Abstract
Background
We examined de‐functionalization and temporal functional recovery of C‐nociceptor evoked pain after topical 8% capsaicin applied for 4 consecutive days.
Methods
Capsaicin and placebo patches were applied to human forearm skin (n = 14). Cold, warmth and heat pain thresholds, pain NRS to electrical and thermal (48°C, 5 s) stimuli and axon reflex flare were recorded weekly for 49 days. Mechanical and heat sensitive (‘polymodal’) nociceptors were activated by single electrical half‐period sinusoidal pulses (0.5 s, 1 Hz). Mechanical and heat insensitive (‘silent’) nociceptors were activated by 4 Hz sinusoidal stimuli.
Results
Capsaicin abolished heat pain. Sensation to electrical sinusoidal stimulation was reduced but never abolished during the treatment. Pain to electrical 1 Hz ‘polymodal’ nociceptor stimulation took longer to recover than pain ratings to 4 Hz 2.5 s sinusoidal stimulation activating ‘polymodal’ and ‘silent’ nociceptors (35 vs. 21 days). Heat pain was indifferent to placebo from day 21–49. Axon reflex flare was abolished during capsaicin and only recovered to ~50% even after 49 days.
Conclusions
Capsaicin abolishes heat transduction at terminal nociceptive endings, whereas small‐diameter axons sensitive to sinusoidal electrical stimulation can still be activated. 1 Hz depolarizing stimuli evoke burst discharges, as demonstrated before, and recover slower after capsaicin than single pulses induced by 4 Hz. The difference in recovery suggests differential time course of functional regeneration for C‐nociceptor sub‐types after capsaicin. All sensations recovered completely within 7 weeks in healthy subjects. Our findings contrast analgesia lasting for months in spontaneous neuropathic pain patients treated with 8% capsaicin.
Significance
Sinusoidal electrical stimulation can still activate small diameter axons desensitized to heat after 4 consecutive days of topical 8% capsaicin application and reveals differential temporal functional regeneration of C‐nociceptor sub‐types. Electrical sinusoidal stimulation may detect such axons that no longer respond to heat stimuli in neuropathic skin.
1. INTRODUCTION
Topical 8% capsaicin applied for 1 h causes an intra‐epidermal nerve fibre reduction (Kennedy et al., 2010) and a progressive de‐functionalization of nociceptors (Anand & Bley, 2011). Treating neuropathic pain patients with 8% capsaicin can induce pain relief for 12 weeks or longer (Anand & Bley, 2011; Backonja et al., 2008). C‐nociceptors, particularly hyper‐excitable ‘silent’ nociceptors, have been associated with neuropathic pain pathology (Orstavik et al., 2003), and long‐term suppression of peripheral nociceptor activity by 8% capsaicin may contribute to the analgesic response. Mechanistically, the partial denervation of the epidermis following capsaicin treatment (Nolano et al., 1999) is linked to tonic activation of the transient receptor potential vanilloid receptor 1 (TRPV1) (Caterina et al., 1997), expressed in human nociceptors (Tavares‐Ferreira et al., 2022). It is also associated with a calcium2+/calpain‐dependent local degeneration (Wang et al., 2017). Neuronal degeneration by capsaicin might also lead to a ‘pruning’ effect, i.e. an increased regeneration of ‘normal’ intraepidermal innervation, and this might correlate to the analgesic effects of topical capsaicin in patients with diabetic‐ and chemotherapy‐induced peripheral neuropathic pain (Anand et al., 2019, 2022).
After 8% capsaicin treatment, a striking temporal mismatch can be observed between the transient reduction of nociceptive thresholds in healthy volunteers (Lo Vecchio et al., 2018) and the analgesic effect lasting months in neuropathic pain patients (Anand & Bley, 2011; Baron et al., 2017). A slower regeneration of the intraepidermal nerve fibre density has been shown to be indicative of disease progression in diabetic patients (Khoshnoodi et al., 2019). Differences in temporal nociceptor recovery could provide a basis for long‐lasting analgesia observed clinically in neuropathic pain patients. Therefore, electrical nociceptor stimulation might be a valuable tool to assess axonal excitability. The present study aimed to investigate the pattern of nociceptor de‐functionalization after 8% capsaicin patch application in healthy volunteers. We recorded the temporal dynamics of nociceptor‐sub‐type‐specific responses following capsaicin‐evoked denervation over a period of 7 weeks. Changes in sensory transduction were assessed by tonic heat tests. Axonal excitation profiles of specific nociceptor classes were explored by sinusoidal electrical stimulation which does not depend on sensory transduction. A single 500 ms half‐sine wave‐shaped pulse (first half of a sinusoidal pulse of 1 Hz) induces a burst of action potentials (APs) in ‘polymodal’ C‐nociceptors, but ‘silent’ C‐nociceptors remain largely unresponsive (Rukwied et al., 2020). Application of 4 Hz sinusoidal pulses activate ‘polymodal’ and ‘silent’ C‐nociceptors with one AP per cycle (Rukwied et al., 2020) and minimum recruitment of A‐fibres (Jonas et al., 2018) or unmyelinated sympathetic efferent neurons (Jonas et al., 2020). Activation of ‘silent’ C‐nociceptors was confirmed by recording superficial skin blood flow changes around the 4 Hz sine wave stimulation site (‘axon reflex flare’) (Schmelz et al., 2000a).
2. METHODS
Experimental procedures carried out on healthy human subjects were approved by the Ethics Committee II of Heidelberg University, Medical Faculty Mannheim under approval number 2022‐545 and in accord with the Helsinki Declaration of 1975, as revised in 1983.
2.1. Human subjects
Overall, 14 right‐handed healthy volunteers (4 female and 10 male, age range 23–34 (female) and 22–58 (male), overall average 32 ± 3 years) were recruited according to their temporal convenience either face‐to‐face or via email. Exclusion criteria of the subjects comprised prevailing neurological or dermatological disorders and use of pain medication. Subjects were informed about the procedure and the experimental protocol and signed a consent form prior to starting the experiments. All subjects were introduced to the test setup and took part in a training session to familiarize them with the electrical stimulation paradigms and the use of the numeric rating scale (NRS) with the endpoints 0 (no pain) and 10 (maximum pain imaginable). Data from the training session were not included in the analysis. Experiments were performed in a quiet room with an ambient air temperature of 20 ± 2°C. Subjects were seated comfortably in a chair and tests were conducted on the volar surface of the forearms that rested in supine position on a table.
2.2. Experimental protocol
Baseline measures of sensory thresholds to electrical stimuli, electrical pain assessment, axon reflex flare, thermal thresholds, and continuous heat pain were conducted at least 24 h after the information and training session (day 0). The stimulation protocol and sequence of sensory tests investigated at one skin site are depicted in Figure 1. Sensory tests were performed in randomized order on the left and right forearm on each of the 4 consecutive days of topical 8% capsaicin/placebo treatment (Days 1–4) and after that weekly over 49 days (day 7–14–21–28–35–42–49, Figure 1a). Electrical and thermal stimuli were delivered transcutaneously. Rationales for the different tests were: Identify thresholds to electrical 0.1 ms rectangular pulses of 20 Hz for A‐fibre recruitment (Torebjork & Hallin, 1973) and electrical 4 Hz sinusoidal stimulation for C‐fibre activation (Jonas et al., 2018) with the endpoints ‘perception’—‘pain’—‘pain NRS 3’ for both electrical stimulation paradigms. Pain intensity (NRS) was recorded after single 1 Hz half‐period sinusoidal pulses (0.2–1 mA, 500 ms), which activate polymodal C‐nociceptors resulting in a burst of APs (Figure 1b, ‘nerve fiber discharge’) (Rukwied et al., 2020). For the activation of both polymodal and ‘silent’ C‐nociceptors, 10 pulses of 4 Hz sinusoidal stimuli (2.5 s) were applied at amplitudes of 0.05–0.4 mA causing AP discharges at 4 Hz (Figure 1b) (Jonas et al., 2018). When supra‐threshold 4 Hz sinusoidal stimuli were delivered continuously for 60 s, healthy volunteers reported profound pain habituation (‘accommodation’ of C‐nociceptors), whereas about 50% of neuropathic pain patients reported increasing pain (Jonas et al., 2018; Landmann et al., 2022). Accordingly, we investigated whether the pattern of C‐nociceptor accommodation changes after capsaicin treatment. Laser Doppler Imaging (LDI) of superficial skin blood flow for ‘axon reflex flare’ recordings was performed as an objective assessment of ‘silent’ C‐nociceptor activation (Schmelz et al., 2000a) (not shown in Figure 1b). Thermal thresholds were assessed for cold sensation (A‐delta fibre function (Lewis & Griffith, 2022)), warm sensation (C‐fibre function (Paricio‐Montesinos et al., 2020)), and heat pain (dependent on polymodal C‐nociceptors (Campero et al., 2009)), all of which are dependent on transient receptor potential (TRP) sensory transduction mechanisms. Continuous warming (42°C, 60 s), and finally tonic supra‐threshold heat stimuli (48°C, 5 s) were applied to explore temperature effects on C‐nociceptors located in deeper skin layers. The time required for each test and the intervals (pauses) are depicted in Figure 1c. Prior to each assessment, the skin surface temperature was determined at the capsaicin test site using an infrared thermometer (Raytek Raygner ST™, Berlin, Germany, not shown in Figure 1b). Pilot experiments, in which 8% capsaicin patches were applied for 2 consecutive days, similar to a previous protocol (Henrich et al., 2015; Magerl et al., 2001), did not abolish pain by C‐fibre‐specific electrical sinusoidal stimulation (data not shown). We therefore increased the duration of 8% capsaicin application to 4 consecutive days.
FIGURE 1.
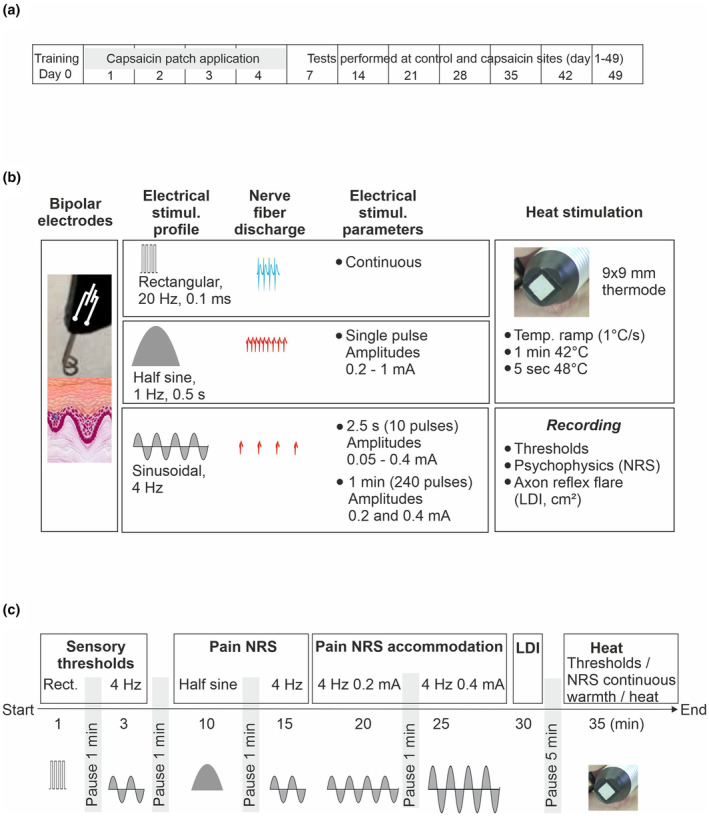
Overall timeline of the experimental design (a), schematic diagram of the stimulation protocol (b), and the timing for test sequences (c) performed in the order from left to right (Start to End). The diagram and schedule apply to one test site only. For details, see Materials and Methods section 2.
2.3. Topical application of capsaicin (8%)
An 8% capsaicin (Qutenza®) patch of 1.5 × 1.5 cm was applied onto one forearm (balanced across subjects between left and right) and a placebo vehicle patch onto the contra‐lateral arm. Patches were secured on the skin with medical tape (Leukosilk®, BSN Medical Hamburg, Germany), and both patches were renewed every 24 h over four consecutive days. Subjects were requested to note maximum pain ratings on each of the 4 days of capsaicin application and to avoid showering the skin patches.
2.4. Electrical stimulation
Constant current stimuli were delivered via a pair of blunt pin platinum wire electrodes (0.4 mm diameter, Cephalon A/S, Nørresundby, Denmark), positioned 3 mm apart from one another and mounted in an applicator printed on a 3D‐printer. Electrodes were positioned on the volar surface of the forearm at either the capsaicin or placebo site. Rectangular and sinusoidal profiles of constant currents were used for transcutaneous electrical stimulation (Figure 1b). The current intensities required for rectangular and sinusoidal stimuli to elicit sensory detection, pain, and to reach pain levels of NRS3 were assessed.
2.4.1. Rectangular pulses
Electrical stimuli were delivered by a constant current stimulator (DS7A, Digitimer Ltd, Welwyn Garden City, UK) and applied as brief (0.1 ms duration) rectangular pulses at a frequency of 20 Hz (triggered by a pulse generator PG1, Rimkus Medizintechnik, Parsdorf, Germany). Current amplitudes of rectangular pulse stimuli were increased in steps of 0.5 mA per second and subjects reported (1) their first sensation (detection threshold), (2) pain threshold, and (3) a pain intensity of NRS3 (supra‐threshold pain).
2.4.2. 1 Hz and 4 Hz sinusoidal stimulation
Sinusoidal pulses were delivered by a constant current stimulator (DS5, Digitimer Ltd., Welwyn Garden City, UK) connected to a digital–analogue converter (DAQ) (NI USB‐6221, National Instruments, Texas, USA) controlled by custom‐written software (Dapsys 8, © Brian Turnquist, Minnesota, USA) to generate the timing, intensity and signal profile of the sinusoidal pulses.
Sinusoidal current at 1 Hz (500 ms half‐period sine wave profile) was applied at amplitudes of 0.2–1 mA (in increments of 0.2 mA) in a randomized order. Single half‐period pulses were delivered at 10 s intervals and each intensity was presented twice. Subjects were requested to estimate the magnitude of pain on a numerical rating scale (NRS 0–10) for each pulse.
Sinusoidal current profiles of 4 Hz were applied for 2.5 s (10 sinusoidal cycles) with increasing current intensities (0.005–0.01 to 0.025–0.05 mA, then increments of 0.05 mA to a maximum of 0.4 mA) to obtain sensory (detection) threshold, a pain NRS of 1 (pain threshold), and a pain NRS of 3 (supra‐threshold pain). Thereafter, an intensity‐response profile to 2.5 s 4 Hz sinusoidal stimulation was generated using current intensities of 0.05–0.1–0.2–0.4 mA (delivered in randomized order) and volunteers were requested to estimate pain intensity (NRS, 0–10). Each stimulus intensity was presented twice. Finally, sinusoidal current profiles of 4 Hz were applied continuously for 60 s at amplitudes of 0.2 mA and 0.4 mA (ascending order, Figure 1c). Subjects were instructed to report pain intensity every 5 s for 20 s and thereafter at 10 s intervals.
2.5. Assessment of the pain intensity
Subjects were asked to rate the intensity of their pain sensations evoked by 1 Hz and 4 Hz sinusoidal electrical stimuli or tonic heat on an 11‐point numerical rating scale (NRS, 0–10) with anchor points at 0 for no pain and 10 for the most excruciating pain imaginable. Subjects were also asked to categorize the quality of pain sensations into burning or stinging in nature and to report any accompanying sensations, such as itch or vibration/pulsation.
2.6. Assessment of skin blood flow with laser Doppler imaging (LDI)
Electrical sinusoidal pulses cause widespread vasodilation (axon reflex flare) of the skin surrounding the stimulation site (Jonas et al., 2018). The area of this axon reflex‐mediated flare response was assessed by LDI (Moor LDI, Moor Instruments Ltd, Axminster, UK). The LDI scanner head was mounted perpendicular and at a 50 cm distance to the skin surface. The scan area covered 25 cm2 and required 1 min for image capture (scan speed 4 ms/pixel). Skin blood flow scans were acquired before and after each bout of sine wave stimulation (2.5 s intensity‐response / 60 s 0.2 mA / 60 s 0.4 mA). The resulting sequence of skin blood flow images was stored on a computer and analysed off‐line using dedicated software supplied by Moor Instruments Ltd. Average baseline skin blood flow and standard deviation across all pixels were calculated and the threshold for an increase in blood flow was set at the mean flux plus two standard deviations. The area of the axon reflex flare was calculated within each image post‐stimulation by simply counting the number of pixels exceeding the flux threshold and lying within a contiguous region of interest (ROI) surrounding the electrical stimulation site.
2.7. Thermal stimulation
A 9 × 9 mm Peltier‐based contact thermode (Somedic Modular Sensory Analyser (MSA), Sösdala, Sweden) was placed onto the skin within the treated area. Thermal thresholds were assessed by dedicated software (Somedic SenseLab version 6.52) and the sequence for testing the capsaicin‐ or the placebo‐site was randomized. Tonic warmth and heat pulses were delivered with SenseLab Exposure3.0 program (Somedic, Sweden).
2.7.1. Cold, warmth and heat pain threshold (HPT)
From a basal holding temperature of 32°C, the temperature of the thermode was set to change by 1°C/s, and subjects instructed to press a hand‐held trigger as soon as the temperature was perceived as cold (cold detection threshold, CDT), warm (warm detection threshold, WDT), or painfully hot (HPT). Each paradigm of cold, warmth, and HPT was performed in triplicate at sites within both the capsaicin and placebo patch areas.
2.7.2. Prolonged warming
The temperature of the thermode was set to deliver a constant temperature of 42°C for 1 min and placed on the patch‐treated skin surface. During this minute, the subject was asked if any sensations were felt, including warmth, tingling, pricking, or itch. If there was burning pain, they were requested to estimate the magnitude of pain on the NRS (0–10). Tests were performed randomized for the patch areas treated with capsaicin and placebo.
2.7.3. Tonic supra‐threshold heat stimulation
A temperature of 48°C was applied for 5 s and randomized to the patch area treated with capsaicin and placebo. Volunteers were requested to estimate the maximum pain sensation perceived during the 5 s stimulation period (NRS, 0–10).
2.8. Time interval between sensory tests
The time kept between threshold tests to electrical stimulation, magnitude of pain assessment to electrical sinusoidal stimulation, and pain accommodation to 0.2 mA and 0.4 mA to 60 s 4 Hz sinusoidal stimulation was at least 1 min (‘pause’ in Figure 1c). An interval of 5 min was kept between skin blood flow assessment (LDI) and the start of thermal tests.
2.9. Time course of changes in sensory function
To assess the time course of impairment and recovery of the sensory function over the 49 days, we calculated the differences between the capsaicin and placebo‐treated sites (∆ values) in each individual subject and day of assessment for pain NRS values to 1 Hz and 4 Hz sinusoidal electrical stimulation, HPTs and tonic heat pain. Thereby, ∆ NRS values in response to the two highest amplitudes (0.8 + 1 mA for 1 Hz and 0.2 + 0.4 mA for 4 Hz stimuli) were used as an index of supra‐threshold electrically induced pain. We compared electrically induced pain, HPTs, and tonic heat pain for each day of assessment to identify similarities or differences in the time course of the different sensory tests (Figure 6a,b). Notably, if HPTs were beyond the thermode cut‐off temperature of 50°C, we used a value of 51°C as HPT for the statistical analysis. In addition, we calculated the time course of somato‐sensory changes utilizing the percentage recovery of the axon reflex flare compared to placebo to 60 s 4 Hz sinusoidal stimulation (Figure 6c). This was used as an additional objective marker for the activation of ‘silent’ (peptidergic) C‐nociceptors.
FIGURE 6.
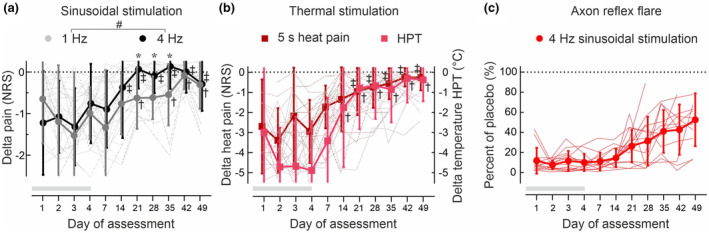
Delta (∆) pain NRS values calculated between the capsaicin‐ and placebo‐treated skin (n = 14) upon (a) supra‐threshold stimulation with 1 Hz half‐period sinusoidal amplitudes (0.8 and 1 mA, grey circles) and 2.5 s of 4 Hz sinusoidal amplitudes (0.2 and 0.4 mA, black circles). Delta (∆) pain NRS to continuous heat (5 s 48°C, red squares) and (∆)HPTs (°C, pink squares) were calculated between the capsaicin‐ and placebo‐treated skin (b). Note that for HPT values >50°C (see Table S3) a fixed HPT value of 51°C was set to allow for ∆ HPT calculation. The axon reflex flare (c) recorded from the capsaicin treatment site upon 4 Hz sinusoidal stimulation (0.2 and 0.4 mA, 60 s stimulation) was calculated in % of the flare area from the placebo patch site. For absolute flare area values see Figure S1 and Table S2. Note that flare responses recovered to only 50% of the placebo value by day49, even though sensory indices (see a and b) already had normalized. The dotted line indicates no difference between placebo‐ and capsaicin‐treated skin site, the grey horizontal bar indicates the duration of patch application (day1‐day4). Hashes (a) indicate significant stimulation × day interaction (p < 0.05, GLM) and asterisks (a) a significant difference between 1 Hz and 4 Hz ∆ pain NRS (p < 0.05, GLM). Single crosses (1 Hz, HPT) and double crosses (4 Hz, continuous heat pain) indicate no significant difference from a theoretical value of zero (p > 0.9, GLM).
2.10. Statistical analyses
Statistical tests were performed in STATISTICA 7.1 (StatSoft Inc, Tulsa, OK, USA). Sphericity and normal distribution of the data were confirmed by Mauchly's and Shapiro–Wilk‐test. A sample size of n = 13 was calculated by the G*Power software package for differences between two dependent means (2 groups of ‘treatment’, matched pairs), with an effect size of 0.8, a significance level alpha of 0.05, and a power of 0.85. Generalized linear mixed models (GLM) were used for the statistical analysis with ‘day’ as a covariate to the main outcome. Bonferroni corrections were made for multiple comparisons to minimize the risk of type I errors outweighing potential type II errors. All data of each individual are presented and the corresponding mean ± SD. p‐values <0.05 were considered to indicate statistically significant differences between the groups and their interactions.
3. RESULTS
3.1. Capsaicin patch‐induced pain
Capsaicin patch‐induced maximum pain was NRS 3.6 ± 1.2 on day1 and diminished significantly over time to NRS 0.7 ± 1 by day4 (F(3,52) = 19.6, p < 0.00001, GLM, Table S1). No pain (NRS 0) was reported from the placebo patch‐treated skin site.
3.2. Electrical stimulation—Sensory thresholds
3.2.1. Rectangular pulses
Sensory thresholds to A‐fibre optimized electrical stimuli (20 Hz, rectangular, 0.1 ms duration) were not significantly different between the capsaicin and placebo patch‐treated skin sites (treatment × day interaction F(10,274) = 0.2, p > 0.9, GLM), but increased in comparison to day0 (baseline) at the placebo site by about 0.5 mA to 1.7 ± 0.3 mA at day49 (F(11,150) = 3.2, p < 0.001, GLM, Figure 2a and Table S1).
FIGURE 2.
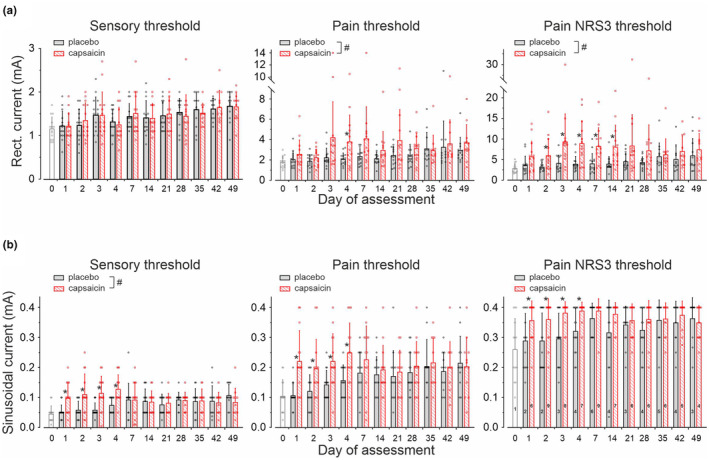
Current intensities required for electrical (a) rectangular stimuli of 20 Hz and 0.1 ms pulse width and (b) for electrical 4 Hz sinusoidal stimuli to evoked a sensation (sensory threshold, left panels), to induce pain (pain threshold, middle panels) or to cause pain of NRS 3 on a NRS (right panels). Note that maximum current intensities for sinusoidal pulses were set 0.4 mA. Numbers inside the columns (bottom right panel) are the number of subjects with ratings NRS <3 to the maximum 4 Hz sinusoidal amplitude. Values are given as mean ± SD. Hashes indicate significant interaction for treatment × day of assessment (p < 0.05, GLM) and asterisks significant differences between capsaicin and placebo sites (p < 0.05, GLM).
Current amplitudes for pain thresholds to rectangular pulses were significantly affected by capsaicin when compared to placebo (F(1,294) = 20.4, p < 0.0001, GLM), but without treatment × day interaction (F(10,274) = 0.99, p > 0.4, GLM).
Similarly, rectangular currents required to evoke pain NRS3 were significantly increased after capsaicin treatment (7.2 ± 4.9 mA, average day1‐49) when compared to placebo (4.5 ± 2.2 mA, average day1‐49, F(1,292) = 45.5, p < 0.0001, GLM), but again without significant treatment × day interaction (F(10,272) = 0.9, p > 0.5, GLM, Figure 2a; Table S1).
3.2.2. 4 Hz sinusoidal pulses
Sensory thresholds to 4 Hz sinusoidal stimuli were 0.05 ± 0.03 mA prior to patch application (baseline condition, day0) and increased significantly throughout the 49 days of assessment at the placebo (F(11,150) = 4, p < 0.0001, GLM) and capsaicin patch site (F(11,150) = 2.9, p < 0.002, GLM) with strong treatment × day interaction (F(10,274) = 3.2, p < 0.001, GLM, Figure 2b and Table S1).
Sinusoidal current intensities required for pain induction (NRS = 1) were 0.1 ± 0.05 mA at baseline, which increased at the placebo site to 0.22 ± 0.09 mA (F(11,150) = 4.2, p < 0.0001, GLM) and at the capsaicin site to 0.2 ± 0.1 mA (F(11,150) = 2.2, p < 0.02, GLM) by day49. There was no treatment × day interaction (F(10,274) = 1.8, p > 0.06, GLM), not even when assessing day1‐4 of treatment only (F(3, 104) = 0.4, p > 0.7, GLM).
Sinusoidal currents required to induce suprathreshold pain of NRS3 were 0.26 ± 0.1 mA under baseline condition (n = 13 subjects, 1 subject >0.4 mA) and increased significantly after placebo treatment to 0.37 ± 0.07 mA by day49 (n = 11 subjects, 3 subjects >0.4 mA, F(11,150) = 2.4, p < 0.01, GLM, Table S1). Current amplitude needed to elicit pain NRS3 at the capsaicin‐treated skin could not be determined in n = 7 subjects at day4 (>0.4 mA, Table S1) and was recorded in the remaining volunteers at 0.37 ± 0.05 mA during day1‐4 on average (Figure 2b). No treatment × day interaction was found neither day1–4 (F(3,104) = 0.1, p > 0.9,GLM) nor across all days of assessment (F(10,274) = 1.6, p > 0.1, GLM).
3.3. Electrical stimulation—Pain intensity
3.3.1. 1 Hz sinusoidal pulses (500 ms half‐period sine wave cycle)
We investigated the current intensity (mA) – response (NRS) relation to 500 ms half‐sine wave stimulation (1 Hz sinusoidal cycle) at amplitudes of 0.2–1 mA (Figure 3a). Each intensity was delivered in duplicate and randomized order. All stimuli induced a ‘burning’ and ‘stinging’ sensation. We did not find a significant effect of the order, independent of current intensity and treatment (placebo (F(1,20) = 0.03, p > 0.8, GLM); capsaicin (F(1,20) = 0.002, p > 0.9, GLM)). Therefore, NRS values of the two repetitions were averaged for statistical analysis.
FIGURE 3.
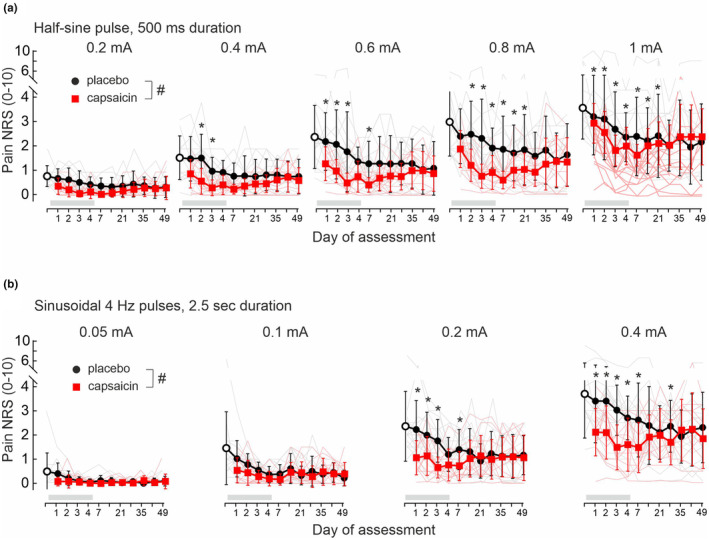
Pain NRS (0–10) in response to 1 Hz half‐period sinusoidal pulses (500 ms) of 0.2–1 mA amplitudes (a) and 4 Hz sinusoidal pulses of 0.05–0.4 mA amplitudes (b) delivered for 2.5 s. Stimuli were applied prior to patch application (baseline day0, open circles), to placebo patch‐treated skin (grey lines) and to the 8% capsaicin patch treatment site (red lines) of 14 volunteers. Average values are depicted as mean ± SD for placebo (black solid circles) and capsaicin‐treated skin (red solid squares). Both patches were renewed each day and applied for 4 days (indicated by grey horizontal bar). Hashes indicate significant treatment × day interaction (p < 0.05, GLM) and asterisks significance between capsaicin and placebo (p < 0.05, Bonferroni posthoc).
Pain ratings increased dependent on current intensity for 1 Hz half‐sine pulses at baseline condition (day0, F(4,52) = 44.1, p < 0.0001, GLM), but gradually decreased at the placebo site during the 49 days of assessment (F(11,149) = 2.1, p < 0.03, GLM, Figure 3a). No interaction for stimulus intensity × day was recorded upon placebo patch application (F(44,596) = 1, p > 0.4). Following capsaicin treatment, a significant stimulus intensity × day interaction was recorded (F(44,596) = 3.35, p < 0.0001) with significantly lowered NRS scores for 1 mA amplitudes at day 3–14 in comparison to day0 (p < 0.05, Bonferroni correction). Compared to the placebo site, pain ratings to half‐sine pulses were significantly reduced in the area of capsaicin treatment (Figure 3a) and in a current intensity‐dependent fashion (interaction stimulus intensity × treatment, F(4,1168) = 21.4, p < 0.0001, GLM). Capsaicin application reduced NRS levels to 2.2 ± 0.9 (1 mA stimuli) at day1 and NRS 1.2 ± 0.8 (1 mA) at day4 (Figure 3a). Maximal inhibition after capsaicin was recorded on day7 (NRS score 0.8 ± 0.5 at 1 mA), and thereafter NRS levels gradually increased again to 1.6 ± 1.2 by day49 (placebo site NRS 2.1 ± 1.6 at 1 mA, Figure 3a). Pain NRS to low‐intensity 1 Hz stimulation (e.g. 0.2 mA amplitude) was reduced at the capsaicin sites when compared to placebo across all days of assessment (F(1,292) = 25.3, p < 0.0001, GLM).
3.3.2. Intensity‐response 2.5 s 4 Hz sinusoidal stimulation
Pain in response to 4 Hz sinusoidal currents (Figure 3b) delivered for 2.5 s at intensities from 0.05 to 0.4 mA (each intensity tested in duplicate and randomized order) was described by the subjects as ‘continuous burning’. Again, we did not find an effect of the test order on pain magnitudes (placebo site (F(1,20) = 0.01, p > 0.9, GLM) and capsaicin site (F(1,26) = 0.01, p > 0.9, GLM)) and used the average NRS values for statistical analysis.
4 Hz sinusoidal stimuli evoked a current intensity‐dependent pain at day0 (F(3,39) = 69.7, p < 0.0001, GLM) that was gradually declining during the 49 day period at the placebo site (F(11,149) = 3.6, p < 0.001, GLM, Figure 3b). There was no significant interaction between stimulus intensity × day after placebo (F(30,408) = 1.4, p > 0.06, GLM) or capsaicin treatment (F(30,408) = 0.63, p > 0.9, GLM). Pain to sinusoidal current stimuli was significantly different between the treatment sites (interaction treatment × stimulus intensity F(3,868) = 20.2, p < 0.0001, GLM, Figure 3b). Significantly lower pain ratings were recorded after capsaicin compared to placebo skin sites already at intensities of 0.05 mA (F(1,292) = 5.3, p < 0.03, GLM). Similarly, pain NRS upon high stimulation intensity (0.2 mA) was significantly different between capsaicin and placebo (interaction treatment × day F(10,272) = 2.1, p < 0.03, GLM) and in particular until day7 (F(1,26) = 5.9, p < 0.03, GLM). During capsaicin application, 4 Hz stimuli of 0.4 mA were perceived as less painful with NRS 2.1 ± 1 on day1 and NRS 1.6 ± 1.1 at day 4, but without treatment × day interaction (F(10,272) = 1.6, p > 0.1, GLM) across 49 days (Figure 3b).
3.3.3. Continuous 60 s 4 Hz sinusoidal stimulation
We delivered 4 Hz sinusoidal pulses continuously for 60 s at amplitudes of 0.2 (Figure 4a) and 0.4 mA (Figure 4b) in ascending order. Pain ratings were attained every 5 s for 20 s and thereafter at 10 s intervals. Maximum pain at baseline (day0) was higher when compared to the ratings recorded from the placebo site upon 0.4 mA during the 7 weeks observation period (F(11,149) = 2.9, p < 0.01, GLM). Pain was reported to be strongest within 15–20 s of stimulation and ratings fell to about 50% by the end of the 60 s period of stimulation (Figure 4a,b). This particular C‐nociceptor accommodation pattern was recorded at both placebo‐ and capsaicin‐treated skin sites with significant treatment × stimulation time interaction for 0.2 mA across all days of assessment (F(7,2044) = 5.1, p < 0.0001, GLM, Figure 4a). NRS ratings recorded throughout the 60 s of 0.2 mA sinusoidal stimulation at 4 Hz were significantly reduced after capsaicin treatment when compared to placebo (interaction treatment × assessment day F(10,272) = 2.65, p < 0.005, GLM), but the capsaicin test site was not completely analgesic to the applied stimuli as volunteers reported pain NRS ~1 throughout this period (Figure 4a). Similarly, pain to 60 s 4 Hz stimulation at 0.4 mA revealed a significant treatment × stimulation time interaction across all assessment days (F(7,2044) = 8.2, p < 0.0001, GLM, Figure 4b). Pain NRS was significantly lower upon capsaicin treatment (interaction treatment × assessment day F(10,272) = 2, p < 0.05, GLM), but sinusoidal stimulation still induced an average pain NRS of 1–2 (Figure 4b).
FIGURE 4.
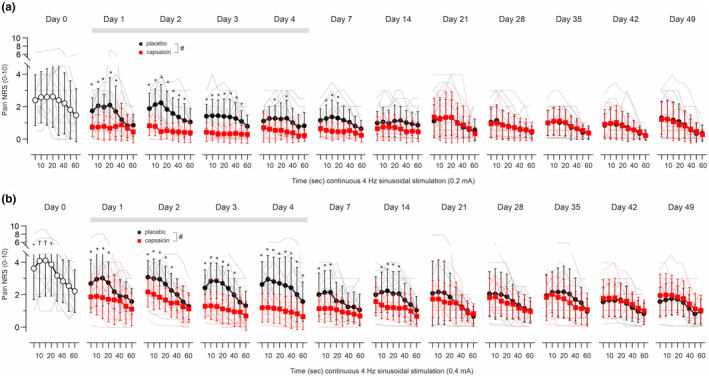
Pain NRS (0–10) in response to 4 Hz sinusoidal amplitudes of (a) 0.2 mA and (b) 0.4 mA delivered continuously for 60 s prior to patch application (baseline day0, open circles) and in placebo patch (black solid circles) and 8% capsaicin patch‐treated skin (red solid squares) of 14 volunteers recorded for 7 weeks (12 volunteers day 35–49). Both patches were renewed each day and applied for 4 days (indicated by grey horizontal bar). Individual values are depicted in grey (placebo) and red (capsaicin) lines, average values are given as mean ± SD for placebo (black solid circles) and capsaicin‐treated skin (red solid squares). Hashes indicate significant treatment × day interaction (p < 0.05, GLM) and asterisks significance between capsaicin and placebo (p < 0.05, Bonferroni posthoc).
3.4. Axon reflex flare
At the placebo‐treated skin sites, 4 Hz sinusoidal electrically‐induced axon reflex flare area was on average (day1‐49) 7.1 ± 3.2 cm2 for 2.5 s, 0.05–0.4 mA, 7.5 ± 2.9 cm2 (60 s, 0.2 mA) and 8.9 ± 3 cm2 (60 s, 0.4 mA, Table S2) without stimulus × day interaction (F(20,274) = 0.85, p > 0.6, GLM). Capsaicin treatment abolished the flare response (<1 cm2) evoked by electrical sinusoidal stimuli from day1 to day7 (Table S2 and Figure S1). Thereafter, electrically induced flare increased gradually in size from day14‐49 (Figure S1), but responses recovered only to about 50% at the final assessment day when compared to the placebo patch site (interaction treatment × stimulus F(2,44) = 4.1, p < 0.03, GLM).
3.5. Thermal stimulation
The skin surface temperature at the site of the capsaicin patch was 34 ± 0.8°C (day1‐4), similar to the skin temperature before (34.5 ± 1.4°C) or after placebo patch application (34.1 ± 0.9°C, F(3,96) = 0.2, p > 0.9, GLM, Table S3).
3.5.1. Cold, warmth and HPTs
Temperature for cold detection threshold (CDT) was significantly elevated at the placebo patch site compared to capsaicin (F(1,292) = 16.2, p < 0.0001, GLM) but without treatment × day interaction (F(10,272) = 1.67, p > 0.08, Figure 5a).
FIGURE 5.
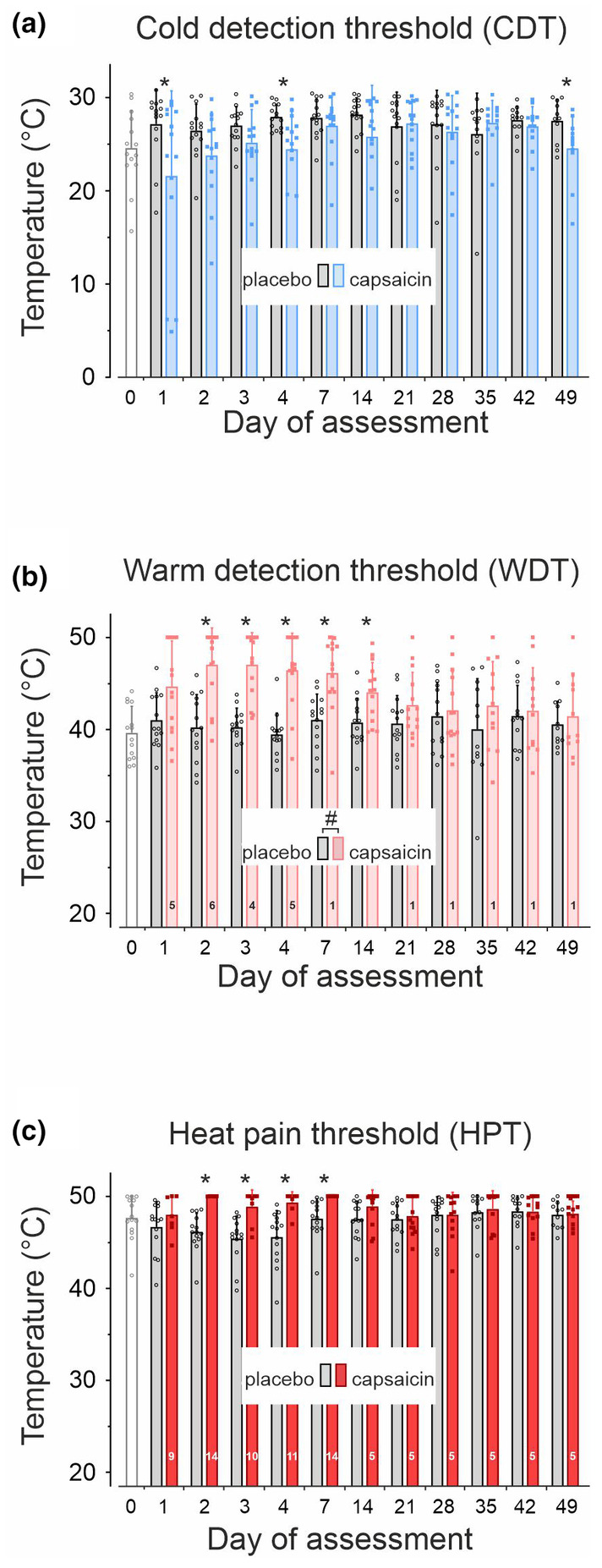
Thermal thresholds for cold sensation (CDT, a), perception of warmth (WDT, b) and for heat pain (HPT, c) prior to patch administration (day0, open columns), at the placebo patch site (grey columns) and at capsaicin‐treated skin (blue and red columns). Numbers inside the columns in panel b (WDT) and c (HPT) indicate the number of subjects with thresholds >50°C (thermode temperature cut off). Values are given as mean ± SD, hashes indicate significant treatment × day interaction and asterisks significant differences between capsaicin and placebo (p < 0.05, GLM).
Warm detection threshold (WDT) was 39.6 ± 2.8°C prior to patch administration (baseline, day0 of assessment) and increased significantly in response to capsaicin (F(11,123) = 3.89, p < 0.0001, GLM). During capsaicin application, WDT was above the cut‐off temperature of the thermode (>50°C) in n = 6 subjects on day2 (Figure 5b). Amongst the remaining 8 subjects (Table S3), WDT was significantly higher compared to placebo (interaction treatment × day F(10,246) = 2.5, p < 0.01, GLM).
HPTs were 47.7 ± 2.4°C at baseline and 47.8 ± 1.8°C at day1‐49 on average (Figure 5c). HPTs were slightly lower during the placebo patch application resulting in a significant time effect (F(10,132) = 3, p < 0.01, GLM). Following capsaicin patch treatment, HPTs were above the thermode cut‐off temperature in all 14 subjects on day2 and day7 (HPT >50°C, Table S3), but returned to values of 48.6 ± 1.8°C during day14‐49 in n = 9 subjects (Figure 5c), not significantly different to placebo (F(5,112) = 0.42, p > 0.8, GLM). Notably, in n = 5 subjects HPT could not be assessed even at day49 after capsaicin treatment (Table S3).
3.5.2. Sensitivity to continuous warmth and supra‐threshold tonic heat stimulation
A constant temperature of 42°C delivered for 1 min (continuous warmth) was perceived as warm for an average duration of 26 ± 8 s across all days of assessment in n = 11 subjects and not significantly different to the placebo patch site (duration 31 ± 7 s, n = 11, F(1,20) = 2.2, p > 0.1, data not shown). A heat pain sensation during continuous warmth was reported by 1 of 14 subjects at baseline, by 3 of 14 subjects after placebo, and by 2 of 14 subjects after capsaicin patch administration.
Maximum pain recorded during 5 s of tonic heat (48°C) was NRS 3.5 ± 2 at baseline and NRS 2.5 ± 1.5 at the placebo sites (average across day1‐49, Table S3). Tonic heat pain reported at the capsaicin‐treated site was rated by 5 subjects with an NRS of 0.5 ± 0.9 on day1 and all 14 subjects perceived tonic heat as not painful (NRS 0) between day2‐7 (Table S3). Thereafter, the number of subjects who felt tonic heat as painful steadily increased from n = 10 on day14 to n = 13 on day49 reporting an NRS of 1.5 ± 1.4 (average day14‐49).
3.6. Comparison of the time course for sensory impairments
Electrical 1 Hz and 4 Hz sinusoidal pain (Figure 6a), HPTs, and continuous heat pain (Figure 6b), as well as electrically induced axon reflex flare size (Figure 6c), were maximally reduced during application of the capsaicin patch (day1 to day4). The time course of delta (∆) pain NRS‐values differed significantly between supra‐threshold 1 Hz (0.8 and 1 mA) and 4 Hz (0.2 and 0.4 mA) sinusoidal pain (Figure 6a, interaction sinusoidal stimulation × day F(10,137) = 2.2, p < 0.02, GLM). Pain NRS recovery to placebo levels (horizontal dotted lines in Figure 6) was significantly slower for 1 Hz compared to 4 Hz sinusoidal stimulation between day21 (F(1,13) = 7.3, p < 0.02, GLM) and day35 (F(1,11) = 7.1, p < 0.03, GLM, Figure 6a). Thresholds for heat pain (∆ HPT) and pain NRS to continuous heat (Figure 6b) recovered to placebo values after day14 (∆ HPT, p > 0.9, GLM) and day21 (continuous heat, p > 0.9, GLM), respectively, and showed a similar profile to the ∆ pain NRS scores for 1 Hz and 4 Hz sinusoidal stimulation from day14 to day49 (∆ HPT interaction 1 Hz stimulus × day F(5,72) = 0.4, p > 0.8, GLM and 4 Hz stimulus × day F(5,72) = 0.6, p > 0.6, GLM). Notably, all sensory indices after capsaicin had normalized to placebo values by day49, which is in contrast to the electrically induced axon reflex flare in capsaicin‐treated skin that had recovered only to ~50% on that day (Figure 6c and Figure S1).
4. DISCUSSION
Prolonged topical 8% capsaicin abolished heat pain after 24 h, whereas superficial skin nociceptors could still be activated by electrical sinusoidal stimuli, albeit causing less pain. Sinusoidal 4 Hz electrical pain recovered faster as compared to pain upon 1 Hz sinusoidal stimulation, which suggests a differential regeneration of axonal branches between ‘polymodal’ nociceptors (activated by both) and ‘silent’ C‐fibres (activated only by 4 Hz stimulation).
4.1. Assessment of de‐sensitized nociceptors
As reported before (Henrich et al., 2015), we found that sensory transduction of supra‐threshold heat (48°C, 5 s) was abolished by topical 8% capsaicin. Superficial nociceptors could still be excited with electrical transcutaneous stimuli (Tables S1 and S3). Absence of heat pain after capsaicin only reflects impaired transduction, whereas spike generation in nociceptors is still possible. Hence, this would allow detection of such heat‐unresponsive nociceptive endings also in neuropathy patients.
4.2. Sensory profile during 8% capsaicin patch application
Most nociceptors in humans express TRPV1 (Schmelz, Schmidt, et al., 2000; Tavares‐Ferreira et al., 2022) and their ‘de‐functionalization’ by capsaicin would explain the loss of heat sensitivity. The residual electrical pain might be encoded by a set of TRPV1‐negative neurons (Henrich et al., 2015). Intradermal injection of capsaicin also causes an ‘analgesic bleb’ (Baumann et al., 1991). Thus, nociceptive responses are completely abolished if local capsaicin concentrations are sufficiently high. In contrast, by using transdermal capsaicin application, the intradermal concentration is sufficient to abolish heat transduction but leaves axons responsive to electrical depolarization (Henrich et al., 2015). An alternative explanation would suggest that TRPV1‐positive neurons remain electrically excitable, perhaps at deeper or more proximal regions requiring higher currents for excitation (Table S1), while nociceptive transduction at superficial terminals is completely abolished.
4.3. Time course of sensation recovery after 8% capsaicin patch application
We applied slowly depolarizing electrical 1 Hz and 4 Hz sinusoidal stimuli to differentially assess the temporal recovery of ‘polymodal’ and ‘silent’ C‐nociceptors after capsaicin. It was hypothesized that sensory endings of polymodal mechano‐heat nociceptors are located more superficially (Tillman et al., 1995). There are no neuronal markers that could differentiate silent from polymodal nociceptors in human skin, so we only have indirect evidence suggesting a deeper location of sensory endings of silent nociceptors: ‘silent’ nociceptors have higher electrical activation thresholds for rectangular pulses when stimulated transcutaneously (Obreja et al., 2018; Weidner et al., 1999). Even though some of these fibres are insensitive to superficial heat stimulation, they still respond to intracutaneous capsaicin injections (Schmelz et al., 2000b). Obviously, topical capsaicin would be expected to impair superficially located nociceptors first and indeed, heat pain was almost gone on day1 of capsaicin application. However, electrically induced pain via polymodal nociceptors (1 Hz sinusoidal pulses) was only moderately reduced (~30% NRS reduction), whereas pain reduction to the 4 Hz sinusoidal stimulus (~50% NRS reduction) was more pronounced. The recovery of pain ratings to 1 Hz stimuli over 7 weeks was considerably more protracted than the recovery to 4 Hz sinusoidal pain. It might be suggested that the electrical stimulation profile could reveal the different time course of nociceptor de‐functionalization and recovery, perhaps based on the biophysical property of the recruited nociceptor. The 4 Hz sinusoidal stimulation is likely to depolarize axons such that a single AP is evoked per 250 ms cycle (Jonas et al., 2018). This stimulus preferentially activates small diameter axons that have a membrane time constant close to 100 ms (Bostock et al., 2003). It might be expected that, based on their high surface‐to‐volume ratio, small‐diameter axons would be those to be most sensitive to functional degeneration associated with capsaicin‐induced calcium increase. Thus, the smallest diameter axons would be particularly sensitive to early de‐functionalization by capsaicin, which could explain the early reduction of pain ratings to 4 Hz sinusoidal stimulation. In contrast, the 500 ms depolarization elicits volleys of APs in polymodal nociceptors (Rukwied et al., 2020). The number of evoked APs upon depolarization increases with more complex branching of sensory terminals (Barkai et al., 2020). This has been considered to facilitate supra‐threshold spontaneous depolarizing fluctuations in human nociceptors linked to the generation of spontaneous activity and ongoing clinical pain (Tian et al., 2024). Also, inhibition of axonal transport upon capsaicin (Kawakami et al., 1993; Szallasi & Blumberg, 1999) could explain a longer time course of inhibitory effects and a protracted regeneration of pain to the 1 Hz electrical sinusoidal stimulation.
The degeneration of TRPV1‐positive epidermal nerve fibres by capsaicin is followed by a slow regrowth over months (Anand et al., 2019; Anand & Bley, 2011). We expect the first axonal sprouts reinnervating the epidermis to have very small diameters rendering them more sensitive to 4 Hz sinusoidal stimulation. Regeneration of the complete axonal branching will presumably take more time. The arborization of the nociceptive terminal tree has a considerable impact on the orthograde AP firing frequency (Barkai et al., 2020) and also likely affects bouts of 1 Hz sinusoidal depolarization. Thus, the protracted recovery of 1 Hz compared to 4 Hz sinusoidal pain thus might reflect the additional time required for a more complete regeneration of the terminal branches.
4.4. Axon reflex flare recovery after capsaicin 8% patch application
Already 1 day after capsaicin application, electrically evoked vasodilation did not exceed 1 cm2 and only recovered to about 50% of the pre‐capsaicin values even after 7 weeks. Of note, APs induce only short‐lasting calcium transients that will release only small portions of locally stored CGRP. Tonic inflow of calcium via TRPV1 channels at the site of continuous capsaicin application, in contrast, results in extreme calcium concentrations and thereby completely depletes the axons from neuropeptide vesicles. The time required for their replenishment may explain the slow axon reflex flare recovery over months.
4.5. Limitations
We did not investigate neuropathic pain patients or provide morphological/neuropathological data supporting our notion that a protracted regeneration of terminal branches might be linked to lasting analgesic effects upon 8% capsaicin. Morphological data based on immunofluorescence staining, however, cannot differentiate regeneration and functional return of specific C‐nociceptor sub‐classes. We aimed to address this issue by the functional selectivity of our electrical 1 Hz and 4 Hz sinusoidal stimulation protocols. Both electrical profiles can activate also non‐nociceptive ‘C‐touch’ fibres (Rukwied et al., 2020). Although there is no overt sensation upon their activation (Olausson et al., 2002), we cannot exclude modulating pain processing (Habig et al., 2017). The short‐term effect observed at the placebo site at day one is probably linked to skin swelling under patch occlusion. The reduction of pain at the placebo site over 7 weeks is most likely due to habituation, as reported before upon repetitive thermal painful stimuli (LeBlanc & Potvin, 1966; Rennefeld et al., 2010).
4.6. Capsaicin 8% patch and clinical perspective
A single 1‐h treatment with topical 8% capsaicin can provide pain relief for periods of 12 weeks and beyond in neuropathic patients (Anand & Bley, 2011; Backonja et al., 2008). The regeneration of intra‐ and sub‐epidermal nerve fibres and their functional regeneration after 8% capsaicin‐induced degeneration of innervation (‘pruning’ (Anand et al., 2019; Anand et al., 2022)) has been associated with pain amelioration. Thus, lasting pain amelioration might depend on the temporal rate of axonal regenerative sprouting in patients. Temporal regeneration of the terminal branches of nociceptors, which are particularly linked to the generation of spontaneous activity (Barkai et al., 2020), could be protracted and thereby contribute to prolonged analgesia in neuropathic pain patients induced by topical capsaicin. However, such hypothetic differences could not be detected by classic sensory testing as patients with and without neuropathic pain do not differ in their sensory thresholds or ‘sensory phenotypes’ (Forstenpointner et al., 2021; Raputova et al., 2017). In contrast, pain to ongoing 4 Hz sinusoidal stimulation does not accommodate in about 50% of patients with neuropathic pain when tested in their symptomatic skin but habituates in healthy volunteers (Jonas et al., 2018; Landmann et al., 2022). Here, the ability of axons to respond to 4 Hz sinusoidal stimuli after capsaicin showed a pain habituation profile similar to normal skin, thus it does not mirror the feature of pain accommodation in neuropathic pain states. Moreover, the distinct temporal recovery pattern of nociceptors observed here cannot explain the lasting pain reduction in patients after a single 1‐h 8% capsaicin application. Yet, the high frequent nociceptor discharge to a single 1 Hz sinusoidal pulse required more time to recover after 8% capsaicin than the regular firing to 4 Hz stimulation. We can only speculate whether this is a structural effect based on the spatial branching of the capsaicin‐targeted nociceptors. However, functionally, it reflects the ability of nociceptors to generate bursts upon depolarization, a key feature identified for primary nociceptors in chronic neuropathic pain (North et al., 2022; Tian et al., 2024). Thus, irrespective of the structural basis, testing the ability of skin nociceptors to generate bursts upon slow depolarizing electrical stimulation may provide information on clinically relevant aspects of nociceptor excitability and may therefore be considered as a functional screening test in neuropathy patients including those treated with 8% capsaicin.
FUNDING INFORMATION
This work was supported by the Deutsche Forschungsgemeinschaft DFG [project 397846571 to RR, SFB1158 to RC and MS].
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Figure S1. Axon reflex flare area (cm2) to continuous 4 Hz sinusoidal stimulation (60 s) of 0.2 mA (circles) and 0.4 mA (squares) amplitudes delivered to skin sites treated with placebo patch (grey and black) and 8% capsaicin (pink and red) recorded for 49 assessment days. The flare response recorded from naïve skin at day0 prior to path application is depicted with open symbols. The grey bar indicates the 4 days of patch application and values are depicted as mean ± SD. Hashes indicate significant treatment × day interaction (p < 0.05, GLM).
Table S1. Pain NRS (0–10) during 8% capsaicin patch application (day1‐day4) and thresholds (mA) for the induction of a sensation (sensory thresholds), for pain, and for pain NRS3 intensity to rectangular pulses (20 Hz, 0.1 ms duration) and 4 Hz sinusoidal stimulation. Significant differences to baseline condition (day0, before patch application) are indicated by asterisks (p < 0.05, GLM). Bold numbers indicate significant differences between the capsaicin and the placebo patch site (p < 0.05, GLM). Values are presented as mean ± SD (in brackets the number of subjects, minimum and maximum values).
Table S2. Area of the axon reflex flare (cm2, mean ± SD, in brackets minimum and maximum values) assessed after 4 Hz sinusoidal amplitudes of 0.05–0.4 mA for 2.5 s, 0.2 mA for 60 s, and 0.4 mA for 60 s. Significant differences to baseline condition (day0, before patch application) are indicated by asterisks (p < 0.05, GLM), bold numbers indicate significant differences between the capsaicin and the placebo patch site (p < 0.05, GLM).
Table S3. Skin surface temperature (°C), cold detection thresholds (CDT,°C), warm detection threshold (WDT,°C), heat pain threshold (HPT,°C) and pain NRS (0–10) to 5 s heat (9 × 9 mm thermode, 48°C) recorded prior to patch application (baseline, day0) and at skin sites treated with 8% capsaicin and placebo patches. Values are presented as mean ± SD (in brackets number of subjects with minimum and maximum values), significant differences to baseline condition are indicated by asterisks (p < 0.05, GLM), and bold numbers indicate significant differences between the capsaicin and the placebo patch site (p < 0.05, GLM). Note that WDT could not be detected in n = 6 subjects at day2 of capsaicin application and HPT in capsaicin‐treated skin was indeterminate in all 14 subjects at day2 and 7. HPT could not be recorded in n = 5 subjects even at day49 (cut‐off temperature of the thermode 50°C).
ACKNOWLEDGEMENTS
We are grateful to all subjects that generously volunteered for this study and Ilona Rossbach for careful proofreading. Open Access funding enabled and organized by Projekt DEAL.
Tumbala Gutti, D. , Carr, R. , Schmelz, M. , & Rukwied, R. (2025). Slow depolarizing electrical stimuli reveal differential time courses of nociceptor recovery after prolonged topical capsaicin in human skin. European Journal of Pain, 29, e4726. 10.1002/ejp.4726
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author upon reasonable request.
REFERENCES
- Anand, P. , & Bley, K. (2011). Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high‐concentration capsaicin 8% patch. British Journal of Anaesthesia, 107, 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand, P. , Elsafa, E. , Privitera, R. , Naidoo, K. , Yiangou, Y. , Donatien, P. , Gabra, H. , Wasan, H. , Kenny, L. , Rahemtulla, A. , & Misra, P. (2019). Rational treatment of chemotherapy‐induced peripheral neuropathy with capsaicin 8% patch: From pain relief towards disease modification. Journal of Pain Research, 12, 2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand, P. , Privitera, R. , Donatien, P. , Fadavi, H. , Tesfaye, S. , Bravis, V. , & Misra, V. P. (2022). Reversing painful and non‐painful diabetic neuropathy with the capsaicin 8% patch: Clinical evidence for pain relief and restoration of function via nerve fiber regeneration. Frontiers in Neurology, 13, 998904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backonja, M. , Wallace, M. S. , Blonsky, E. R. , Cutler, B. J. , Malan, P., Jr. , Rauck, R. , & Tobias, J. (2008). NGX‐4010, a high‐concentration capsaicin patch, for the treatment of postherpetic neuralgia: A randomised, double‐blind study. Lancet Neurology, 7, 1106–1112. [DOI] [PubMed] [Google Scholar]
- Barkai, O. , Butterman, R. , Katz, B. , Lev, S. , & Binshtok, A. M. (2020). The input‐output relation of primary nociceptive neurons is determined by the morphology of the peripheral nociceptive terminals. The Journal of Neuroscience, 40, 9346–9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron, R. , Treede, R. D. , Birklein, F. , Cegla, T. , Freynhagen, R. , Heskamp, M. L. , Kern, K. U. , Maier, C. , Rolke, R. , Seddigh, S. , Sommer, C. , Ständer, S. , & Maihöfner, C. (2017). Treatment of painful radiculopathies with capsaicin 8% cutaneous patch. Current Medical Research and Opinion, 33, 1401–1411. [DOI] [PubMed] [Google Scholar]
- Baumann, T. K. , Simone, D. A. , Shain, C. N. , & LaMotte, R. H. (1991). Neurogenic hyperalgesia: The search for the primary cutaneous afferent fibers that contribute to capsaicin‐induced pain and hyperalgesia. Journal of Neurophysiology, 66, 212–227. [DOI] [PubMed] [Google Scholar]
- Bostock, H. , Campero, M. , Serra, J. , & Ochoa, J. (2003). Velocity recovery cycles of C fibres innervating human skin. The Journal of Physiology, 553, 649–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campero, M. , Baumann, T. K. , Bostock, H. , & Ochoa, J. L. (2009). Human cutaneous C fibres activated by cooling, heating and menthol. The Journal of Physiology, 587, 5633–5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina, M. J. , Schumacher, M. A. , Tominaga, M. , Rosen, T. A. , Levine, J. D. , & Julius, D. (1997). The capsaicin receptor: A heat‐activated ion channel in the pain pathway. Nature, 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Forstenpointner, J. , Ruscheweyh, R. , Attal, N. , Baron, R. , Bouhassira, D. , Enax‐Krumova, E. K. , Finnerup, N. B. , Freynhagen, R. , Gierthmühlen, J. , Hansson, P. , Jensen, T. S. , Maier, C. , Rice, A. S. C. , Segerdahl, M. , Tölle, T. , Treede, R. D. , & Vollert, J. (2021). No pain, still gain (of function): The relation between sensory profiles and the presence or absence of self‐reported pain in a large multicenter cohort of patients with neuropathy. Pain, 162, 718–727. [DOI] [PubMed] [Google Scholar]
- Habig, K. , Schanzer, A. , Schirner, W. , Lautenschlager, G. , Dassinger, B. , Olausson, H. , Birklein, F. , Gizewski, E. R. , & Kramer, H. H. (2017). Low threshold unmyelinated mechanoafferents can modulate pain. BMC Neurology, 17, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich, F. , Magerl, W. , Klein, T. , Greffrath, W. , & Treede, R. D. (2015). Capsaicin‐sensitive C‐ and A‐fibre nociceptors control long‐term potentiation‐like pain amplification in humans. Brain, 138, 2505–2520. [DOI] [PubMed] [Google Scholar]
- Jonas, R. , Namer, B. , Schnakenberg, M. , Soares, S. , Pakalniskis, J. , Carr, R. , Schmelz, M. , & Rukwied, R. (2020). Sympathetic efferent neurons are less sensitive than nociceptors to 4 Hz sinusoidal stimulation. European Journal of Pain, 24, 122–133. [DOI] [PubMed] [Google Scholar]
- Jonas, R. , Namer, B. , Stockinger, L. , Chisholm, K. , Schnakenberg, M. , Landmann, G. , Kucharczyk, M. , Konrad, C. , Schmidt, R. , Carr, R. , McMahon, S. , Schmelz, M. , & Rukwied, R. (2018). Tuning in C‐nociceptors to reveal mechanisms in chronic neuropathic pain. Annals of Neurology, 83, 945–957. [DOI] [PubMed] [Google Scholar]
- Kawakami, T. , Hikawa, N. , Kusakabe, T. , Kano, M. , Bandou, Y. , Gotoh, H. , & Takenaka, T. (1993). Mechanism of inhibitory action of capsaicin on particulate axoplasmic transport in sensory neurons in culture. Journal of Neurobiology, 24, 545–551. [DOI] [PubMed] [Google Scholar]
- Kennedy, W. R. , Vanhove, G. F. , Lu, S. P. , Tobias, J. , Bley, K. R. , Walk, D. , Wendelschafer‐Crabb, G. , Simone, D. A. , & Selim, M. M. (2010). A randomized, controlled, open‐label study of the long‐term effects of NGX‐4010, a high‐concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. The Journal of Pain, 11, 579–587. [DOI] [PubMed] [Google Scholar]
- Khoshnoodi, M. , Truelove, S. , & Polydefkis, M. (2019). Effect of diabetes type on long‐term outcome of epidermal axon regeneration. Annals of Clinical Translational Neurology, 6, 2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann, G. , Stockinger, L. , Gerber, B. , Benrath, J. , Schmelz, M. , & Rukwied, R. (2022). Local hyperexcitability of C‐nociceptors may predict responsiveness to topical lidocaine in neuropathic pain. PLoS One, 17, e0271327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc, J. , & Potvin, P. (1966). Studies on habituation to cold pain. Canadian Journal of Physiology and Pharmacology, 44, 287–293. [DOI] [PubMed] [Google Scholar]
- Lewis, C. M. , & Griffith, T. N. (2022). The mechanisms of cold encoding. Current Opinion in Neurobiology, 75, 102571. [DOI] [PubMed] [Google Scholar]
- Lo Vecchio, S. , Andersen, H. H. , & Arendt‐Nielsen, L. (2018). The time course of brief and prolonged topical 8% capsaicin‐induced desensitization in healthy volunteers evaluated by quantitative sensory testing and vasomotor imaging. Experimental Brain Research, 236, 2231–2244. [DOI] [PubMed] [Google Scholar]
- Magerl, W. , Fuchs, P. N. , Meyer, R. A. , & Treede, R. D. (2001). Roles of capsaicin‐insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain, 124, 1754–1764. [DOI] [PubMed] [Google Scholar]
- Nolano, M. , Simone, D. A. , Wendelschafer‐Crabb, G. , Johnson, T. , Hazen, E. , & Kennedy, W. R. (1999). Topical capsaicin in humans: Parallel loss of epidermal nerve fibers and pain sensation. Pain, 81, 135–145. [DOI] [PubMed] [Google Scholar]
- North, R. Y. , Odem, M. A. , Li, Y. , Tatsui, C. E. , Cassidy, R. M. , Dougherty, P. M. , & Walters, E. T. (2022). Electrophysiological alterations driving pain‐associated spontaneous activity in human sensory neuron Somata parallel alterations described in spontaneously active rodent nociceptors. The Journal of Pain, 23, 1343–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obreja, O. , Rukwied, R. , Nagler, L. , Schmidt, M. , Schmelz, M. , & Namer, B. (2018). Nerve growth factor locally sensitizes nociceptors in human skin. Pain, 159, 416–426. [DOI] [PubMed] [Google Scholar]
- Olausson, H. , Lamarre, Y. , Backlund, H. , Morin, C. , Wallin, B. G. , Starck, G. , Ekholm, S. , Strigo, I. , Worsley, K. , Vallbo, A. B. , & Bushnell, M. C. (2002). Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience, 5, 900–904. [DOI] [PubMed] [Google Scholar]
- Orstavik, K. , Weidner, C. , Schmidt, R. , Schmelz, M. , Hilliges, M. , Jorum, E. , Handwerker, H. , & Torebjörk, H. E. (2003). Pathological C‐fibres in patients with a chronic painful condition. Brain, 126, 567–578. [DOI] [PubMed] [Google Scholar]
- Paricio‐Montesinos, R. , Schwaller, F. , Udhayachandran, A. , Rau, F. , Walcher, J. , Evangelista, R. , Vriens, J. , Voets, T. , Poulet, J. F. A. , & Lewin, G. R. (2020). The sensory coding of warm perception. Neuron, 106, 830–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raputova, J. , Srotova, I. , Vlckova, E. , Sommer, C. , Uceyler, N. , Birklein, F. , Rittner, H. L. , Rebhorn, C. , Adamova, B. , Kovalova, I. , Kralickova Nekvapilova, E. , Forer, L. , Belobradkova, J. , Olsovsky, J. , Weber, P. , Dusek, L. , Jarkovsky, J. , & Bednarik, J. (2017). Sensory phenotype and risk factors for painful diabetic neuropathy: A cross‐sectional observational study. Pain, 158, 2340–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennefeld, C. , Wiech, K. , Schoell, E. D. , Lorenz, J. , & Bingel, U. (2010). Habituation to pain: Further support for a central component. Pain, 148, 503–508. [DOI] [PubMed] [Google Scholar]
- Rukwied, R. , Thomas, C. , Obreja, O. , Werland, F. , Kleggetveit, I. P. , Jorum, E. , Carr, R. W. , Namer, B. , & Schmelz, M. (2020). Slow depolarizing stimuli differentially activate mechanosensitive and silent C‐nociceptors in human and pig skin. Pain, 161, 2119–2128. [DOI] [PubMed] [Google Scholar]
- Schmelz, M. , Michael, K. , Weidner, C. , Schmidt, R. , Torebjörk, H. E. , & Handwerker, H. O. (2000). Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport, 11, 645–648. [DOI] [PubMed] [Google Scholar]
- Schmelz, M. , Schmidt, R. , Handwerker, H. O. , & Torebjork, H. E. (2000). Encoding of burning pain from capsaicin‐treated human skin in two categories of unmyelinated nerve fibres. Brain, 123, 560–571. [DOI] [PubMed] [Google Scholar]
- Szallasi, A. , & Blumberg, P. M. (1999). Vanilloid (capsaicin) receptors and mechanisms. Pharmacological reviews, 51, 159–212. [PubMed] [Google Scholar]
- Tavares‐Ferreira, D. , Shiers, S. , Ray, P. R. , Wangzhou, A. , Jeevakumar, V. , Sankaranarayanan, I. , Cervantes, A. M. , Reese, J. C. , Chamessian, A. , Copits, B. A. , Dougherty, P. M. , Gereau, R. W. , Burton, M. D. , Dussor, G. , & Price, T. J. (2022). Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Science Translational Medicine, 14, eabj8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, J. , Bavencoffe, A. G. , Zhu, M. X. , & Walters, E. T. (2024). Readiness of nociceptor cell bodies to generate spontaneous activity results from background activity of diverse ion channels and high input resistance. Pain, 165, 893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman, D. B. , Treede, R. D. , Meyer, R. A. , & Campbell, J. N. (1995). Response of C fibre nociceptors in the anaesthetized monkey to heat stimuli: Estimates of receptor depth and threshold. The Journal of Physiology, 485(Pt 3), 753–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torebjork, H. E. , & Hallin, R. G. (1973). Perceptual changes accompanying controlled preferential blocking of a and C fibre responses in intact human skin nerves. Experimental Brain Research, 16, 321–332. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Wang, S. , Asgar, J. , Joseph, J. , Ro, J. Y. , Wei, F. , Campbell, J. N. , & Chung, M. K. (2017). Ca(2+) and calpain mediate capsaicin‐induced ablation of axonal terminals expressing transient receptor potential vanilloid 1. The Journal of Biological Chemistry, 292, 8291–8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner, C. , Schmelz, M. , Schmidt, R. , Hansson, B. , Handwerker, H. O. , & Torebjork, H. E. (1999). Functional attributes discriminating mechano‐insensitive and mechano‐responsive C nociceptors in human skin. The Journal of neuroscience, 19, 10184–10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Axon reflex flare area (cm2) to continuous 4 Hz sinusoidal stimulation (60 s) of 0.2 mA (circles) and 0.4 mA (squares) amplitudes delivered to skin sites treated with placebo patch (grey and black) and 8% capsaicin (pink and red) recorded for 49 assessment days. The flare response recorded from naïve skin at day0 prior to path application is depicted with open symbols. The grey bar indicates the 4 days of patch application and values are depicted as mean ± SD. Hashes indicate significant treatment × day interaction (p < 0.05, GLM).
Table S1. Pain NRS (0–10) during 8% capsaicin patch application (day1‐day4) and thresholds (mA) for the induction of a sensation (sensory thresholds), for pain, and for pain NRS3 intensity to rectangular pulses (20 Hz, 0.1 ms duration) and 4 Hz sinusoidal stimulation. Significant differences to baseline condition (day0, before patch application) are indicated by asterisks (p < 0.05, GLM). Bold numbers indicate significant differences between the capsaicin and the placebo patch site (p < 0.05, GLM). Values are presented as mean ± SD (in brackets the number of subjects, minimum and maximum values).
Table S2. Area of the axon reflex flare (cm2, mean ± SD, in brackets minimum and maximum values) assessed after 4 Hz sinusoidal amplitudes of 0.05–0.4 mA for 2.5 s, 0.2 mA for 60 s, and 0.4 mA for 60 s. Significant differences to baseline condition (day0, before patch application) are indicated by asterisks (p < 0.05, GLM), bold numbers indicate significant differences between the capsaicin and the placebo patch site (p < 0.05, GLM).
Table S3. Skin surface temperature (°C), cold detection thresholds (CDT,°C), warm detection threshold (WDT,°C), heat pain threshold (HPT,°C) and pain NRS (0–10) to 5 s heat (9 × 9 mm thermode, 48°C) recorded prior to patch application (baseline, day0) and at skin sites treated with 8% capsaicin and placebo patches. Values are presented as mean ± SD (in brackets number of subjects with minimum and maximum values), significant differences to baseline condition are indicated by asterisks (p < 0.05, GLM), and bold numbers indicate significant differences between the capsaicin and the placebo patch site (p < 0.05, GLM). Note that WDT could not be detected in n = 6 subjects at day2 of capsaicin application and HPT in capsaicin‐treated skin was indeterminate in all 14 subjects at day2 and 7. HPT could not be recorded in n = 5 subjects even at day49 (cut‐off temperature of the thermode 50°C).
Data Availability Statement
Data are available from the corresponding author upon reasonable request.


