Abstract
We report a case of an adult woman of African ancestry who was hospitalized with statin induced‐ rhabdomyolysis. The patient presented to the emergency room with a 2‐week history of worsening muscle pain, nausea, vomiting and low oral intake, 1 month after starting 40 mg daily dose of rosuvastatin. Sequencing of SLCO1B1 coding regions revealed the patient was heterozygous for two SLCO1B1 deleterious variants, c.481+1G>T and c.1463G>C (*9), which are more prevalent in patients of African ancestry. This highlights the importance of pharmacogenetic testing in SLCO1B1, which includes a broader range of genetic variants for patients of African ancestry.
Keywords: statins, rhabdomyolysis, pharmacogenetics, SLCO1B1
1. CASE REPORT
An adult female, of African ancestry, with a history of type 2 diabetes, hypertension and ischaemic stroke presents with a 2‐week history of progressively worsening leg, shoulder and back pain, which became severe 3 days prior to the emergency room visit, along with nausea, vomiting and low oral intake.
In the emergency department, the patient was noted to have markedly abnormal pH (6.97), lactate (>17.0 mmol/L), beta‐hydroxybutyrate (8.00 mmol/L) and creatinine (734 μM/L), whereas a month prior, her serum creatinine was 64 μM/L. Her serum creatine kinase level was >22 000 U/L (Figure 1). She was admitted to the intensive care unit (ICU), and was thought to have acute kidney injury (AKI) secondary to statin‐induced rhabdomyolysis and volume depletion from euglycaemic diabetic ketoacidosis; her lactic acidosis was thought to have resulted from metformin accumulation/toxicity in the context of the severe AKI. She was started on haemodialysis, an insulin infusion and intravenous fluids, with improvement in her metabolic disturbances. Rosuvastatin was held, as was her metformin, empagliflozin and sitagliptin. She was transferred out of the ICU on post‐admission Day 3, discharged home on Day 10, and haemodialysis discontinued about 1 month later.
FIGURE 1.
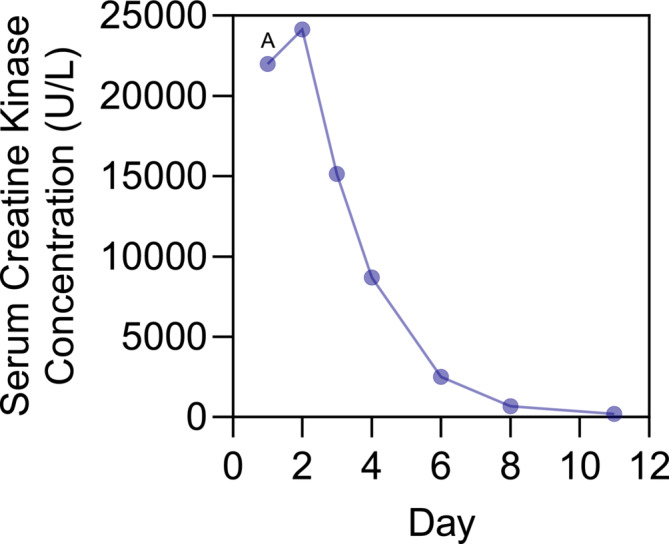
Patient's serum creatine kinase concentration after hospital admission. Plasma concentration of serum creatine kinase (U/L). Day 1 is day presenting to the emergency room. A, exact concentrations of creatine kinase could not be determined on admission to the emergency room and was reported as >22 000 U/L.
Interestingly, 1 month prior, she was briefly admitted with dysarthria and right‐sided weakness, and MR head demonstrated a subacute left parasagittal pontine stroke. She was previously prescribed 10 mg rosuvastatin daily, though she was only taking it perhaps 3 days/week; after her new stroke, rosuvastatin dose was increased to 40 mg daily and she was diligent about compliance.
After the patient recovered from rhabdomyolysis, she was reviewed in our pharmacogenomics (PGx) clinic to better elucidate a potential genetic basis of this statin‐induced rhabdomyolysis. The patient's medication list was reviewed for any potentially relevant drug–drug interactions, and none were identified. Sanger sequencing of the coding and flanking intronic region of SLCO1B1 was performed, using the conditions and primers detailed in Tables S1 and S2. She was found to be heterozygous for four SLCO1B1 single nucleotide variants (SNVs), c.388A>G (*37), c.481+1G>T, c.597C>T and c.1463G>C (*9), of which the minor allele frequency (MAF) and in silico predicted function are reported in Table 1. Additionally, the patient was wildtype for the ABCG2 SNVs c.34G>A (rs2231137) and c.421C>A (rs2231142), which have previously been associated with statin induced toxicity. 1 Note that the patient provided informed consent for pharmacogenomic testing as a part of our study approved by the Health Sciences Research Ethics Board at Western University (REB# 15586). The patient also provided a separate signed consent for this case report.
TABLE 1.
SLCO1B1 genetic variant found in patients with rhabdomyolysis.
| rs number | Nucleotide change | Protein change | CADD score a | Minor allele frequency (MAF) b | ||||
|---|---|---|---|---|---|---|---|---|
| Total | African | European | East Asian | South Asian | ||||
| rs2306283 | c.388A>G (*37) | p.Asn130Asp | 0.546 | 0.4185 | 0.7739 | 0.3709 | 0.7025 | 0.4663 |
| rs77271279 | c.481+1G>T | 33 | 0.0009 | 0.0286 | 0 | 0 | 0.00005 | |
| rs2291075 | c.597C>T | p.Phe199= | 7.10 | 0.3820 | 0.5600 | 0.3867 | 0.4398 | 0.1905 |
| rs59502379 | c.1463G>C (*9) | p.Gly488Ala | 24.2 | 0.0011 | 0.0394 | 0 | 0 | 0.00007 |
Combined Annotation Dependent Depletion (CADD), deleterious variants >20.
Minor Allele Frequency (MAF) from gnomAD exomes, retrieved April 29, 2024.
Additionally, coproporphyrin I (CPI), a known biomarker of SLCO1B1 activity in vivo, 2 , 3 was measured in plasma. The plasma CPI level of this patient was found to be 1.84 ng/mL (2.23 nM). This is relatively higher than previously published ranges in 356 healthy individuals of 0.1–1.6 ng/mL with a geometric mean of 0.57 ng/mL and similar to the geometric mean CPI levels for patients with loss of function SLCO1B1 haplotype *15/*15, 1.65 ng/mL3.
2. DISCUSSION
Statins, including rosuvastatin, are commonly prescribed HMG‐CoA reductase inhibitors that reduce cholesterol production. Although musculoskeletal side effects are common during statin therapy, these represent a spectrum, with severe events such as rhabdomyolysis being rare. 4 A recent narrative report, which included 12 studies, reported the rate of statin‐induced rhabdomyolysis as 0.009% (102/11294770). 5 Rhabdomyolysis results from damage to the skeletal muscle, leading to the release of contents such as creatine kinase (CK), myoglobin and lactate dehydrogenase, which can result in acute kidney injury (AKI). 4
The drug uptake transporter, organic anion‐transporting peptide 1B1 (OATP1B1; encoded by the gene SLCO1B1) is responsible for the uptake of a number of endogenous substrates as well as xenobiotics such as statins into the liver (Figure 2). 6 , 7 Loss of OATP1B1 function, whether due to genetic variation or drug interactions, results in elevated plasma statin levels, thereby increasing the risk for statin‐associated muscle injury. Our laboratory was the first to identify a number of loss‐of‐function genetic variants in SLCO1B1, previously known as OATP‐C, including the variant c.521T>C. 8 This genetic variant is relatively common among patients of European or Asian ancestry, associated with increased plasma statin levels. 4 The estimated plasma exposure of 10 and 40 mg of rosuvastatin is increased by 72 and 117% in patients with c.521C/C compared to wildtype (T/T). 1 Additionally, a GWAS study demonstrated a strong association of c.521T>C variant with statin myopathy in patients on high‐dose simvastatin therapy. 9 This effect has been further confirmed in multiple meta‐analyses. 10 , 11 These findings have led to international guidelines for dosing of statins in patients with SLCO1B1 genetic variation, as a way of reducing the risk for statin‐associated muscle symptoms (SAMS). 1
FIGURE 2.
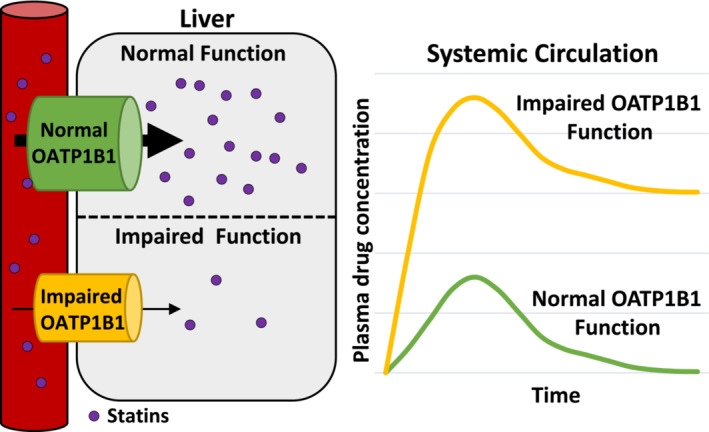
Schematic of OATP1B1 involvement of statins uptake into the liver. Statins are transported into the liver by OATP1B1 (gene name; SLCO1B1). Genetic variants in SLCO1B1 can result in loss or decreased function of OATP1B1 statin transport into the liver, leading to increased plasma statin concentrations.
This patient experienced a rare but life‐threatening rhabdomyolysis shortly after starting 40 mg rosuvastatin on a consistent daily basis. Since the commonly assessed c.521T>C in SLCO1B1 is rare among those of African descent, exon sequencing of SLCO1B1 was performed to identify potentially rare or novel genetic variant(s) in SLCO1B1. In this patient, four SNVs were identified, c.388A>G (*37, formerly called *1b) and c.597C>T, as well as two potentially causal SNVs, c.481+1G>T and c.1463G>C (*9). The SLCO1B1 SNV c.481+1G>T is predicted to result in disrupted splicing, leading to a loss of function protein. Furthermore, this loss of function SNV has previously been reported in patients deficient in OATP1B1/1B3 with Rotor syndrome. 12 Additionally, a recent case of a patient who experienced rhabdomyolysis on high dose atorvastatin (80 mg/day) was found to be heterozygous for c.481+1G>T on a genetic analysis, which was suggested to be the causal variant. 13
Interestingly, c.1463G>C is a genetic variation that our group had first discovered in 2001 from DNA of African Americans, where we demonstrated marked loss of function in vitro due to reduced cell surface trafficking of the encoded OATP1B1 transporter. 8 Remarkably, studies on the association of c.1463G>C on statin‐induced rhabdomyolysis in patients are lacking. In vitro, we had shown that the presence of this SNV led to a marked loss in the cellular uptake of rosuvastatin. 8 , 14 Moreover, the effect of c.1463G>C SNV was comparable to the well‐known loss of function SLCO1B1 variant c.521T>C (*5). 8 , 14 Lastly, a preprint meta‐analysis using two electronic health record‐linked biobanks, which contained 773 patients who self‐identified as Black, concluded that both SLCO1B1 c.481+1G>T (odds ratio [OR] = 3.27, 95% confidence interval [CI] 1.43–7.46) and c.1463G>C (OR = 2.42, 95% CI 1.04–5.78) increased the risk of severe myopathy. 15
Importantly, the allele frequency of both these reported SNVs has been shown to be ethnicity‐specific. The estimated percentage of patients with one allele of c.481+1G>T or c.1463G>C is extremely low at 0.01 and 0.014%, respectively, in Caucasians, but are much more prevalent in patients with African ancestry at 5.72 and 7.9%, respectively. Additionally, it is essential to recognize that many of the most common SLCO1B1 SNVs are more prevalent in patients with African ancestry (Table S3), and yet little research has been carried out in relation to these variants, potentially increasing the risk of severe statin‐associated side effects in this population. Currently, pharmacogenetic guidelines by the Clinical Pharmacogenetics Implementation Consortium (CPIC) for recommendations of statin dosing based on SLCO1B1 SNV are based on studies in largely European or Caucasian populations. 1 Thus, more attention needs to be paid to understanding the clinical importance and functional relevance of SNVs, including those noted in this case study, in other populations. This is in agreement with Yee et al. who suggest that the inclusion of Afrocentric variants into pre‐emptive pharmacogenomic testing would significantly decrease the risk of statin‐associated myopathy and rhabdomyolysis. 15
In conclusion, we report the case of a patient with severe rhabdomyolysis who was later discovered to be heterozygous for two deleterious SLCO1B1 SNVs, c.481+1G>T and c.1463G>C, which are more common in patients with African ancestry. Therefore, future clinical guidelines for genetic variants associated with statin‐associated myopathy or rhabdomyolysis should consider the inclusion of a broader range of SLCO1B1 variants that better reflect all populations.
2.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2023/24.
AUTHOR CONTRIBUTIONS
SEG and RBK were involved in the patient's care. SM and RD carried out targeted gene sequencing and data analysis. SM and RBK drafted the manuscript. All authors approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
Supporting information
Table S1. Primers used for PCR amplification of SLCO1B1 coding exons.
Table S2. Thermocycling conditions for Sanger sequencing preparation.
Table S3. Minor allelic frequency of 10 most common SLCO1B1 single nucleotide variants by ethnicity.
Medwid S, Deckert R, Gryn SE, Kim RB. SLCO1B1 variants in a patient of African ancestry presenting with rosuvastatin‐induced rhabdomyolysis: A case report. Br J Clin Pharmacol. 2025;91(1):232‐235. doi: 10.1111/bcp.16329
Funding information Canadian Institutes of Health Team Grant: Personalized Health (FRN 178435), and Wolfe Medical Research Chair in Pharmacogenomics to R.B.K.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cooper‐DeHoff RM, Niemi M, Ramsey LB, et al. The Clinical Pharmacogenetics Implementation Consortium guideline for SLCO1B1, ABCG2, and CYP2C9 genotypes and statin‐associated musculoskeletal symptoms. Clin Pharmacol Ther. 2022;111(5):1007‐1021. doi: 10.1002/cpt.2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neuvonen M, Tornio A, Hirvensalo P, Backman JT, Niemi M. Performance of plasma coproporphyrin I and III as OATP1B1 biomarkers in humans. Clin Pharmacol Ther. 2021;110(6):1622‐1632. doi: 10.1002/cpt.2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kunze A, Ediage EN, Dillen L, Monshouwer M, Snoeys J. Clinical investigation of coproporphyrins as sensitive biomarkers to predict mild to strong OATP1B‐mediated drug‐drug interactions. Clin Pharmacokinet. 2018;57(12):1559‐1570. doi: 10.1007/s40262-018-0648-3 [DOI] [PubMed] [Google Scholar]
- 4. Turner RM, Pirmohamed M. Statin‐related myotoxicity: a comprehensive review of pharmacokinetic, pharmacogenomic and muscle components. J Clin Med. 2019;9(1):22. doi: 10.3390/jcm9010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Safitri N, Alaina MF, Pitaloka DAE, Abdulah R. A narrative review of statin‐induced rhabdomyolysis: molecular mechanism, risk factors, and management. Drug Healthc Patient Saf. 2021;13:211‐219. doi: 10.2147/DHPS.S333738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim RB. 3‐Hydroxy‐3‐methylglutaryl‐coenzyme A reductase inhibitors (statins) and genetic variability (single nucleotide polymorphisms) in a hepatic drug uptake transporter: what's it all about? Clin Pharmacol Ther. 2004;75(5):381‐385. doi: 10.1016/j.clpt.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 7. Gong IY, Kim RB. Impact of genetic variation in OATP transporters to drug disposition and response. Drug Metab Pharmacokinet. 2013;28(1):4‐18. doi: 10.2133/dmpk.dmpk-12-rv-099 [DOI] [PubMed] [Google Scholar]
- 8. Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP‐C: identification of multiple allelic variants associated with altered transport activity among European‐ and African‐Americans. J Biol Chem. 2001;276(38):35669‐35675. doi: 10.1074/jbc M103792200 [DOI] [PubMed] [Google Scholar]
- 9. Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin‐induced myopathy—a genomewide study. N Engl J Med. 2008;359(8):789‐799. doi: 10.1056/NEJMoa0801936 [DOI] [PubMed] [Google Scholar]
- 10. Carr DF, Francis B, Jorgensen AL, et al. Genomewide association study of statin‐induced myopathy in patients recruited using the UK Clinical Practice Research Datalink. Clin Pharmacol Ther. 2019;106(6):1353‐1361. doi: 10.1002/cpt.1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiang Q, Chen SQ, Ma LY, et al. Association between SLCO1B1 T521C polymorphism and risk of statin‐induced myopathy: a meta‐analysis. Pharmacogenomics J. 2018;18(6):721‐729. doi: 10.1038/s41397-018-0054-0 [DOI] [PubMed] [Google Scholar]
- 12. van de Steeg E, Stránecký V, Hartmannová H, et al. Complete OATP1B1 and OATP1B3 deficiency causes human rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J Clin Invest. 2012;122(2):519‐528. doi: 10.1172/JCI59526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kiage J, Venkatanarayan A, Roth M, Elam M. Atorvastatin‐associated rhabdomyolysis in a patient with a novel variant of the SLCO1B1 gene: a case report. J Clin Lipidol. 2022;16(1):23‐27. doi: 10.1016/j.jacl.2021.11.007 [DOI] [PubMed] [Google Scholar]
- 14. Ho RH, Tirona RG, Leake BF, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130(6):1793‐1806. doi: 10.1053/j.gastro.2006.02.034 [DOI] [PubMed] [Google Scholar]
- 15. Yee SW, Haldar T, Kvale M, Yang J, Douglas MP, Oni‐Orisan A. Functional variants and statin‐induced myopathy in people with recent genealogical ancestors from Africa: a population‐based real‐world study. medRxiv. 2023:2023.12.02.23299324. doi: 10.1101/2023.12.02.23299324 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used for PCR amplification of SLCO1B1 coding exons.
Table S2. Thermocycling conditions for Sanger sequencing preparation.
Table S3. Minor allelic frequency of 10 most common SLCO1B1 single nucleotide variants by ethnicity.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


