Abstract
Background
As a novel blocker of vascular endothelial growth factor receptor (VEGFR), fruquintinib has been approved for treating colorectal cancer (CRC). However, its dosage and therapeutic efficacy are limited by its widespread adverse reactions. Venetoclax, recognized as the initial inhibitor of B-cell lymphoma protein 2 (BCL2), has shown potential in boosting the effectiveness of immunotherapy against CRC. This study investigated the efficacy and mechanisms of fruquintinib combined with venetoclax in treating CRC.
Methods and Materials
We developed a colon cancer mouse model with the CT26 colon cell line to demonstrate fruquintinib and venetoclax’s efficacy against tumors. Then we employed various techniques to evaluate different aspects of the experimental outcomes. Immunohistochemistry was used to detect cell proliferation and angiogenesis in tumor tissues. Western blot analysis was utilized to examine the occurrence of cell apoptosis, and flow cytometry to quantitate immune cells within the tumor tissues. Moreover, immunofluorescence was employed to measure cytokine levels.
Results
The strongest inhibition on tumor growth was achieved by the combination of fruquintinib with venetoclax, as opposed to individual drug use. Venetoclax was found to amplify the impact of fruquintinib, leading to decreased cancer cell proliferation, increased cancer cell apoptosis, lowered angiogenesis, better vascular structure normalization, and improved immune cell infiltration.
Conclusion
Our findings indicate that the addition of venetoclax enhances the impact of fruquintinib on vascular normalization and modulation of the tumor immune microenvironment. Our study presents the justification for utilizing the fruquintinib and venetoclax combination in treating CRC. Venetoclax holds promise in being assimilated into anticancer medications for CRC.
Keywords: Colorectal cancer (CRC), B-cell lymphoma protein 2 (BCL2), Venetoclax, Vascular endothelial growth factor receptor (VEGFR), Fruquintinib, Anti-angiogenesis, Immunotherapy
Introduction
Colorectal cancer (CRC) ranks as the third most common and the second most lethal form of malignant tumors, presenting a significant global health challenge [1]. At the time of diagnosis, about a quarter of CRC patients are already battling metastases, and nearly half will ultimately develop metastatic disease. The prognosis for patients with metastatic colorectal cancer (mCRC) is grim, with a five-year survival rate ranging from a mere 5% to 15% [2]. Systemic therapies are recommended for advanced-stage CRC, typically involving first-line and second-line drugs, such as fluorouracil, oxaliplatin, irinotecan, bevacizumab, and cetuximab [3]. Despite these options, the effectiveness of such treatments is significantly hampered by the emergence of drug resistance and the occurrence of adverse reactions, often leading to the recurrence and progression of metastasis.
In recent years, the introduction of immune checkpoint inhibitors targeting the programmed death receptor 1 (PD-1)/programmed cell death ligand 1 (PD-L1) axis and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) has broadened the spectrum of therapeutic strategies for CRC, notably for its metastatic variant (mCRC). Anti-PD-1 shows its superiority in dealing with tumors displaying the microsatellite instability-high (MSI-H) phenotype, a characteristic found in only 4–6% of cases of advanced colorectal cancer. Patients with microsatellite stable (MSS) mCRC have not shown favorable responses to monotherapy with these immunotherapies in previous clinical trials [4–6]. Moreover, after undergoing more than three-line therapies, many patients experience a marked deterioration in their physical well-being, rendering them unable to continue with prolonged treatment regimens. Consequently, there is a pressing need to develop later-line treatment options for mCRC that not only minimize adverse effects but also enhance therapeutic efficacy.
Currently, the standard third-line drugs recommended for mCRC include regorafenib, fruquintinib, and TAS-102 [7–9]. Fruquinitinib, an oral tyrosine kinase inhibitor (TKI), is highly selective in inhibiting VEGFR 1, 2, and 3. Fruquinitinib can effectively suppress VEGFR phosphorylation, which in turn blocks tumor angiogenesis and tumor growth. Fruquintinib has received approval from the United States Food & Drug Administration and is endorsed by the National Comprehensive Cancer Network guidelines for the management of mCRC in adults who have previously received a fluoropyrimidine, oxaliplatin, and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if having a RAS wild type and being medically suitable, an anti-EGFR therapy. Many clinical studies have shown that fruquintinib monotherapy can effectively prolong the survival time of patients with advanced CRC [7,10]. Fruquintinib also enhances the antitumor immune response against PD-1 in CRC [11–13].
Programmed cell death, a complex cellular dynamics, is involved in antitumor immune responses. The family of B-cell lymphoma protein 2 (BCL2) regulates apoptotic signaling pathways enriched in immune cell development, homeostasis, and responses [14]. Apoptosis, a key mechanism within programmed cell death, is vital for maintaining tissue development. Any disruption in cell apoptosis may trigger the progression of tumors [15]. Members of the BCL2 gene family, owing to their close tie with cell apoptosis, have slipped into the research hotspot. Some BCL2 members can effectively inhibit programmed apoptosis in cells and then induce malignant tumors [16]. BCL2, cloned from the breakpoint of the human B-cell lymphoma translocation, is the first identified family member [17]. Numerous studies have indicated that BCL2 is differentially expressed in human tumors, exerting a direct or indirect impact on the proliferation, migration, invasion, and development of tumor cells at different stages of apoptosis [18]. Studies have shown that BCL2 expression is linked to cancer progression, specifically liver metastases of CRC [19].
Venetoclax, also known by its names ABT-199, Venclexta, and Venclyxto, is an orally administered, highly selective BCL2 inhibitor capable of triggering tumor cell apoptosis. The US Food and Drug Administration has endorsed venetoclax for treating chronic lymphocytic leukemia and acute myeloid leukemia [20]. Venetoclax can selectively target to inhibit BCL2, restore the apoptotic process, replace pro-apoptotic proteins, and impart cytotoxicity on BCL2-overexpressed tumor cells [21,22], ultimately contributing to the killing of tumor cells [23]. Encouraging efficacy has been achieved by therapies combining multiple BCL2 family inhibitors simultaneously, or BCL2 family inhibitors with standard anticancer drugs. Currently, venetoclax is most preferred for blood system diseases such as leukemia; however, relevant clinical trials for solid tumors, such as small cell lung cancer, colorectal cancer, and breast cancer, are also underway.
In our study, a syngeneic CRC model was developed to evaluate the effectiveness of combining a BCL2 inhibitor with an antiangiogenic drug. The therapeutic mechanism related to immunosuppressive tumor microenvironment was also elucidated. This combination might serve as a therapeutic strategy for CRC.
Materials and Methods
Reagents
Fruquintinib used in this study was supplied generously by Hutchison MediPharma Limited (Shanghai, China). Venetoclax was purchased from AbbVie Inc. (North Chicago, Illinois, USA). The TUNEL Cell Apoptosis Detection Kit (G1501-50) was obtained from Servicebio Technology (Wuhan, Hubei, China). CD45-BV421 (103134), CD4-PE/Dazzle 594 (100456), CD8-FITC (100706), and IFN-γ-APC (505809) used for flow cytometry were obtained from Biolegend (San Diego, California, USA). In the immunohistochemistry and immunofluorescence, antibodies were used as follows: anti-PCNA (sc-56; Santa Cruz, California, USA), anti-CD31 (28083-1-AP; Proteintech, Rosemont, Chicago, USA), anti-α-SMA (55135-1-AP; Proteintech), anti-Ki67 (343569; Abmart, Shanghai, China), anti-granzyme B (10345; Sino Biological, Beijing, China), and anti-IFN-γ (105995; Sino Biological). For immunofluorescence, the secondary antibody used was CoraLite488-conjugated goat anti-rabbit IgG (SA00013-2) from Proteintech. Immunohistochemistry (GK500705) was operated on the GTVision kit (GeneTech Company, Shanghai, China). The monoclonal antibodies (mAbs) for western blot analysis included Cleaved Caspase 3 (AC030-1; Beyotime, Shanghai, China), β-actin (T0022; Affinity Biosciences, Changzhou, Jiangsu, China), anti-mouse IgG (H+L) (5220-0341; Milford, Massachusetts, USA) and anti-rabbit IgG (H+L) (5220-0336; Milford). Other reagents for our experiments were purchased from Beyotime.
Cell culture
The CT26 cells were sourced from the Shanghai Institute of Cell Biology and cultured in RPMI 1640 medium (11875-093, Gibco) containing 10% heat-inactivated fetal bovine serum (C04001-500, Vivacell), penicillin (100 U/mL), and streptomycin (C0222, Beyotime, China) (100 mg/mL) at 37°C in a 5% CO2 and humidified environment. Cell quality analysis for the CT26 cells was performed by a third-party company (Jiangsu KeyGEN BioTECH Corp., Ltd). For mycoplasma testing, the Certificate of Analysis for CT26 cells indicated that the cells did not have mycoplasma infection.
Animal model establishment
24 Seven-week-old BALB/c mice were acquired from SPF BIOtechnology Co. LTD in Suzhou, Jiangsu, China, kept in plastic cages, and given free access to pellet food and water at 21°C ± 2°C in a 12-h light/dark cycle. The animal experiment protocol was approved by the Animal Experiment Ethical Review Committee of Nanjing Medical University, China (Approval number: IACUC-2210010). All animal experiments were conducted according to the guidelines of the Animal Experimentation Center and under the review and supervision of Nanjing Medical University (Approval number: SYXK2023-0029). Minimized animals were used and their suffering was maximally controlled. Tumors were induced by inoculation of CT26 cells (1 × 106) into the right flank of BALB/c mice for three days. After the tumor reached 50 mm3 in volume, the mice were split into four groups with 6 mice in each group. Fruquintinib dosed at 1.5 mg/kg was dissolved in 0.9% sodium carboxymethyl cellulose and given once daily, while venetoclax dosed at 50 mg/kg was dissolved in 0.9% sodium carboxymethyl cellulose and also given once daily. Both drugs, 100 μL each, were simultaneously used in the combination group. The vehicle group received 100 μL of 0.9% sodium carboxymethyl cellulose only. Tumor volume was calculated by a ∗ b ∗ b ∗ 0.5, where “a” represents the maximal orthogonal length and “b” represents the maximal orthogonal width, which was measured every two days using a vernier caliper. After delivery of drugs for 13 days. The mice were weighed and euthanized on the 13th day, and their tumors were removed and weighed.
Preparation of tumor-infiltrating cells and intracellular staining
Levels of tumor-infiltrating cells in each group were measured as previously reported [24]. The mouse tumor tissues were harvested, followed by mechanical and enzymatical dissociation using a dissociation kit (130-096-730, Miltenyi Biotec, Germany). The suspensions were filtered through a 70-μm cell strainer. Single-cell suspensions were labeled with different surface monoclonal antibodies, such as anti-CD45 conjugated with BV421, anti-CD4 conjugated with PE/Dazzle 594, and anti-CD8 conjugated with FITC. To analyze IFN-γ, the cells were pre-cultured in 24-well, flat-bottom plates with a stimulation mixture under optimized conditions. Then the cells were fixed and permeabilized. After permeabilization, the cells were labeled with intracellular markers of APC-IFN-γ. At the end of staining, cells were washed once using PBS and then flow cytometry was performed.
Western blot analysis
Proteins were extracted from whole tumor tissues using RIPA lysis (P0013B, Beyotime, Shanghai, China) buffer mixed with protease inhibitor cocktail and kept on ice. Proteins in an equal amount determinated by a BCA protein assay kit (23228, Thermo, USA) were isolated using SDS-PAGE, and then shifted to polyvinylidene difluoride membranes, which were then blocked with 3% BSA (B0012-100, SunShine Bio, Nanjing, China), incubated with primary antibodies (1:1000) at 4°C overnight, and then with secondary antibodies (1:10000, RT). Later on, immunoreactive bands were identified by using an ECL kit (180-506, Tanon, Shanghai, China), following the guidelines provided by the manufacturer.
Hematoxylin and eosin (H&E) staining, immunohistochemistry (IHC), immunofluorescence (IF) and TUNEL assay
After deparaffinization, rehydration, and rinsing with 1% PBS-Tween 20, the sections were subjected to IHC, in which hydrogen peroxide (2%) was introduced to block endogenous peroxidases, followed by blocking with goat serum (C0265, Beyotime, Shanghai, China) (3%), as well as incubation with specific primary Abs (1:200) at 4°C overnight. After another round of incubation with streptavidin–HRP for 40 min, the sections were first stained with diaminobenzidine substrate and then counter-stained with hematoxylin. Afterward, immunofluorescence and TUNEL were performed by staining the sections with fluorescence-labeled TUNEL-FITC (1:100), followed by counter-staining with DAPI (C1002, Beyotime, Shanghai, China) for 5 min. Imaging was done using light microscopy and fluorescence microscopy (BX51, Olympus, Tokyo, Japan).
Statistical analysis
All statistical analyses were conducted using GraphPad Prism version 9.0 (La Jolla, CA, USA). Results were expressed as mean±SEM, with significance set at p < 0.05. Two-tailed Student’s t-test was used for analyzing continuous variables. Wilcoxon test was employed for comparing two groups, while the Kruskal-Wallis test was utilized for evaluating differences among three groups. Non-repeated measures ANOVA followed by Scheffe test was applied for cases with more than three groups.
Results
The growth of transplanted CRC in mice was significantly inhibited by combining venetoclax with fruquintinib
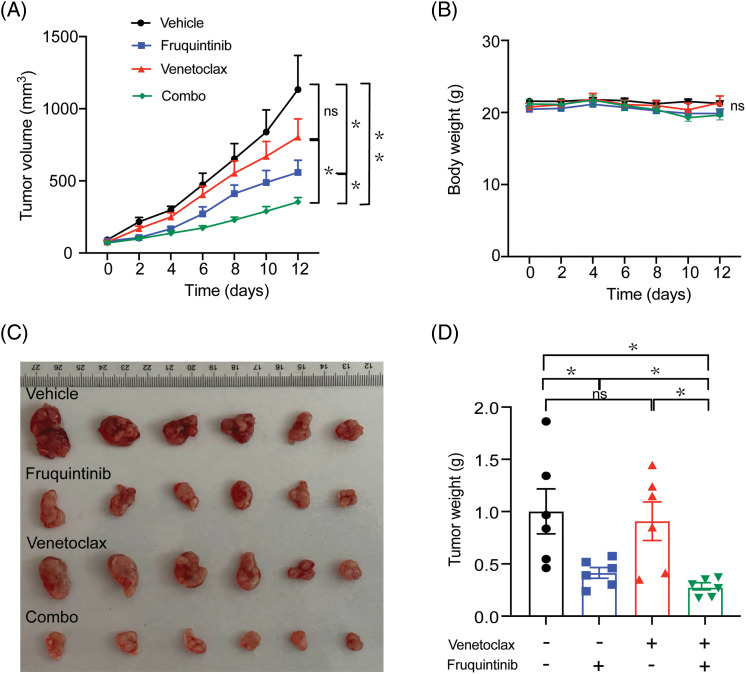
A xenograft murine model was established by transplanting CT26 mouse CRC cells subcutaneously. Venetoclax was delivered at a dose of 50 mg/kg, and fruquintinib at a dose of 1.5 mg/kg, both orally once every day. Mice treated with the combination of venetoclax with fruquintinib exhibited slower tumor growth and a lower tumor weight, compared to the control group (Figs. 1A, 1C, and 1D). Notably, fruquintinib reduced tumor weight by 50.7%, venetoclax by 29.1%, and their combination by 68.76%. During the administration period, there was no significant change in body weight observed in all the combination groups (Fig. 1B). This suggested that the combination of venetoclax and fruquintinib had little to no side effects in the mice.
Figure 1. The growth of transplanted tumors in mice was significantly inhibited by the combination of fruquintinib with venetoclax. The weight of the bodies and volumes of tumors were measured every other day (A and B). Following sacrifice, solid tumors from the mouse were isolated, photographed, and weighed (C and D). Results from six mice per group were indicated as mean ± SEM. *p < 0.05, **p < 0.01 compared to indicated values. ns, not significant.
A combination of venetoclax with fruquintinib inhibited the proliferation of CRC cells in vivo
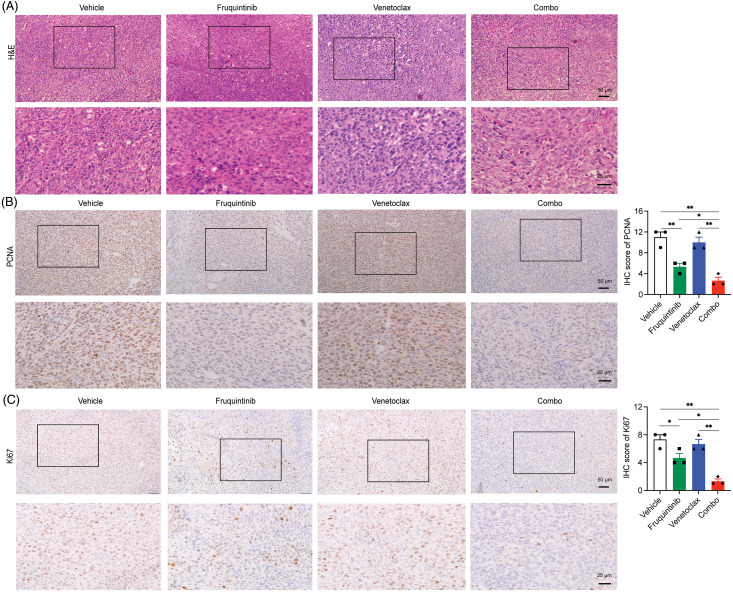
H&E staining revealed that the combination distorted tumor cell morphology significantly, including nuclear shrinkage, sparse arrangement, and cell fragmentation (Fig. 2A). Through further immunohistochemical analysis, the protein levels of PCNA and Ki67 in the tumor tissues were lower in the combination group than in the fruquintinib single-agent group (Figs. 2B and 2C). These findings confirmed that the addition of venetoclax enhanced the tumor-inhibitory effect of fruquintinib.
Figure 2. Combination of venetoclax with fruquintinib inhibited the proliferation of tumor cells in vivo. (A) Tumor tissues were examined using H&E staining on paraffin sections. (B) The assessment of PCNA-positive cells was conducted via IHC with ImageJ in three random fields. (C) We analyzed the quantification of Ki67-positive cells through immunohistochemistry (IHC) with ImageJ in three random fields. The data of three mice per group were indicated as the mean ± SEM. *p < 0.05, **p < 0.01, vs. as indicated.
Venetoclax enhanced the effects of fruquininib on inhibiting tumor neovascularization and inducing apoptosis of cancer cells in vivo
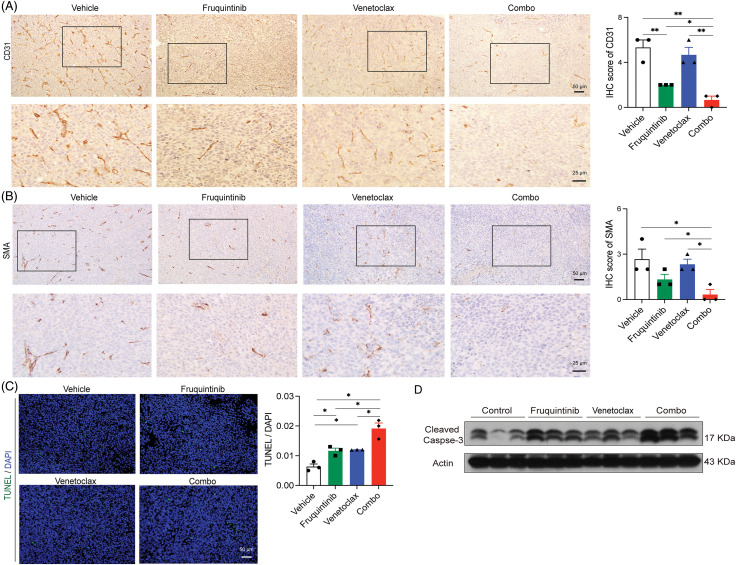
Next, we investigated whether venetoclax can enhance the effect of VEGFR-targeting fruquintinib. IHC was performed to detect the PECAM-1 (CD31) and α-SMA expression. PECAM-1 (CD31) is a tumor neovascularization marker and α-SMA is a vascular smooth muscle cell marker in mouse colon cancer tissues. The results showed that although fruquintinib itself has a good anti-angiogenic effect, the addition of venetoclax further reduced the level of PECAM-1 (CD31) and reduced the expression of α-SMA (Figs. 3A and 3B). Furthermore, TUNEL staining and Western blot analysis of cleaved Caspase 3 confirmed that the combination inflicted tumor cells with extensive apoptosis (Figs. 3C and 3D). These findings suggested that the addition of venetoclax enhanced the anti-angiogenic effects of fruquintinib and induced cancer cell apoptosis in mouse CRC tissues.
Figure 3. The addition of venetoclax promoted the antiangiogenic effect of fuquintinib, and the combination of both made the effect of promoting apoptosis superimposed. (A) IHC-examined expression of PECAM-1 (CD31) in CT26 tumor tissues was examined by IHC. (B) IHC-examined expression of α-SMA in CT26 tumor tissues was examined by IHC. (C) TUNEL staining of CT26 tumor tissues. (D) Western blot analysis of cleaved-Caspase 3. *p < 0.05.
Venetoclax promoted the anti-tumor immune function of fruquininib
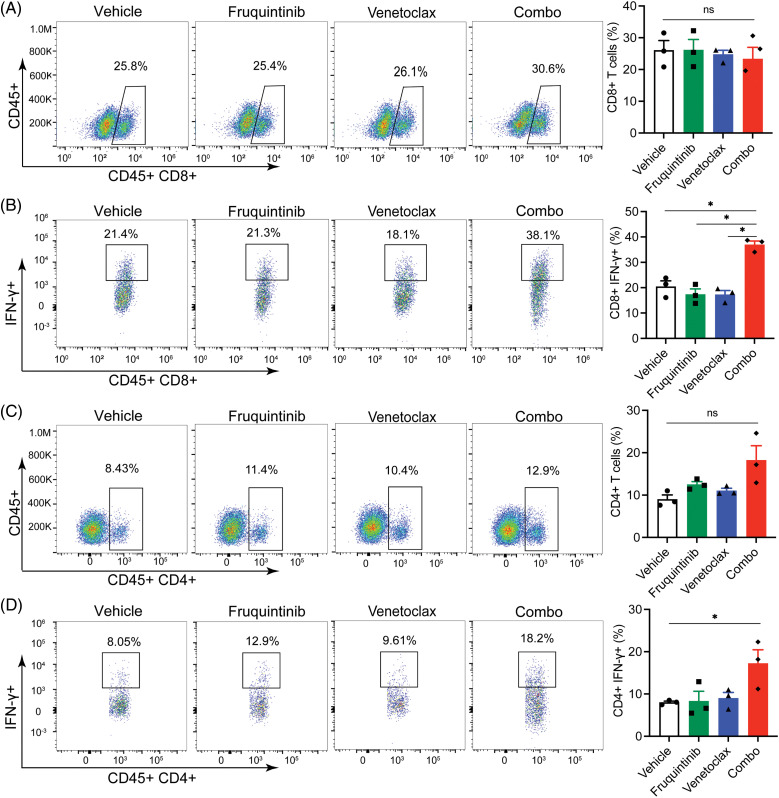
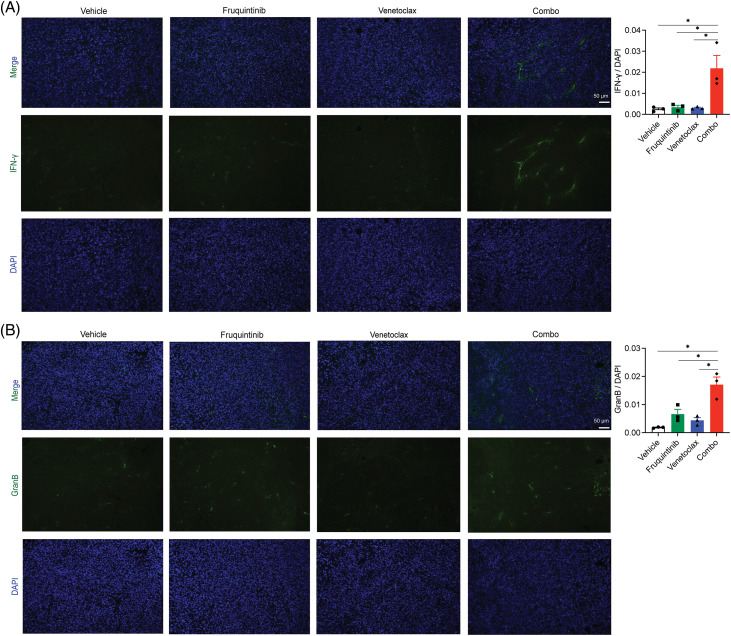
We further profiled the abundance and functions of immune cells within tumor tissues. No significant changes were witnessed in the levels of CD45+CD8+ T cells and CD45+CD4+ T cells (Figs. 4A and 4C), but those of CD45+CD8+IFN-γ+ T cells and CD45+CD4+IFN-γ+ T cells increased obviously (Figs. 4B and 4D), indicating activation of these immune cells in the tumor microenvironment (TME) upon the induction from the combination of venetoclax and fruquintinib. Furthermore, the abundance of markers of cytotoxic lymphocytes, such as IFN-γ and granzyme B, rose in the fruquintinib-treated mice and peaked after administration of both drugs (Figs. 5A and 5B). The findings suggested that the co-treatment of venetoclax and fruquintinib might provoke immune activation in the TME to realize its antineoplastic effects.
Figure 4. Combination of venetoclax with fruquintinib substantially activated CD4+ and CD8+ cells in tumor tissues. Percentages of CD45+CD8+ (A) and CD45+CD4+ T cells (C) in a viable lymphocyte gate. Counts of CD45+CD8+IFN-γ+ (B) and CD45+CD4+IFN-γ+ T cells (D) in a viable lymphocyte gate. The data of three mice per group were indicated as the mean ± SEM. *p < 0.05, vs. as indicated. ns, not significant.
Figure 5. Venetoclax increased the expression of IFN-γ and Granzyme B caused by fruquintinib. Representative images of IFN-γ (A) and Granzyme B (B) (green) immunostaining and DAPI nuclear staining (blue) in tumor tissues. The data were expressed as the mean ± SEM of three mice per group. *p < 0.05.
Discussion
MSS-CRC is the most common type of tumor in both nonmetastatic and metastatic situations. Thus far, single use of anti-PD-1/PD-L1 has never demonstrated effectiveness in treating these tumors. Fruquintinib, a blocker of VEGFR, commonly utilized in the treatment of mCRC, restores the vessels of tumors in a dependent manner of dose and time, which is a crucial factor in the impact of combining fruquintinib with cytotoxic medications. Several international and multicentre clinical studies showed that treatment with fruquinib demonstrated a notable and important improvement in survival outcomes among individuals diagnosed with advanced colorectal cancer who did not respond to previous therapies [7,10,25]. Studies have demonstrated that the concomitant administration of fruquintinib and an anti-PD-1 antibody powerfully inhibits CRC growth [11,26]. This combination works by modifying the tumor immune microenvironment, ultimately supporting its use in the treatment of advanced CRC. However, fruquintinib presents a variety of adverse drug reactions, including hypertension, hand and foot skin reaction (HFSR), diarrhea, and thrombocytopenia, which are frequently observed in clinical practice [7,25]. Although these adverse reactions are relatively controllable, it is not easy to improve the efficacy of fruquintinib by increasing the dose.
Apoptosis is a meticulously regulated fundamental cellular process that takes place in both physiological and pathological situations, aiding in the removal of abnormal cells to uphold homeostasis. Apoptosis is also a crucial process in immune system development and self-regulation. Traditionally viewed as an active biological mechanism, apoptosis has been a focal point in cancer research for prevention and treatment [27,28]. Oncoprotein BCL2 is essential for tumor development and the activation of the immune system [14,29,30]. Aside from hematopoietic malignancies, the BCL2 gene exhibits activity in various types of solid tumors, including but not limited to breast cancer, lung cancer, pancreatic cancer, and sarcoma. Therefore, BCL2 inhibitors may be manipulated to treat solid tumors. In recent years, BCL2 inhibitors have made great progress in the treatment of hematologic malignancies. However, the effectiveness of monotherapy with BCL2 inhibitors in solid tumors is currently limited, with the majority of studies still in the preclinical stage. Therefore, it is imperative to assess the potential of combination therapy as a promising future direction [31]. Our study indicates that combining the BCL2 inhibitor venetoclax with fruquintinib could enhance immune responses in the TME, potentially inhibiting CRC progression. It was reported that venetoclax could enhance antitumor T-cell responses when used in combination with immune checkpoint inhibitors [32]. Meanwhile, combinations of BCL2 inhibitors with other anticancer drugs have also been reported. For example, a combination of BCL2 inhibitor Navitoclax with a third-generation EGFR inhibitor osimertinib has demonstrated high safety and feasibility in patients with advanced EGFR-mutated lung cancer [33]. In another study, introducing BCL2 inhibitors into chemotherapy achieves favorable effects in patients with esophageal cancer, suggesting their potential to be widely used in solid tumor treatments. In vitro experiments have shown that BCL2 inhibitor AT-101 can reduce tumor cell proliferation and enhance the apoptotic effect of docetaxel. Additionally, preclinical and clinical data suggest that AT-101 can repress tumor cell proliferation and promote docetaxel-induced apoptosis, potentially overcoming resistance in gastroesophageal cancers [34]. Studies have shown that increased expression of BCL2 comes with T-cell activation and cellular adhesion [35]. BCL2 is implicated in regulating T-cell homeostasis and effector responses [14]. Mouse model studies show that BCL2 knockout reduces lymphocyte counts, but effector lymphocytes, once survived, can still proliferate upon immune stimulation, indicating BCL2’s partial role in immune responses [36,37]. However, the role of BCL2 in these processes remains controversial. In the mouse model, venetoclax enhances the ability of immune checkpoint inhibitors in fighting against tumors and increases PD-1+ T memory effector cell levels without impairing T cell function or antagonizing anti-PD-1-induced activation. Estrogen receptor (ER)-positive breast cancer is the first solid tumor type to undergo clinical trials with venetoclax. In 2013, venetoclax was found to significantly improve tumor responses to tamoxifen and mitigate specific adverse effects of tamoxifen in xenografts of ER-positive breast cancer [38]. In a study on small cell lung cancer (SCLC) characterized by high invasiveness and a poor prognosis, researchers found that the upregulation of BCL2 expression was common and could be used as a predictor of drug sensitivity [39]. Another study found that the combination of the BET inhibitor ABBV-075 and venetoclax showed a strong synergistic inhibitory effect on SCLC, and this effect was positively correlated with the expression of BCL2 [40].
The TME consists of tumor cells, extracellular matrix, immune cells, cytokines, blood vessels, and lymphatic networks. Tumor cells are a key component of the TME, serving as both the main body of the tumor and the initiator of tumor development. Tumor cells exhibit characteristics such as excessive proliferation and inhibition of apoptosis [41,42]. Excessive proliferation often leads to hypoxia, mitochondrial damage, and the expression of Reactive oxygen species (ROS) and transcription factor 1α (HIF-1α). HIF-1α triggers the production of pro-angiogenic factors like VEGF, promoting new blood vessel formation, as well as transcription activation of TGF-β3 and epidermal growth factor (EGF) to facilitate tumor metastasis to oxygen-rich tissues. Additionally, the migration ability of tumor cells can promote local invasion, lymphatic migration, or blood migration [43]. Metabolically, tumor cells primarily rely on aerobic glycolysis, generating lactic acid and creating an acidic microenvironment that suppresses the immune response, further enhancing tumor metastasis and recurrence. Early in 1998, a study suggested that BCL2 possibly controls the development of tumor angiogenesis with putative mediation by VEGF in lung cancer [44]. A study demonstrated that increased levels of HIF-1α are linked to elevated levels of BCL-xl, a member of the BCL2 family, primarily as a result of its anti-apoptotic characteristics, leading to the development of resistance to chemotherapy and radiation [45].
It has been reported that BCL2 expression in lymphoma cells induces hypoxia-inducible factor-1 alpha (HIF1α)-mediated expression of VEGF, an angiogenic factor that promotes endothelial cell proliferation and migration. There was evidence that BCL2 prevents HIF-1α degradation in a contact-dependent process under non-hypoxic conditions, and this binding promotes dimerization of HIF-1α and HIF-1β, subsequently stimulating through interactions facilitated by the BH4 region, the physical connection between BCL2 and HIF-1α serves to underscore the potential impact of BCL2 mutations on the signaling pathways of HIF-1α and VEGF [46]. The specific mechanism of venetoclax combined with fruquintinib was not thoroughly researched in our study, and the combination of the two drugs is expected to be further explored in clinical practice in the future.
In summary, there is currently no regimen for the combined use of antiangiogenic drugs and BCL2 inhibitors. Our study examined the effects of venetoclax and assessed its combined efficacy with the VEGFR inhibitor fruquintinib in treating CRC [47]. Our findings showed that combining venetoclax with fruquintinib more effectively inhibited tumor growth, reduced tumor cell proliferation, and induced apoptosis than venetoclax alone, while also hindering new blood vessel formation fruquintinib alone. Although the combination therapy did not significantly increase T cell infiltration, it enhanced T lymphocyte activity. VEGFR inhibitors block angiogenesis, and nutrient delivery to tumors, and induce cancer cell death and tumor antigen release. The addition of venetoclax promotes these effects. BCL2 may play a dual role in tumor immunotherapy. On the one hand, it may be an anti-immune factor of the tumor, and on the other hand, it may be a potential target of immunotherapy, which can enhance the effect of immunotherapy by inhibiting its function. Together, our findings and previous studies indicate that combining a BCL2 inhibitor with a VEGFR inhibitor could be a promising strategy for CRC treatment.
Conclusion
This is the first time that the addition of venetoclax enhances the impact of fruquintinib on vascular normalization and modulation of the tumor immune microenvironment. This study presents the justification for utilizing the fruquintinib and venetoclax combination in treating CRC. Venetoclax may potentially be integrated into anticancer therapies.
Acknowledgments
Not applicable.
Contributor Information
WENJIE GUO, Email: guowj@nju.edu.cn.
YANHONG GU, Email: guyhphd@163.com.
Funding Statement
This study was supported by the National Natural Science Foundation of China (82072675, 82273197, 82173933). Fundamental Research Funds for the Central Universities (020814380160).
Author Contributions
The authors confirm contribution to the paper as follows: study conception and design: Yanhong Gu, Wenjie Guo; data collection: Wei Zhang, Weicheng Wang, Rui Wang; analysis and interpretation of results: Weicheng Wang, Xiao Han; draft manuscript preparation: Wei Zhang, Lijun Zhu. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
Data are available upon reasonable request.
Ethics Approval
The animal experiment protocol was approved by the Animal Experiment Ethical Review Committee of Nanjing Medical University, China (Approval number: IACUC-2210010). All animal experiments were conducted according to the guidelines of the Animal Experimentation Center and under the review and supervision of Nanjing Medical University (Approval number: SYXK2023-0029).
Conflicts of Interest
The authors declare no conflicts of interest to report regarding the present study.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708; [DOI] [PubMed] [Google Scholar]
- 2.Bonney GK, Chew CA, Lodge P, Hubbard J, Halazun KJ, Trunecka P, et al. Liver transplantation for non-resectable colorectal liver metastases: the International Hepato-Pancreato-Biliary Association consensus guidelines. Lancet Gastroenterol Hepatol. 2021;6(11):933–46. doi: 10.1016/S2468-1253(21)00219-3; [DOI] [PubMed] [Google Scholar]
- 3.Rouanet P, Rullier E, Lelong B, Maingon P, Tuech JJ, Pezet D, et al. Tailored treatment strategy for locally advanced rectal carcinoma based on the tumor response to induction chemotherapy: preliminary results of the French phase II multicenter GRECCAR4 trial. Dis Colon Rectum. 2017;60(7):653–63. doi: 10.1097/DCR.0000000000000849; [DOI] [PubMed] [Google Scholar]
- 4.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Neil BH, Wallmark JM, Lorente D, Elez E, Raimbourg J, Gomez-Roca C, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12(12):e0189848. doi: 10.1371/journal.pone.0189848; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Wang S, Desai J, Trapani JA, Neeson PJ. Therapeutic strategies to remodel immunologically cold tumors. Clin Transl Immunol. 2020;9(12):e1226. doi: 10.1002/cti2.v9.12; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. 2018;319(24):2486–96. doi: 10.1001/jama.2018.7855; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437–48. doi: 10.1016/S1470-2045(18)30739-3; [DOI] [PubMed] [Google Scholar]
- 9.Grothey A, van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12. doi: 10.1016/S0140-6736(12)61900-X; [DOI] [PubMed] [Google Scholar]
- 10.Lavacchi D, Roviello G, Guidolin A, Romano S, Venturini J, Caliman E, et al. Evaluation of fruquintinib in the continuum of care of patients with colorectal cancer. Int J Mol Sci. 2023;24(6):5840. doi: 10.3390/ijms24065840; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Wei B, Gao J, Cai X, Xu L, Zhong H, et al. Combination of fruquintinib and anti-PD-1 for the treatment of colorectal cancer. J Immunol. 2020;205(10):2905–15. doi: 10.4049/jimmunol.2000463; [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Cheng X, Zhou C, Tang Y, Li F, Zhang B, et al. Fruquintinib enhances the antitumor immune responses of anti-programmed death receptor-1 in colorectal cancer. Front Oncol. 2022;12:841977. doi: 10.3389/fonc.2022.841977; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gou M, Qian N, Zhang Y, Yan H, Si H, Wang Z, et al. Fruquintinib in combination with PD-1 inhibitors in patients with refractory non-MSI-H/pMMR metastatic colorectal cancer: a real-world study in China. Front Oncol. 2022;12:851756. doi: 10.3389/fonc.2022.851756; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsden VS, Strasser A. Control of apoptosis in the immune system: bcl-2, BH3-only proteins and more. Annu Rev Immunol. 2003;21:71–105. doi: 10.1146/immunol.2003.21.issue-1. [DOI] [PubMed] [Google Scholar]
- 15.Liu PF, Hu YC, Kang BH, Tseng YK, Wu PC, Liang CC, et al. Expression levels of cleaved caspase-3 and caspase-3 in tumorigenesis and prognosis of oral tongue squamous cell carcinoma. PLoS One. 2017;12(7):e0180620. doi: 10.1371/journal.pone.0180620; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bleicken S, Hantusch A, Das KK, Frickey T, Garcia-Saez AJ. Quantitative interactome of a membrane Bcl-2 network identifies a hierarchy of complexes for apoptosis regulation. Nat Commun. 2017;8(1):73. doi: 10.1038/s41467-017-00086-6; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226(4678):1097–9. doi: 10.1126/science.6093263; [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20(3):175–93. doi: 10.1038/s41580-018-0089-8; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melucci E, Cosimelli M, Carpanese L, Pizzi G, Izzo F, Fiore F, et al. Decrease of survivin, p53 and Bcl-2 expression in chemorefractory colorectal liver metastases may be predictive of radiosensivity radiosensivity after radioembolization with yttrium-90 resin microspheres. J Exp Clin Cancer Res. 2013;32(1):13. doi: 10.1186/1756-9966-32-13; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diepstraten ST, Anderson MA, Czabotar PE, Lessene G, Strasser A, Kelly GL. The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat Rev Cancer. 2022;22(1):45–64. doi: 10.1038/s41568-021-00407-4; [DOI] [PubMed] [Google Scholar]
- 21.King AC, Peterson TJ, Horvat TZ, Rodriguez M, Tang LA. Venetoclax: a first-in-class oral BCL-2 inhibitor for the management of lymphoid malignancies. Ann Pharmacother. 2017;51(5):410–6. doi: 10.1177/1060028016685803; [DOI] [PubMed] [Google Scholar]
- 22.Mihalyova J, Jelinek T, Growkova K, Hrdinka M, Simicek M, Hajek R. Venetoclax: a new wave in hematooncology. Exp Hematol. 2018;61:10–25. doi: 10.1016/j.exphem.2018.02.002; [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Yang Q, Zha J, Deng M, Zhou Y, Fu G, et al. Preclinical evaluation of a regimen combining chidamide and ABT-199 in acute myeloid leukemia. Cell Death Dis. 2020;11(9):778. doi: 10.1038/s41419-020-02972-2; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Cai P, Li X, Wu X, Gao J, Liu W, et al. Inhibition of NLRP3 inflammasome activation in myeloid-derived suppressor cells by andrographolide sulfonate contributes to 5-FU sensitization in mice. Toxicol Appl Pharmacol. 2021;428:115672. doi: 10.1016/j.taap.2021.115672; [DOI] [PubMed] [Google Scholar]
- 25.Dasari A, Lonardi S, Garcia-Carbonero R, Elez E, Yoshino T, Sobrero A, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet. 2023;402(10395):41–53. doi: 10.1016/S0140-6736(23)00772-9; [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Zhang W, Ying J, Zhang Y, Pan Y, Qiu W, et al. Phase 1b/2 trial of fruquintinib plus sintilimab in treating advanced solid tumours: the dose-escalation and metastatic colorectal cancer cohort in the dose-expansion phases. Eur J Cancer. 2023;181:26–37. doi: 10.1016/j.ejca.2022.12.004; [DOI] [PubMed] [Google Scholar]
- 27.Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7(1):196. doi: 10.1038/s41392-022-01046-3; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang R, Xu J, Zhang B, Liu J, Liang C, Hua J, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13(1):110. doi: 10.1186/s13045-020-00946-7; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5(3):189–200. doi: 10.1038/nri1568; [DOI] [PubMed] [Google Scholar]
- 30.Lee YG, Guruprasad P, Ghilardi G, Pajarillo R, Sauter CT, Patel R, et al. Modulation of BCL-2 in both T cells and tumor cells to enhance chimeric antigen receptor T-cell immunotherapy against cancer. Cancer Discov. 2022;12(10):2372–91. doi: 10.1158/2159-8290.CD-21-1026; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Dong X, Huang DCS, Xu P, Zhao Q, Chen B. Current advances and future strategies for BCL-2 inhibitors: potent weapons against cancers. Cancers. 2023;15(20):4957. doi: 10.3390/cancers15204957; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohlhapp FJ, Haribhai D, Mathew R, Duggan R, Ellis PA, Wang R, et al. Venetoclax increases intratumoral effector T cells and antitumor efficacy in combination with immune checkpoint blockade. Cancer Discov. 2021;11(1):68–79. doi: 10.1158/2159-8290.CD-19-0759; [DOI] [PubMed] [Google Scholar]
- 33.Bertino EM, Gentzler RD, Clifford S, Kolesar J, Muzikansky A, Haura EB, et al. Phase IB study of osimertinib in combination with navitoclax in EGFR-mutant NSCLC following resistance to initial EGFR therapy (ETCTN 9903). Clin Cancer Res. 2021;27(6):1604–11. doi: 10.1158/1078-0432.CCR-20-4084; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song S, Chen Q, Li Y, Lei G, Scott A, Huo L, et al. Targeting cancer stem cells with a pan-BCL-2 inhibitor in preclinical and clinical settings in patients with gastroesophageal carcinoma. Gut. 2021;70(12):2238–48. doi: 10.1136/gutjnl-2020-321175; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L, Cheng X, Yang H, Lian S, Jiang Y, Liang J, et al. BCL-2 expression promotes immunosuppression in chronic lymphocytic leukemia by enhancing regulatory T cell differentiation and cytotoxic T cell exhaustion. Mol Cancer. 2022;21(1):59. doi: 10.1186/s12943-022-01516-w; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakayama K, Nakayama K, Negishi I, Kuida K, Sawa H, Loh DY. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci U S A. 1994;91(9):3700–4. doi: 10.1073/pnas.91.9.3700; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204(7):1665–75. doi: 10.1084/jem.20070618; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaillant F, Merino D, Lee L, Breslin K, Pal B, Ritchie ME, et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell. 2013;24(1):120–9. doi: 10.1016/j.ccr.2013.06.002; [DOI] [PubMed] [Google Scholar]
- 39.Lochmann TL, Floros KV, Naseri M, Powell KM, Cook W, March RJ, et al. Venetoclax is effective in small-cell lung cancers with high BCL-2 expression. Clin Cancer Res. 2018;24(2):360–9. doi: 10.1158/1078-0432.CCR-17-1606; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam LT, Lin X, Faivre EJ, Yang Z, Huang X, Wilcox DM, et al. Vulnerability of small-cell lung cancer to apoptosis induced by the combination of BET bromodomain proteins and BCL2 inhibitors. Mol Cancer Ther. 2017;16(8):1511–20. doi: 10.1158/1535-7163.MCT-16-0459; [DOI] [PubMed] [Google Scholar]
- 41.Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 2023;8(1):198. doi: 10.1038/s41392-023-01460-1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059; [DOI] [PubMed] [Google Scholar]
- 43.Masoud GN, Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–89. doi: 10.1016/j.apsb.2015.05.007; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontanini G, Boldrini L, Vignati S, Chine S, Basolo F, Silvestri V, et al. Bcl2 and p53 regulate vascular endothelial growth factor (VEGF)-mediated angiogenesis in non-small cell lung carcinoma. Eur J Cancer. 1998;34(5):718–23. doi: 10.1016/S0959-8049(97)10145-9; [DOI] [PubMed] [Google Scholar]
- 45.Fu ZJ, Wang ZY, Xu L, Chen XH, Li XX, Liao WT, et al. HIF-1alpha-BNIP3-mediated mitophagy in tubular cells protects against renal ischemia/reperfusion injury. Redox Biol. 2020;36:101671. doi: 10.1016/j.redox.2020.101671; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh K, Briggs JM. Functional Implications of the spectrum of BCL2 mutations in Lymphoma. Mutat Res Rev Mutat Res. 2016;769:1–18. doi: 10.1016/j.mrrev.2016.06.001; [DOI] [PubMed] [Google Scholar]
- 47.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–64. doi: 10.1016/j.cell.2019.01.021; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.