | 0.0512 |
| Pure error | 87389.01 | 42 | 2080.69 | ||
| Cor total | 5.31E+05 | 62 |
3.3.4. Correlations between neutral xylanase activity and various straws of H. erinaceus
The regression equation between neutral xylanase activity and each main material is expressed as: Y=3646.14X1 + 3595.03X2 + 5551.18X3 + 4034.24X4 + 6659.82X5 + 16182.29X1X2 + 8650.69X1X3 + 20049.46X1X4 - 1477.11X1X5 + 1735.73X2X3 - 10076.61X2X4 - 13000.07X2X5 + 4892.99X3X4 - 4816.13X3X5 - 7639.03X4X5 - 3.971E + 006X1 2X2X3 - 2.700E + 006X1 2X2X4 + 3.354E + 006X1 2X2X5 + 3.235E + 006X1 2X3X4 - 83254.70X1X2 2X3, correlation coefficient of R2 = 0.9777. (4)
Based on the variance analysis of the quadratic multiple regression model for neutral xylanase activity ( Table 5 ), the P-values of the linear mixed model and the quadratic regression model were both less than 0.0001, suggesting both models fitted the relationship between the main ingredients and neutral xylanase activity well, and the data could be used in the subsequent analysis. From the regression coefficients (K value) of the regression equation, it can be inferred that the effects of five kinds of straw on neutral xylanase activity ranked in the following decreasing order: rapeseed straw (KX5 = 6659.82) > cob (KX3 = 5551.18) > peanut straw (KX4 = 4034.24) > rice straw (KX1 = 3646.14) > soybean straw (KX2 = 3595.03). As shown in Table 5 , except for X1X5, X2X3, and X1X2 2X3, the p-values of other f interaction terms were less than 0.01, indicating that other interaction terms all can significantly affect the neutral xylanase activity of H. erinaceus. Based on the regression equation and contour plot analysis ( Figure 2IV ), for X1X2(k=16182.29, red), X1X3(k=8650.69, red), X1X4(k=20049.46, red), X1 2X2X3 (k=0.06, red), X1 2X2X4 (k=0.06, red), X1 2X2X5 (k=0.06, red) and X1 2X3X4(k=0.06, red), adding X2, X3, X4, X2-X3, X2-X4, X2-X5 or X3-X4 to the formula can enhance the effect of X1 on neutral xylanase activity, indicating that their interaction was beneficial for the hemicellulose degradation; for X2, the interaction effect of adding X4 (k=-10076.61, blue) or X5 (k=-13000.07, blue) to the formula was not conducive to hemicellulose degradation; for X3X4 (k=4892.99, red) and X3X5 (k=-4816.13, blue), the effect of X3 on neutral xylanase activity was associated with the amount of X4 and X5 in the formula; for X4, the interaction effect (k=-7639.03, blue) of adding X5 to the formula was not conducive to hemicellulose degradation.
Table 5.
ANOVA for the fitted quadratic polynomial model of neutral xylanase activity.
| Source | Sun of squares | df | Mean square | F | P |
|---|---|---|---|---|---|
| Model | 2.13E+08 | 19 | 1.12E+07 | 99.29 | < 0.0001 |
| Linear mixed model | 6.07E+07 | 4 | 1.52E+07 | 134.06 | < 0.0001 |
| X1X3 | 9.36E+06 | 1 | 9.36E+06 | 82.7 | < 0.0001 |
| X1X4 | 5.03E+07 | 1 | 5.03E+07 | 444.23 | < 0.0001 |
| X1X5 | 2.73E+05 | 1 | 2.73E+05 | 2.41 | 0.1278 |
| X2X3 | 3.77E+05 | 1 | 3.77E+05 | 3.33 | 0.075 |
| X2X4 | 1.27E+07 | 1 | 1.27E+07 | 112.21 | < 0.0001 |
| X2X5 | 2.11E+07 | 1 | 2.11E+07 | 186.77 | < 0.0001 |
| X3X4 | 2.99E+06 | 1 | 2.99E+06 | 26.46 | < 0.0001 |
| X3X5 | 2.90E+06 | 1 | 2.90E+06 | 25.63 | < 0.0001 |
| X4X5 | 7.30E+06 | 1 | 7.30E+06 | 64.49 | < 0.0001 |
| X1 2X2X3 | 4.94E+06 | 1 | 4.94E+06 | 43.64 | < 0.0001 |
| X1 2X2X4 | 2.95E+06 | 1 | 2.95E+06 | 26.07 | < 0.0001 |
| X1 2X2X5 | 7.34E+06 | 1 | 7.34E+06 | 64.85 | < 0.0001 |
| X1 2X3X4 | 2.89E+06 | 1 | 2.89E+06 | 25.54 | < 0.0001 |
| X1X2 2X3 | 67375.87 | 1 | 67375.87 | 0.6 | 0.4445 |
| Residual | 4.87E+06 | 43 | 1.13E+05 | ||
| Lack of fit | 2.36E+05 | 1 | 2.36E+05 | 2.14 | 0.1507 |
| Pure error | 4.63E+06 | 42 | 1.10E+05 | ||
| Cor total | 2.18E+08 | 62 |
3.4. Formula optimization and validation test
Based on the above regression equations, the expected response values of the evaluation indices were analyzed and set and then the formula with wood replaced by grass for the mycelial growth of H. erinaceus was optimized as follows: 16.3% rice straw, 59.7% cob, 20.0% wheat bran, 2.0% gypsum, 1.0% sucrose, and 1.0% calcium superphosphate. The validation results of plate test and mushroom production ( Table 6 ) showed that mycelial growth rate, laccase activity, cellulase activity, and neutral xylanase activity of the optimized formula were better than that of control formula. The differences in agronomic traits or fruiting body morphology between the optimized formula and the control formula were not significant, but the differences in the contents of nutrients in fruiting bodies were significant. The contents of crude fats, crude proteins, K, P, and Se in the optimized formula were significantly higher than those in the control, but the contents of crude fibers, crude polysaccharides, Ca, Fe, Zn, and Cu in the optimized formula were lower. The results indicated that the optimized formula could replace the conventional wood chip formula for the cultivation of H. erinaceus.
Table 6.
Comparison between optimized formula and control formula.
| Categories | Test items | VF | CK |
|---|---|---|---|
| Mycelia stage | Mycelia growth rate (mm/d) | 4.55 ± 032 | 4.43 ± 0.11 |
| Laccase activity (U/g) | 80.67 ± 1.76 | 68.29 ± 2.02 | |
| Cellulase activity (U/g) | 858.98 ± 4.19 | 816.05 ± 8.25 | |
| Neutral xylanase activity (U/g) | 6075.54 ± 78.45 | 2357.59 ± 61.12 | |
| Mushroom emergence period | Primordium formation time (d) | 7 | 8 |
| Average fresh weight (g) | 445.69 ± 5.49 | 462.45 ± 3.87 | |
| The fertility period of the first mushroom crop | 50-53 | 57-59 | |
| Average biological conversion rate (%) | 89.14 ± 1.23 | 92.49 ± 3.46 | |
| Fruiting body color | white | white | |
| Fruiting body shape | Round, monkey head-shaped | Round, monkey head-shaped | |
| Fungal spines | Long | Long | |
| Firmness of fruiting body | Tight and compact | Tight and compact | |
| Disease resistance | Strong | Strong | |
| Ingredients of fruiting body | Crude fats (%) DW | 6.10 ± 0.11 | 5.28 ± 0.12 |
| Crude proteins (g/kg) DW | 152.02 ± 0.39 | 82.03 ± 0.59 | |
| Crude fiber (%) DW | 15.11 ± 0.13 | 17.00 ± 0.39 | |
| Ash content (%) DW | 7.78 ± 0.14 | 7.00 ± 0.08 | |
| Crude polysaccharides (mg/g) DW | 343.1 ± 0.55 | 435.13 ± 3.15 | |
| Water content (%) | 65.72 ± 0.07 | 64.26 ± 0.11 | |
| K (g/kg) DW | 19.71 ± 0.28 | 10.03 ± 0.09 | |
| Ca (mg/kg) DW | 240.63 ± 10.34 | 340.63 ± 10.34 | |
| Fe (mg/kg) DW | 88.04 ± 1.13 | 111.21 ± 2.20 | |
| Zn (mg/kg) DW | 10.45 ± 0.22 | 28.53 ± 0.85 | |
| Mn (mg/kg) DW | 4.37 ± 0.18 | 4.87 ± 0.06 | |
| Cu (mg/kg) DW | 8.10 ± 0.06 | 10.10 ± 0.34 | |
| P (g/kg) DW | 2.48 ± 0.15 | 1.53 ± 0.12 | |
| Se (ug/kg) DW | 6.06 ± 4.42 | 2.08 ± 0.27 |
4. Discussion
The cultivation substrate for edible fungus was usually composed of main material, auxiliary materials and water, providing carbon source, nitrogen source, and trace elements for the growth and development of mycelia and fruiting bodies. It was one of the three elements (strains, cultivation substrate, and cultivation technology) in the production of edible fungi. An appropriate cultivation formula can improve the growth state of edible fungi and reduce the cultivation cost. In recent years, the technology of replacing wood by grass has been used to optimize the cultivation formula of edible fungi. The technology resolved the resource conflicts between mushroom cultivation and forest industry and reduced the production cost. H. erinaceus is an important edible and medicinal mushroom and its cultivation area gradually increases. Therefore, the optimization of cultivation formula of H. erinaceus is important. In this study, corn straw, cob, rice straw, wheat straw, soybean straw, peanut straw, and rapeseed straw were collected in China and used in the optimization of mycelial growth formula of H. erinaceus, in order to obtain a high-yield and high-quality straw cultivation formula for the sustainable development of H. erinaceus industry.
With Simplex-lattice method, the quantitative relationships between matrix ratios and evaluation indexes were firstly explored, and expected response values were then set based on the quantitative relationships and actual production demands in order to optimize the formula. Simplex-lattice method has been successfully used to optimize the formulas of Waffle ice cream cones (Mahulkar et al., 2024) and novel adsorbent materials (Ghorbani et al., 2021) and the concentrations of O2 and CO2 for the purposes of maintaining the quality of mango fruits and extending the shelf life (Ntsoane et al., 2020). In recent years, the method has also been well applied in edible fungus cultivation. With the method, Wu et al. (2019a) optimized a high-yield formula of Pleurotus pulmonarius, which increased biological efficiency by 15.2% and shortened the fertility period by 6 days. Song et al. (2018) used the method to optimize a high-yield formula of Grifola frondosa, which increased the yield by 39.97% and the biological efficiency by 38.53% compared to its control formula. In this study, we also optimized a formula with wood replaced by grass for the mycelial growth of H. erinaceus by setting the expected response value of each evaluation index according to the major crop straw resources in Jilin Province. The biological efficiency of this optimized formula (89.14%) was much higher than that (69.77%) of the wheat straw-based formula (Jahedi et al., 2024). The fertility period of the first mushroom crop was shortened by 7 to 9 days, and even 1 to 2 days shorter than the results of Atila (2019). Its average fresh biomass was also higher than that cultivated from rice straw by Bunroj et al. (2017). In short, the optimized formula could be used as an advantageous formula for H. erinaceus cultivation.
The quantitative relationship between each main material and evaluation indexes is the key to further optimize the cultivation formula. In the designed formulas of five kinds of agricultural straw selected in this study, it was found that the interactions between different kinds of straw affected the evaluation indexes such as mycelial growth rate, laccase activity, cellulase activity, and neutral xylanase activity. The interactions between two kinds of straw (soybean straw-rapeseed straw; peanut straw-rapeseed straw) positively contributed to the mycelial growth of H. erinaceus and the above combinations of two kinds of straw could maximize the contribution. With the same method, Zhang (2023) studied sand-washing residual mud-based low-carbon gelling materials and also found the interaction between various factors. Wu et al. (2019b) also obtained the same results in the study on the formula of Pleurotus djamor. The positive interaction between different kinds of straw might be interpreted as follows. The straw combination provided a more suitable C/N ratio and physicochemical conditions for the mycelial growth of edible fungi (Feng, 2011). Carbon and nitrogen sources are important factors affecting lignin degradation and production of extracellular enzymes by fungi. When various media with different C/N were used to cultivate Pleurotus geesterani, the activities of extracellular enzymes, such as laccase and hemicellulase showed significant differences. The limited supply of carbon and nitrogen nutrients could stimulate the fungus to synthesize lignin-degrading enzymes. The mixture of carbon sources showed the more significant stimulation action than a single carbon source (Wang, 2019).
In mushroom formulas studies, the quality and nutrient contents of fruiting bodies were also important agronomic traits for evaluate formulas. Cultivation substrates affect the nutrient composition and nutrient contents of mushroom (Bhattacharjya et al., 2015; Meng et al., 2019; Elkanah et al., 2022). In our study, we found that the contents of K, P and other trace elements in the fruiting bodies of optimized formulas were significantly improved because H. erinaceus might better absorb trace elements from cob and rice straw and convert them into own nutritional components. Edible fungi have always been an important source of high-quality proteins (Zied et al., 2017; Yao et al., 2019) and the protein content in H. erinaceus is even higher than that in most edible fungi (Zhang et al., 2024). Atila et al. (2021) found that the protein contents of H. erinaceus are greatly affected by fruiting temperature. Each strain has the ability to produce fruiting bodies at different temperatures (15 °C, 20 °C, and 25 °C), but the protein content varies greatly. Among them, the protein content of strain He Ankara reached a maximum of 19.7% at 20 °C. Jahedi et al. (2024) cultivated H. erinaceus with different crop formulas, and found that carbon nitrogen ratio (C/N) was an important factor affecting the protein content of fruiting bodies. When the nitrogen content in the formula is high, the protein content of the fruiting body is also high (up to 19.33%). In the fruiting bodies obtained with the optimized straw cultivation formula in this study, the content of crude proteins was 152.02 g/kg (15.2%), significantly higher than that of the conventional wood chip formula. The differences between the results of Atila and Jahedi may be caused by differences in strains, fruiting temperature, and the carbon nitrogen ratio of formulas. In the future, we can achieve high protein targeted improvement of H. erinaceus 20190111 by adjusting the temperature and formula carbon nitrogen ratio, providing high-quality strain for the H. erinaceus industry.
5. Conclusion
In this study, a straw cultivation formula was optimized with Simplex-lattice method: 16.3% rice straw, 59.7% cob, 20.0% wheat bran, 2.0% gypsum, 1.0% sucrose, and 1.0% calcium superphosphate. We also found that the mixtures of different kinds of straw as the main material would produce the interactions and affect the mycelial growth rate, laccase activity, cellulase activity, and neutral xylanase activity. In the mushroom production validation experiments, the biological efficiency of the optimized formula was as high as 89% and the fertility period of the first crop of mushrooms was shortened by 7 - 9 days. In addition, the contents of crude proteins and crude fats were also significantly increased. The optimized formula can be used in the production of H. erinaceus. This study lays the foundation for expanded cultivation and targeted breeding of varieties of H. erinaceus, and is conducive to the rapid development of H. erinaceus industry.
Acknowledgments
We thank TopPaper for its linguistic assistance during the preparation of this manuscript.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Basic Scientific Research Projects in Provincial Departmental Budgets (2022-2024), the Jilin Province Agricultural Key Core Technology Demonstration and Promotion (Industrial Technology System) Projects (JARS-2024-0601), and the Central Public-interest Scientific Institution Basal Research Fund (No. 1630042022003, No. 1630042022020).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZL: Writing – original draft, Software, Methodology, Data curation, Conceptualization. LL: Writing – original draft, Methodology, Data curation. ZR: Writing – original draft, Methodology. SH: Writing – original draft, Methodology, Data curation. YW: Writing – original draft, Methodology. SJ: Writing – original draft, Methodology. XW: Writing – original draft, Methodology. ZD: Writing – original draft, Methodology. YL: Writing – original draft, Methodology. YY: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. YSY: Writing – review & editing, Methodology, Conceptualization.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Agustinho B. C., Daniel J., Zeoula L. M., Alcalde C., Machado E., Bragatto J., et al. (2021). Enzymatic effects of Pleurotus ostreatus spent substrate on whole-plant corn silage and performance of lactating goats. J. Dairy Sci. 104, 11660–11672. doi: 10.3168/jds.2021-20775 [DOI] [PubMed] [Google Scholar]
- Atila F. (2019). Lignocellulosic and proximate based compositional changes in substrates during cultivation of Hericium erinaceus mushroom. Sci. Hortic. 258, 108779. doi: 10.1016/j.scienta.2019.108779 [DOI] [Google Scholar]
- Atila F., Tüzel. Y., Peken A., Faz Cano A., Fernández J. A. (2021). The effect of different fruiting temperatures on the yield and nutritional parameters of some wild and hybrid Hericium isolates. Sci. Hortic 280, 109915. doi: 10.1016/j.scienta.2021.109915 [DOI] [Google Scholar]
- Ball A. S., Jackson A. M. (1995). The recovery of lignocellulose-degrading enzymes from spent mushroom compost. Bioresour. Technol. 54, 311–314. doi: 10.1016/0960-8524(95)00153-0 [DOI] [Google Scholar]
- Bhattacharjya S. S. D. K., Paul R. K., Miah M. N., Ahmed K. U. (2015). Comparative study on nutritional composition of oyster mushroom (Pleurotus ostreatus fr.) Cultivated on different sawdust substrates. Biores. Commun. 1(2). [Google Scholar]
- Bunroj A., Sawasdikarn J., Rassami W. (2017). Research and development project of monkey’ s head mushroom (Hericium erinaceus) cultivation in east of Thailand. Int. J. Agric. Technology. 13, 1529–1535. [Google Scholar]
- Cesur A., Yamamoto R., Asada Y., Watanabe A. (2022). Relationship between fruiting body development and extracellular laccase production in the edible mushroom Flammulina velutipes . Biochem. Biophys. Rep. 29, 101204. doi: 10.1016/j.bbrep.2022.101204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F., Gao X., Zhang J., Liu M., Zhang C., Xu N., et al. (2016). Protective effects of extracellular and intracellular polysaccharides on hepatotoxicity by Hericium erinaceus sg-02. Curr. Microbiol. 73, 379–385. doi: 10.1007/s00284-016-1073-1 [DOI] [PubMed] [Google Scholar]
- Dedousi M., Melanouri E., Karayannis D., Kaminarides E. I., Diamantopoulou P. (2024). Utilization of spent substrates and waste products of mushroom cultivation to produce new crops of Pleurotus ostreatus, Pleurotus eryngii and Agaricus bisporus . Carbon Resour. Convers. 7 (2), 1. doi: 10.1016/j.crcon.2023.08.001 [DOI] [Google Scholar]
- Elkanah F. A., Oke M. A., Adebayo E. A. (2022). Substrate composition effect on the nutritional quality of Pleurotus ostreatus (mk751847) fruiting body. Heliyon 8, e11841. doi: 10.1016/j.heliyon.2022.e11841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z. (2011). Studies on optimization of culture formulation and physiological-biochemical characteristics for Pleurotus nebrodensis . Beijing, China: Chinese Academy of Agricultural Sciences. [Google Scholar]
- Friedman M. (2015). Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (lion’s mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 63, 7108–7123. doi: 10.1021/acs.jafc.5b02914 [DOI] [PubMed] [Google Scholar]
- Ghislain M. M., Gerard O. B., Emeric T. N., Adolphe M. I. (2022). Improvement of environmental characteristics of natural monoesters for use as insulating liquid in power transformers. Environ. Technol. Innov. 27, 102784. doi: 10.1016/j.eti.2022.102784 [DOI] [Google Scholar]
- Ghorbani M., Mohammadi P., Keshavarzi M., Saghi M.H., Mohammadi M., Shams A., et al. (2021). Simultaneous determination of organophosphorus pesticides residues in vegetable, fruit juice, and milk samples with magnetic dispersive micro solid-phase extraction and chromatographic method; Recruitment of simplex lattice mixture design for optimization of novel sorbent composites. Anal. Chim. Acta 1178, 338802. doi: 10.1016/j.aca.2021.338802 [DOI] [PubMed] [Google Scholar]
- Gong M., An J., Lü H., Wu C., Li Y., Cheng J., et al. (2004). Effects of denaturation and amino acid modification on fluorescence spectrum and hemagglutinating activity of Hericium erinaceum lectin. Acta Biochim Biophys Sin (Shanghai). 36(005), 343–350. doi: 10.1093/abbs/36.5.343 [DOI] [PubMed] [Google Scholar]
- He K., Zhang J., Feng J., Hu T., Zhang L. (2016). The impact of social capital on farmers’ willingness to reuse agricultural waste for sustainable development. Sustain. Dev. 24, 101–108. doi: 10.1002/sd.1611 [DOI] [Google Scholar]
- He K., Zhang J., Zeng Y. (2018). Rural households’ willingness to accept compensation for energy utilization of crop straw in China. Energy 165, 562–571. doi: 10.1016/j.energy.2018.09.023 [DOI] [Google Scholar]
- Jahedi A., Ahmadifar S., Mohammadigoltapeh E. (2024). Revival of wild edible-medicinal mushroom (Hericium erinaceus) based on organic agro-industrial waste- achieving a commercial protocol with the highest yield; Optimum reuse of organic waste. Sci. Hortic. 323, 112510. doi: 10.1016/j.scienta.2023.112510 [DOI] [Google Scholar]
- Jeswani G., Chablani L., Gupta U., Sahoo R., Nakhate K., Ajazuddin, et al. (2021). Development and optimization of paclitaxel loaded eudragit/plga nanoparticles by simplex lattice mixture design: exploration of improved hemocompatibility and in vivo kinetics. Biomed. Pharmacother. 144, 112286. doi: 10.1016/j.biopha.2021.112286 [DOI] [PubMed] [Google Scholar]
- Jiang S., Wang Y., Zhang X. (2016). Comparative studies on extracts from Hericium erinaceus by different polarity reagents to gain higher antioxidant activities. Exp. Ther. Med. 12, 513–517. doi: 10.3892/etm.2016.3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabel M. A., Jurak E., Makela M. R., De Vries R. (2017). Occurrence and function of enzymes for lignocellulose degradation in commercial Agaricus bisporus cultivation. Appl. Microbiol. Biotechnol. 101 (11), 4363–4369. doi: 10.1007/s00253-017-8294-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhou W., Kim E., Shim S., Kang H., Kim Y. (2015). Isolation and identification of aromatic compounds in lion’s mane mushroom and their anticancer activities. Food Chem. 170, 336–342. doi: 10.1016/j.foodchem.2014.08.078 [DOI] [PubMed] [Google Scholar]
- Liu J. Y., Hou X. X., Li Z. Y., Shan S., Chang M., Feng C., et al. (2020). Isolation and structural characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its arrest of cell cycle at s-phage in colon cancer cells. Int. J. Biol. Macromol. 157, 288–295. doi: 10.1016/j.ijbiomac.2020.04.162 [DOI] [PubMed] [Google Scholar]
- Mahulkar K. C., Patil A., Bhalerao P. P., Dabade A., Hundare S., Bhushette P., et al. (2024). Optimization of formulation millets flours for waffle ice cream cone using simplex lattice design: characterization and shelf life study. Food Chem. Adv. 4, 100600. doi: 10.1016/j.focha.2023.100600 [DOI] [Google Scholar]
- Majumder R., Banik S. P., Khowala S. (2016). Akp from mushroom termitomyces clypeatus is a proteoglycan specific protease with apoptotic effect on hepg2. Int. J. Biol. Macromol. 91, 198–207. doi: 10.1016/j.ijbiomac.2016.05.034 [DOI] [PubMed] [Google Scholar]
- Meng L., Fu Y., Li D., Sun X., Chen Y., Li X., et al. (2019). Effects of corn stalk cultivation substrate on the growth of the slippery mushroom (Pholiota microspora). Rsc Adv. 9, 5347–5353. doi: 10.1039/c8ra10627d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero G., Coronado M. A., Torres R., Jaramillo B. E., Valenzuela E. (2016). Higher heating value determination of wheat straw from baja california, Mexico. Energy 109, 612–619. doi: 10.1016/j.energy.2016.05.011 [DOI] [Google Scholar]
- Moonmoon M., Uddin M. N., Ahmed S. (2010). Cultivation of different strains of king oyster mushroom (pleurotus eryngii) on saw dust and rice straw in Bangladesh. Saudi J. Biol. Sci. 17, 341–345. doi: 10.1016/j.sjbs.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntsoane M. L., Sivakumar D., Mahajan P. V. (2020). Optimisation of O2 and CO2 concentrations to retain quality and prolong shelf life of ‘shelly’ mango fruit using a simplex lattice mixture design. Biosyst. Eng. 192, 14–23. doi: 10.1016/j.biosystemseng.2020.01.009 [DOI] [Google Scholar]
- Pedri Z. C., Lozano L. M., Hermann K. L., Helm C. V., Peralta R. M., Tavares L. B. B. (2015). Influence of nitrogen sources on the enzymatic activity and grown by lentinula edodes in biomass eucalyptus benthamii. Braz. J. Biol. 75, 940–947. doi: 10.1590/1519-6984.03214 [DOI] [PubMed] [Google Scholar]
- Ren J., Danchana K., Sasaki K., Kaneta T. (2023). Fluorometric assay of laccase in mushroom extracts and comparisons with absorption spectrophotometry. J. Food Compos. Anal. 123, 105627. doi: 10.1016/j.jfca.2023.105627 [DOI] [Google Scholar]
- Sangtitanu T., Sangtanoo P., Srimongkol P., Saisavoey T., Reamtong O., Karnchanatat A. (2020). Peptides obtained from edible mushrooms: Hericium erinaceus offers the ability to scavenge free radicals and induce apoptosis in lung cancer cells in humans. Food Funct. 11, 4927–4939. doi: 10.1039/d0fo00227e [DOI] [PubMed] [Google Scholar]
- Shrestha B., Lee W. H., Han S. K., Sung J. M. (2006). Observations on some of the mycelial growth and pigmentation characteristics of Cordyceps militaris isolates. Mycobiology 34, 83–91. doi: 10.4489/MYCO.2006.34.2.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B., Ye J., Sossah F. L., Li C., Li D., Meng L., et al. (2018). Assessing the effects of different agro-residue as substrates on growth cycle and yield of grifola frondosa and statistical optimization of substrate components using simplex-lattice design. Amb Express 8, 46. doi: 10.1186/s13568-018-0565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Wei Y., Kou J., Han Z., Shi Q., Liu L., et al. (2021). Improve spent mushroom substrate decomposition, bacterial community and mature compost quality by adding cellulase during composting. J. Clean Prod. 299, 126928. doi: 10.1016/j.jclepro.2021.126928 [DOI] [Google Scholar]
- Tada H., Kawahara K., Osawa H., Song L. T., Numazaki K., Kawai J., et al. (2022). Hericium erinaceus ethanol extract and ergosterol exert anti-inflammatory activities by neutralizing lipopolysaccharide-induced pro-inflammatory cytokine production in human monocytes. Biochem. Biophys. Res. Commun. 636, 1–9. doi: 10.1016/j.bbrc.2022.10.090 [DOI] [PubMed] [Google Scholar]
- Wang X. (2019). Effects of carbon and nitrogen on liquid culture and extracel lularenzyme activity of Pleurotus gees teranusaa. (Anhui, China: Anhui Agricultural University; ). [Google Scholar]
- Wang Q., Meng L., Wang X., Zhao W., Shi X., Wang W., et al. (2022). The yield, nutritional value, umami components and mineral contents of the first-flush and second-flush Pleurotus pulmonarius mushrooms grown on three forestry wastes. Food Chem. 397, 133714. doi: 10.1016/j.foodchem.2022.133714 [DOI] [PubMed] [Google Scholar]
- Wu N., Tian F. H., Jia C., Wang Y., Li C. (2019. b). Substrate optimization for mycelium growth of Pleurotus djamor in the concept of “replacing wood by grass. Microbiology 46, 1390–1403. doi: 10.13344/j.microbiol.China.180509 [DOI] [Google Scholar]
- Wu N., Tian F., Moodley O., Song B., Jia C., Ye J., et al. (2019. a). Optimization of agro-residues as substrates for Pleurotus pulmonarius production. Amb Express 9, 184. doi: 10.1186/s13568-019-0907-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Chen L., Xiong R., Jiang J., Liu Y., Tan X., et al. (2023). Long-term straw return improves cooked indica rice texture by altering starch structural, physicochemical properties in south China. Food Chemistry: X 20, 100965. doi: 10.1016/j.fochx.2023.100965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Liu Y., Ma Z. F., Zhang X. (2019). Analysis of nutritional quality of black fungus cultivated with corn stalks. J. Food Qual. 2019, 1–5. doi: 10.1155/2019/9590251 [DOI] [Google Scholar]
- Yao W., Zhang J., Dong C., Zhuang C., Hirota S., Inanaga K., et al. (2015). Effects of amycenone on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 136, 7–12. doi: 10.1016/j.pbb.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Yuan E., Nie S., Liu L., Ren J. (2021). Study on the interaction of Hericium erinaceus mycelium polysaccharides and its degradation products with food additive silica nanoparticles. Food Chemistry: X 12, 100172. doi: 10.1016/j.fochx.2021.100172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. (2023). Optimization of low-carbon cementitious material mix ratio based on sand washing residue mud based on optimal mixture design. Rec. Materials. 12, 41–46. doi: 10.16009/j.cnki.cn13-1295/tq.2023.12.020 [DOI] [Google Scholar]
- Zhang B., Cha L., Zhao Y., Zhang M., Yu P., Xu B., et al. (2024). Hypoglycemic activities of proteins and enzymatic hydrolyzed products from 12 species of edible fungi. Mycosystema 43, 230322. doi: 10.13346/j.mycosystema.230322 [DOI] [Google Scholar]
- Zhang Y., Yao A., Song K. (2016). Torrefaction of cultivation residue of auricularia auricula-judae to obtain biochar with enhanced fuel properties. Bioresour. Technol. 206, 211–216. doi: 10.1016/j.biortech.2016.01.099 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Liu S., Zha L., Zhang Y., Zhang Y., Li Z., Yu C. (2020). Influence of cellulase gene expression and cellulolytic activity on cellulose utilization in different Volvariella volvacea strains. Sci. Hortic. 261, 108956. doi: 10.1016/j.scienta.2019.108956 [DOI] [Google Scholar]
- Zhu Y., Li Q., Mao G., Zou Y., Feng W., Zheng D., et al. (2014). Optimization of enzyme-assisted extraction and characterization of polysaccharides from Hericium erinaceus. Carbohydr. Polym. 101, 606–613. doi: 10.1016/j.carbpol.2013.09.099 [DOI] [PubMed] [Google Scholar]
- Zhu L., Wu D., Zhang H., Li Q., Zhang Z., Liu Y., et al. (2019). Effects of atmospheric and room temperature plasma (ARTP) mutagenesis on physicochemical characteristics and immune activity in vitro of Hericium erinaceus polysaccharides. Molecules. 24 (2), 262. doi: 10.3390/molecules24020262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zied D. C., Pardo J. E., Tomaz R. S., Miasaki C. T., Pardo-Giménez A. (2017). Mycochemical characterization of agaricus subrufescens considering their morphological and physiological stage of maturity on the traceability process. BioMed. Res. Int. 2017, 2713742. doi: 10.1155/2017/2713742 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.