Abstract
Background
Chronic pain is one of the most common health conditions among older adults, triggering various disruptions in information processing across attentional, emotional, and somatosensory domains. However, there is insufficient information about how these aspects interact and their potential contribution to the vulnerability of older adults to chronic pain. This study aimed to investigate potential alterations induced by chronic pain during aging in attentional aspects of tactile stimulation and to observe the influence of affective context.
Method
Twenty‐six older adults with chronic pain (70.00 ± 5.07 years; 11 males), 28 pain‐free older adults (69.57 ± 3.96 years; 13 males) and 27 healthy younger adults (21.48 ± 1.80 years; 14 males) participated in the study. We compared the somatosensory evoked potentials elicited by frequent and deviant stimulation (probability 14%) applied when participants were viewing blocks of pleasant, unpleasant, and neutral images from the International Affective Picture System.
Results
During frequent stimulation, older adults with chronic pain showed higher P50 and N100 amplitudes compared to pain‐free older adults and younger individuals. Furthermore, the older group with pain exhibited higher P300 amplitude during emotional contexts compared to neutral scenarios. During deviant stimulation, older adults with chronic pain exhibited higher P50 and N100 amplitudes compared to pain‐free older adults but displayed typical age‐related flattening during P300.
Conclusions
These findings indicate that chronic pain leads to a decline in the ability to habituate to non‐painful irrelevant somatosensory stimuli, especially when it is presented in an emotional context.
Significance Statement
In the present study, we have observed how older individuals suffering from chronic pain exhibit a decline in the habituation capacity of irrelevant somatosensory information. Furthermore, we have observed how the affective context in which these individuals are situated leads to an exacerbation of this deficit. Enhancing our comprehension of how aging and chronic pain interact to impact somatosensory processing could facilitate the tailoring of novel intervention strategies.
1. INTRODUCTION
Epidemiological studies suggest that more than 50% of older adults over 65 years suffer from chronic pain (Dahlhamer et al., 2018), provoking disturbances in the processing of information at the cognitive, affective, and sensory levels (Domenichiello & Ramsden, 2019). However, there is no information about how these disturbances interact in aging, and their contribution to vulnerability to chronic pain in older adults.
The oddball paradigm is a key experimental method that examine habituation and attention capability, which consist in presenting mostly identical stimuli (frequent) with occasional deviant stimuli. Healthy individuals exhibit enhanced cortical activation during deviant stimulation (Sitges et al., 2010) while demonstrating pronounced habituation during frequent stimulation (Montoya & Sitges, 2006). These attentional deflections are reflected in augmentations and reductions, respectively, of both early (P50, N100) and late (P300) evoked potentials, which are related to initial stimulus processing and attentional orientation (Freedman et al., 1987; Näätänen & Picton, 1987), and stimulus evaluation (Picton, 1992).
Interestingly, during this task, individuals with chronic pain and aging populations show similar alterations. Chronic pain patients exhibit reduced habituation during frequent stimulation, as seen in enhanced N100 across sensory domains (Choi et al., 2015, 2016; Coppola et al., 2013). Likewise, an age‐related augmentation of the N100 has been observed during somatosensory (Bolton & Staines, 2012), and visual (Czigler et al., 2006) stimulation.
Conflicting results exist regarding deviant stimulation in chronic pain patients. One study reported a reduced P300 response to auditory stimuli (Gubler et al., 2021), while another found no changes during somatosensory stimulation (Sitges et al., 2010). Nevertheless, chronic pain patients are assumed to have impaired attentional processes due to pain's demand for cognitive resources (Eccleston & Crombez, 1999). Older adults demonstrate a similar decline in attentional response, reflected in reduced P300 across sensory domains (Bolton & Staines, 2012; Polich, 1996), typically interpreted as a marker of diminished cognitive processing.
Furthermore, research shows that affective context influence attentional processing during the oddball paradigm, indicating that Somatosensory Evoked Potentials (SEPs) are diminished during an unpleasant context, underscoring the competition for attentional resources (Montoya & Sitges, 2006). Indeed, it is well‐known that chronic pain patients exhibit a negativity bias (Duschek et al., 2014), leading to an abnormal processing of somatosensory information when somatic signals arise from the body within an aversive context (Montoya et al., 2005). Affective modulation during oddball paradigms hasn't been studied in aging. However, the “positivity effect” is well‐established in older individuals, involving a tendency to prioritize positive stimuli over negative ones (Mikels et al., 2014).
Therefore, the present study aims to investigate the interaction between aging and chronic pain in SEPs, and their modulation during an oddball paradigm with different affective contexts, among older with chronic pain (POA), pain‐free older (OA), and healthy younger (YA). We expect POA to show decreased habituation, as seen in increased N100 during frequent stimulation. Additionally, we hypothesize POA will show reduced P300 during deviant stimulation. Lastly, due to chronic pain's affective symptomatology, POA may lack the positivity effect, displaying heightened attentional bias to unpleasant contexts.
2. METHODS AND MATERIALS
2.1. Participants
Eighty‐seven volunteers were initially recruited for the study. Younger participants were mainly recruited from the University of the Balearic Islands, while older adults were recruited from a senior program of the same university and from different senior citizen associations in Mallorca. However, 6 participants had to be excluded either because of technical problems during the electroencephalography (EEG) session (1 POA) or due to excessive artefacts in EEG recordings (>30% rejected trials after preprocessing; 2 POA, 3 YA). Therefore, the final sample was composed of 26 older adults with chronic pain (POA) (70.00 ± 5.07 years; 11 males), 28 pain‐free older adults (OA) (69.57 ± 3.96 years; 13 males), and 27 healthy younger adults (YA) (21.48 ± 1.80 years; 14 males) (see Table 1).
TABLE 1.
Sociodemographic, clinical and self‐reported data.
| Younger | Pain‐free older | Older with chronic pain | Statistics | |
|---|---|---|---|---|
| Mean ± SD (range) | Mean ± SD (range) | Mean ± SD (range) | ||
| Age | 21.48 ± 1.80 (18–25) | 69.57 ± 3.96 (62–77) | 70.00 ± 5.07 (62–82) | F(2,78) = 1419.502, p < 0.001 a , b |
| Male n (%) | 14 (51.8) | 13 (46.4) | 11 (42.3) | χ 2(2,81) = 0.488, p = 0.783 |
| Female n (%) | 13 (48.2) | 15 (53.6) | 15 (57.7) | |
| PANAS‐positive | 37.88 ± 3.87 (28–47) | 40.75 ± 5.59 (32–50) | 38.03 ± 6.69 (27–49) | F(2,78) = 2.363, p = 0.101 |
| PANAS‐negative | 13.96 ± 4.30 (10–25) | 12.35 ± 3.25 (10–21) | 13.50 ± 5.10 (10–27) | F(2,78) = 1.035, p = 0.360 |
| PCS‐Rumination | 2.11 ± 2.25 (0–16) | 0.82 ± 1.44 (0–7) | 2.24 ± 2.53 (0–16) | F(2,77) = 3.753, p = 0.028 c |
| PCS‐ Magnification | 2.69 ± 4.13 (0–10) | 0.42 ± 1.37 (0–5) | 3.60 ± 4.83 (0–8) | F(2,77) = 5.162, p = 0.008c |
| PCS‐Helpless | 3.14 ± 3.64 (0–17) | 0.64 ± 1.41 (0–5) | 3.68 ± 4.74 (0–16) | F(2,77) = 5.825, p = 0.004a,c |
| PHQ‐9 | 4.25 ± 2.63 (0–9) | 1.75 ± 2.48 (0–7) | 3.96 ± 3.16 (0–9) | F(2,78) = 6.734, p = 0.002 a , c |
| GAD‐7 | 4.74 ± 2.75 (0–11) | 1.57 ± 2.16 (0–7) | 3.65 ± 3.09 (0–11) | F(2,78) = 9.887, p < 0.001a,c |
| Medication | ||||
| Cholesterol | N = 0 | N = 2 | N = 3 | χ 2(2,81) = 3.11, p = 0.211 |
| Hyperthension | N = 0 | N = 6 | N = 12 | χ 2(2,81) = 16.340, p < 0.001 a , b |
| Anxiolytic | N = 0 | N = 1 | N = 3 | χ 2(2,81) = 3.927, p = 0.140 |
| Antidepressant | N = 0 | N = 4 | N = 5 | χ 2(2,81) = 5.40, p = 0.067 |
| Anti‐inflammatory | N = 0 | N = 0 | N = 5 | χ 2(2,81) = 11.27, p = 0.004b,c |
| Others | N = 1 | N = 11 | N = 12 | |
Note: Means, standard deviation and range values are displayed for younger and older participants with and without chronic pain. Statistical values and significance of group differences are shown.
Abbreviations: PANAS, Positive and Negative Affect Schedule; PCS, Pain Catastrophizing Scale; PHQ‐9, Patient Health Questionnaire; GAD‐7, Generalized Anxiety Disorder Assessment.
Differences between Younger adults and Pain‐free older adults.
Differences between Younger adults and Older adults with chronic pain.
Differences between Older adults with chronic pain and Pain‐free older adults.
Participants were invited to the EEG session, on condition that they did not present with: (1) any psychiatric or neurological condition (including having a score >9 on PHQ‐9 or >11 on GAD‐7); (2) uncontrolled hypertension, heart failure, or history of acute myocardial infarction; (3) systemic rheumatic disorders (i.e., rheumatoid arthritis, systemic lupus, fibromyalgia); (4) chronic opioid use; (5) suffered cancer or undergoing treatment in the last 5 years; (6) suffered COVID in the last 3 months; (7) having undergone a surgical intervention in the last 6 months; (8) cognitive impairment (Mini Mental State Examination <27 (Lobo et al., 1999)); (9) left‐handedness and; (10) acute or chronic pain (only for OA and YA). Inclusion in the POA group required self‐reported suffering from chronic musculoskeletal pain, defined as the presence of pain in at least 1 location of moderate or severe intensity (minimum rating of 3 out of 10) in the previous 6 months, some, most, or all the time (Buckalew et al., 2010; Ezzati et al., 2014; McCarthy et al., 2009). These criteria are consistent with the definition of chronic pain issued by the International Association for the Study of Pain (Nicholas et al., 2019).
Regarding clinical features of pain, participants in the POA group reported as their worst pain zone the upper or lower back (n = 11), shoulders/neck (n = 5), knee (n = 6), or other zones (hip, hand, and abdomen) (n = 4). The mean age of pain onset was 58.48 ± 13.70 years and the mean pain duration was 11.51 ± 12.79 years. Maximum, mean and minimum clinical pain intensity measured with a numerical rating scale (NRS, ranging from 0 to 10) during the week prior to the experiment reported by the participants were 5.76 ± 2.17, 4.57 ± 1.36, and 2.11 ± 1.86, respectively.
2.2. Procedure
Prior to the EEG recording day, all participants underwent an initial assessment session. This laboratory appointment started with a semi‐structured interview regarding the medical and psychological history (see information about medication in Table 1). In addition, anxiety and depression were evaluated by the Spanish versions of the Generalized Anxiety Disorder Assessment (GAD‐7) (García‐Campayo et al., 2010) and the Patient Health Questionnaire (PHQ‐9) (Kroenke et al., 2001), respectively. Chronic pain patients also completed a pain history interview (location, duration, intensity, daily interference of pain, etc.) as well as the Spanish version of the Brief Pain Inventory (BPI) (de Andrés Ares et al., 2015). Handedness laterality was evaluated with the Edinburgh Handedness Inventory (Oldfield, 1971).
Participants were requested not to take analgesics or anti‐inflammatory drugs on the day of the EEG session. On arrival in the laboratory, and prior to the EEG recording, they completed the Positive and Negative Affect Schedule (PANAS) (Watson et al., 1988) to assess their mood. Finally, after the EEG recording (to avoid generating possible expectancies), participants completed the Spanish version of the Pain Catastrophizing Scale (PCS) (Sullivan & Pivik, 1995).
All individuals were naive to the experiment and gave written informed consent prior to participating. The study was conducted in accordance with the Declaration of Helsinki (1991) and was approved by the Ethics Committee of the Balearic Islands (IB 4281/20).
2.3. Somatosensory stimulation
SEPs elicited on tactile stimulation were recorded following an oddball paradigm, adapting the used by Montoya et al. (2005, 2006). During this experimental task, two types of stimuli were presented in a random series such that one of them occurs infrequently (oddball or deviant stimuli). In the present experiment, 630 (86%) stimuli were applied to the right hand (frequent stimuli) and 105 (14%) stimuli were applied to the left hand (infrequent stimuli). All stimuli were delivered using a pneumatic stimulator consisting of a small membrane attached to the index finger by a plastic clip. Participants received three stimulation blocks in counterbalanced order, during which they viewed either pleasant, unpleasant, or neutral images from the International Affective Picture System (IAPS) (Lang et al., 2008). Therefore, in each block 35 pictures of the same affective context were presented for 6 s followed by a 500‐millisecond blank screen. One deviant and six frequent tactile stimuli were delivered during each picture. Thus, a total of 245 tactile stimuli (210 even, 35 odd) of 100 ms duration with a constant pressure of two bar and a variable interstimulus interval of 925 ms (±50 ms) were presented during each emotional condition. Pneumatic stimulation was pseudorandomized; odd stimuli never appeared first in the sequence to avoid coinciding with the appearance of a new image from the IAPS. This was done to ensure that the presence of the new visual stimulus would not affect the amplitude of the deviant somatosensory stimulus. Moreover, two odd stimuli were never presented successively. None of the participants reported discomfort associated with the tactile stimulation. At the end of the EEG session, participants were asked to rate IAPS images regarding valence (from 1 = very unpleasant to 9 = very pleasant) and arousal (1= very low to 9 = very high) using a Self‐Assessment Manikin (SAM). The images were selected based on adaptations for the Spanish and older population (Moltó et al., 2013). Arousal ratings for images presented in the pleasant and unpleasant blocks were matched (see Data S1 for the code of the images used and their valence and arousal values according to the norms). During the experiment, participants were instructed to ignore the tactile stimulation, to pay attention to the images, and to try and imagine experiencing themselves in the situations portrayed in the pictures. They were seated in front of a computer screen in a sound‐attenuated room.
2.4. EEG recording
EEG signals were recorded using a QuickAmp amplifier (BrainProducts GmbH, Munich, Germany) from 60 scalp electrodes placed according to the International 10/20 System. Electrode signals were recorded using an average reference calculated by the amplifier. An electrooculogram (EOG) signal was obtained by placing one electrode above and another below the right eye. Electrode impedance was kept below 10K Ω. EEG and EOG signals were recorded with a sampling rate of 1000 Hz using Brain Vision Recorder software (BrainProducts GmbH, Munich, Germany).
EEG signals were further processed offline using Brain Vision Analyser (BrainProducts GmbH). Due to the persistent presence of artefacts in frontopolar (Fp1, Fpz, Fp2) and antero‐frontal (AF3, AF1, AF2, AF4) electrodes, these electrodes were excluded from the analyses, resulting in a total of 53 electrodes. A frequency band‐pass filter of 0.1 to 35 Hz was applied. Eye movement artefacts were corrected using the Gratton et al. (1983) (Gratton et al., 1983). For analyses of evoked potentials elicited by tactile stimuli, EEG waveforms were segmented in epochs of 900 ms duration (–100 to 800 ms relative to stimulus onset) and baseline corrected (from –100 to 0 ms). Thereafter, an artefact rejection protocol with the following criteria was applied: maximal allowed voltage step/sampling point = 75 mV, minimal allowed amplitude = –75 mV, maximal allowed amplitude = 75 mV, and maximal allowed absolute difference in the epoch = 75 mV. Afterwards, EEG waveforms elicited by the tactile stimuli were averaged separately for the type of stimulus (deviant and frequent) and the emotional block (pleasant, unpleasant and neutral) during which the stimulus occurred. All average waves were digitally filtered (30 Hz low pass) and baseline‐corrected before statistical measures of component amplitude were computed. Peak amplitudes were computed for the following SEP components: P50 (30–70 ms after stimulus onset) (Montoya & Sitges, 2006), N100 (60–110 ms) (Montoya & Sitges, 2006), and P300 (160–320 ms) (Picton, 1992).
Specifically, both P50 and N100 are typically located primarily in centroparietal electrodes in the contralateral hemisphere where the stimulation was received (Montoya & Sitges, 2006). On the other hand, P300 is usually observed bilaterally at the centroparietal electrodes (Polich, 2007). In addition, visual evoked potentials (VEP) elicited by pleasant, unpleasant and neutral pictures were also analysed. The averaged window spanned 700 ms from 100 ms prior to picture onset. P1 (80–130 ms), N1(130–170 ms), P3 (280–380 ms) and LPP (300–600 ms) (Hajcak et al., 2010; Olofsson et al., 2008) were determined.
2.5. Data analysis
Analyses were conducted using SPSS (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY, USA: IBM Corp). Sex distribution in the sample was analysed with Chi‐Square Tests. Age and questionnaire responses were analysed with one‐way ANOVAs with the factor GROUP (OA, YA, POA). Valence and arousal ratings from the IAPS evaluation were analysed with repeated measures ANOVAs with the within‐subject factor EMOTION (pleasant, unpleasant, neutral) and the between‐subject factor GROUP (OA, YA, POA).
For the SEPs analysis, we calculated a pool of left frontal (F3, F5), right frontal (F4, F6), left frontocentral (FC3, FC5), right frontocentral (FC4, FC6), left central (C3, C5), right central (C4, C6), left centroparietal (CP3, CP5), right centroparietal (CP4, CP6), left parietal (P3, P5) and right parietal (P4, P6) electrodes. Furthermore, we took into consideration whether the activity was located on the contralateral or ipsilateral side of the tactile stimulation. For deviant stimulation (left hand), activity in electrodes located on the right side was considered contralateral, and ipsilateral in the left hemisphere. The opposite applied for frequent stimulation (right hand). Taking this into consideration, SEP amplitudes were statistically analysed using repeated measures ANOVAs with the within‐subject factors EMOTION (unpleasant vs. neutral vs. pleasant), CONDITION (frequent vs. deviant), ZONE (contralateral to the stimulation side vs. ipsilateral), BRAIN (frontal, frontocentral, central, centroparietal, parietal) and the between‐subject factor GROUP (OA, YA, POA).
VEPS amplitudes were statistically analysed for the Oz electrode (Dan et al., 2020) using a multivariate ANOVA for repeated measures with the factors EMOTION (unpleasant vs. neutral vs. pleasant pictures) as within‐subject factor, and GROUP (OA, YA, POA) as between‐subject factor.
All ANOVAs were adjusted using Greenhouse–Geisser corrections for the degrees of freedom, and Bonferroni corrections were applied when necessary.
Finally, Spearman's correlations were computed to investigate if P50, N100 and P300 amplitudes showing significant differences between groups were associated with valence and arousal ratings, PHQ‐9 and GAD‐7. Bonferroni correction using as criteria the number of IAPS ratings and questionnaires was applied, setting the new p = 0.012 (0.05/4). Additionally, in the POA group correlations with the characteristics of clinical pain (pain intensity and pain duration) were also performed.
3. RESULTS
3.1. Self‐report measures
Regarding PCS scale, POA reported higher Rumination and Magnification and Helpless scores than OA. YA reported higher Helpless scores than OA (see Table 1 for statistics, means and standard deviations). OA showed reduced scores in GAD‐7 and PHQ‐9 than POA and YA. No other significant differences between groups were found (see Table 1 for description and statistics).
3.2. IAPS evaluation
The ANOVA for valence ratings showed a main effect of EMOTION (F(2, 136) = 1051,291, p < 0.001) and a significant EMOTION*GROUP interaction (F(4, 136) = 7.297, p < 0.001). Post‐hoc analyses showed that POA rated pleasant pictures as more positive than YA (p = 0.022). Moreover, OA and POA rated unpleasant pictures as more negative than YA (p = 0.004, p < 0.001, respectively). Furthermore, all groups considered unpleasant pictures as the most negative stimuli, followed by neutral and pleasant pictures (all ps < 0.001) (see Figure 1).
FIGURE 1.
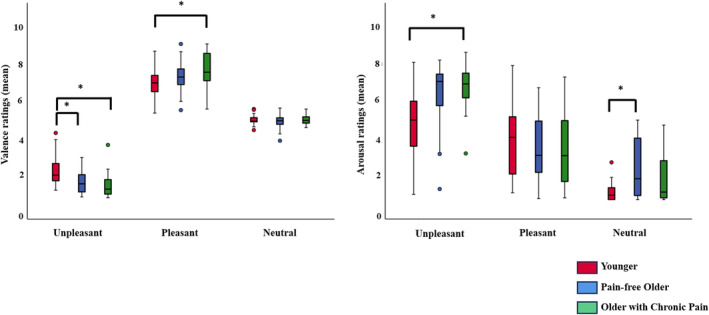
IAPS evaluation. Valence and arousal ratings of IAPS pictures from younger adults (red), pain‐free older adults (blue) and older adults with chronic pain (green) (*<0.05).
The ANOVA for arousal ratings showed a main effect of EMOTION (F(2, 136) = 199.898, p < 0.001) and a significant EMOTION*GROUP interaction (F(4, 136) = 8.869, p < 0.001). Post‐hoc analysis showed that POA and OA perceived unpleasant images as more arousing than YA (p < 0.001, p = 0.003, respectively). OA also perceived neutral images as more arousing than YA (p = 0.001). In addition, both groups of older adults rated unpleasant images as the most arousing, followed by pleasant, and finally neutral images (all ps < 0.001). YA rated pleasant and unpleasant pictures as more arousing than neutral ones (all ps < 0.001), while no differences were found between pleasant and unpleasant images (p = 0.121) (see Figure 1).
3.3. EEG analyses
3.3.1. SEPs
SEP waveforms generated by both stimulus types exhibited a positive peak (P50), succeeded by a negative peak (N100) and a subsequent positive peak (P300) (refer to Figure 2). The scalp topography of P50 and N100 components indicated that brain activity evoked by tactile stimulation was more pronounced over centroparietal regions, and over electrodes contralateral to the site of stimulation in comparison to ipsilateral ones. P300 exhibited a centroparietal‐dominant scalp distribution, observed across both contralateral and ipsilateral areas. For simplicity purposes, we will report our results focusing on the type of stimulation (deviant and frequent) for each of the components comprising the SEPs and limit ourselves to reporting the post‐hoc comparisons of interactions that involve the GROUP factor. For more details on the topographic characteristics of the potentials and to verify all post‐hoc effects obtained from our ANOVAs, please consult Data S2.
FIGURE 2.
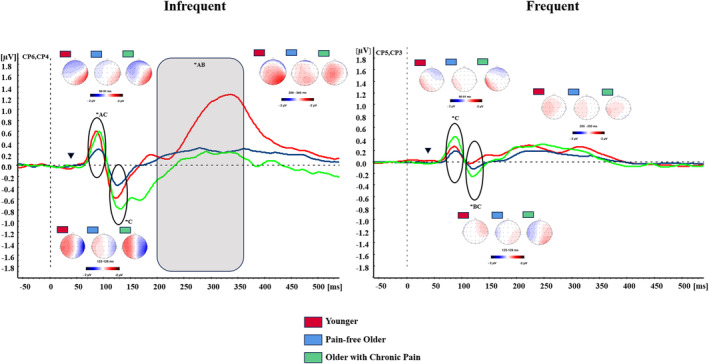
Somatosensory evoked potentials (SEPs). Grand averages of SEPs elicited by frequent and infrequent stimulation in their contralateral centroparietal electrodes for younger adults (red lines), pain‐free older adults (blue lines) and older adults with chronic pain (green lines). Inverted triangle shows stimulation onset. Circles and grey areas underlie time windows showing statistically significant differences between groups. Maps represent the scalp distribution at each peak (P50, N100, P300). ADifferences between Younger adults and Pain‐free older adults (p < 0.05). BDifferences between Younger adults and Older adults with chronic pain (p < 0.05). CDifferences between Older adults with chronic pain and Pain‐free older adults (p < 0.05).
The multivariate ANOVA on P50 amplitudes revealed significant main effects of ZONE (F(1,78) = 17.051, p < 0.001), BRAIN (F(4,312) = 21.026, p < 0.001), CONDITION (F(1,78) = 36.400, p < 0.001) and GROUP (F(2,78) = 6.281, p = 0.003), as well as, significant CONDITION*GROUP (F(8,78) = 3.557, p < 0.033), ZONE*BRAIN (F(4,312) = 19.878, p < 0.001), ZONE*BRAIN*GROUP (F(8,312) = 2.473, p = 0.032) and BRAIN*CONDITION*GROUP (F(8,312) = 2.592, p = 0.026) interactions. The examination of the BRAIN*CONDITION*GROUP interaction showed that during deviant stimulation OA participants showed reduced amplitudes in SEPs in comparison to POA and YA at centroparietal (p = 0.044, p = 0.007, respectively) and parietal (p = 0.038, p = 0.040, respectively) electrodes. During frequent stimulation, POA showed larger amplitudes than OA at central (p = 0.012), centroparietal (p = 0.009) and parietal (p = 0.006) electrodes. Furthermore, YA showed higher amplitudes than OA at frontocentral (p = 0.024) electrodes. No significant differences between POA and YA were observed (all ps > 0.05) (see Figure 2).
The multivariate ANOVA on N100 amplitudes displayed significant main effects of ZONE (F(1,78) = 52.212, p < 0.001), BRAIN (F(4,312) = 4.976, p = 0.006), CONDITION (F(1,78) = 97.116, p < 0.001) and GROUP (F(2,78) = 5.442, p = 0.006). Furthermore, a significant BRAIN*CONDITION (F(4,312) = 3.543, p = 0.008), ZONE*BRAIN (F(4,312) = 6.930, p < 0.001), CONDITION*GROUP (F(2,78) = 3.186, p = 0.047), ZONE*BRAIN*CONDITION (F(4,312) = 3.908, p = 0.004), and ZONE*CONDITION*GROUP (F(2,78) = 9.714, p = 0.010) interactions were found. The examination of ZONE*CONDITION*GROUP showed that during deviant stimulation POA showed higher amplitudes than OA contralaterally (p = 0.033), while YA showed higher amplitudes than POA ipsilaterally. During frequent stimulation, POA showed higher amplitudes than OA (p = 0.005) and YA (p = 0.005) contralaterally. No significant differences between groups were observed ipsilaterally (all ps > 0.05) (see Figure 2).
The multivariate ANOVA on P300 amplitudes revealed main effects and interactions of BRAIN (F(4,312) = 10.479, p < 0.001), CONDITION (F(1,78) = 9.331, p = 0.003), BRAIN*GROUP (F(8,312) = 3.404, p = 0.013), ZONE*BRAIN (F(4,312) = 8.367, p < 0.001), ZONE*BRAIN*GROUP (F(8,312) = 4.094, p = 0.003), BRAIN*CONDITION (F(4,312) = 17.830, p < 0.001), BRAIN*CONDITION*GROUP (F(8,312) = 8.940, p < 0.001), ZONE*CONDITION*BRAIN*GROUP (F(8,312) = 2.848, p = 0.021). Post‐hoc analyses of ZONE*CONDITION*BRAIN*GROUP revealed that during deviant stimulation, YA displayed higher amplitudes than OA and POA at centroparietal (p = 0.011, p = 0.002, respectively) and parietal (p < 0.001) contralateral electrodes. YA also showed larger amplitudes than OA (p = 0.018) and POA at frontal ipsilateral electrodes (p = 0.004) (see Figure 2). Finally, a significant CONDITION*EMOTION*GROUP (F(4,156) = 4.183, p = 0.007) interaction was also found. Post‐hoc analyses showed that during frequent stimulation, POA displayed higher amplitudes during the unpleasant (p = 0.030) and pleasant (p = 0.011) context in comparison to the neutral context, while no differences in OA and YA were found (all ps > 0.05) (see Figure 3). No significant differences between groups were observed when considering the emotional context (all ps > 0.05).
FIGURE 3.
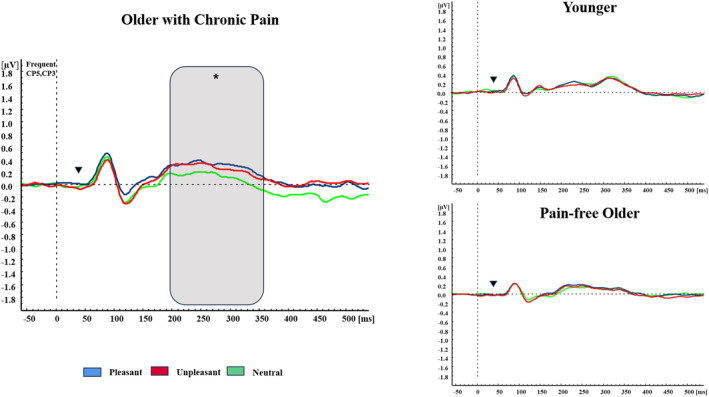
Affective modulation in SEPs. Grand averages of SEPs elicited by frequent stimulation in contralateral centroparietal electrodes for unpleasant context (red lines), pleasant context (blue lines) and neutral context (green lines) in all the groups. Inverted triangle shows stimulation onset. Grey area displays differences between conditions (*<0.05).
3.4. VEPs
The multivariate ANOVA for P3 amplitudes revealed a GROUP*EMOTION (F(4,156) = 2.547, p = 0.043) interaction, indicating that POA showed higher amplitudes during unpleasant (p = 0.009) and pleasant (p = 0.05) pictures in comparison to neutral pictures (see Figure 4). No significant differences regarding the affective context in YA and OA groups neither between groups were found. No significant differences in P1, N1 and LPP were found (all ps > 0.05).
FIGURE 4.
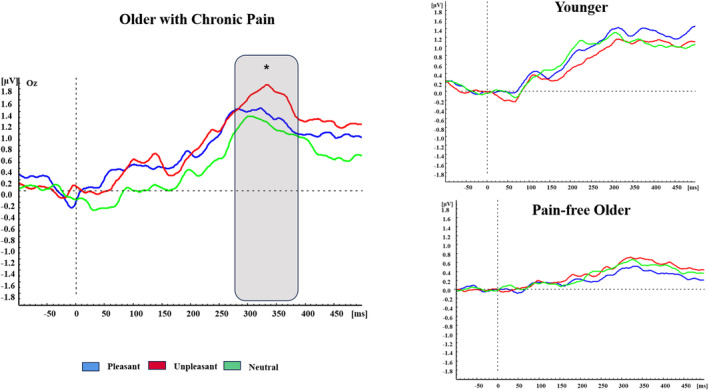
Affective modulation in VEPs. Grand averages of VEPs in Oz electrode for unpleasant context (red lines), pleasant context (blue lines) and neutral context (green lines) in all the groups. Grey area displays differences between conditions (*<0.05).
3.4.1. Correlation analyses
We found no significant correlations between any ERP (SEPs and VEPs) amplitudes and SAM ratings (valence and arousal), or with clinical pain characteristics in the POA group (all ps > 0.012).
4. DISCUSSION
This study aimed to explore alterations in somatosensory processing during an oddball paradigm in older adults with chronic pain, to understand how brain changes caused by chronic pain and aging combine to modify somatosensory function and attentional processes. Furthermore, we wanted to explore how affective context modulates somatosensory processing in older adults with chronic pain. We found that, for frequent stimuli, POA showed higher SEP amplitudes than OA and YA, specifically for the P50 (OA) and N100 (OA and YA). These results, as we hypothesized, may reflect that chronic pain in older adults leads to an enhanced habituation impairment. Second, during deviant stimulation, OA exhibited lower amplitudes in early SEPs compared to both POA and YA, specifically for the P50 (POA and YA) and N100 (POA), reflecting the expected age‐related decline in SEPs. This age‐related decline was not evident in POA during early latencies; it became prominent in later latencies, with significantly reduced P300 responses in both older adult groups (POA, OA) compared to YA. Third, differences in affective modulation were observed. Specifically, only the POA group showed higher P300 amplitudes during pleasant and unpleasant contexts compared to neutral in P300 during frequent stimulation. In summary, our results suggest that chronic pain during aging exacerbates the habituation deficit typically reported in aging studies. This inhibition deficit of irrelevant somatosensory information coexists with the general cognitive deficit in aging, reflected in reduced P300 to deviant stimulation. These alterations can be particularly pronounced under affective demands. These findings have important implications for the characterization and treatment of pain in aging, as discussed below.
Regarding frequent stimulation, POA displayed higher P50 amplitude than OA and higher N100 than OA and YA. As hypothesized, our findings suggest that the POA group is unable to engage in inhibitory responses to sensory repetition. Habituation is the capability of the central nervous system to disregard unimportant sensory information, marked by a decrease in neural activity after repeated exposure to identical stimuli (Näätänen & Picton, 1987). This phenomenon is seen as the brain's protective mechanism against sensory overload. Dysfunction in this mechanism is attributed to an overwhelmed higher‐order system for sensory perception and cognition (Freedman et al., 1987). Different studies have shown that a habituation deficit is common in patients with different chronic pain modalities (Choi et al., 2016; Coppola et al., 2013; De Tommaso et al., 2011, 2014; Lowén et al., 2015). Although similar results regarding habituation have also been found in aging (Bolton & Staines, 2012), our results suggest that chronic pain causes deficits in habituation capacity that exceed those produced by the aging process.
During deviant stimulation, POA and YA showed higher amplitudes in an early potential than OA. P50 is an early cortical response to somatic stimulation in the primary somatosensory cortex (Hari & Reinikainen, 1984). Despite cognitive and affective influences on P50 (Montoya & Sitges, 2006; Yee & White, 2001), it is linked to initial somatosensory encoding. On the other side, N100 is linked to attention allocation (Näätänen & Picton, 1987). In this sense, our results showed that chronic pain in aging enhances early processing during deviant simtuli, achieving the same amplitude as YA, not showing the typical age‐related flattening. These results suggest that POA exhibited increased attentional processing of somatosensory stimuli, consistent with the literature indicating that chronic pain patients generally show a heightened attentional focus on their bodily sensations (Horsburgh et al., 2024). Moreover, classic studies demonstrate how chronic pain is related to this increased attentional focus on somatosensory processing (Eccleston et al., 1997). However, this conclusion contrasts with the results for the P300, where we observed the typical bilateral flattening associated with aging in both groups of older adults, compared to YA. This reflects an aging effect on the attentional/evaluative somatosensory processing, aligning with the existing literature (Bolton & Staines, 2012; Fjell & Walhovd, 2004; Kamp, 2020). Therefore, our hypothesis positing reduced somatosensory processing in the POA compared to the OA, attributed to resource consumption by pain according to Gubler et al. (2021), is not supported by our findings. Our results are more in line with those observed by Sitges et al. (2010), where no effect on P300 was observed due to the presence of chronic pain. Indeed, we observed an increase in early latencies and a reduction in later latencies, that may indicate an initial attentional bias toward somatosensory stimuli that diminishes as their harmlessness is recognized. This pattern suggests that when pain accompanies aging, there may be a combination of reduced habituation, characteristic of chronic pain, and an age‐related flattening of the P300 response to somatosensory stimuli.
Regarding the affective context effect on attentional demand in the oddball paradigm, our results partially support our hypothesis. We observed affective modulation of SEPs only in POA. Specifically, during frequent stimulation, POA showed higher P300 amplitudes for both pleasant and unpleasant contexts compared to neutral, contrary to our expectation of a negative context impact only. These results align with studies involving healthy participants showing an increased P300 in response to emotionally salient stimuli (pleasant or unpleasant) compared to neutral stimuli (Carretié et al., 2003; Cuthbert et al., 2000; Schlüter & Bermeitinger, 2017). The theoretical explanation for this phenomenon is that highly activating stimuli mimic biological arousal, increasing their attentional salience (Polich, 2007). These conclusions align with VEPs results, where we found affective modulation (higher P300 amplitudes in pleasant and unpleasant conditions) only in POA. This suggests that older adults with chronic pain are more sensitive activating affective contexts, leading to an increased deterioration of habituation capacity to irrelevant stimulation.
Our results contrast with previous findings in healthy young individuals showing SEPs modulation in affective contexts (Montoya & Sitges, 2006). In those studies, SEPs exhibited reduced amplitude during unpleasant contexts compared to pleasant scenarios. This difference may stem from using adapted images based on older population norms, (Moltó et al., 2013), generally making them less impactful. Support for this hypothesis comes from participants' ratings of IAPS images. Both older groups rated unpleasant images more negatively than YA. This is consistent with the suggestion that adaptation has made the images less attentionally salient, preventing affective modulation from taking place in the YA group.
Despite insights from this study, acknowledging and addressing limitations is crucial for interpreting findings. First, although one strength of this study is having a second control group consisting of healthy younger individuals, having a sample of younger individuals with chronic pain could have enriched our results. However, our main objective is to observe how chronic pain combines with the aging process, rather than exploring the differences in experiencing chronic pain across the lifespan. Second, due to the clinical characteristics of the pain group, the two groups of older adults differed in anti‐inflammatory medication intake, which can influence affective states (Husain et al., 2017). Nonetheless, participants were instructed not to take pain relievers or anti‐inflammatories before the EEG session. Moreover, although GAD‐7 and PHQ‐9 scores obtained for all the participants are considered normative (Kroenke & Spitzer, 2002; Spitzer et al., 2006), we found that OA showed better mood states (reduced GAD‐7, PHQ‐9 and Helpless scores) than the other groups. These differences can be explained by the aforementioned “positivity effect” present in the older population (Mikels et al., 2014), and did not affect our results as shown by the ANCOVA performed controlling by these scores (Data S3). Third, we found no significant correlations between ERPs and IAPS evaluations, or between ERPs and other clinical data, such as mood, pain‐related cognitions, or clinical pain characteristics in POA. This limits the clinical interpretation of our results. Finally, we conducted an extensive topographic analysis of our data (see Data S2). Although this analysis may have reduced our statistical power, it has enhanced the robustness of our results. Furthermore, our findings indicate that in POA, changes during somatosensory processing are observed in centro‐parietal regions, which are commonly associated with this type of stimulation. Therefore, our study does not support the well‐documented compensatory over‐recruitment of frontal brain areas observed in aging (Li et al., 2018; Spreng & Turner, 2019), possibly because this phenomenon was observed during higher‐order cognitive tasks, whereas our study focuses on most basic attentional processes during somatosensory processing.
In conclusion, we have found that POA exhibits an increase in the amplitude of early SEPs during the processing of frequent stimuli. These results suggest that chronic pain leads to a deterioration in the habituation capacity to non‐painful somatosensory stimulation, which surpasses that produced by aging alone. On the other hand, POA shows similar cognitive‐related alterations (reduced P300 during deviant stimuli) as observed in OA. Furthermore, our findings indicate that alterations in somatosensory processing among POA may be amplified in affective contexts, likely due to eliciting greater activation within this group. This enhanced activation could in turn heighten the evaluative processing of otherwise neglected somatosensory stimuli. A better understanding of how aging and chronic pain combine in influencing somatosensory processing may also help to adapt new intervention protocols, considering the heightened vigilance toward bodily sensations and sensitivity to affective contexts in older individuals suffering from chronic pain.
AUTHORS CONTRIBUTIONS
All the authors have read and approved the paper. AG conceptualized, designed and supervised the experiment; AD collected data; AD and AG analysed the data; AG and AD drafted the original manuscript; CS and MM revised the manuscript. We declare having no conflict of interest for this study.
FUNDING INFORMATION
Study supported by the Spanish Ministry of Science and Innovation (PID2019‐110096GB‐I00/AEI/10.13039/501100011033, PRE2020‐092706).
CONFLICT OF INTEREST STATEMENT
All authors declare that they have no conflicts of interest.
Supporting information
Data S1: Supporting Information
Data S2: Supporting Information
Data S3: Supporting Information
Dorado, A. , Sitges, C. , van der Meulen, M. , & González‐Roldán, A. M. (2025). Impaired somatosensory habituation in older adults with chronic pain during an affective oddball task. European Journal of Pain, 29, e4732. 10.1002/ejp.4732
DATA AVAILABILITY STATEMENT
Data available on reasonable request.
REFERENCES
- Bolton, D. A. E. , & Staines, W. R. (2012). Age‐related loss in attention‐based modulation of tactile stimuli at early stages of somatosensory processing. Neuropsychologia, 50, 1502–1513. [DOI] [PubMed] [Google Scholar]
- Buckalew, N. , Haut, M. W. , Aizenstein, H. , Morrow, L. , Perera, S. , Kuwabara, H. , & Weiner, D. K. (2010). Differences in brain structure and function in older adults with self‐reported disabling and non‐disabling chronic low Back pain. Pain Medicine, 11, 1183–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié, L. , Hinojosa, J. A. , & Mercado, F. (2003). Cerebral patterns of attentional habituation to emotional visual stimuli. Psychophysiology, 40, 381–388. [DOI] [PubMed] [Google Scholar]
- Choi, W. , Lim, M. , Kim, J. S. , & Chung, C. K. (2016). Habituation deficit of auditory N100m in patients with fibromyalgia. European Journal of Pain (United Kingdom), 20, 1634–1643. [DOI] [PubMed] [Google Scholar]
- Choi, W. , Lim, M. , Kim, J. S. , Kim, D. J. , & Chung, C. K. (2015). Impaired pre‐attentive auditory processing in fibromyalgia: A mismatch negativity (MMN) study. Clinical Neurophysiology, 126, 1310–1318. [DOI] [PubMed] [Google Scholar]
- Coppola, G. , Di Lorenzo, C. , Schoenen, J. , & Pierelli, F. (2013). Habituation and sensitization in primary headaches. Journal of Headache and Pain, 14, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert, B. N. , Schupp, H. T. , Bradley, M. M. , Birbaumer, N. , & Lang, P. J. (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52, 95–111. [DOI] [PubMed] [Google Scholar]
- Czigler, I. , Pató, L. , Poszet, E. , & Balázs, L. (2006). Age and novelty: Event‐related potentials to visual stimuli within an auditory oddball‐visual detection task. International Journal of Psychophysiology, 62, 290–299. [DOI] [PubMed] [Google Scholar]
- Dahlhamer, J. , Lucas, J. , Zelaya, C. , Nahin, R. , Mackey, S. , DeBar, L. , Kerns, R. , Von Korff, M. , Porter, L. , & Helmick, C. (2018). Prevalence of chronic pain and high‐impact chronic pain among adults — United States, 2016. MMWR. Morbidity and Mortality Weekly Report, 67, 1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan, O. , Zreik, G. , & Raz, S. (2020). The relationship between individuals with fearful‐avoidant adult attachment orientation and early neural responses to emotional content: An event‐related potentials (ERPs) study. Neuropsychology, 34, 155–167. [DOI] [PubMed] [Google Scholar]
- de Andrés Ares, J. , Cruces Prado, L. M. , Canos Verdecho, M. A. , Penide Villanueva, L. , del Valle Hoyos, M. , Herdman, M. , Traseira Lugilde, S. , & Velázquez Rivera, I. (2015). Validation of the short form of the brief pain inventory (BPI‐SF) in Spanish patients with non‐cancer‐related pain. Pain Practice, 15, 643–653. [DOI] [PubMed] [Google Scholar]
- De Tommaso, M. , Ambrosini, A. , Brighina, F. , Coppola, G. , Perrotta, A. , Pierelli, F. , Sandrini, G. , Valeriani, M. , Marinazzo, D. , Stramaglia, S. , & Schoenen, J. (2014). Altered processing of sensory stimuli in patients with migraine. Nature Reviews, Neurology, 10, 144–155. [DOI] [PubMed] [Google Scholar]
- De Tommaso, M. , Federici, A. , Santostasi, R. , Calabrese, R. , Vecchio, E. , Lapadula, G. , Iannone, F. , Lamberti, P. , & Livrea, P. (2011). Laser‐evoked potentials habituation in fibromyalgia. Journal of Pain, 12, 116–124. [DOI] [PubMed] [Google Scholar]
- Domenichiello, A. F. , & Ramsden, C. E. (2019). The silent epidemic of chronic pain in older adults. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 93, 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duschek, S. , Werner, N. S. , Limbert, N. , Winkelmann, A. , & Montoya, P. (2014). Attentional bias toward negative information in patients with fibromyalgia syndrome. Pain Medicine, 15, 603–612. [DOI] [PubMed] [Google Scholar]
- Eccleston, C. , & Crombez, G. (1999). Pain demands attention: A cognitive‐affective model of the interruptive function of pain. Psychological Bulletin, 125, 356–366. [DOI] [PubMed] [Google Scholar]
- Eccleston, C. , Crombez, G. , Aldrich, S. , & Stannard, C. (1997). Attention and somatic awareness in chronic pain. Pain, 72, 209–2015. [DOI] [PubMed] [Google Scholar]
- Ezzati, A. , Zimmerman, M. E. , Katz, M. J. , Sundermann, E. E. , Smith, J. L. , Lipton, M. L. , & Lipton, R. B. (2014). Hippocampal subfields differentially correlate with chronic pain in older adults. Brain Research, 1573, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell, A. M. , & Walhovd, K. B. (2004). Life‐span changes in P3a. Psychophysiology, 41, 575–583. [DOI] [PubMed] [Google Scholar]
- Freedman, R. , Adler, L.£. , Gerhardt, G. A. , Waldo, M. , Baker, N. , Rose, G. M. , Drebing, C. , Nagamoto, H. , Bick‐Ford‐Wimer, P. , & Franks, R. (1987). Neurobiological studies of sensory gating in schizophrenia. Schizophrenia Bulletin, 13, 669–678. [DOI] [PubMed] [Google Scholar]
- García‐Campayo, J. , Zamorano, E. , Ruiz, M. A. , Pardo, A. , Pérez‐Páramo, M. , López‐Gómez, V. , Freire, O. , & Rejas, J. (2010). Cultural adaptation into Spanish of the generalized anxiety disorder‐7 (GAD‐7) scale as a screening tool. Health Qual Life Outcomes, 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton, G. , Coles, M. G. H. , & Donchin, E. (1983). A new method for off‐line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55, 468–484. [DOI] [PubMed] [Google Scholar]
- Gubler, D. A. , Zeiss, S. , Egloff, N. , Frickmann, F. , Goetze, B. , Holtforth, M. , Harnik, M. , Streitberger, K. , & Troche, S. J. (2021). The effect of chronic pain on voluntary and involuntary capture of attention: An event‐related potential study. Behavioral Neuroscience, 136, 195–205. [DOI] [PubMed] [Google Scholar]
- Hajcak, G. , Macnamara, A. , & Olvet, D. M. (2010). Event‐related potentials, emotion, and emotion regulation: An integrative review. Developmental Neuropsychology, 35, 129–155. [DOI] [PubMed] [Google Scholar]
- Hari, R. , Reinikainen, K. , Kaukoranta, E. , Hämäläinen, M. , Ilmoniemi, R. , Penttinen, A. , Salminen, J. , & Teszner, D. (1984). Somatosensory evoked cerebral magnetic fields from SI and SII in man. Electroencephalography and Clinical Neurophysiology, 57, 254–263. [DOI] [PubMed] [Google Scholar]
- Horsburgh, A. , Summers, S. J. , Lewis, A. , Keegan, R. J. , & Flood, A. (2024). The relationship between pain and Interoception: A systematic review and meta‐analysis. Journal of Pain, 25, 104476. [DOI] [PubMed] [Google Scholar]
- Husain, M. I. , Strawbridge, R. , Stokes, P. R. A. , & Young, A. H. (2017). Anti‐inflammatory treatments for mood disorders: Systematic review and meta‐analysis. Journal of Psychopharmacology, 31, 1137–1148. [DOI] [PubMed] [Google Scholar]
- Kamp, S. M. (2020). Preceding stimulus sequence effects on the oddball‐P300 in young and healthy older adults. Psychophysiology, 57, e13593. [DOI] [PubMed] [Google Scholar]
- Kroenke, K. , Spitzer, R. , & Williams, J. (2001). The PHQ‐9: Validity of a brief depression severity measure. Journal of General Inernal Medicine, 16, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke, K. , & Spitzer, R. L. (2002). The PHQ‐9: A new depression diagnostic and severity measure. Psychiatric Annals, 32, 509–515. [Google Scholar]
- Lang, P. J. , Bradley, M. M. , & Cuthbert, B. N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. University of Florida. [Google Scholar]
- Li, K. Z. H. , Bherer, L. , Mirelman, A. , Maidan, I. & Hausdorff, J. M. (2018). Cognitive involvement in balance, gait and dual‐tasking in aging: A focused review from a neuroscience of aging perspective. Frontiers in Neurology, Article 913, 10.3389/fneur.2018.00913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo, A. , Saz, P. , Marcos, G. , Día, J. , de la Cámara, C. , Ventura, T. , Morales‐Asín, F. , Fernando‐Pascual, L. , Montañes, J. , & Aznar, S. (1999). Revalidation and standardization of the cognition mini‐exam (first Spanish version of the mini‐mental status examination) in the general geriatric population. Medicina Clínica (Barcelona), 112, 767–774. [PubMed] [Google Scholar]
- Lowén, M. B. O. , Mayer, E. , Tillisch, K. , Labus, J. , Naliboff, B. , Lundberg, P. , Thorell, L. H. , Ström, M. , Engström, M. , & Walter, S. (2015). Deficient habituation to repeated rectal distensions in irritable bowel syndrome patients with visceral hypersensitivity. Neurogastroenterology and Motility, 27, 646–655. [DOI] [PubMed] [Google Scholar]
- McCarthy, L. H. , Bigal, M. E. , Katz, M. , Derby, C. , & Lipton, R. B. (2009). Chronic pain and obesity in elderly people: Results from the einstein aging study. Journal of the American Geriatrics Society, 57, 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels, J. , Reed, A. , Hardy, L. , & Lóckenhoff, C. (2014). Positive emotions across the adult life span. In Tugade M. M., Shiota M. N., & Kirby L. D. (Eds.), Handbook of Positive Emotions (pp. 256–271). The Guilford Press. [Google Scholar]
- Moltó, J. , Segarra, P. , López, R. , Esteller, À. , Fonfría, A. , Pastor, M. C. , & Poy, R. (2013). Adaptación española del “International Affective Picture System” (IAPS). Tercera Parte. Anales de Psicologia, 29, 965–984. [Google Scholar]
- Montoya, P. , & Sitges, C. (2006). Affective modulation of somatosensory‐evoked potentials elicited by tactile stimulation. Brain Research, 1068, 205–212. [DOI] [PubMed] [Google Scholar]
- Montoya, P. , Sitges, C. , García‐Herrera, M. , Izquierdo, R. , Truyols, M. , Blay, N. , & Collado, D. (2005). Abnormal affective modulation of somatosensory brain processing among patients with fibromyalgia. Psychosomatic Medicine, 67, 957–963. [DOI] [PubMed] [Google Scholar]
- Näätänen, R. , & Picton, T. (1987). The N1 wave of the human electric and Manfetic response to sound: A review and an analysis of the component structure. Psychophysiology, 24, 375–425. [DOI] [PubMed] [Google Scholar]
- Nicholas, M. , Vlaeyen, J. W. S. , Rief, W. , Barke, A. , Aziz, Q. , Benoliel, R. , Cohen, M. , Evers, S. , Giamberardino, M. A. , Goebel, A. , Korwisi, B. , Perrot, S. , Svensson, P. , Wang, S. J. , & Treede, R. D. (2019). The IASP classification of chronic pain for ICD‐11: Chronic primary pain. Pain, 160, 28–37. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessmentand analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Olofsson, J. K. , Nordin, S. , Sequeira, H. , & Polich, J. (2008). Affective picture processing: An integrative review of ERP findings. Biological Psychology, 77, 247–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton, T. W. (1992). The P300 wave of the human event‐related potential. Journal of Clinical Neurophysiology, 9, 456–479. [DOI] [PubMed] [Google Scholar]
- Polich, J. (1996). Meta‐analysis of P300 normative aging studies. Psychophysiology, 33, 334–353. [DOI] [PubMed] [Google Scholar]
- Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118, 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter, H. , & Bermeitinger, C. (2017). Emotional oddball: A review on variants, results, and mechanisms. Review of General Psychology, 21, 179–222. [Google Scholar]
- Sitges, C. , Bornas, X. , Llabrés, J. , Noguera, M. & Montoya, P. (2010). Linear and nonlinear analyses of EEG dynamics during non‐painful somatosensory processing in chronic pain patients. International Journal of Psychophysiology, 77(2), 176–183. [DOI] [PubMed] [Google Scholar]
- Spitzer, R. L. , Kroenke, K. , Williams, J. B. W. , & Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD‐7. Archives of Internal Medicine, 166, 1092–1097. [DOI] [PubMed] [Google Scholar]
- Spreng, R. N. , & Turner, G. R. (2019). The Shifting Architecture of Cognition and Brain Function in Older Adulthood. Perspectives on Psychological Science, 14(4), 523–542. 10.1177/1745691619827511 [DOI] [PubMed] [Google Scholar]
- Sullivan, M. , & Pivik, J. (1995). The pain Catastrophising scale: Development and validation. Psychological Assesment, 4, 524–532. [Google Scholar]
- Watson, D. , Clark, L. A. , & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Yee, C. M. , & White, P. M. (2001). Experimental modification of p50 suppression. Psychophysiology, 38, 531–539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting Information
Data S2: Supporting Information
Data S3: Supporting Information
Data Availability Statement
Data available on reasonable request.


