Abstract
Diabetic nephropathy (DN), as the most serious minor vascular complication of diabetes, imposes a significant socioeconomic and medical cost around the world, and its prevention and treatment are a major challenge in the current medical community. Observational studies and randomized controlled trials have revealed protective and risk factors for some DN. However, the conclusions of these researches may be influenced by several types of confounding. Mendelian randomization is a new epidemiological method mainly used to infer the causal relationship between exposure and outcome. Many Mendelian randomization studies have found potential causal relationships between DN and some diseases and lifestyle habits, thus providing valuable data for future mechanistic studies as well as the development and implementation of clinical prevention strategies. As a result, the purpose of this review is to evaluate the published Mendelian randomization study of DN, using the bibliometric research method, analyze the current research status and hot spots, and further summarize the genetic evidence about the potential protection of DN and risk factors to provide new inspiration for the etiology of DN and as a reference for clinical intervention.
Keywords: diabetic nephropathy, Mendelian randomization, bibliometrics analysis, genetic variation, causal relationship
1. Introduction
Due to ongoing changes in dietary habits and social structures, diabetes has become a major global health concern. Epidemiological surveys indicate that the number of people with diabetes worldwide will surpass 783 million by 2045 (1), and the prevalence rate has increased exponentially. Diabetic nephropathy (DN), also known as chronic kidney disease (CKD) caused by diabetes, is a microvascular complication frequently accompanied by massive proteinuria and retinopathy. Each year, more than one-third of newly diagnosed patients develop DN. DN accounts for 30% to 50% of end-stage renal disease cases, which is a leading cause of death and disability among diabetic patients (2).
Although the pathogenesis of DN is not clear, however, many randomized controlled trials (RCTs) and observational studies have found that genetic factors, intestinal flora, dietary lifestyle, and other factors are closely related to DN. However, promoting RCT testing is challenging due to discharge requirements and medical ethics constraints, and observational studies cannot eliminate many confounding factors, potentially leading to bias in the results (3). Consequently, no current studies can provide high-quality medical evidence to support preventive and treatment plans for DN.
The large sample Mendelian randomization (MR) research method, based on genome-wide association studies (GWAS), has gained widespread attention for studying high-risk factors of various diseases. To avoid the biased effects of confounding factors and reverse causality, MR analysis uses single nucleotide polymorphisms (SNPs) or genetic variations as instrumental variables (IVs) for causal inferences between risk factors and disease.
Using MR analysis to investigate high-risk factors for diabetes complications has become a research hotspot in recent years, driven by the public release of numerous large-scale GWAS studies. Among these, MR analysis related to DN is the most prominent and holds significant clinical value for understanding the etiology of DN. This review, therefore, focuses on the current situation of MR analysis in DN etiology to provide the basis and reference for the clinical prevention and treatment of DN.
2. An overview of the MR principle
Mendelian’s law of inheritance serves as the theoretical foundation for MR: during meiosis, genetic variation is assigned randomly to children and remains constant thereafter. MR analysis uses whole genome sequencing data and genetic variation as instrumental factors to determine the causal link between exposure and outcome.
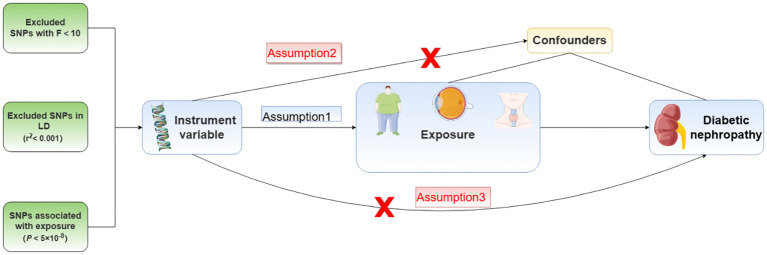
MR analysis requires that the selection of instrument variables should meet the three core assumptions of association, independence, and exclusivity: Assumption 1: instrument variables must be strongly associated with exposure factors; Assumption 2: instrument variables cannot be associated with any confounding factors associated with “exposure-outcome”; Assumption 3: instrument variables can only influence the outcome variables through exposure factors. Because genetic variation is independent of social environment, diet, and other factors, and the formation of genetic variation must precede the occurrence and change of various confounding factors and disease outcomes, using it as an instrumental variable can theoretically avoid the interference of confounding factors on the results while also avoiding the role of reverse causality ( Figure 1 ).
Figure 1.
Mendelian randomization assumption model.
MR analysis has gradually gained popularity in the medical field over the last decade, giving high-quality etiological evidence for a wide range of complicated clinical disorders as well as scientific guidance and reference for disease prevention and treatment.
3. Application and exploration of MR in the study of DN etiology
3.1. Bibliometric analysis of MR analysis related to DN
Bibliometric analysis can help to identify research trends and hotspots in existing fields, as well as inspire future studies.
This review was conducted by searching the Web of Science Core Collection (WOSCC). The search strategy is “((TS=(Diabetic kidney disease) OR TS=(Diabetic nephropathy)) AND TS=(Mendelian randomization)”, The search was finished by May 6, 2024, and after two scientists manually evaluated and removed papers unrelated to measuring risk factors for DN using MR analysis, a total of 90 articles matched the criteria. Based on R-Bibliometrix and Stork, the annual number of publications, published country map, and author cooperation network map were generated.
3.1.1. Annual scientific publications
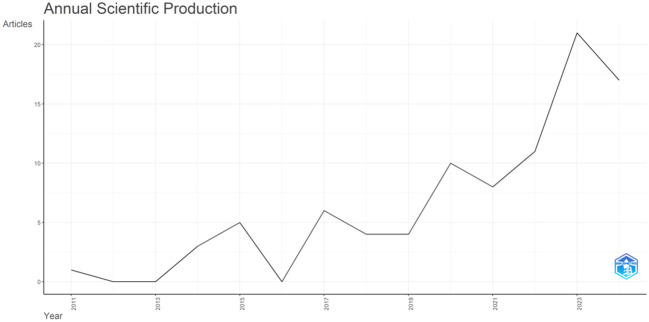
Studies using MR analysis to explore the etiology of DN began in 2011. The number of publications has increased steadily over the last 13 years, with an overall yearly growth rate of 24.35%. From 2019 to 2024, the blowout stage of this field, the number of publications increased dramatically compared to previous years, peaking in 2023 (21 papers). Since the search was undertaken in May 2024, the 2024 publications count is incomplete; nonetheless, as of May, the publications count had reached 17 ( Figure 2 ). It demonstrates that the use of MR analysis to investigate the high-risk variables of DN has gained widespread recognition.
Figure 2.
Annual scientific production of DN-related MR analysis.
3.1.2. Country scientific production
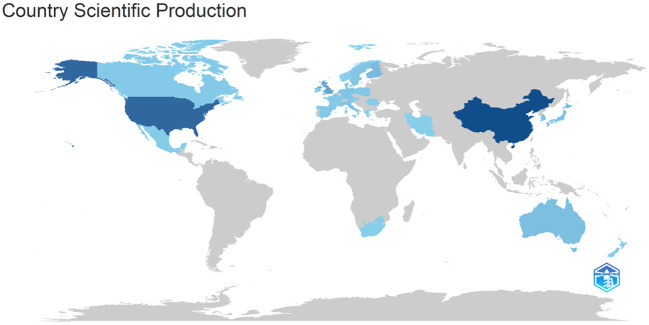
Using MR analysis to explore DN risk factors research primarily in North America, Europe, and Asia, including China’s largest post (44 papers), followed by the United States (13 papers) and Singapore (5 papers), according to the research map analysis, the field national research level difference is larger, with plenty of room for future development ( Figure 3 ).
Figure 3.
Country scientific production of DN-related MR analysis Darker colors indicate a higher number of productions; lighter colors indicate a lower number of production.
3.1.3. WordCloud analysis
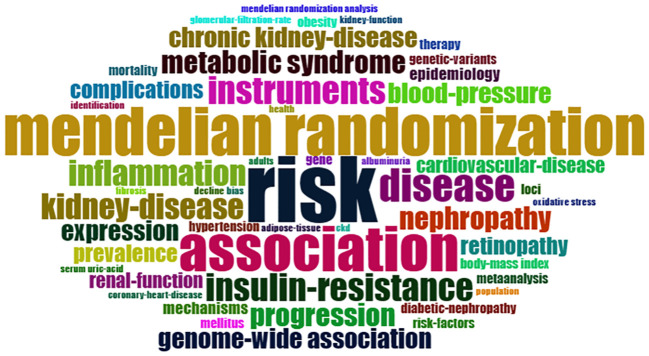
Analyzing the keyword co-occurrence map can reveal the research hotspot in the current study topic. There is no doubt that “Mendelian randomization”, “risk” and “association” are the core keywords, but also include “inflammation”, “blood-pressure”, “insulin-resistance”, “cardiovascular-disease “ and other keywords ( Figure 4 ), indicating that researchers have explored the causal relationship of various diseases and DN, trying to analyze the high-risk factors of DN progression provide a reliable basis and guidance for clinical preventive treatment.
Figure 4.
WordCloud of DN-related MR analysis.
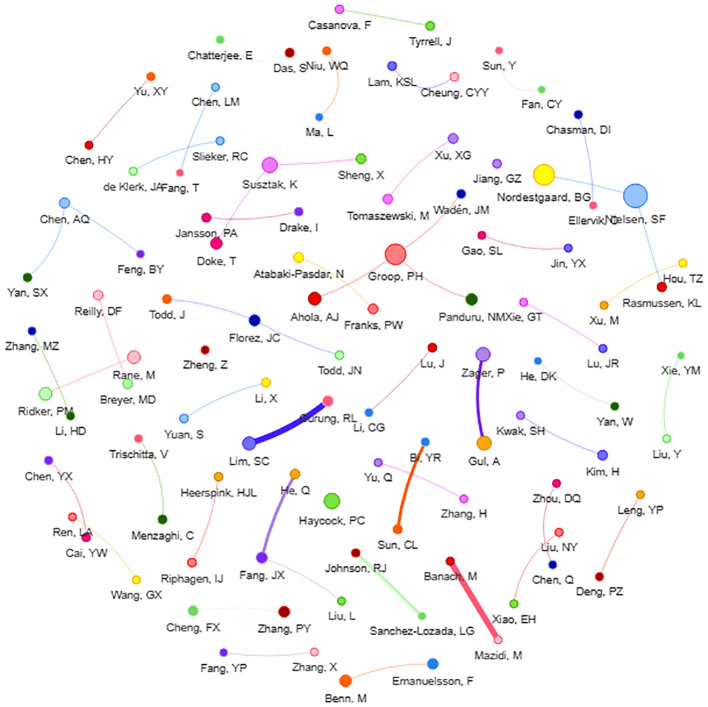
3.1.4. Researcher collaboration network
Analyzing the researcher collaboration network can reveal the core authors in this subject, and knowing the important research of the core authors can aid in understanding the field ‘s future research trends. Liu is the most published author in this field (8 papers). Liu ‘s research mainly focuses on the study of micro-exposures such as leukocyte telomere length (4) and soluble receptors of advanced glycation end products (5), and their association with the risk of DN. The second was Groop (6 papers), who used the MR to analyze the risk of DN associated with various factors such as obesity (6) and serum uric acid concentration (7). Furthermore, this review discovered that researchers are closely cooperative, but only within their own region, and there is very little cooperation between researchers from various nations, implying that regional academic cooperation in this sector should be improved in future research processes ( Figure 5 ).
Figure 5.
Researcher collaboration network of DN-related MR analysis Each dot represents a researcher, and the larger dot indicates higher output. The line between dots indicates that the researchers have cooperation, and the thicker line indicates the cooperation is closer.
3.1.5. Publishing journal analysis
Analyzing publication journals offers a better understanding of key journals on the subject, which can be widely referenced in future research. The study finds that “Frontiers in Endocrinology” has the highest publication volume (15 papers), while “Kidney International” and “Diabetes Care” have the highest impact factor (IF = 14.8). Overall, journals publishing articles connected to Mendelian randomization analysis of DN display high standards, giving high-quality references for further related studies ( Table 1 ).
Table 1.
Main research journals of DN-related MR analysis (2023).
| Journal | Count | Impact Factor | Journal Citation Reports |
|---|---|---|---|
| Frontiers in Endocrinology | 15 | 3.9 | Q2 |
| Diabetes | 7 | 6.2 | Q1 |
| Journal of Clinical Endocrinology & Metabolism | 4 | 5.0 | Q1 |
| Diabetologia | 3 | 8.4 | Q1 |
| Kidney International | 2 | 14.8 | Q1 |
| Diabetes Care | 2 | 14.8 | Q1 |
| Diabetes Metabolic Syndrome and Obesity | 2 | 2.8 | Q3 |
| Frontiers in Microbiology | 2 | 4 | Q2 |
| International Urology and Nephrology | 2 | 1.8 | Q3 |
| BMJ Open Diabetes Research & Care | 2 | 4.1 | Q2 |
3.2. Causal relationship between micro-exposures and DN
3.2.1. Gut microbiota
As a complex ecosystem, the intestinal microflora has a significant impact on the internal environment. The existence of the gut-kidney axis provides a possibility for the gut microbiota to contribute to the development of DN, and related studies have also found a close relationship between gut microbiota and DN (8–10). Therefore, disruptions in the gut microbiota are considered a risk factor for DN. However, various biases exist in observational studies, and the causal relationship remains unclear. Yan (11) obtained genetic data on gut microbiota from the publicly available GWAS data of the MiBioGen consortium, which includes 24 cohorts and 18,340 samples. Their study conducted an MR analysis on the relationship between 12 microbial taxa in gut microbiota and DN. The results showed that Akkermansia, Verrucomicrobia, Peptostreptococcaceae, Butyricimonas, Catenibacterium, and Marvinbryantia significantly increase the risk of DN. Among them, the lack of Akkermansia is closely related to obesity, diabetes, inflammation, and tumors (12). However, some studies have found that its abundance was positively correlated with serum creatinine (SCr) and blood urea nitrogen (BUN) levels (13). Therefore, it is speculated that Akkermansia may reduce the risk of developing diabetes by regulating glucose metabolism and intestinal function. However, with a prolonged duration of diabetes, Akkermansia may increase the risk of DN by affecting renal function. Verrucomicrobia and Akkermansia belong to the same bacterial group, and the mechanisms by which they increase the risk of developing DN may be similar. Therefore, further experiments are needed in the future to verify the role of gut microbiota at different stages of the disease. The above results were confirmed in the latest MR analysis by Yan (14).
3.2.2. Blood biomarkers
As a complication of metabolic disease, observational studies have found that the progression of DN is closely associated with other metabolic markers in the body, including vitamin D, blood uric acid level, and serum albumin level, but the interference of confounding factors cannot be excluded. Therefore, conducting MR analysis can further elucidate the relationship between exposure and outcome, enabling better prediction of the progression of DN in the future.
Firstly, vitamin D participates in various signaling pathways within the body, including inflammation, apoptosis, proliferation, etc, in the form of 25-hydroxyvitamin D. Previous studies believe that vitamin D can improve glucose metabolism and inhibit the activation of the renin-angiotensin system (RAS) to achieve the purpose of preventing DN (15, 16). However, He et al. (17) conducted an MR analysis using SNP closely associated with vitamin D extracted from a publicly available GWAS involving 79,366 individuals of European descent. The study ultimately found no causal relationship between vitamin D and DN. Therefore, currently, from a genetic perspective, there is no support for using vitamin D supplementation as an effective strategy for preventing DN.
Secondly, uric acid, as the end product of purine metabolism, circulates through the kidneys and is excreted from the body, closely related to renal function. Epidemiological and observational studies have found an association between uric acid levels and the progression of DN (18, 19). However, in an MR analysis conducted by Ahola et al. (7) in 2014, contradictory results to previous studies were found. The study showed that there was no causal relationship between blood uric acid levels and the occurrence of DN in patients with type 1 diabetes (defined based on glomerular filtration rate) (OR: 2.24, 95% CI: -17.29 to 21.77, P = 0.631). Subsequently, Feng et al. (20) utilized the latest publicly available GWAS data from the FinnGen and UK Biobank to conduct an MR analysis, confirming these findings: there is no causal relationship between blood uric acid levels and DN. Our study speculated that uric acid may contribute to the progression of CKD by generating nitric oxide, activating the RAS, and stimulating vascular smooth muscle cell proliferation. However, for DN, simply lowering serum uric acid levels may not be beneficial for the outcome of DN.
Thirdly, serum albumin is the most abundant protein in the plasma. Previous cohort studies are ambiguous on the relationship between serum albumin and the onset of diabetes mellitus (21, 22). However, for DN, some relevant retrospective studies have found that the decreased serum albumin level is an independent risk indicator of DN (23, 24). Therefore, MR analysis is needed to avoid the possible error bias in previous studies to clarify the causal relationship. Cai et al. (25) combined prospective studies with bidirectional two-sample MR analysis. In the prospective study, it was found that for every 10 g/l increase in serum albumin levels, the hazard ratio (HR) of DN was 0.42 (95% CI: 0.30 to 0.58). Regarding the MR analysis, the researchers extracted 19 SNPs associated with serum albumin from the UK Biobank as instrumental variables. The MR analysis ultimately found a causal relationship between serum albumin levels and the occurrence of diabetes, which was negatively correlated (OR: 0.990, 95% CI: 0.984 to 0.995, P = 2.33×10-4), thus validating the results of the observational study. The protection mechanism of serum albumin for diabetes and DN may lie in its ability to regulate colloidal osmotic pressure and capillary membrane permeability. Additionally, it can scavenge free radicals and play an antioxidant role (26), In addition, the glycation of serum albumin may take precedence over the glycation of hemoglobin. Therefore, an increase in serum albumin levels can lead to enhanced competitive glycation inhibition, thus reducing the level of hemoglobin A1c (27). Ultimately, this contributes to lowering blood glucose levels and delaying the progression of microvascular complications.
3.2.3. Inflammatory mediators
With the emergence of the inflammatory theory in the field of diabetic complications research, it has been receiving increasing attention. A growing body of experimental evidence emphasizes the significant role of inflammatory factors in the occurrence and progression of DN. Activated inflammatory cells migrate and infiltrate the kidneys, locally producing inflammatory mediators that exacerbate kidney damage in a high-glucose environment. Therefore, the elevation of inflammatory mediators may be an upstream event in the progression of DN. Clarifying the relationship between inflammatory mediators and DN is crucial for clinical prevention and treatment. An et al. (28) utilized two-sample MR to investigate the causal relationships between 41 inflammatory factors and DN. The final results revealed that Interferon-γ (IFN-γ) (OR: 1.33, 95% CI:1.09 to 1.63, P = 0.005) and Stem Cell Factor (SCF) (OR: 1.25, 95%CI: 1.02 to 1.52, P = 0.027) has a positive causal relationship with increased risk of DN, Meanwhile, Macrophage inflammatory protein-1β (MIP-1β) (OR: 0.92,95%CI: 0.85 to 0.98, P = 0.022) and interleukin-16 (IL-16) (OR: 0.89, 95%CI: 0.81 to 0.99, P = 0.043) were found to have a causal relationship with reduced risk of DN. Furthermore, Lin et al. (29) conducted an observational study and used genetic data provided by the Taiwan Biobank for MR analysis. The final results of both studies indicate a causal relationship between levels of high-sensitivity C-reactive protein (hs-CRP) and the occurrence of DN.
This review analyzed the roles of relevant inflammatory mediators in DN as follows: First, IFN-γ overexpression increases IFN-regulatory factors (IRFs), as well as the secretion of nuclear factor kappa-B (NF-κB) and signal transducer and activator of transcription-1 (STAT-1), selectively promoting the polarization of M1 macrophages (30). This leads to increased secretion of inflammatory cytokines. Furthermore, IFN-γ can also enhance the expression of vascular endothelial growth factor (VEGF). Through the combined action of these mechanisms, continuous damage to the renal microvasculature may occur. Observational studies further confirmed that elevated IFN- γ is a predictor of DN onset (31). For SCF, the SCF/c-kit signaling pathway leads to the aggregation of endothelial progenitor cells and promotes angiogenesis. Animal experiments have found a significant positive correlation between the expression levels of SCF and c-kit and the degree of mast cell infiltration. Mast cell infiltration promotes renal interstitial fibrosis, thereby increasing the risk of DN (32). Finally, CRP is a nonspecific marker of inflammation and tissue damage. CRP can promote renal inflammation through the CD32b-NF-κB signaling pathway and induce renal fibrosis via the CD32b-Smad3-mTOR signaling pathway (33). Additionally, elevated CRP levels increase the production of pro-inflammatory cytokines, leading to mesangial cell proliferation, excessive matrix production, and increased vascular permeability, which in turn results in renal function impairment and albuminuria (34). The aforementioned MR results emphasize that early intervention targeting certain inflammatory mediators can play a positive role in protecting patients with DN.
3.2.4. Leukocyte telomere length
Telomeres are DNA-protein structures at the ends of chromosomes, and their length shortens with each cell division. Telomeres protect the genome from damage and serve as important markers of organismal aging and cellular apoptosis. A study has found that controlling telomere length is crucial for maintaining telomere stability (35). A cohort observational study found that relatively short telomere length (RTL) is closely associated with faster CKD progression in diabetic patients (HR: 1.16, 95% CI: 1.01 to 1.34, P = 0.03) (36). Another prospective study based on multiple Asian ethnicities similarly found that T2DM patients with shorter leukocyte telomere length (LTL) had more than double the risk of albuminuria (OR: 2.10, 95% CI: 1.15 to 3.83, P = 0.016) (37). These studies suggest that short LTL may be a novel biomarker for the progression of DN. To further clarify the causal relationship, Gurung et al. (4) extracted 16 SNPs closely associated with leukocyte telomere length (LTL) from a GWAS in the Singapore Chinese Health Study (SCHS) cohort and conducted a two-sample MR analysis. The final results validated the conclusions of previous observational studies: genetically determined shorter LTL is closely associated with an increased risk of CKD in T2DM patients (OR: 1.51, 95% CI: 1.12 to 2.12, P = 0.007). Therefore, preventing premature telomere shortening will be an important strategy in the prevention and treatment of DN.
3.2.5. Drug target
Drug-target MR analysis is emerging as an effective tool for inferring the effect of various drugs acting on encoded proteins on disease risk (38). Unlike traditional MR studies, in drug-target MR analysis, genetic variants are selected from the gene of interest or a neighboring genomic region. The wide application of drug-target MR can help to better identify the potential targets of drug action on diseases in order to facilitate drug development and improve clinical efficacy.
For the onset and progression of DN, the renin-angiotensin-aldosterone system (RAAS) plays an important role, and inhibition of the RAAS can have many positive effects on the prevention of DN (39). RAAS inhibitors, with Angiotensin Converting Enzyme Inhibitors (ACEI) and Angiotensin Receptor Blockers (ARB), are the most widely used in clinical practice. However, a follow-up study found that DN patients who have been treated with ARB, are still at high risk of end-stage renal disease (ESRD) outcomes (40). In addition to this, another study found that combining an ACEI with an ARB increased the risk of hyperkalemia and acute kidney injury (41). Thus, drug-target MR offers the possibility to explore the target mechanism of action of RAAS inhibitors in DN. Zhou (42) conducted a network pharmacology combined with a drug-target MR study, and the final MR results demonstrated the positive effects of CTSC (IVW, OR: 0.861, P=0.041) and PDE5A (IVW, OR: 0.842, P=0.018), the key targets of RAAS inhibitors in the treatment of DN, in protecting DN. CTSC has been shown in previous studies to be a human DN uroprotein gene, which may play a role by participating in the regulation of urinary proteins (43). PDE5A belongs to the phosphodiesterase family, and an animal study has shown that targeted regulation of PDE5 exerts a significant anti-fibrotic and nephroprotective effect on the kidney (44).
In addition, statins are widely used in the diabetic population because diabetic patients are often prone to comorbid lipid metabolism abnormalities (45). However, previous studies have differed on the use of statins in patients with DM. A multicenter cohort study conducted in China showed that statin use was associated with a lower risk of DN events (HR: 0.72, 95% CI = 0.62 to 0.83) (46). A retrospective cohort study, however, found that statin use in diabetic patients was associated with a moderately elevated risk of kidney disease progression (OR: 1.16, 95% CI:1.12 to 1.20) (47). Therefore, drug-target MR studies need to be introduced to avoid the influence of various confounding factors on the results. Zhao et al. (48) found a significant correlation between HMGCR inhibition, the main pathway of action of statins, and a high risk of DN (OR: 1.79, 95% CI:1.14 to 2.78, P = 0.01). The results of MR are usually interpreted as lifetime exposure, so the final results can be interpreted as a negative effect of long-term HMGCR inhibitors on DN. This study provides new insights into the selection of lipid-lowering medications for patients with clinical DN.
3.2.6. Proteomic
Proteomic MR is an emerging research direction with similarities to drug-target MR, as proteins often have specific binding sites or regions that can be targeted by biologics, so with the identification of thousands of protein quantitative trait loci (pQTLs) for plasma proteins by GWAS, carrying out proteomic MR can help us to identify the potential therapeutic sites for DN and identify drug targets in advance. Fan et al. (49) conducted a proteomic MR by extracting pQTL of plasma proteins from seven different proteomic GWAS and found that higher levels of MICB (OR: 1.46, 95% CI 1.27 to 1.67; P = 3.94×10-8), GZMA (OR: 1.34, 95% CI 1.17 to 1.53; P = 1.86×10-5), and CLIC5 (OR: 1.45, 95% CI 1.04 to 2.03, P = 2.99×10-2) may promote the progression of DN, whereas CTSS (OR: 0.90, 95% CI 0.83 to 0.97, P = 5.78×10-3) may play a protective role in the progression of DN. Zhang et al. (50) subsequently combined proteomic MR with co-localization analysis and external validation in an attempt to find key targets for the treatment of DN, resulting in the novel identification of potential drug target properties of COL6A2, CBLN1, TGFBI, and ITIH3 for the treatment of DN. Gurung et al. (51) conducted an MR analysis based on young Asian T2DM DN patients and found that higher plasma ANG levels were associated with an increased risk of DN in young Asian T2DM (OR: 4.03, 95% CI 1.28 to 12.68, P = 0.017).
These proteomic MR studies offer the possibility of new protein targets for the prevention and treatment of DN.
3.3. Causal relationship between macro-exposures and DN
3.3.1. The underlying diseases
The occurrence and progression of DN are regulated and influenced by the endocrine system, immune system, and other factors. Using MR analysis to study the causal relationship between various diseases and DN contributes to the early diagnosis and screening of DN in populations with related diseases, enabling early and effective intervention.
Firstly, Diabetic Retinopathy (DR) is another typical microvascular complication of diabetes mellitus, apart from DN. Meta-analysis has shown that the comprehensive sensitivity and specificity of diabetic retinopathy in predicting DN are 0.65 (95% CI: 0.62 to 0.68) and 0.75 (95% CI: 0.73 to 0.78), respectively. Duan et al. (52) further conducted MR analysis by extracting SNPs closely related to DR and DN from FinnGen and UK Biobank. They ultimately found a causal relationship between DR and DN occurrence (OR: 2.89, 95% CI: 1.76 to 4.75, P<0.001), validating the conclusions of previous observational study (53). Therefore, it is recommended to promptly perform comprehensive renal function tests in DR patients to prevent the occurrence of DN.
Thyroid hormone receptors are abundantly present in the vascular endothelium, and fluctuations in thyroid hormone levels can affect vascular function. Although current observational studies have found a certain correlation between thyroid function and renal function (54, 55), there is still insufficient evidence to prove a direct causal relationship between thyroid function and DN. Therefore, Li et al. (56) conducted an MR analysis based on a European population sample to investigate the causal relationship between thyroid function and DN. They found that thyroid-stimulating hormone (TSH) was positively correlated with the risk of DN (OR: 1.44, 95% CI: 1.04 to 2.41, P = 0.033). Additionally, TSH was negatively correlated with the estimated glomerular filtration rate (eGFR) in diabetic patients (β: -0.031, 95% CI: -0.063 to -0.001, P = 0.047). This suggests that an increase in TSH may raise the risk of DN and simultaneously decrease the eGFR in patients with T2DM. This study speculates that this is because TSH can stimulate leptin secretion, thereby increasing hepatic glucose output and enhancing gluconeogenesis to stimulate endogenous glucose production, which in turn reduces hepatic insulin sensitivity. Additionally, TSH inhibits insulin synthesis in β-cells, ultimately raising blood glucose levels (57). Moreover, abnormal secretion of thyroid hormones combined with a high-glucose environment will further exacerbate damage to the vascular endothelium (58). Although MR analysis has demonstrated the causal relationship between them, further basic research is still needed to clarify the underlying mechanisms in the future.
Obesity has been considered a starting point for multiple diseases and is closely associated with the occurrence of complications in diabetes. Assessment of obesity includes indicators such as body mass index (BMI), waist circumference (WC), trunk fat content, etc. Several MR analyses have now demonstrated a significant causal relationship between obesity and DN (6, 59–61). Our study suggests that obesity leads to reduced secretion of adiponectin in the body. Adiponectin has various beneficial effects, such as anti-atherosclerosis and anti-inflammatory properties (62). Consequently, reduced adiponectin levels exacerbate endothelial damage and inflammation. Additionally, excessive visceral adipose tissue (VAT) can disrupt metabolism and exacerbate insulin resistance. Meanwhile, lymphocytes and macrophages infiltrate adipose tissue, leading to the release of inflammatory cytokines and reactive oxygen species, exacerbating the body’s inflammatory response and oxidative stress levels, and ultimately promoting the progression of DN (63, 64).
Additionally, after MR analysis, diseases such as inflammatory bowel disease (65), sarcopenia (66), and periodontitis (67) were found to lack a significant causal relationship with DN from a biogenetic perspective.
3.3.2. Lifestyle
Since the 21st century, there has been a significant change in human lifestyle compared to previous times. Factors such as diet and daily routines constantly influence bodily functions, leading to the emergence of a new discipline: Lifestyle Medicine. This discipline aims to study the significance of lifestyle changes in the prevention and treatment of chronic diseases (68). Using MR analysis to explore the relationship between lifestyles and DN effectively avoids errors caused by confounding, reverse causation, and bias. It has become an essential tool in epidemiological research today.
Coffee, currently the most widely consumed beverage worldwide, contains main components such as caffeine, chlorogenic acid, and hydroxy-hydroquinone (69). Previous epidemiological studies and meta-analyses have not reached consensus on the relationship between coffee consumption and diabetes and its complications. Some studies suggest that coffee can significantly reduce the risk of developing T2DM while delaying the occurrence of diabetic complications (70–72). However, a prospective study has found that consuming more than 2 cups of caffeine-containing beverages per day increases the risk of eGFR decline by 1.19 times (OR: 1.19, 95% CI: 1.01 to 1.41) (73). Therefore, further research is needed to clarify the relationship. In 2021, Mazidi et al. (74) conducted an MR analysis on the relationship between coffee intake and kidney function, finding no causal relationship between coffee intake and eGFR in diabetic patients. Additionally, in 2023, an MR analysis based on the latest GWAS data from the UK Biobank revealed a causal relationship between coffee consumption and the risk of DN, showing a positive correlation (OR: 1.939, 95% CI: 1.012 to 3.712, P = 0.045) (75). Therefore, based on the results of MR analysis and genetic perspective, it is suggested that coffee does not provide a protective effect against the occurrence of DN. Moreover, excessive intake of coffee may increase the risk of DN. This review suggests that this is because caffeine, as an adenosine receptor antagonist, binds to adenosine receptors upon intake (76), influencing adenosine’s anti-inflammatory properties and glomerular hemodynamics, leading to glomerular remodeling, sclerosis, and the occurrence of proteinuria (77).
Sleep plays a crucial role in the regulation of endocrine functions (78). Previous observational studies have found significant associations between both long and short sleep durations and the occurrence of DN, as well as increased levels of urinary albumin-to-creatinine ratio (UACR) (79, 80). To further clarify the role of sleep in DN, Mazidi et al. (81) selected 78 SNPs closely related to sleep duration from the Biobank as instrumental variables for MR analysis. The final results, however, revealed no causal relationship between sleep duration and eGFR levels in T2DM patients. However, the study also found that, in non-diabetic populations, longer sleep durations were causally associated with lower eGFR levels (IVW: β: -0.024, SE = 0.011, P = 0.020). This suggests that prolonged sleep may have potential adverse effects on renal function. Our analysis suggests that sleep duration is closely related to the levels of various inflammatory factors (tumor necrosis factor-alpha, IL-1, CRP, etc.) (82). At the same time, disruption of sleep rhythms can negatively impact the RAS, sodium-potassium excretion system, and thereby damage renal function (83). A study in animal models has found that circadian disruption (including prolonged, shortened, or interrupted sleep) leads to proteinuria, glomerulosclerosis, tubular hyperplasia, and renal fibrosis (84).
4. Limitations and prospects of using MR in DN etiology research
Although MR analysis has been widely applied in the field of DN etiology research, providing definite advantages for exploring DN risk factors, there are still certain limitations and challenges in its future application. The summary is as follows: First of all, MR is dependent on the gene-exposure-disease chain, and if the effect of one link is weak, the effectiveness of the overall analysis will be affected. GWAS is a genome-wide association study of genes and phenotypes, and therefore, large samples and high representativeness of GWAS data are required for the genetic data to have authenticity and persuasive power. However, the number of cases of GWAS data is relatively small, resulting in fewer instrumental variables to be included in the study, which will reduce the effectiveness and specificity of the analysis. Furthermore, most of the current GWAS data are derived from UK Biobank, whose study population is predominantly of European origin, which may further limit the extrapolation of the results. Therefore, the generalizability of the results and the impact of possible sample overlap on the results need to be considered when conducting MR. In future studies, GWAS data with larger sample sizes should be preferred for analyses, and the sample sizes of different populations and cohorts with relevant exposures and DN should also be continuously expanded to guarantee the authenticity and reliability of the study. Secondly, due to the inconsistency of diagnostic criteria for DN, the gold standard for pathology is still needed to assess whether CKD is due to diabetes. Therefore, further improvement of DN phenotype information as well as data on its genetic variants is needed in the future. Finally, although MR can reveal the causal relationship between exposure and outcome, it is still unable to elaborate and analyze the specific mechanism of action, so in the future, it is still necessary to combine with basic studies, RCT, etc. to explore the specific pathways and targets of exposure so as to better guide the prevention and control of disease and to provide high-quality evidence-based evidence for the diagnosis and treatment of disease in the later stage.
5. Conclusion
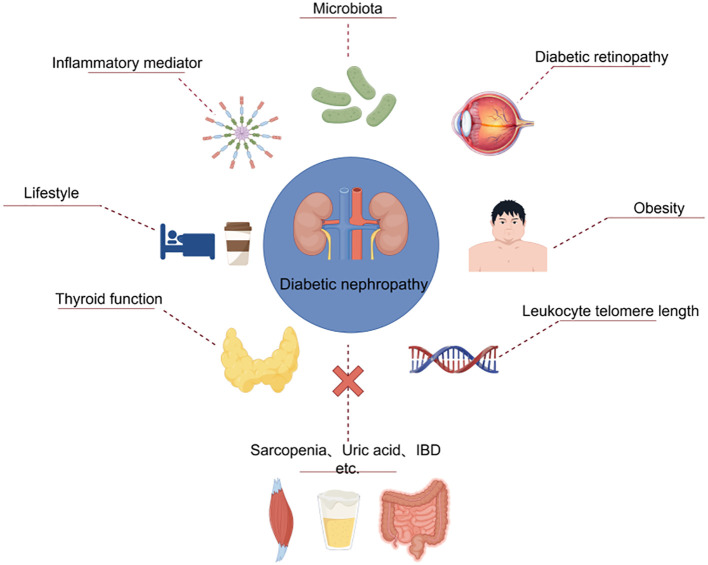
The article provides a review of recent studies on the application of MR analysis in DN epidemiology research, summarizing the causal relationships between various exposure factors and the risk of DN. Ultimately, this review found that gut flora such as Akkermansia and Verrucomicrobia, serum albumin levels, inflammatory mediators such as IFN-γ and CRP, leukocyte telomere length, protein, diabetic retinopathy, thyroid dysfunction, obesity, coffee intake, and sleep were all causally associated with the development of DN ( Figure 6 ). However, based on current genetic data, MR analysis failed to prove that vitamin D, uric acid, inflammatory bowel disease, sarcopenia, periodontitis, etc. are risk factors for DN ( Table 2 ). MR analysis plays a groundbreaking role in enhancing researchers’ understanding of DN etiology and developing new treatment approaches. Based on this, early intervention and prevention of relevant risk factors in clinical diagnosis and treatment processes can be conducted. The ultimate goal is to help prevent DN in high-risk populations while slowing the progression of the disease in people with DN.
Figure 6.
Risk factors for DN in the MR analysis.
Table 2.
Mendelian randomization studies of various exposures with DN as the outcome.
| Exposures | Year | Authors | Population of exposure | Population of outcome | SNPs, n | Effect | P-value |
|---|---|---|---|---|---|---|---|
| Verrucomicrobia | 2023 | Yan W et al. (11) | 18,340 adult individuals, European | DN, 210463 controls and 3283 cases, European | 12 | OR = 1.390(1.10–1.75); | P= 0.005 |
| Akkermansia | 2023 | Yan W et al. (11) | 18,340 adult individuals, European | DN, 210463 controls and 3283 cases, European | 12 | OR=1.390(1.10–1.75) | P= 0.005 |
| Peptreptococcaceae | 2023 | Yan W et al. (11) | 18,340 adult individuals, European | DN, 210463 controls and 3283 cases, European | 14 | OR=1.284(1.03–1.59) | P= 0.012 |
| Butyricimonas | 2023 | Yan W et al. (11) | 18,340 adult individuals, European | DN, 210463 controls and 3283 cases, European | 16 | OR = 1.261( 1.02–1.55) | P= 0.031 |
| Catenibacterium | 2023 | Yan W et al. (11) | 18,340 adult individuals, European | DN, 210463 controls and 3283 cases, European | 4 | OR=1.278(1.02-1.59) | p=0.030 |
| Marvinbryantia | 2023 | Yan W et al. (11) | 18,340 adult individuals, European | DN, 210463 controls and 3283 cases, European | 10 | OR = 1.369(1.04–1.79) | P= 0.022 |
| Class Verrucomicrobiae | 2024 | Yan S. et al. (14) | 18,340 adult individuals, European | T2DN, 283224 controls and 2394 cases, European | 9 | OR=1.822 (1.241-2.676) | P=0.013 |
| Eubacterium protogenes | 2024 | Yan S. et al. (14) | 18,340 adult individuals, European | T1DN, 283224 controls and 1441 cases, European | 13 | OR=0.407(0.241-0.688) | P=0.002 |
| 25(OH) vitamin D | 2023 | He M. et al. (17) | 79,366 adult individuals, European | T1DN (early), 67452 controls and 3399 cases, European | 3 | OR=0.587(0.03 -11.458) | P=0.726 |
| 25(OH) vitamin D | 2023 | He M. et al. (17) | 79,366 adult individuals, European | T1DN (later), 67452 controls and 4352 cases, European | 3 | OR=1.517(0.114-20.208) | P=0.752 |
| 25(OH) vitamin D | 2023 | He M. et al. (17) | 79,366 adult individuals, European | T2DN (early),2238 controls and 1989 cases, European | 3 | OR=0.039(0.114-20.208) | P=0.109 |
| 25(OH) vitamin D | 2023 | He M. et al. (17) | 79,366 adult individuals, European | T2DN (later), 2372 controls and 1339 cases, European | 3 | OR=1.870(0.389-8.990) | P=0.435 |
| Serum uric acid | 2017 | Ahola AJ. et al. (7) | >140,000 adult individuals ,European | T1DN (eGFR), 2720 cases, European | 23 | OR=2.24 (-17.29-21.77) | P= 0.631 |
| Serum uric acid | 2022 | Feng B. et al. (20) | 336619 adult individuals, European | DN, 210463 controls and 3282 cases, European | 188 | OR=1.06(0.91-1.24) | P=0.428 |
| Serum Albumin | 2023 | Cai YW. et al. (25) | 115,060 adult individuals, European | T2DM, 439,238 controls and 22,340 cases, European | 19 | OR=0.990 (0.984-0.995) | P=2.33×10-4 |
| IFN-γ | 2024 | An L. et al. (28) | 8293 adult individuals, European | DN, 210463 controls and 3282 cases, European | 13 | OR=1.33(1.09-1.63) | P=0.005 |
| SCF | 2024 | An L. et al. (28) | 8293 adult individuals, European | DN, 210463 controls and 3282 cases, European | 9 | OR=1.25(1.02-1.52) | P=0.027 |
| MIP-1β | 2024 | An L. et al. (28) | 8293 adult individuals, European | DN, 210463 controls and 3282 cases, European | 17 | OR=0.92(0.85-0.98) | P=0.022 |
| IL-16 | 2024 | An L. et al. (28) | 8293 adult individuals, European | DN, 210463 controls and 3282 cases, European | 10 | OR=0.89(0.81-0.99) | P=0.043 |
| hs-CRP | 2023 | Lin CC. et al. (29) | 2332 adult individuals, Chinese | DN, 2332 controls and 256 cases,chinese | 4 | OR=1.67(1.40-1.98) | NOT REPORT |
| Leukocyte telomere length | 2021 | Gurung RL. et al. (4) | 25,273 East Asians and 37,505 European | T2DN, 2005 controls and 498 cases,East Asians | 16 | OR=1.51(1.12-2.12) | P=0.007 |
| Diabetic retinopathy | 2023 | Duan J. et al. (52) | 95752 adult individuals, European | DN, 210463 controls and 3283 cases, European | 4 | OR=2.89(1.76-4.75) | P<0.001 |
| TSH | 2023 | Li H. et al. (56) | 39282 adult individuals, European | DKD, 3,676 cases and 283,456 controls, European | 39 | OR=1.44(1.04-2.41) | P=0.033 |
| FT4 | 2023 | Li H. et al. (56) | 72,167 adult individuals, European | DKD, 3,676 cases and 283,456 controls, European | 16 | OR=0.830.67-1.03) | P = 0.093 |
| TPOAb | 2023 | Li H. et al. (56) | >40000 adult individuals, European | DKD, 3,676 cases and 283,456 controls, European | 4 | OR=1.17 (0.57-2.38) | P = 0.672 |
| Obesity | 2015 | Todd JN. et al. (6) | 249796 adult individuals, European | T1DN macroalbuminuria, 2347 cases and 6049 controls, European | NOT REPORT | OR 1.28(1.11-1.45) | P = 0.001 |
| Obesity | 2015 | Todd JN. et al. (6) | 249796 adult individuals, European | T1DN ESDR,2347 cases and 6049 controls, European | NOT REPORT | OR 1.43(1.20-1.72) | P < 0.001 |
| Obesity | 2015 | Todd JN. et al. (6) | 249796 adult individuals, European | T1DKD ,2347 cases and 6049 controls, European | NOT REPORT | OR 1.33(1.17-1.51) | P < 0.001 |
| Body Mass Index | 2023 | Huang Y. et al. (59) | 461460 adult individuals, European | DN, 3283 cases and 210463 controls, European | 376 | OR=1.74(1.47-2.07) | P=0.000000000217 |
| Waist circumference | 2023 | Huang Y. et al. (59) | 462166 adult individuals, European | DN, 3283 cases and 210463 controls, European | 315 | OR=2.03(1.62-2.55) | P=0.0000000011 |
| Body Mass Index | 2022 | Wang M. et al. (60) | 681275 adult individuals, European | DN, 3,283 ncase 181,704 controls, European | 441 | OR=1.99 (1.47–2.69) | p = 7.89 × 10−6 |
| Waist circumference | 2022 | Wang M. et al. (60) | 232101 adult individuals, European | DN, 3,283 ncase 181,704 controls, European | 214 | OR=2.48 (1.40–4.42) | p = 1.93 × 10−3 |
| Trunk fat mass | 2022 | Wang M. et al. (60) | 454588 adult individuals, European | DN, 3,283 ncase 181,704 controls, European | 34 | OR=1.80 (1.28–2.53) | p = 6.84 × 10−4 |
| Body Mass Index | 2022 | Lu J. et al. (61) | 158284 adult individuals,Japanese | DN,1314 cases and 2658 controls,chinese | 56 | OR=3.76(1.88-7.53) | P < 0.001 |
| Inflammatory bowel disease | 2023 | Lian X. et al. (65) | 86640 adult individuals, European | DN, 3,283 ncase 181,704 controls,European | 129 | OR=1.01(1.00-1.02) | P=0.5 |
| Appendicular lean mass | 2023 | Ren L. et al. (66) | 244730 adult individuals, European | DN, 3,283 ncase 181,704 controls,European | 424 | OR= 0.863(0.767-0.971) | P = 0.014 |
| Grip strength left | 2023 | Ren L. et al. (66) | 461026 adult individuals, European | DN, 3,283 ncase 181,704 controls,European | 147 | OR=1.119(0.688-1.820) | P=0.650 |
| Grip strength right | 2023 | Ren L. et al. (66) | 461089 adult individuals, European | DN, 3,283 ncase 181,704 controls,European | 164 | OR=0.847(0.552-1.300) | P= 0.447 |
| Walking speed | 2023 | Ren L. et al. (66) | 459915 adult individuals, European | DN, 3,283 ncase 181,705 controls,European | 56 | OR=0.495(0.206-1.189) | P=0.116 |
| Periodontitis | 2024 | Yan P. et al. (67) | 461031 adult individuals, European | DN, 3,283 ncase 210463 controls,European | 6 | OR=1.02(0.91–1.14) | P=0.77 |
| coffee intake | 2021 | Mazidi M. et al. (74) | 91462 adult individuals, European | DM eGFR,n = 133,413 individuals with replication in up to 42,166 individuals | 5 | beta=-0.00645 | P=0.478 |
| coffee consumption | 2023 | Fang J. et al. (75) | 428860 adult individuals, European | DN, 3,283 ncase 210463 controls,European | 33 | OR:1.939 (1.012-3.712) | P =0.045 |
| coffee consumption | 2023 | Fang J. et al. (75) | 428860 adult individuals, European | T2DM with renal complications, 1,296 cases and 183,185 European-descent controls | 35 | OR=2.787 (0.926-8.394) | P = 0.047 |
| coffee consumption | 2023 | Fang J. et al. (75) | 428860 adult individuals, European | T1DM diabetes with renal complications, 963 cases and 183,185 controls | 36 | OR = 2.667 (0.796-8.929) | P = 0.112 |
| coffee consumption | 2023 | Fang J. et al. (75) | 428860 adult individuals, European | Urinary albumin-to-creatinine ratio in diabetes, 5,825 cases and 46061 controls of European individuals | 30 | OR=0.884 (0.395-1.802) | P=0.661 |
| Sleep duration | 2021 | Mazidi M. et al. (81) | 446118 adult individuals, European | eGFR in the total population, n = 133,413 individuals with replication in up to 42,166 individuals | 78 | beta=-0.019 | p = 0.047 |
| CTSC | 2024 | Zhou D. et al. (42) | 31684 adult individuals, European | Diabetic nephropathy 1,032 case 451,248 control | 12 | OR=0.861 | P=0.041 |
| PDE5A | 2024 | Zhou D. et al. (42) | 19173 adult individuals, European | Diabetic nephropathy 1,032 case 451,248 control | 9 | OR=0.842 | P=0.018 |
| inhibition of HMGCR | 2024 | Zhao R. et al. (48) | NOT REPORT | DN: males and females with 3283 cases and 181,704 controls | 19 | OR= 1.79 | P = 0.01 |
| COL6A2 | 2024 | Zhang W. et al. (50) | 49,708 individuals of Icelandic descent | DN:287,132 Finnish adult participants (3,676 cases and 283,456 controls) | 23 | OR=1.588 (1.284-1.963) | P=0.0000198 |
| CBLN1 | 2024 | Zhang W. et al. (50) | 49,708 individuals of Icelandic descent | DN:287,132 Finnish adult participants (3,676 cases and 283,456 controls) | 162 | OR=1.141 (1.039-1.253) | P=0.00571 |
| TGFBI | 2024 | Zhang W. et al. (50) | 49,708 individuals of Icelandic descent | DN:287,132 Finnish adult participants (3,676 cases and 283,456 controls) | 75 | OR=1.284 (1.118-1.475) | P=0.000402 |
| ITIH3 | 2024 | Zhang W. et al. (50) | 49,708 individuals of Icelandic descent | DN:287,132 Finnish adult participants (3,676 cases and 283,456 controls) | 164 | OR=1.179 (1.089-1.277) | P=0.0000506 |
| ANG | 2024 | Gurung RL. et al. (51) | 1,000 adult individuals,European+338 participants with Arab and Asian ethnicities. | YT2D DN(:≤40YAsian participants) Control=546, Case=321 | 1 | OR=4.03 (1.28-12.68) | P = 0.017 |
DN, diabetic nephropathy; DKD, diabetic kidney disease; ESDR, End stage renal disease; YT2D, young onset of type 2 diabetes; eGFR, estimated glomerular filtration rate.
Acknowledgments
Figures 1 and 6 materials are provided by Figdraw (www.figdraw.com) and SCIfig (www.sciFigurecom) Figures 2 – 5 are provided by R-bibliometrix and Stork (www.storkapp.me).
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This article was funded by innovation team and talents cultivation program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202209), Talent Support Program of State Administration of Traditional Chinese Medicine: Qihuang Scholar (National Education Letter of Traditional Chinese Medicine (2022) No.6), Sichuan Provincial Administration of Traditional Chinese Medicine: Chinese Medicine Prevention and Treatment of Endocrine Metabolic Diseases Science and Technology Industry Innovation Team (2022C012). This article was also funded by the Natural Science Foundation of Sichuan Province (24NSFSC5627).
Author contributions
QH: Writing – original draft, Visualization, Writing – review & editing. CA: Visualization, Writing – original draft, Writing – review & editing. ST: Conceptualization, Writing – review & editing. YLL: Methodology, Writing – review & editing. YZ: Writing – original draft. BW: Writing – original draft. YH: Writing – original draft. YL: Writing – original draft. CX: Funding acquisition, Writing – review & editing, Writing – original draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Magliano DJ, Boyko EJDA. IDF DIABETES ATLAS. Brussels: International Diabetes Federation; (2021). [PubMed] [Google Scholar]
- 2. Anders HJ, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. (2018) 14:361–77. doi: 10.1038/s41581-018-0001-y [DOI] [PubMed] [Google Scholar]
- 3. Cortinovis M, Perico N, Remuzzi G. Should we still believe in randomized controlled trials in nephrology? Nephron Clin Pract. (2017) 136:281–86. doi: 10.1159/000450618 [DOI] [PubMed] [Google Scholar]
- 4. Gurung RL, Dorajoo R, M Y, Wang L, Liu S, Liu JJ, et al. Association of leukocyte telomere length with chronic kidney disease in East Asians with type 2 diabetes: a Mendelian randomization study. Clin Kidney J. (2021) 14:2371–76. doi: 10.1093/ckj/sfab067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lim SC, Dorajoo R, Zhang X, Wang L, Ang SF, Tan C, et al. Genetic variants in the receptor for advanced glycation end products (RAGE) gene were associated with circulating soluble RAGE level but not with renal function among Asians with type 2 diabetes: a genome-wide association study. Nephrol Dial Transplant. (2017) 32:1697–704. doi: 10.1093/ndt/gfw263 [DOI] [PubMed] [Google Scholar]
- 6. Todd JN, Dahlstrom EH, Salem RM, Sandholm N, Forsblom C, McKnight AJ, et al. Genetic evidence for a causal role of obesity in diabetic kidney disease. Diabetes. (2015) 64:4238–46. doi: 10.2337/db15-0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahola AJ, Sandholm N, Forsblom C, Harjutsalo V, Dahlstrom E, Groop PH. The serum uric acid concentration is not causally linked to diabetic nephropathy in type 1 diabetes. Kidney Int. (2017) 91:1178–85. doi: 10.1016/j.kint.2016.11.025 [DOI] [PubMed] [Google Scholar]
- 8. Cai K, Ma Y, Cai F, Huang X, Xiao L, Zhong C, et al. Changes of gut microbiota in diabetic nephropathy and its effect on the progression of kidney injury. Endocrine. (2022) 76:294–303. doi: 10.1007/s12020-022-03002-1 [DOI] [PubMed] [Google Scholar]
- 9. Deng L, Yang Y, Xu G. Empagliflozin ameliorates type 2 diabetes mellitus-related diabetic nephropathy via altering the gut microbiota. Biochim Biophys Acta Mol Cell Biol Lipids. (2022) 1867:159234. doi: 10.1016/j.bbalip.2022.159234 [DOI] [PubMed] [Google Scholar]
- 10. Ni Y, Zheng L, Nan S, Ke L, Fu Z, Jin J. Enterorenal crosstalks in diabetic nephropathy and novel therapeutics targeting the gut microbiota. Acta Biochim Biophys Sin (Shanghai). (2022) 54:1406–20. doi: 10.3724/abbs.2022140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan W, Ge Y, Wang L, Wang Y, He D. Causal relationship of gut microbiota with diabetic nephropathy: a Mendelian randomization analysis. Front Microbiol. (2023) 14:1281361. doi: 10.3389/fmicb.2023.1281361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cani PD, Depommier C, Derrien M, Everard A, de Vos WM. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol. (2022) 19:625–37. doi: 10.1038/s41575-022-00631-9 [DOI] [PubMed] [Google Scholar]
- 13. Ren Z, Fan Y, Li A, Shen Q, Wu J, Ren L, et al. Alterations of the human gut microbiome in chronic kidney disease. Adv Sci (Weinh). (2020) 7:2001936. doi: 10.1002/advs.202001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan S, Wang H, Feng B, Ye L, Chen A. Causal relationship between gut microbiota and diabetic nephropathy: a two-sample Mendelian randomization study. Front Immunol. (2024) 15:1332757. doi: 10.3389/fimmu.2024.1332757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lei M, Liu Z, Guo J. The emerging role of vitamin D and vitamin D receptor in diabetic nephropathy. BioMed Res Int. (2020) 2020:4137268. doi: 10.1155/2020/4137268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernandez-Juarez G, Luno J, Barrio V, de Vinuesa SG, Praga M, Goicoechea M, et al. 25 (OH) vitamin D levels and renal disease progression in patients with type 2 diabetic nephropathy and blockade of the renin-angiotensin system. Clin J Am Soc Nephrol. (2013) 8:1870–76. doi: 10.2215/CJN.00910113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He M, Yang T, Zhou P, Bu P, Yang X, Zou Y, et al. A Mendelian randomization study on causal effects of 25(OH) vitamin D levels on diabetic nephropathy. BMC Nephrol. (2023) 24:192. doi: 10.1186/s12882-023-03186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes. (2009) 58:1668–71. doi: 10.2337/db09-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ficociello LH, Rosolowsky ET, Niewczas MA, Maselli NJ, Weinberg JM, Aschengrau A, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. (2010) 33:1337–43. doi: 10.2337/dc10-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feng B, Lu Y, Ye L, Yin L, Zhou Y, Chen A. Mendelian randomization study supports the causal association between serum cystatin C and risk of diabetic nephropathy. Front Endocrinol (Lausanne). (2022) 13:1043174. doi: 10.3389/fendo.2022.1043174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu F, Lou Y, Shi J, Cao L, Wang C, Ma J, et al. Baseline serum albumin and its dynamic change is associated with type 2 diabetes risk: A large cohort study in China. Diabetes Metab Res Rev. (2020) 36:e3296. doi: 10.1002/dmrr.3296 [DOI] [PubMed] [Google Scholar]
- 22. Liu M, Tang J, Zeng J, He Y. Higher serum albumin was related with diabetes incidence and the impact of BMI changes: Based on cohort study of 18,384 Chinese male elderly. J Diabetes Complications. (2017) 31:1663–68. doi: 10.1016/j.jdiacomp.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 23. Zhang J, Deng Y, Wan Y, He S, Cai W, Xu J. Association between serum albumin level and microvascular complications of type 2 diabetes mellitus. Diabetes Metab Syndr Obes. (2022) 15:2173–82. doi: 10.2147/DMSO.S373160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang J, Zhang R, Wang Y, Li H, Han Q, Wu Y, et al. The level of serum albumin is associated with renal prognosis in patients with diabetic nephropathy. J Diabetes Res. (2019) 2019:7825804. doi: 10.1155/2019/7825804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cai YW, Zhang HF, Gao JW, Cai ZX, Cai JW, Gao QY, et al. Serum albumin and risk of incident diabetes and diabetic microvascular complications in the UK Biobank cohort. Diabetes Metab. (2023) 49:101472. doi: 10.1016/j.diabet.2023.101472 [DOI] [PubMed] [Google Scholar]
- 26. Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. (2012) 33:209–90. doi: 10.1016/j.mam.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 27. Feng X, Yang Y, Zhuang S, Fang Y, Dai Y, Fu Y, et al. Influence of serum albumin on hbA1c and hbA1c-defined glycemic status: A retrospective study. Front Med (Lausanne). (2021) 8:583093. doi: 10.3389/fmed.2021.583093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. An L, Ren X, Pan Y, Gao W, Ren L, Wang J, et al. IFN-gamma, SCF, MIP1b and IL-16 were associated with risk of diabetic nephropathy: A mendelian randomization study. Diabetes Metab Syndr Obes. (2024) 17:851–56. doi: 10.2147/DMSO.S452227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin CC, Li CI, Liu CS, Liao LN, Yang CW, Lin CH, et al. Association of high-sensitivity C-reactive protein and diabetic nephropathy in patients with type 2 diabetes: a Mendelian randomization study. BMJ Open Diabetes Res Care. (2023) 11. doi: 10.1136/bmjdrc-2022-003197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chistiakov DA, Myasoedova VA, Revin VV, Orekhov AN, Bobryshev YV. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology. (2018) 223:101–11. doi: 10.1016/j.imbio.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 31. Fathy SA, Mohamed MR, Ali M, El-Helaly AE, Alattar AT. Influence of IL-6, IL-10, IFN-gamma and TNF-alpha genetic variants on susceptibility to diabetic kidney disease in type 2 diabetes mellitus patients. Biomarkers. (2019) 24:43–55. doi: 10.1080/1354750X.2018.1501761 [DOI] [PubMed] [Google Scholar]
- 32. Yin DD, Luo JH, Zhao ZY, Liao YJ, Li Y. Tranilast prevents renal interstitial fibrosis by blocking mast cell infiltration in a rat model of diabetic kidney disease. Mol Med Rep. (2018) 17:7356–64. doi: 10.3892/mmr.2018.8776 [DOI] [PubMed] [Google Scholar]
- 33. You YK, Huang XR, Chen HY, Lyu XF, Liu HF, Lan HY. C-Reactive Protein Promotes Diabetic Kidney Disease in db/db Mice via the CD32b-Smad3-mTOR signaling Pathway. Sci Rep. (2016) 6:26740. doi: 10.1038/srep26740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sinha SK, Nicholas SB, Sung JH, Correa A, Rajavashisth TB, Norris KC, et al. hs-CRP is associated with incident diabetic nephropathy: findings from the jackson heart study. Diabetes Care. (2019) 42:2083–89. doi: 10.2337/dc18-2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rivera T, Haggblom C, Cosconati S, Karlseder J. A balance between elongation and trimming regulates telomere stability in stem cells. Nat Struct Mol Biol. (2017) 24:30–9. doi: 10.1038/nsmb.3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raschenberger J, Kollerits B, Ritchie J, Lane B, Kalra PA, Ritz E, et al. Association of relative telomere length with progression of chronic kidney disease in two cohorts: effect modification by smoking and diabetes. Sci Rep. (2015) 5:11887. doi: 10.1038/srep11887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gurung RL MY, Liu S, Liu JJ, Lim SC. Short leukocyte telomere length predicts albuminuria progression in individuals with type 2 diabetes. Kidney Int Rep. (2018) 3:592–601. doi: 10.1016/j.ekir.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gordillo-Maranon M, Zwierzyna M, Charoen P, Drenos F, Chopade S, Shah T, et al. Validation of lipid-related therapeutic targets for coronary heart disease prevention using human genetics. Nat Commun. (2021) 12:6120. doi: 10.1038/s41467-021-25731-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ruggenenti P, Cravedi P, Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol. (2010) 6:319–30. doi: 10.1038/nrneph.2010.58 [DOI] [PubMed] [Google Scholar]
- 40. van der Sande NG, Dorresteijn JA, Visseren FL, Dwyer JP, Blankestijn PJ, van der Graaf Y, et al. Individualized prediction of the effect of angiotensin receptor blockade on renal and cardiovascular outcomes in patients with diabetic nephropathy. Diabetes Obes Metab. (2016) 18:1120–27. doi: 10.1111/dom.12708 [DOI] [PubMed] [Google Scholar]
- 41. Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. (2013) 369:1892–903. doi: 10.1056/NEJMoa1303154 [DOI] [PubMed] [Google Scholar]
- 42. Zhou D, Zhou T, Tang S, Li Q, Li W, Gan G, et al. Network pharmacology combined with Mendelian randomization analysis to identify the key targets of renin-angiotensin-aldosterone system inhibitors in the treatment of diabetic nephropathy. Front Endocrinol (Lausanne). (2024) 15:1354950. doi: 10.3389/fendo.2024.1354950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Teumer A, Tin A, Sorice R, Gorski M, Yeo NC, Chu AY, et al. Genome-wide association studies identify genetic loci associated with albuminuria in diabetes. Diabetes. (2016) 65:803–17. doi: 10.2337/db15-1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fang L, Radovits T, Szabo G, Mozes MM, Rosivall L, Kokeny G. Selective phosphodiesterase-5 (PDE-5) inhibitor vardenafil ameliorates renal damage in type 1 diabetic rats by restoring cyclic 3’,5’ guanosine monophosphate (cGMP) level in podocytes. Nephrol Dial Transplant. (2013) 28:1751–61. doi: 10.1093/ndt/gfs391 [DOI] [PubMed] [Google Scholar]
- 45. Brandts J, Tittel SR, Bramlage P, Danne T, Brix JM, Zimny S, et al. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol in type 1 diabetes and type 2 diabetes: Lipid goal attainment in a large German-Austrian diabetes registry. Diabetes Obes Metab. (2023) 25:3700–08. doi: 10.1111/dom.15264 [DOI] [PubMed] [Google Scholar]
- 46. Zhou S, Su L, Xu R, Li Y, Chen R, Cao Y, et al. Statin initiation and risk of incident kidney disease in patients with diabetes. Cmaj. (2023) 195:E729–38. doi: 10.1503/cmaj.230093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mansi IA, Chansard M, Lingvay I, Zhang S, Halm EA, Alvarez CA. Statins and renal disease progression, ophthalmic manifestations, and neurological manifestations in veterans with diabetes: A retrospective cohort study. PloS One. (2022) 17:e269982. doi: 10.1371/journal.pone.0269982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao R, Wang W, Zhang W, Lu J, Liu Y, Guo J, et al. Effects of genetically proxied statins on diabetic nephropathy and retinopathy: a Mendelian randomization study. Sci Rep. (2024) 14:16885. doi: 10.1038/s41598-024-67800-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fan C, Gao Y, Sun Y. Integrated multiple-microarray analysis and mendelian randomization to identify novel targets involved in diabetic nephropathy. Front Endocrinol (Lausanne). (2023) 14:1191768. doi: 10.3389/fendo.2023.1191768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang W, Ma L, Zhou Q, Gu T, Zhang X, Xing H. Therapeutic targets for diabetic kidney disease: proteome-wide mendelian randomization and colocalization analyses. Diabetes. (2024) 73:618–27. doi: 10.2337/db23-0564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gurung RL, Zheng H, Koh H, Yiamunaa M, Liu JJ, Liu S, et al. Plasma proteomics of diabetic kidney disease among Asians with younger-onset type 2 diabetes. J Clin Endocrinol Metab. (2024). doi: 10.1210/clinem/dgae266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Duan J, Liu D, Zhao Z, Liang L, Pan S, Tian F, et al. Short-term duration of diabetic retinopathy as a predictor for development of diabetic kidney disease. J Transl Int Med. (2023) 11:449–58. doi: 10.2478/jtim-2022-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hung CC, Lin HY, Hwang DY, Kuo IC, Chiu YW, Lim LM, et al. Diabetic retinopathy and clinical parameters favoring the presence of diabetic nephropathy could predict renal outcome in patients with diabetic kidney disease. Sci Rep. (2017) 7:1236. doi: 10.1038/s41598-017-01204-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang L, Yang G, Su Z, Yang J. Correlation between subclinical hypothyroidism and renal function in patients with diabetes mellitus. Nephrol (Carlton). (2017) 22:790–95. doi: 10.1111/nep.12852 [DOI] [PubMed] [Google Scholar]
- 55. Furukawa S, Yamamoto S, Todo Y, Maruyama K, Miyake T, Ueda T, et al. Association between subclinical hypothyroidism and diabetic nephropathy in patients with type 2 diabetes mellitus. Endocr J. (2014) 61:1011–18. doi: 10.1507/endocrj.ej14-0206 [DOI] [PubMed] [Google Scholar]
- 56. Li H, Li M, Dong S, Zhang S, Dong A, Zhang M. Assessment of the association between genetic factors regulating thyroid function and microvascular complications in diabetes: A two-sample Mendelian randomization study in the European population. Front Endocrinol (Lausanne). (2023) 14:1126339. doi: 10.3389/fendo.2023.1126339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duntas LH, Orgiazzi J, Brabant G. The interface between thyroid and diabetes mellitus. Clin Endocrinol (Oxf). (2011) 75:1–09. doi: 10.1111/j.1365-2265.2011.04029.x [DOI] [PubMed] [Google Scholar]
- 58. Stefanowicz-Rutkowska MM, Baranowska-Jurkun A, Matuszewski W, Bandurska-Stankiewicz EM. Thyroid dysfunction in patients with diabetic retinopathy. Endokrynol Pol. (2020) 71:176–83. doi: 10.5603/EP.a2020.0013 [DOI] [PubMed] [Google Scholar]
- 59. Huang Y, Zhang X, Li B, Zhu X, Li C, Zhou C, et al. Association of BMI and waist circumference with diabetic microvascular complications: A prospective cohort study from the UK Biobank and Mendelian randomization analysis. Diabetes Res Clin Pract. (2023) 205:110975. doi: 10.1016/j.diabres.2023.110975 [DOI] [PubMed] [Google Scholar]
- 60. Wang M, Li X, Mei H, Huang ZH, Liu Y, Zhu YH, et al. Genetically predicted body fat mass and distribution with diabetic kidney disease: A two-sample Mendelian randomization study. Front Genet. (2022) 13:872962. doi: 10.3389/fgene.2022.872962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lu J, Liu X, Jiang S, Kan S, An Y, Zheng C, et al. Body mass index and risk of diabetic nephropathy: A mendelian randomization study. J Clin Endocrinol Metab. (2022) 107:1599–608. doi: 10.1210/clinem/dgac057 [DOI] [PubMed] [Google Scholar]
- 62. Snijder MB, Flyvbjerg A, Stehouwer CD, Frystyk J, Henry RM, Seidell JC, et al. Relationship of adiposity with arterial stiffness as mediated by adiponectin in older men and women: the Hoorn Study. Eur J Endocrinol. (2009) 160:387–95. doi: 10.1530/EJE-08-0817 [DOI] [PubMed] [Google Scholar]
- 63. Kessler C. Pathophysiology of obesity. Nurs Clin North Am. (2021) 56:465–78. doi: 10.1016/j.cnur.2021.08.001 [DOI] [PubMed] [Google Scholar]
- 64. Tao M, Zhou G, Liu J, He M, Luo X, Wang C, et al. Visceral adipose tissue and risk of diabetic nephropathy: A Mendelian randomization study. Diabetes Res Clin Pract. (2024) 209:111586. doi: 10.1016/j.diabres.2024.111586 [DOI] [PubMed] [Google Scholar]
- 65. Lian X, Wang Y, Wang S, Peng X, Wang Y, Huang Y, et al. Does inflammatory bowel disease promote kidney diseases: a mendelian randomization study with populations of European ancestry. BMC Med Genomics. (2023) 16:225. doi: 10.1186/s12920-023-01644-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ren L, Wang Y, Ju F, Sun M, Gang X, Wang G. Causality between sarcopenia and diabetic nephropathy: a bidirectional Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1188972. doi: 10.3389/fendo.2023.1188972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yan P, Ke B, Fang X. Bioinformatics reveals the pathophysiological relationship between diabetic nephropathy and periodontitis in the context of aging. Heliyon. (2024) 10:e24872. doi: 10.1016/j.heliyon.2024.e24872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kushner RF, Sorensen KW. Lifestyle medicine: the future of chronic disease management. Curr Opin Endocrinol Diabetes Obes. (2013) 20:389–95. doi: 10.1097/01.med.0000433056.76699.5d [DOI] [PubMed] [Google Scholar]
- 69. Butt MS, Sultan MT. Coffee and its consumption: benefits and risks. Crit Rev Food Sci Nutr. (2011) 51:363–73. doi: 10.1080/10408390903586412 [DOI] [PubMed] [Google Scholar]
- 70. Ma L, Hu Y, Alperet DJ, Liu G, Malik V, Manson JE, et al. Beverage consumption and mortality among adults with type 2 diabetes: prospective cohort study. Bmj. (2023) 381:e73406. doi: 10.1136/bmj-2022-073406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kolb H, Martin S, Kempf K. Coffee and lower risk of type 2 diabetes: arguments for a causal relationship. Nutrients. (2021) 13:1144. doi: 10.3390/nu13041144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bellou V, Belbasis L, Tzoulaki I, Evangelou E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PloS One. (2018) 13:e194127. doi: 10.1371/journal.pone.0194127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Diaz-Lopez A, Paz-Graniel I, Ruiz V, Toledo E, Becerra-Tomas N, Corella D, et al. Consumption of caffeinated beverages and kidney function decline in an elderly Mediterranean population with metabolic syndrome. Sci Rep. (2021) 11:8719. doi: 10.1038/s41598-021-88028-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mazidi M, Mikhailidis DP, Dehghan A, Jozwiak J, Covic A, Sattar N, et al. The association between coffee and caffeine consumption and renal function: insight from individual-level data, Mendelian randomization, and meta-analysis. Arch Med Sci. (2022) 18:900–11. doi: 10.5114/aoms/144905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fang J, Song K, Zhang D, Liang Y, Zhao H, Jin J, et al. Coffee intake and risk of diabetic nephropathy: a Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1169933. doi: 10.3389/fendo.2023.1169933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McLellan TM, Caldwell JA, Lieberman HR. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev. (2016) 71:294–312. doi: 10.1016/j.neubiorev.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 77. Wierema TK, Houben AJ, Kroon AA, Postma CT, Koster D, van Engelshoven JM, et al. Mechanisms of adenosine-induced renal vasodilatation in hypertensive patients. J Hypertens. (2005) 23:1731–36. doi: 10.1097/01.hjh.0000180160.89264.9d [DOI] [PubMed] [Google Scholar]
- 78. Steiger A. Sleep and endocrinology. J Intern Med. (2003) 254:13–22. doi: 10.1046/j.1365-2796.2003.01175.x [DOI] [PubMed] [Google Scholar]
- 79. Meng LL, Liu Y, Geng RN, Tang YZ, Li DQ. Association of diabetic vascular complications with poor sleep complaints. Diabetol Metab Syndr. (2016) 8:80. doi: 10.1186/s13098-016-0195-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ohkuma T, Fujii H, Iwase M, Ogata-Kaizu S, Ide H, Kikuchi Y, et al. Association between sleep duration and urinary albumin excretion in patients with type 2 diabetes: the Fukuoka diabetes registry. PloS One. (2013) 8:e78968. doi: 10.1371/journal.pone.0078968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mazidi M, Shekoohi N, Katsiki N, Banach M. Longer sleep duration may negatively affect renal function. Int Urol Nephrol. (2021) 53:325–32. doi: 10.1007/s11255-020-02624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. (2013) 5:93–107. doi: 10.2147/NSS.S31063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Solocinski K, Gumz ML. The circadian clock in the regulation of renal rhythms. J Biol Rhythms. (2015) 30:470–86. doi: 10.1177/0748730415610879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. (2008) 294:R1675–83. doi: 10.1152/ajpregu.00829.2007 [DOI] [PubMed] [Google Scholar]