Abstract
Green tea has garnered increasing attention across age groups due to its numerous health benefits, largely attributed to Epigallocatechin 3-gallate (EGCG), its key polyphenol. EGCG exhibits a wide spectrum of biological activities, including antioxidant, anti-inflammatory, antibacterial, anticancer, and neuroprotective properties, as well as benefits for cardiovascular and oral health. This review provides a comprehensive overview of recent findings on the therapeutic potential of EGCG in various human diseases. Neuroprotective effects of EGCG include safeguarding neurons from damage and enhancing cognitive function, primarily through its antioxidant capacity to reduce reactive oxygen species (ROS) generated during physiological stress. Additionally, EGCG modulates key signaling pathways such as JAK/STAT, Delta-Notch, and TNF, all of which play critical roles in neuronal survival, growth, and function. Furthermore, EGCG is involved in regulating apoptosis and cell cycle progression, making it a promising candidate for the treatment of metabolic diseases, including cancer and diabetes. Despite its promising therapeutic potential, further clinical trials are essential to validate the efficacy and safety of EGCG and to optimize its delivery to target tissues. While many reviews have addressed the anticancer properties of EGCG, this review focuses on the molecular mechanisms and signaling pathways by which EGCG used in specific human diseases, particularly cancer, neurodegenerative and metabolic diseases. It serves as a valuable resource for researchers, clinicians, and healthcare professionals, revealing the potential of EGCG in managing neurodegenerative disorders, cancer, and metabolic diseases and highlighting its broader therapeutic values.
Keywords: Epigallocatechin gallate, Neurological disorders, Cancer therapy, Neuroinflammation, Targeted therapy, Natural products
Introduction
Polyphenols are a group of bioactive compounds found in foods and beverages, mainly present in green tea. They act as an antioxidant, anti-inflammatory, and neuroprotective agent, which belongs to catechins [1]. Among many other compounds, the potential and major biochemical compound is Epigallocatechin-3-gallate (EGCG), the most abundant flavone-3-ol polyphenol present in green tea. Structurally, EGCG contains eight free hydroxyl groups responsible for its bioactivities properties [2]. Nowadays, the consumption of green tea among the youth has increased in folds, providing numerous benefits to human health [3–5]. Polyphenols represent therapeutic agents against various symptoms. EGCG has inhibitory functions in multiple diseases that arise due to abnormal changes or modifications with an effect as anti-inflammatory properties and antibacterial effects that appear within its effective range. In green tea, EGCG accounts for about 59% of the total catechins and exhibits a broad spectrum of biological activities, such as oral disease linked with microbes. EGCG plays crucial roles against pathogenic microorganisms, such as several Gram-positive and Gram-negative bacteria, and a wide range of anti-infective drugs [6, 7]. Studies have been conducted that proved its role in anti-oxidant, anti-cancer, anti-diabetic, anti-inflammatory, anti-microbial, anti-oxidant, anti-diabetic, neuroprotective activities, anti-cancer, anti-inflammatory, anti-microbial, etc. [8]. Thus, therapeutic advantages from EGCG utilization were observed in oral infections, cancer, obesity, diabetes, neurodegenerative, and inflammatory diseases (Fig. 1).
Fig. 1.
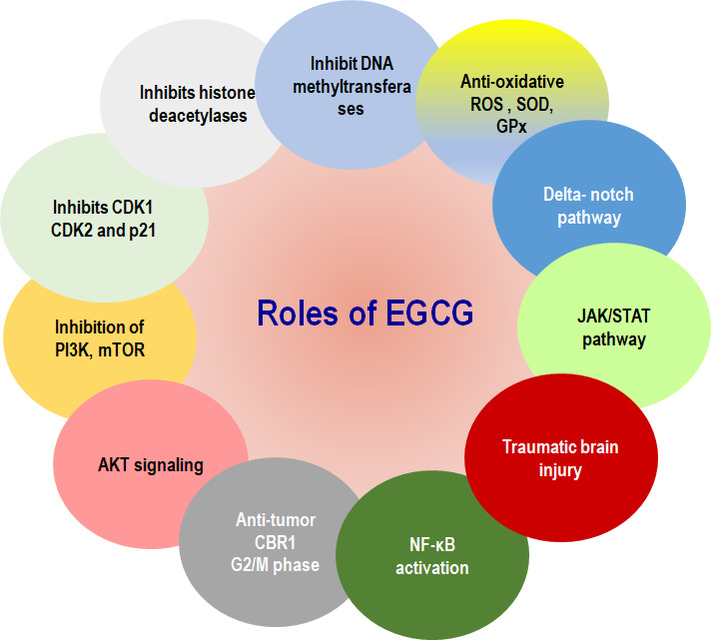
Mechanistic Overview of Modulation of Cellular Signaling Pathways by EGCG. This figure illustrates the multifaceted mechanism of EGCG and its interactions with key signaling and molecular pathways. EGCG is shown to regulate various target genes and proteins, including JAK/STAT, NF-κB, AKT, and Notch pathways, highlighting its critical roles in cellular processes such as apoptosis, proliferation, and survival. The diagram emphasizes the biological functions of EGCG, including its anti-cancer, anti-inflammatory, and antioxidant activities. By inhibiting oxidative stress, reducing inflammation, and promoting apoptosis in cancer cells, EGCG showcases its therapeutic potential in combating diseases like cancer, cardiovascular , and neurodegenerative diseases
EGCG ameliorates oral health by blocking the deterioration of periodontitis dentin erosion and defending oral mucosa against oral tumor cells. Dose-dependent mechanisms for flora-related diseases such as caries and periodontal EGCG have been found to be highly effective. High doses of EGCG act as bactericidal, i.e., kill bacteria by destroying their structures, whereas low doses exert antibacterial effects by reducing virulence, which often leads to bacterial membrane disruption and growth inhibition [9].
EGCG is a promising therapeutic molecule to prevent neurodegenerative diseases (NDs). It has effective antioxidant and anti-inflammatory effects and subsequent therapeutic potential [10, 11]. EGCG can modulate several targets associated with the pathogenesis of several chronic diseases [12–14]. EGCG is promising for promoting healthy aging by recovering the morphologic and functional modifications that happen in a naturally aging brain, suppressing cognitive dysfunction and diminishing oxidative injury in the brain. EGCG possesses neuroprotective effects against several neural injuries [15].
The neuroprotective effects of EGCG were reported in Alzheimer's (AD) and Parkinson's disease (PD) [10, 13]. EGCG has the potential to attenuate cell death, repress ROS accumulation and free intracellular calcium, modify signalling cascades, and consequently decrease oxidative stress. However, in the case of AD, ROS accumulation leads to β-amyloid degradation and repression in the phosphorylation of tau protein [16]. EGCG, with its amyloid precursor protein (APP) processing ability, offers an alternative strategy for AD prevention. Studies have been performed on different cell lines, including MC65, EOC 13.31, SweAPP N2a, and N2a/APP695, proving its anti-neuroinflammatory capacity by inhibiting microglia-induced cytotoxicity [17].
EGCG is a well-explored phytochemical with numerous health benefits that can improve the human lifestyle [18–21]. Its clinical application remains limited due to its poor physicochemical stability and low oral bioavailability. While EGCG exhibits favourable and strong advantages against biochemical and molecular-related diseases, its effectiveness in clinical settings does not fully match that of first-line chemotherapy agents. There is still a certain gap that prevents the real potential of EGCG.
This review focuses on biochemical metabolism, and physiological role in humans. Here, we aimed to compile and present the latest research on biological activities, including the antioxidant, anti-inflammatory, anti-microbial, and neuroprotective properties of EGCG. We further explored the underlying mechanism by which EGCG modulates various cellular signalling pathways, such as those involved in inflammation, apoptosis, and epigenetic modifications, to exert its therapeutic effects. In addition, we summarize the recent findings on the molecular mechanisms and therapeutic effects of EGCG in oral-related and neurological diseases. We discuss the potential of EGCG as a promising therapeutic drug for treating these conditions.
Biological and pharmacological effects of EGCG
The biological action of EGCG lies in its phenolic hydroxyl groups with molecular structures that are implicated in food production. EGCG has a protecive effect on inflammation due to its antioxidant properties [22, 23]. It prevents cellular oxidation and inhibits cell-free radical damage [24–26]. EGCG attenuates oxidative breakdown under elevated temperatures and alkali states and accelerates the degradation rate with the increase in temperature and oxidation concentration [27]. The effects of EGCG could be highly synergistic when combined with other catechins and thus metabolically stimulated to form stronger and more efficient bioactive compounds [28, 29].
A detailed analysis of the biological effects of polyphenolic compounds present in green tea has shown the difference in pharmacokinetics activity in the individual compounds. It was experimentally proven that intake of 1.5 mM of green tea elevates the level of both ((-)-epicatechin-3-gallate) EGC and EGCG, but the variation is observed in the duration of half-life. For instance, EGC plasma level rises quickly with a low elimination half-time of 1.7 h, whereas EGCG levels rise slowly with a higher half-time of 3.9 h [30, 31].
EGCG is absorbed in the intestine when ingested orally, but its bioavailability has always been a question. Due to its oxidation, efflux, and metabolism, there is a low availability in the gut area. Microbiota present in the gut region deconjugate and degrade EGCG. Studies have even found that EGCG undergoes metabolism before absorption. With the help of gut microbes such as Enterobacter aerogenes, Klebsiella pneumoniae subspecies EGCG breaks down to EGC and gallic acid [32]. Later, EGC degrades to 5-(3,5-dihydroxyphenyl)−4-hydroxyvaleric acid, which is the main metabolite of EGCG. The absorbed compound is 5-(3’,5’-dihydroxyphenyl)-γ-valerolactone and its glucuronide form is the major urinary metabolite.
The consumption of EGCG has shown various physiological and pharmacological health benefits [33]. EGCG is one of the oldest polyphenols that have been experimented with to test its efficiency on bacteria. EGCG has a potential effect on the growth of Staphylococcus aureus, especially methicillin-resistant Streptococcus and E. coli [34]. EGCG has shown anti bacterial - activity against a heterogeneity of Gram-positive and Gram-negative pathogens, viruses, fungi, and prions. Hence, it is known to be a prospective anti-infective agent. It has antifungal action against human-pathogenic yeast [35, 36].
EGCG inhibits enoyl-acyl carrier protein reductase (ENR) in Plasmodium falciparum. The mechanism of inhibition is believed to involve a slow-tight binding mechanism. This means that EGCG binds to the enzyme slowly but forms a very stable complex, effectively inhibiting its activity. Inhibiting ENR would disrupt the fatty acid biosynthesis pathway, which is essential for the survival of parasite. This could lead to reduced parasite viability and potentially prevent or treat malaria infections. Targeting ENR represents a novel approach to combating malaria, which is becoming increasingly resistant to traditional antimalarial drugs [37]. Studies have revealed the mechanism by which EGCG inhibits the occurrence of bacterial outbursts in the body. EGCG interacts with σ, thus modulating the activity of RybB and CsgD genes. This interaction downstream disrupts the biofilm found by the bacteria. This is achieved by alternating curli subunit and c-di-GMP required for membrane formation [38], as described in Fig. 2.
Fig. 2.
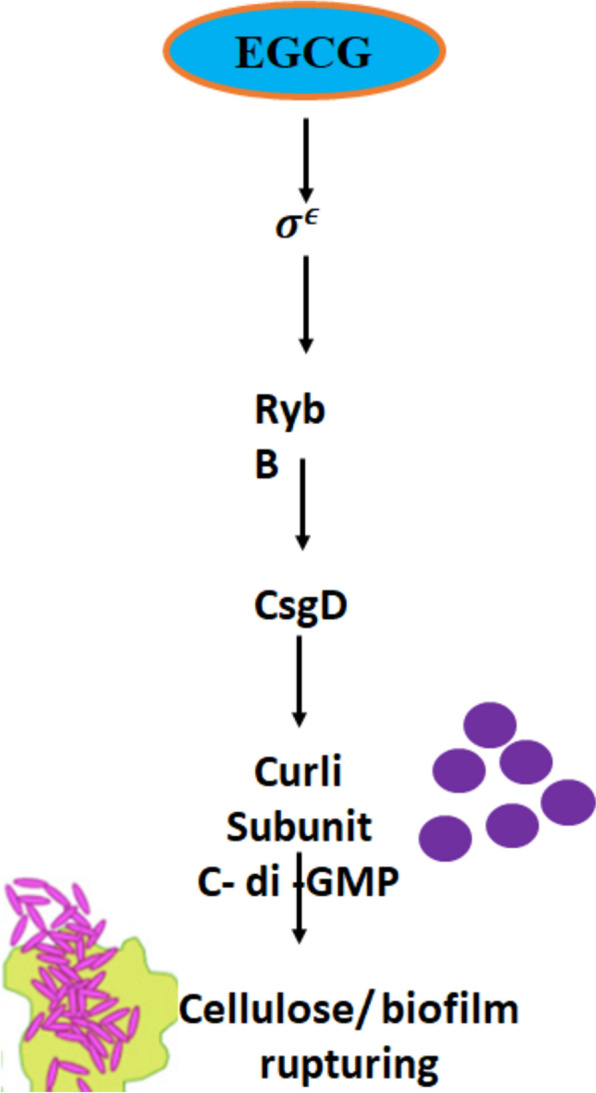
Antimicrobial Action of EGCG through Biofilm Disruption. Showing the antimicrobial mechanism of EGCG, focusing on its ability to disrupt biofilm formation. EGCG targets the σϵ regulatory pathway, which plays a crucial role in bacterial membrane adhesion. By interfering with the production of curli subunits and cyclic di-GMP (c-di-GMP), both essential for biofilm stability and bacterial adherence, EGCG effectively impairs biofilm formation. This disruption weakens bacterial colonization and enhances susceptibility to antimicrobial treatments, highlighting the potential of EGCG as a powerful agent in combating bacterial infections and biofilm-associated resistance
EGCG induces changes in the proteome of Microcystis aeruginosa under stress conditions. It inhibits carbon and nitrogen assimilation along with chlorophyll synthesis and cell division. In contrast, stress response proteins such as superoxide dismutase and glutaredoxin were found to be upregulated. The observed proteomic changes provide insight into the molecular mechanism of the inhibitory activity of EGCG on M. aeruginosa [39].
Experimental evidence has proven the relationship between EGCG and exercise and their effect on the physiology of AD. EGCG and exercise, either separately or in combination, are applied to a 2-month mouse, with the impact of a 4-month wheel-running exercise attenuating or lowering the formation of soluble Aβ1–42 levels in the cortex and hippocampus [40]. Also, EGCG enhanced the effects when formulated as dual-drug loaded PEGylated PLGA nanoparticles [41, 42]. However, oral administration of EGCG/AA NPs in mice affected EGCG gathering in all main organs, such as the brain [43]. EGCG might prevent specific biomedical vital molecular targets, including antiapoptotic Bcl-2 proteins and vascular endothelial growth factor (VEGFR) signaling [28, 44–47]. Pharmacokinetic examination accomplished in human exhibits shows that the physiologically pertinent serum concentrations of EGCG could be in the elevated nanomolar range.
EGCG protects against Aβ-mediated cytotoxicity via the Akt pathway activation [16]. The neuroprotective results against Aβ-mediated neuronal cell death have been generated via its capability to scavenge ROS efficiently. The oral administration of EGCG has identified considerable development in spatial cognitive learning capability. EGCG can reduce Aβ and tau toxicity, explaining its promise for the obstacle of AD. EGCG has shown considerable inhibitory results against oxidative stress-mediated cell death. Hence, EGCG is a therapeutic drug in the management of PD [48, 49].
The anti-oxidation process by EGCG is of great importance to human health care [50]. EGCG mitigates the harmful effects of locally administered bupivacaine, a commonly used for epidural anaesthesia and nerve blocks, by suppressing bupivacaine-induced ROS production in neuroblastoma cells. Briefly, EGCG attenuates bupivacaine-induced ROS generation in neuroblastoma cells, protecting against its toxicity [51]. Structurally, the presence of phenol rings in the EGCG acts as a hunter of free radicals and traps the electrons that inhibit the formation of ROS. This causes a decrease in the damage caused by oxidative stress [52–56]. EGCG is an essential compound that received much recognition for its numerous health benefits because it reduces inflammation and helps to prevent heart and brain disease, assisting in weight loss [52].
EGCG prevents stimulated oxidative stress and neurotoxicity, and EC diminishes Aβ-fibril creation. EGCG can control the proteolytic processing of APP, suggesting that green tea polyphenols may be potential therapeutic agents for PD and AD [16]. A marked reduction in β- and γ-secretase function and BACE1 and APP expression was observed [57]. Despite the encouraging effects of pre-clinical examines, a translational gap exists between fundamental invention and clinical application [58]. Multiple studies in the brains of AD patients revealed a decline of PKC€ action in the membrane fraction.
EGCG acts as a potential inhibitor for the dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A). DYRK1A overexpression induces morphological defects and impairment in the individuals, leading to Down syndrome, Pick's disease, and AD. EGCG displays appreciable inhibition of DYRK1A even at high concentrations (360 mg/kg). Since the stability is low, structurally modified EGCG shows non-competitive properties. Differing from other published inhibitors, EGCG acts as a non-competitive inhibitor [59]. It revealed that prenatal exposure prevents the over-expression of DYRK1A in the brain. EGCG acts as a competitive inhibitor at the ATP-binding site when DYRK1A induces K465R mutation. Interestingly, the K465R mutation in DYRK1A changes the mode of inhibition of EGCG from a non-competitive to a competitive inhibitor at the ATP site. Although EGCG showed promise as a therapeutic agent (Table 1), its clinical application was limited by its low absorption rate and susceptibility to degradation.
Table 1.
Target proteins of EGCG
| S. No | Target protein | Associated disease | Affinity | Reference |
|---|---|---|---|---|
| 1 | Enoyl-acyl carrier protein reductase | Plasmodium falciparum | 8 nM | [60] |
| 2 | 3-hydroxyacyl-[acyl-carrier-protein] dehydratase | Microcystis aeruginosa | 30 nM | [39] |
| 3 | Ser/Thr protein kinase mTOR | Oxidative stress | 320 nM | [61] |
| 4 | DYRK1A | Down syndrome | 330 nM | [59] |
| 5 | Bcl2 | Anti-cancerous | 335 nM | [62] |
| 6 | NAD Kinase | Lung cancer | 3 nM | [63, 64] |
| 7 | Cannabinoid receptor 1 | Cancer | 4 nM | [65, 66] |
| 8 | Disintegrin and metalloproteinase domain 17 | Cancer | 4 nM | [67] |
| 9 | Peptidyl- prolyl cis–trans isomerase | Breast Cancer | 4 nM | [68] |
| 10 | Amyloid-beta precursor protein | Alzheimer's disease | 4.60E + 3 nM | [69] |
Molecular mechanisms of EGCG activity
This section provides insights into the intricate interactions of EGCG with key cellular targets, shedding light on its multifaceted therapeutic potential. Antioxidant properties of EGCG are central to its role in reducing oxidative stress and mitigating neuro-traumatization, particularly in neurodegenerative diseases. By scavenging ROS, EGCG helps maintain cellular homeostasis and protects neurons from oxidative damage. Additionally, EGCG induces apoptosis in cancer cells by modulating several pro-apoptotic and anti-apoptotic proteins, effectively promoting programmed cell death in abnormal cells while preserving healthy tissue.
EGCG reduces ROS oxidative stress
EGCG inhibits cellular mechanisms and tissue oxidative injury by preventing pro-oxidant enzymes. EGCG, having high lipophilic features, illustrated a better affinity for free radicals than the unchanged EGCG [70]. EGCG has been reported as a potent antioxidant and possesses beneficial effects in oxidative stress-related diseases [71].
A study [72] demonstrated the anti-oxidative capabilities of EGCG in H2O2-mediated oxidative stress of myocardial ischemia injury [73]. The levels of superoxide dismutase (SOD) and glutathione peroxidize (GPx) activity ameliorates with the treatment of H2O2-induced control biopsies with EGCG at the concentration of (50 µg/ml) for 24 h. It has also been observed that when H2O2-induced cervical cancer biopsies treated with EGCG (50 µg/ml) suppress the activity of SOD and GPx by 38.54% and 57.04%, respectively. Similar results were obtained with HeLa cells. Improvement in SOD and GPx activity by co-culture of EGCG indicates it to be an effective natural antioxidant combating ROS generation [74]. Thus, it can be rightfully said that EGCG imparts an inhibitory effect and cancer chemo-preventative agent on the activity of many enzymes and metabolic pathways.
EGG modulates the pathway, which directly inhibits cytokines inflammation and formation of ROS in uric acid-induced human umbilical vein endothelial cells [75, 76]. EGCG reduces the mRNA levels of Notch1, Hey1, and Hes1 in a dose-dependent manner, leading to the inhibition of cancer growth. Additionally, EGCG suppresses Notch promoter activity, further contributing to its anticancer effects. In vivo studies demonstrated a significant reduction in both tumor growth and Notch1 expression in mice injected with EGCG. Similar findings were observed in EGCG-treated colorectal cancer cells, where decreased levels of Hes1 and Notch2 were reported. Notably, EGCG was also found to cleave Notch1 in 5-fluorouracil-resistant colorectal cancer cells, indicating its potential role in overcoming drug resistance [77].
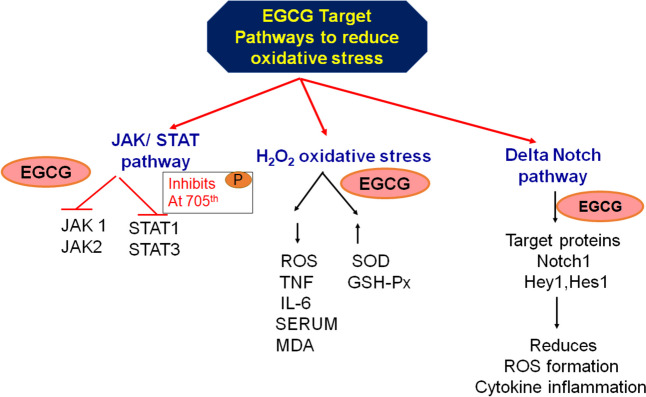
EGCG significantly improves the survival rate of human umbilical vein endothelial cells exposed to H2O2-induced oxidative stress [61]. EGCG was found to upregulate key autophagy-related proteins, including Atg5, Atg7, LC3 II/I, and the Atg5–Atg12 complex. It also inhibits the mTOR signaling pathway, further promoting EGCG-induced autophagy (Fig. 3). In addition, EGCG disrupts the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway at multiple stages by inhibiting STAT phosphorylation, thereby preventing its translocation to the nucleus [78]. Specifically, EGCG inhibits the phosphorylation of the 705th tyrosine residue of STAT, and protein kinase C delta (PKC-delta), effectively blocking STAT1 activity. Moreover, EGCG suppresses the activity of interferon-gamma (IFNγ), JAK1, JAK2, and STAT1/STAT3. Interestingly, EGCG-mono-palmitate, a derivative of EGCG, activate the Src homology 2 domain-containing tyrosine phosphatase-1 (SHP-1) enzyme, which reduced the phosphorylated levels of BCR-ABL and STAT3 in human chronic myeloid leukemia cells [79].
Fig. 3.
EGCG-Mediated Pathways Targeting Oxidative Stress. Showing the diverse molecular pathways through which EGCG mitigates oxidative stress. EGCG inhibits the phosphorylation of STAT3, thereby disrupting the JAK/STAT signaling pathway, which is crucial for cell proliferation and survival. Additionally, EGCG targets key proteins in the Delta-Notch pathway, including Notch, HEY, and HES1, further influencing cellular differentiation and survival mechanisms
EGCG has shown protective effects when administrated to patients with contrast-induced nephropathy. It normalized kidney function markers, such as serum creatinine and blood urea nitrogen. It is responsible for improving renal tissue health, as indicated by histological analysis. Metabolic physiology showed reduced cell death and oxidative stress within the kidneys and alleviated inflammation in the renal tissue. EGCG-loaded chitosan-casino phosphopeptide nanoparticles show free radical scavenging activity [80].
Furthermore, EGCG decreased both the expression and secretion levels of pro-inflammatory cytokine genes, including TNF-α, IL-1β, and IL-6. EGCG also downregulated the expressions of α-SMA, fibronectin, mast cell trypsin, and chymotrypsin, as well as TGF-β1, CTGF, and PAI-1 [81] as an emerging potential phytocompound EGCG has come upon with its recently discovered role in fibrosis. Due to its anti-inflammatory and anti-proliferative role, EGCG promotes wound healing and prevents scar formation. This is achieved by blocking the pathways of VEGF, CTGF, and TGF-β1. It downregulates the formation of skin scars by reducing the production of COL-1 [82]. EGCG may be effective in treating skin scars by decreasing blood flow and skin thickness, reducing mast cell activity and angiogenesis while also enhancing heme oxygenase-1 levels, increasing M2 macrophage counts, and boosting elastin, antioxidant activity, and hydration [81].
EGCG reduces traumatic brain injury
EGCG prevents traumatic brain injury (TBI)-mediated IL-1β and TNF-α mRNA in mice brains [83]. EGCG considerably diminished the levels of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-PX) after TBI. EGCG diminished TBI-mediated NADPH oxidase activation by inhibiting p47 translocation to the plasma membrane [55, 84]. Preclinical trials reflect the therapeutic potential of EGCG in suppressing the occurrence of secondary brain damage after TBI. The intake of EGCG observes a reduction of brain infarction and oedema in rodent models. EGCG preserves blood–brain barrier integrity through the modulation of tight junction proteins, limiting the entry of blood-derived substances into brain parenchyma. It inhibits the activation of microglial, reducing TNF-α, IL-1β, and IL-6 and alters the NF-κB pathway in the injured rat brain. Additionally, it remarkably reduces neutrophil infiltration and cytokine pro-inflammation [85].
Therapeutic benefits of green tea consumption were reported in inflammatory and NDs [86]. EGCG effectively inhibited IL-8 in epithelial cells. IL-8 is responsible for the recruitment of neutrophils and supports the formation of ROS. EGCG acts as an antioxidant by inhibiting the phosphorylation of IκB to prevent IL-1β-induced NF-κB activation [87]. EGCG inhibited IL-1β-mediated expression of iNOS and COX-2 by inhibiting proteasomal degradation of IκBα [88]. EGCG prevents the binding of AP1 to DNA via IL-1β-mediated phosphorylation of c-Jun, thereby downregulating IL-8 and TNF-α progression. Evidence emerged that supports the inhibition of CD4 + T cells, promotes IκB-α, and reduces ROS formation in neurons by EGCG. EGCG prevents the binding of AP1 to DNA via IL-1β-mediated phosphorylation of c-Jun, thereby downregulating IL-8 and TNF-α progression [89].
EGCG acts as a neuroprotective agent against Aβ-induced oxidative stress by increasing the antioxidants in BV2 microglial cells [90]. EGCG reduces the NF-κB signaling activation, obstructing the formation of cytokines and VEGF in U373MG cells [91]. EGCG represses Aβ-induced neuroinflammatory reactions of microglia, including TNFα, IL-1β, and iNOS, and enhances the levels of antioxidants [92]. It repressed Aβ-induced NF-κB and MAPK pathways, including JNK and p38 [93–96]. EGCG blocks inflammatory and oxidative stress markers via the regulation of nuclear receptor PPARγ in N2a/APP695 cells [97].
EGCG induces apoptosis: anti-proliferation
EGCG, combined with chemotherapeutic drugs, experimentally showed higher sensitivity against tumor cells. At the same time, the anti-inflammatory and antioxidant effects of EGCG reduce the negative impact caused by the chemotherapy. Clinically, the nanomodification of EGCG significantly increases anti-tumor activity. One such pathway illustrated with doxorubicin (DOX). EGCG enhances anti-tumor activity by inhibiting the activity of carbonyl reductase 1 (CBR1) and P-gp in the liver cells. Here, EGCG binds to CBR1 and inhibits the conversion of daunorubicinol (DNROL) to DOX, enhancing antitumor activity and inducing cardiotoxicity [98].
Combining EGCG and sulforaphane with cisplatin significantly increases its effectiveness against both sensitive and resistant ovarian cancer cells. This combination treatment reduces cell survival, accelerates cell death (apoptosis), and halts cell division at the G2/M phase in a time- and dose-dependent manner [99]. EGCG reduced EGFR phosphorylation, suppressed AKT signaling in breast cancer cells, and amplified raloxifene-induced apoptosis [100]. In mice, combined EGCG with tamoxifen leads to a decrease in tumor volume by 71% and tumor weight by 80% by inhibiting mTOR and EGFR expression [101] (Fig. 4).
Fig. 4.
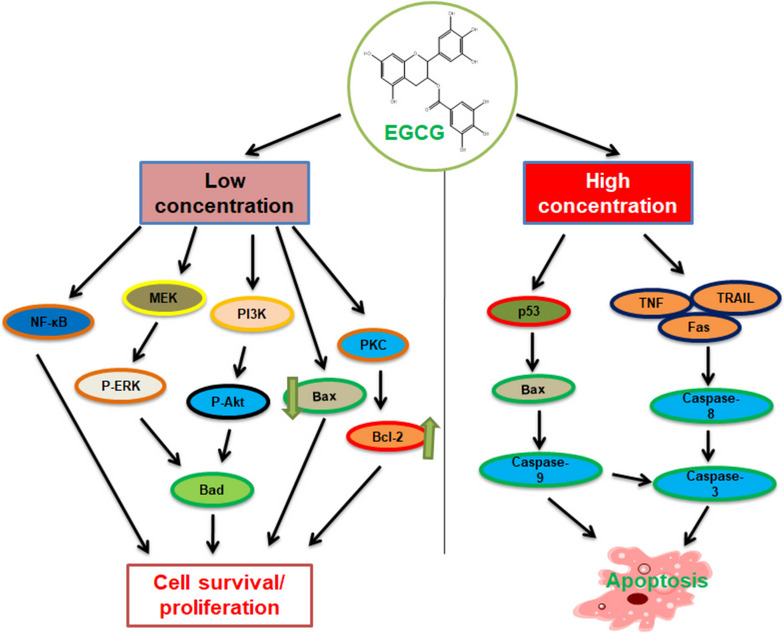
Impact of EGCG on tumor-mediated signaling cascades through apoptosis. EGCG blocks signaling cascade activation and promotes apoptosis. Outline the promising gene targets engaged in anti- and pro-apoptotic activities of low and high EGCG concentration. This effect could be attained via the increased regulation of p53 expression. EGCG enhances the ratio of Bax/Bcl-2 and activates apoptosis
EGCG administration not only extended the lifespan of lethally irradiated mice but also mitigated radiation-induced damage to the intestinal mucosa. Treatment with EGCG significantly increased the population of Lgr5 + intestinal stem cells (ISCs) and their proliferating Ki67 + progeny while simultaneously reducing radiation-induced DNA damage and apoptosis. Moreover, EGCG effectively lowered ROS levels and activated the Nrf2 transcription factor, which in turn upregulated antioxidant proteins such as Slc7A11, HO-1, and GPX4. Consequently, EGCG acts as a protective agent against radiation-induced injury by scavenging ROS and inhibiting both apoptosis and ferroptosis via the Nrf2 signaling pathway [102].
EGCG induces cellular signaling and epigenetic modification
EGCG regulates the cell cycle by modulating cyclin-dependent kinases (CDKs). It inhibits the activity of CDK1 and CDK2 and activates cyclin-dependent protein kinase inhibitors such as p17, p21, and p16 [103]. EGCG exerts growth inhibitor effort on human cancer cell lines without affecting normal cells of the body. The effect is dose-dependent, inhibiting cell growth, G0/G1 phase arrest, and DNA damage. Such physiological role indices apoptosis in many cancer cell lines. Molecular docking studies have revealed CDK-6 as the better ligand for EGCG with binding energy − 12.70 and docking energy − 11.40 kcal/mol, as compared to other CDK-6 inhibitors. This study was confirmed with experimental immunoblot assay analysis, which proved that EGCG is an inhibitor of CDK-6 [104, 105].
The role of EGCG in epigenetic modification has always been appreciated. Sjögren’s syndrome (SS) is an autoimmune disease particularly affecting the exocrine glands. There has been a correlation between the syndrome and EGCG, which is responsible for altering the gene expression of related molecules. Such alterations lead to ameliorated salivary gland damage. The mechanism remains the same, including the reduction in ROS activity and accumulation, thereby inhibiting ROS mediating water aqua channel 5. Eventually, EGCG is responsible for increasing the saliva flow [106].
EGCG can inhibit DNA methyltransferases, the enzymes responsible for adding methyl groups to DNA [107]. This inhibition can lead to the demethylation of certain gene promoters, potentially activating genes that were previously silenced. In addition, EGCG has been observed to affect histone acetylation by inhibiting histone deacetylases. Increased histone acetylation typically leads to a more relaxed chromatin structure, which enhances gene transcription. Furthermore, EGCG can influence the expression of microRNAs, which are small non-coding RNAs that regulate gene expression post-transcriptionally. Changes in miRNA levels can affect various cellular processes and contribute to therapeutic effects of EGCG [108].
EGCG Therapeutic potential in specific human diseases
EGCG is a promising therapeutic agent reported for its diverse biological activities, including antioxidant, anti-inflammatory, and anticancer properties. Many of these interactions require EGCG concentrations far higher than those obtainable through regular green tea consumption or standard green tea extract supplements. Despite this limitation, recent well-conducted clinical trials have demonstrated the efficacy of green tea extracts and purified EGCG products in treating specific conditions. This section focuses on clinically relevant studies, highlighting recent advancements in EGCG-based therapies for diseases. Additionally, this review explores EGCG’s mechanisms of action, examining the existing evidence supporting its therapeutic potential in addressing human diseases.
Oral health and dentistry
Gingivitis and periodontitis are inflammatory mouth infections in which gums turn red and swollen and sometimes bleed. EGCG helps reduce periodontal disease development by inhibiting the growth of Porphyromonas gingivalis, Prevotellani crescent, and Prevotella intermedia. These bacteria are engaged in periodontal tissues, causing periodontitis. The efficiency of catechins defends the gingival epithelium against the invasion of Porphyromonas gingivalis, a potent cause of periodontal disease. Treatment with EGCG blocks the functioning of the Matrix Metalloproteinase-9 (MMP-9) factor and supports osteoclasts in periodontal illness. MMP-2, 8, and 9 present in the dentine region of the oral cavity are responsible for the degradation of tooth decay. EGCG was reported as an MMP inhibitor [109, 110].
Acrolein was found to prevent gingival fibroblasts, usually because of cell disruptions and growth. Hence, this might increase several inflammatory situations in the oral cavity, which could cause gingival and periodontal disease. EGCG may decrease the toxicity of acrolein. Therefore, green tea/EGCG has been confirmed as a wonder drug for oral health [111]. Due to inadequate diets among the youth, the uptake of large amounts of carbohydrates and fermented sugars leads to the accumulation of acid-producing microbiota. Among all microbiota, S. mutans, Lactobacillus, and Actinomyces viscous are dominant and highly implicated in the development of dental caries.
A high dose of EGCG kills bacterial structure whereas a low dose is effective in anti-bacterial nature by inhibiting virulence factors. In either dose, EGCG leads to destruction in the biofilm growth. Such efficacy is prominent against oral Candida. A high dose induces damage to mitochondrial membranes and uncoupling of oxidative phosphorylation. EGCG when combined with other drugs, shows a higher potency towards drug-resistant strains. EGCG is directly involved in the inactivation of oral viruses such as HPV, HSV, and other viral proteins [112].
Tea leaves are rich in fluoride, which improves dental health. Dental caries are induced by oral microflora. Microbial dysbiosis containing Gram‑positive and Gram-negative aerobic and anaerobic bacteria affects the progression of cariogenic dental plaque [112–115]. EGCG inhibits sugar transport and acid formation via lactate dehydrogenase [116]. EGCG is known as a competitive inhibitor of NADPH. In addition, EGCG inhibits the NADPH oxidase translocation and ROS production [117]. Consuming catechins daily efficiently decreases dental caries, and using EGCG/green tea mouthwash diminishes the acidity of saliva and prevents bacterial colonization. The study enhanced salivary pH with EGCG/green tea and decreased dental caries [118, 119].
EGCG acts as the main compound in green tea that was reported to inhibit the growth and virulence factor of microbes of oral pathogens. Though the mechanism is unclear, it has been validated that EGCG is responsible for reducing volatile sulfur compounds by suppressing the mgl gene. Mgl gene is encoded for enzymes L-methionine-α-deamino-γ-mercaptomethane-lyase, responsible for methyl mercaptan (CH3SH) production by oral anaerobes. EGCG inhibits the growth of P. gingivalis and reduces CH3SH production and mgl gene expression [112]. The reaction has been carried out by introducing a methyl sulfonyl group into the B-ring of EGCG. A methylation reaction is set to attach the orthoquinone site via oxidation, which facilitates reduction in halitosis. Hence, green tea helps to lower the oral odor [114].
Solobacterium moorei is an anaerobic bacterium grouped under a volatile sulfide compound (VSC) associated with halitosis. Upon performing a microplate dilution assay, EGCG was found to show anti-bacterial activity against S. moorei. EGCG inhibits the growth of S. moorei, with MIC values of 250 μg/ml [120]. Through transmission electron microscopy and a permeabilization assay, it was found that EGCG is responsible for targeting bacterial cell membranes and attenuating their integrity. Similar results were obtained when an analysis was performed on colonization properties. It was found that EGCG significantly reduces colonization and adherence to oral epithelial cells. In addition, EGCG at ½ MIC significantly decreased the β-galactosidase gene expression [120].
Cancer
Oral cancer
EGCG inhibits cell cycle progression and modulates signalling pathways that cause cancer. Its therapeutic potential has been effective against oral cancer. EGCG imparts the G1 arrest of tumor cells, and its treatment significantly enhances caspase-7, 9, and −3 activity along with increased expression of Bax [121]. Indirectly, EGCG is involved in caspases-induced apoptosis.
EGCG causes cell death in cancerous cells by modulation of several signalling cascades. It diminishes cell proliferation via suppressed Akt, NF-κB, EGFR, and MAPK/ERK1/2 pathways [47]. EGCG acts as a potent drug for tumor chemoprevention capability for cancer transformation of oral premalignant lesions, tumor proliferation blockage, and cell death initiation (Fig. 5).
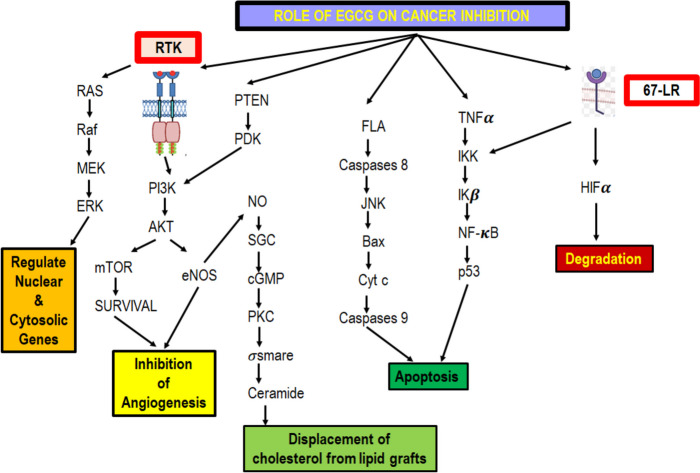
Fig. 5.
Role of EGCG in inhibition of cancer growth. EGCG acts as an anti-cancer agent either by responding through the RTK pathway or by binding to its 67 -ligand-receptor (67-LR). RTK method mediates via RAS pathway regulating nuclear and cytosolic genes. RTK also alter PI3K-AKT pathway thereby inhibiting angiogenesis. EGCG regulates the process of apoptosis via FLA and TNFα
MMP-9 is associated with oral tumour development [122, 123]. EGCG blocks the invasion and migration of human oral tumour cells by downregulating the MMP 2 and 9 [124] (Table 2). By regulating MMP, metastasis is also prevented. A partial decrease in angiogenesis was observed in VEGF receptor phosphorylation in oral tumours [5]. The effects of EGCG in hindering HGF-mediated proliferation of oral cancers have been reported. EGCG blocks HGF-induced Met phosphorylation and the expression of MMP-9 and −2 [125, 126]. It causes an inhibitory effect on cell motility, migration, and adhesion [127]. The outcomes of green tea/EGCG hinder the development of premalignant lesions for the tumor [128].
Table 2.
Effects of EGCG on oral diseases
| Study Design | Results/Effects | Ref |
|---|---|---|
| In vitro | Inhibition of the planktonic growth and the biofilm formation | [129, 130] |
| In vitro | Decrease of S. mutans EPS production | [131, 132] |
| In vitro |
Protected keratinocytes against the TNF- -mediated breakdown of barrier integrity |
[133, 134] |
| In vitro | Prevented bacterial adhesion and F. nucleatum-mediated hemolysis | [135, 136] |
| In vitro |
Blocked S. moorei growth and bacterial adherence Declined of the biofilm formation Repression of bacterial-galactosidase activity |
[135, 137] |
| In vitro and In vivo | Inhibition of p-focal adhesion kinase (p-FAK), p-Src, snail-1, and vimentin | [127, 138] |
| In vitro | Reduced expressions of MMP-2, MMP-9, and uPA in a dose-dependent way | [124, 139] |
| In vitro and In vivo | Decrease the toxicity of acrolein | [111] |
| In vitro | Inactivation of oral viruses such as HPV, HSV and other viral proteins | [140] |
| In vitro | Inhibits the NADPH oxidase translocation and ROS production | [141] |
| In vitro | Inhibits the growth of P. gingivalis and reduces CH3SH production and mgl gene expression | [112] |
| In vitro | Decreased the β-galactosidase gene expression | [142] |
Breast cancer
EGCG has demonstrated a significant potential in the prevention of various types of cancer. Studies revealed that in a concentration-dependent manner, EGCG shows antioxidant or pro-oxidant properties. Such characteristics endure EGCG to block cell cycle progression and modulate signaling pathways during cancer prognosis. EGCG inhibits the transpiration of VEGF by inducing apoptosis and negatively modulates metastasis . Similar results were obtained from in vivo studies on xenograft animal models [143].
EGCG interferes with estrogen receptor signaling, which is critical in estrogen receptor-positive breast cancers, potentially reducing the proliferation of these cancer cells. It inhibits the formation of new blood vessels (angiogenesis), which is essential for tumour growth and metastasis in breast cancer. EGCG enhances the efficacy of common breast cancer treatments, such as tamoxifen, by sensitizing cancer cells to these therapies.
Even the overall expression of cyclins (Cyclin D, Cyclin E, CDK 4, CDK 1, and PCNA) was down-regulated in time-dependent increasing concertation of the EGCG-treated group as compared to the untreated control group by Western blot analysis that blocked the G1 phase of the cell cycle [47]. Experiments on nude mice induced with human MDA-MB-231 breast cancer cells and treated with EGCG show an effective reduction in tumour incidence [144].
EGCG has demonstrated prominent inhibition in the growth of cancer stem cells (CSCs) when used alone and when used in combination with other chemotherapeutic drugs. It reduces the resistance of CSCs and intracellular efflux. Moreover, EGCG increases the drug concentration in CSCs by inhibiting the activity of the intracellular transporter gene family, ABC transporters, at minimal concentration [145]. EGCG does not affect the ABC transporter in glioma CSCs but is able to downregulate P-glycoprotein, thus reducing colony formation and the migration of tumour cells [146]. EGCG at low concentration inhibits colony formation in pancreatic ductal carcinoma, but when used in combination with PDE3A inhibitor trequinsin, it lowers the expression of proteins FOXO3 and CD44 [147]. Upregulation of cGMP expression is observed, which showed a significant reduction in colony formation [148].
Salivary gland tumors
EGCG inhibits β1 integrin, thereby reducing the expression of MMP-2 and MMP-9, thus providing molecular evidence for the inhibitory effect of EGCG on salivary gland cancer metastasis [149]. Studies have reported that EGCG inhibits cell proliferation and expression of EGFR, downregulates Bcl-2, and upregulates Bax;, therefore, inducing apoptosis of adenoid cystic carcinoma [150]. EGCG mitigates inflammatory damage to the salivary glands by suppressing inflammatory cytokines and reducing oxidative stress. Moreover, it promotes the growth and repair of salivary gland tissue. Additionally, EGCG inhibits the metastasis of salivary gland cancer by downregulating MMP proteins [151, 152].
Prostate cancer
EGCG administration leads to the downregulation of cyclin D and cyclin E, which are involved in G1/S progression. This indicates that EGCG induces G1 phase arrest in prostate carcinoma cells. Similar role was observed in breast cancers suggesting chemo-preventiverole of EGCG [153]. Treatment with ECGC on LNCaP prostate cancer cell lines reduces cell proliferation. There was increased expression of androgen receptor and prostate-specific antigens on the cancer cell lines [154]. EGCG induces apoptosis in prostate cancer cells. It activates pro-apoptotic proteins and inhibits anti-apoptotic proteins, leading to the selective killing of cancer cells while sparing normal cells [155]. EGCG can cause cell cycle arrest in prostate cancer cells, particularly at the G1 phase, preventing cells from progressing to the S phase and thereby inhibiting tumor growth. Prostate cancer growth is often driven by androgen receptor signalling. EGCG downregulates androgen receptor expression and function, reducing the proliferation of androgen-dependent prostate cancer cells. In addition, EGCGinhibits the expression of MMP enzymes that degrade the extracellular matrix and facilitate cancer cell invasion and metastasis. By inhibiting MMPs, EGCG reduces the metastatic potential of prostate cancer cells [149].
Others
EGCG exerts anti-cancer effects across various cancer types through multiple mechanisms, including the induction of apoptosis, inhibition of cell proliferation, suppression of metastasis, and modulation of epigenetic changes [58, 156]. Its ability to target specific signaling pathways and enhance the effectiveness of existing treatments for cancer prevention and therapy [157]. EGCG diminished the gefitinib-induced overexpression of CYP1A1, CYP1B1, EGFR, cyclin D1, p-Akt (Ser473), and survivin at both the transcriptional and translational levels. EGCG upregulated phosphorylation p53 at Ser15, thereby enhancing gefitinib's therapeutic efficacy against benzo[a]pyrene-induced lung cancer [158].
EGCG inhibit lung cancer cell growth by causing cell cycle arrest and promoting apoptosis. It reduces the metastatic potential of lung cancer cells by downregulating the expression of MMPs and other factors involved in cell migration and invasion [33]. By reducing oxidative stress, EGCG decreases the risk of lung cancer initiation and progression, especially in smokers and individuals exposed to environmental carcinogens [159].
Due to its numerous health benefits, EGCG has been increasingly used in the treatment of acute and chronic respiratory diseases. EGCG affects tumour initiation and progression by downregulating angiogenesis and metastasis. Its involvement is remarkable in increasing tumor suppressor genes, apoptosis, neoplastic cells, and cell cycle arrest. EGCG downregulates (Intercellular adhesion molecule) ICAM-1 expression and the counts of neutrophils and eosinophils in the bronchoalveolar lavage fluid (BALF) in human pulmonary alveolar epithelial cells [160].
An evident correlation was observed between EGCG and Nonalcoholic fatty liver disease (NAFLD). EGCG effectively ameliorated NAFLD disorder and its phenotypic [161]. It inhibits intestinal barrier and inflammations. This is achieved by increasing the concentration of gut microbes, including short-chain fatty acid (SCFA) producers such as gram-negative Lactobacillus. An increase in SCFA level inhibits the TLR4/NF-κB pathway, thereby alleviating liver inflammation. Thus, it suggests that the role of EGCG on gut microbiota is to reduce the inflammation in hepatocytes [141].
EGCG targets survival pathways such as PI3K/Akt and NF-κB, which are often upregulated in pancreatic cancer [162]. This leads to reduced cell proliferation and increased apoptosis. EGCG enhances the sensitivity of pancreatic cancer cells to chemotherapy, potentially overcoming resistance to conventional treatments [163]. EGCG inhibits the metastatic spread of pancreatic cancer cells by downregulating enzymes and signalling molecules involved in invasion and migration (Fig. 5).
EGCG was reported as a noncompetitive inhibitor of NAD kinase with a Ki of 3.28 ± 0.32 μM. SPR analysis revealed a Kd of 1.78 ± 1.15 μM, while HDX-MS indicated binding at non-substrate sites on NADK [63]. EGCG selectively inhibited the growth of KRAS-mutant lung cancer cells without affecting KRAS wild-type cells [164].
Cardiovascular diseases
Atherosclerosis
Atherosclerosis is a disease of the arteries caused by endothelial dysfunction, inflammatory vascular cells, and lipid accumulation. EGCG-treated mice showed a 23% decline in aortic weights and cholesterol as well as TG [165]. Studies exhibited a considerable decrease in LDL-C, which was examined in EGCG-treated subjects. Therefore, these outcomes recommend that EGCG has the potential to block the progression of CVD and hypertension via an important decrease in LDL-C [166, 167]. Studies revealed that EGCG acts as an inhibitor of HDAC1, leading to better functioning of the heart. It decreases heart/body weight and the ratio of mtDNA/nDNA. In addition to inhibiting HDAC1, EGCG increases the binding of acetylated H3K9 or H3K14 in the promotor regions of peroxisome proliferator-activated receptor-1α and nuclear respiratory factors [168].
Cardiac hypertrophy and heart failure
The protection of cardiac homeostasis comprises hypertrophy and enlargement of heart size, as well as increased protein synthesis, which are the major symptoms of cardiac hypertrophy that generally cause a decline in the heart's capability to pump blood to the organs. The therapeutic potential effect of EGCG in treating hypertension-mediated learning as well as memory impairment is possibly due to its influential antioxidative roles [169]. EGCG is helpful in fighting against aging-mediated cardiac hypertrophy, fibrosis, and cell death [170]. EGCG prevents the development of heart failure in mice via inhibition of myocardial fibrosis and decline of ventricular collagen remodelling [171]. EGCG inhibits NF-κB activation and subsequent TGF overexpression. However, it diminished the expression of fibronectin and the proliferation of rat cardiac fibroblasts [165, 172]. The treatment of EGCG drastically recovered cardiac diastolic role via up-regulating cTnI via preventing histone deacetylase 1/3 expression. EGCG can contribute to the hindrance of cardiac diastolic dysfunction [173].
Neurodegenerative disorders
An effective and well-mentioned role of EGCG is to reduce the formation and accumulation of ROS production and increase pro-apoptotic markers in various tissues. EGCG imparts neuroprotective effects and widens neuro-rescue actions; thus, EGCG shows neuroprotective effects. Some human studies have revealed a dose-dependent relationship between EGCG intake and neuro-related disorders such as AD and PD [10, 174].
PD is a neurodegenerative disorder associated with the accumulation of Fe, oxidative stress, and inflammation. It is distinguished by nigrostriatal degeneration, which may involve α-synuclein (αS) aggregation, dysregulation of redox metal homeostasis, and neurotoxicity. However, tea polyphenols play a vital function, halting the progression of PD development. Tea polyphenols can directly obstruct the aggregation of the αS protein and alter signaling cascades in animal models [175].
EGCG prevents αS aggregation and interferes with PD development. The aggregation has reduced via EGCG in a concentration-dependent way, as revealed through a lack of thioflavin T binding [176, 177]. αS aggregates have been identified via incubation with A-Syn-HiLyte488. This binding has been inhibited via EGCG [176]. The αS amino acid positions that are interrelated with EGCG have been identified on peptide membranes. EGCG attaches to αS through unstable hydrophobic interfaces. EGCG might be a promising altering drug of αS aggregates for the management of PD and other α-synucleinopathies [176].
EGCG post-treatment significantly rescued 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity by increasing the rotational latency and increasing dopamine [178–181]. EGCG has antioxidant and iron-chelating properties. EGCG blocked MPTP-mediated neurotoxicity by raising the locomotor activity, positive neurons, and striatal dopamine (DA) concentrations. A decline in TH action with iron, EGCG inhibited MPTP-induced neurodegeneration and rescued dopaminergic neurons after MPTP treatment in iron excess condition [182–184].
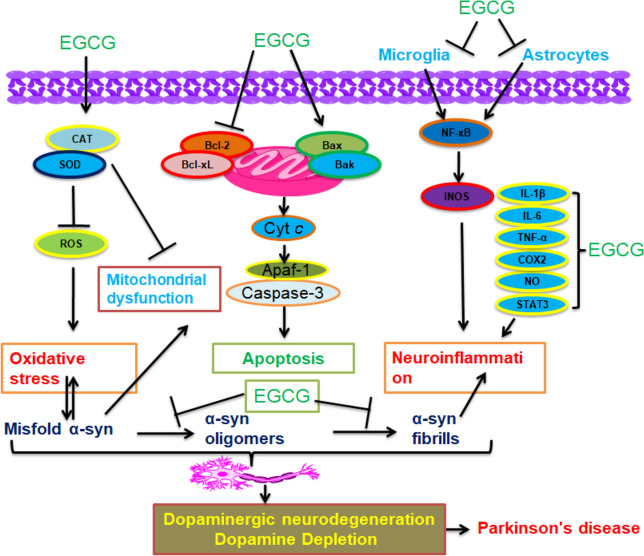
The neuroprotective functions of EGCG were reported in PD models. Because of its multi-targeted activities, EGCG might have the potential to be a new drug for the treatment of PD and for preventing neurodegeneration. The molecular mechanism by which EGCG exerts neuroprotective advantages, such as inducing apoptosis and inhibition of inflammation, oxidative stress, modulation of dopamine making, and the aggregation of αS, is described in Fig. 6.
Fig. 6.
The potential neuroprotective effects of EGCG in PD. The mechanisms of EGCG exert neuroprotective advantages. EGCG can prevent protein misfolding, neuronal apoptosis, oxidative stress, and neuroinflammatory responses
The main pathological characteristics of AD is amyloid β peptide (Aβ) accumulation in the brain [185–187]. Aβ is believed to be a vital neuroinflammatory stimulus to microglia. Treatment with EGCG has been found to reduce the aggregation of amyloid [16]. Aβ aggregates have been diminished after EGCG treatment in APP transgenic animal models. EGCG controlled (amyloid precursor protein) APP processing and subsequently reduced Aβ deposition [16].
EGCG is involved in the non-amyloidogenic procedure via stimulating α-secretase cleavage in SweAPP N2a cells. EGCG enhances sAPP-α and diminished Aβ formation in MC65 cells, hence showing the defensive nature of EGCG on brain edema and neuronal injury[16]. EGCG bypassed the blood–brain barrier (BBB) to accomplish the efficient parts of the brain. EGCG is being considered a promising agent for managing NDs [188–190]. The function of EGCG in treating AD has been studied, demonstrating that EGCG participates in a neuroprotective function and is promising to be utilized as a therapeutic drug for treating AD (Fig. 7).
Fig. 7.
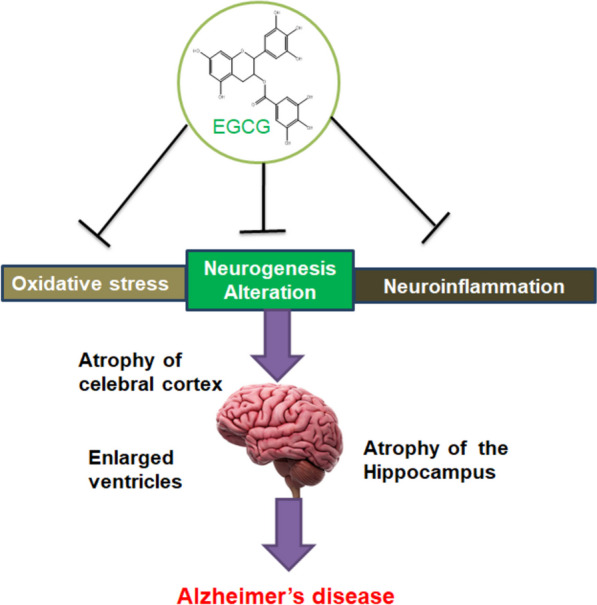
The promising effects of EGCG in AD pathogenesis. EGCG participates in a neuroprotective function and is prospectively utilized as a therapeutic drug for managing AD
EGCG enhances the production of soluble proteins of AβPP, sAβPPα through PKC-dependent α-secretase activation. Cleavage of AβPP to sAβPPα leads to a non-amyloidogenic secretory pathway accomplished via a putative α-secretase, therefore precluding the formation of Aβ, the last is controlled through the sequential activity of β and γ-secretases [191]. The neuro-protecting results of EGCG against Aβ-mediated neuronal loss and tau toxicity in AD models have been identified in multiple studies. EGCG, with its APP processing ability, provides a hopeful and different strategy for AD prevention [16].
Metabolic disorders
Diabetes mellitus
Metabolic diseases (MDs), diabetes, and obesity are the most common disorders [192]. Diabetes mellitus (DM), one of the most frequent metabolic disorders worldwide, is attributed to hyperglycemia caused by either reduced insulin emission or insulin resistance. Multiple studies have revealed that type 2 diabetes mellitus (T2DM) might stimulate various complications, including diabetic cardiovascular complications, diabetic nephropathy, and neuropathy, which were the main reasons for its mortality and morbidity [193]. The chief pathophysiologic factors that cause T2DM are peripheral insulin resistance and the last devastation of insulin creator pancreatic cells [194, 195].
The antioxidant properties of EGCG have been studied in multiple diseases. However, improving the bioavailability of EGCG via nano-formulation might contribute to a more productive treatment of DM metabolic consequences and vascular complications [196]. Although the mechanisms by which EGCG is related to the onset of DM are still unknown, the possibility might explain the hyperglycemia-induced inflammation (Fig. 8). A report has discussed a case of popular interstitial EGCG composed of MMP-9-bearing cells in a type II DM patient. EGCG improves insulin sensitivity and glycaemic control and drastically diminish serum triglycerides and total cholesterol levels following long-term supplementation [142]. Additionally, EGCG declined triglycerides and considerably enhanced HDL and glucagon-like peptide 1 levels in a randomized, double-blinded, placebo-controlled clinical trial linking patients with T2DMs and lipid abnormalities [197].
Fig. 8.
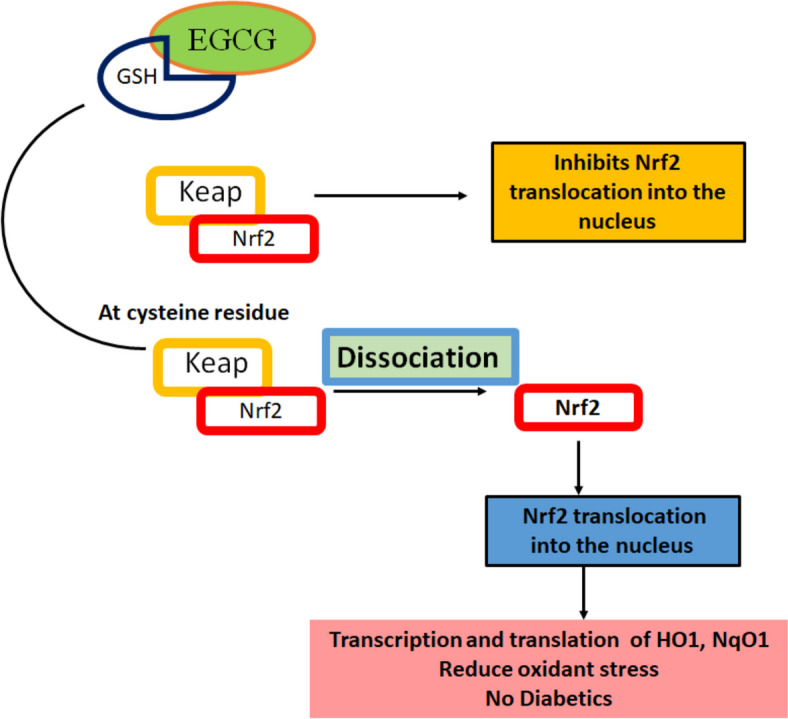
The Role of EGCG in Diabetes Management. Showing the pivotal role of EGCG in diabetes, particularly through its interaction with the KEAP1-Nrf2 signaling pathway. EGCG forms a hybrid complex with glutathione (GSH), which binds to KEAP1, resulting in the dissociation of KEAP1 from Nrf2. This dissociation allows Nrf2 to translocate into the nucleus, where it initiates the transcription of crucial antioxidant proteins, including heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase 1 (NQO1). These antioxidant proteins play an important role in reducing oxidative stress and inflammation, key contributors to diabetic complications
Obesity
Obesity is predominantly the outcome of a positive energy equilibrium determined by enhanced calorie-enrich food utilization and minimal exercise. It is considered a low-grade systematic inflammation disease. It could react quickly and animatedly to modifications in nutrient excess via adipocyte hypertrophy as well as hyperplasia. Obesity is principally motivated by an inequity between energy intake and expenditure; this modification leads to an improvement of the adipose tissue due to the gathering of lipids that happens in peripheral-related organs [198]. EGCG is shown to have anti-obesity as well as anti-diabetic effects [199]. The promising role of ECGC as an antioxidant and anti-inflammatory in the formation of gut microbiota and favours the formation of bacteria such as Alloprevotella and Muribaculaceae [200]. EGCG enables the reduction of negative regulating bacteria, Blautia, hence mining the functions of probiotics to improve intestinal inflammation and treat obesity.
Apart from gut improvement, EGCG shows its prominent role in balancing the level and proportion of gut SCFAs, the expression levels of inflammatory and transcription factors, and alterations in hypothalamic neurotransmitters. This suggests the effect of EGCG on the overall body axis, thereby improving obesity and its induced inflammatory response [201].
Although epidemiological and clinical studies explain the health advantages of EGCG on diabetes and obesity, the mechanisms of its actions are promising based on several laboratory data. EGCG had a considerable outcome on the diminished obesity in body weight gain and decline in epididymal adipose tissue weight, which influenced serum lipid attributes, such as triglyceride and cholesterol [202].
Conclusions and future prospects
EGCG, a primary polyphenol present as a major composition in green tea, has achieved a significant attantion due to its proven importance in maintaining human health. It has a wide range of biological activities in preventing various disease. EGCG has antioxidant, anti-inflammatory, and anti-microbial properties. These activities are connected to its ability to inhibit the growth of cancer cells, prevent tooth decay, reduce inflammation, and protect neurons from damage. EGCG improves overall health in oral dentistry, including bad breath, oral cancer, tooth decay, and oral cavity inflammation.
While preclinical studies highlight the potential of EGCG in treating various diseases, rigorous clinical trials are needed to establish its efficacy and safety in humans. Future research should focus on determining the optimal dosage, treatment duration, and delivery methods to maximize therapeutic benefits while minimizing potential side effects. Efficient delivery of EGCG to target tissues remains a challenge due to its poor bioavailability and rapid metabolism. Future research could explore advanced delivery systems such as nanoparticles, liposomes, or other innovative drug delivery technologies that enhance stability and bioavailability of EGCG, allowing it to reach specific tissues more effectively.
EGCG modulates various signaling pathways and opens avenue for its use in combination with other therapeutic agents. EGCG could be tailored to target specific signaling pathways unique to certain cancer types. This precision approach may lead to more personalized cancer therapies with higher efficacy. Future studies could investigate the synergistic effects of EGCG when combined with other drugs or natural compounds, potentially enhancing treatment outcomes for conditions like cancer, neurodegenerative diseases, and metabolic disorders.
In addition to advancements in genomics and personalized medicine, there is potential to tailor EGCG-based therapies to individual genetic profiles. Although EGCG is generally considered safe, long-term studies are needed to assess its safety profile, particularly at higher doses or with prolonged use. Investigating potential interactions with other medications and understanding the long-term effects on different organ systems will be crucial for its safe therapeutic application.
EGCG has significant potential as a chemopreventive agent, particularly in individuals at high risk of developing certain cancers. Future studies could explore the role of EGCG in preventing cancer initiation and progression, potentially leading to its use in preventive strategies for at-risk populations. The role of EGCG in neuroprotection, cancer prevention, and oral health, future studies could explore its potential in other areas such as cardiovascular health, diabetes management, and immune system modulation. Expanding the scope of research could uncover new therapeutic applications for EGCG. The future of EGCG in disease therapy lies in its potential to serve as both a preventive agent and a therapeutic adjunct. By developing advanced delivery systems, combining EGCG with existing therapies, and conducting rigorous clinical trials, EGCG could become an integral part of newer treatments.
For EGCG to transition from experimental studies to clinical practice, it must undergo rigorous evaluation . EGCG holds significant promise as a therapeutic agent for various diseases, particularly in neuroprotection, cancer prevention, and oral health. However, realizing its full potential requires continued research, clinical validation, and the development of innovative delivery methods. Overall, the available evidence suggests that EGCG has the potential to be a safe and effective treatment for several oral diseases and neurodegenerative diseases. Hence, more investigation is needed to validate these findings and to develop more effective delivery methods for EGCG.
Acknowledgements
MA thanks the Indian Council of Medical Research for financial support (Grant No. 45/6/2020-DDI/BMS). MIH acknowledges the Indian Council of Medical Research for financial support (Grant No. ISRM/12(22)/2020). AS thanks to the Ajman University for the payment of APC.
Abbreviations
- EGCG
Epigallocatechin 3-gallate
- AD
Alzheimer's disease
- PD
Parkinson's disease
- (Aβ)
Amyloid β peptide
- COX-2
Cyclooxygenase 2
- MMP-9
Matrix metalloproteinase-9
- NF-κB
Nuclear Factor Kappa B
- ERK1/2
Extracellular signal-regulated kinases1/2
- H2S
Hydrogen sulfide
- CH3SH
Methyl mercaptan
- ROS
Reactive oxygen species
- H2O2
Hydrogen peroxide
- VEGF
Vascular endothelial growth factor
- NHL
Non-Hodgkin lymphomas
- TNF
Tumor necrosis factor
- EGFR
Epidermal growth factor receptor
Authors’ contributions
All authors have read and agreed to publish the current version of the manuscript. Manzar Alam: Conceptualization, Writing- Original draft preparation, Data curation, Investigation, Methodology; Mehak Gulzar: Writing- Original draft preparation, Investigation, Methodology; Mohammad Salman Akhtar: Writing- Original draft preparation, Investigation, Methodology; Summya Rashid: Writing- Original draft preparation, Data curation, Methodology, Zulfareen: Data curation, Investigation, Methodology; Tanuja: Writing- Original draft preparation, Data curation, Investigation, Methodology; Anas Shamsi: Writing- Original draft preparation, Data curation, Md. Imtaiyaz Hassan: Conceptualization, Writing- Original draft preparation review and editing, Investigation, Supervision, project administration. All authors have read and approved the final version of the manuscript.
Funding
This work is supported by the Central Council for Research in Unani Medicine (CCRUM), Ministry of AYUSH, Government of India (Grant No. 3–69/2020- CCRUM/Tech).
Data availability
All data generated or analyzed during this study are included in this manuscript and are attached to this article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manzar Alam and Mehak Gulzar contributed equally to this work.
Contributor Information
Anas Shamsi, Email: anas.shamsi18@gmail.com.
Md. Imtaiyaz Hassan, Email: mihassan@jmi.ac.in.
References
- 1.Fraga CG, Croft KD, Kennedy DO, Tomás-Barberán FA. The effects of polyphenols and other bioactives on human health. Food Funct. 2019;10(2):514–28. 10.1039/C8FO01997E. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Mao B, Cui S, Zhang Q, Zhao J, Tang X, et al. Absorption, metabolism, bioactivity, and biotransformation of epigallocatechin gallate. Crit Rev Food Sci Nutr. 2024;64(19):6546–66. 10.1080/10408398.2023.2170972. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T, Miyoshi N, Hayakawa S, Imai S, Isemura M, Nakamura Y. Health benefits of tea consumption. Beverage impacts on health and nutrition. Springer; 2016. p. 49–67. 10.1007/978-3-319-23672-8_4
- 4.Hayakawa S, Oishi Y, Tanabe H, Isemura M, Suzuki Y. Tea, Coffee and Health Benefits. Bioactive Molecules in Food; Mérillon, J-M, Ramawat, KG, Eds. 2018:1–58. 10.3390/molecules25194553
- 5.Kciuk M, Alam M, Ali N, Rashid S, Głowacka P, Sundaraj R, et al. Epigallocatechin-3-gallate therapeutic potential in cancer: mechanism of action and clinical implications. Molecules. 2023;28(13):5246. 10.3390/molecules28135246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reygaert WC. Green tea catechins: Their use in treating and preventing infectious diseases. BioMed Res Int. 2018;2018:9105261. 10.1155/2018/9105261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakrawarti L, Agrawal R, Dang S, Gupta S, Gabrani R. Therapeutic effects of EGCG: a patent review. Expert Opin Ther Pat. 2016;26(8):907–16. 10.1080/13543776.2016.1203419. [DOI] [PubMed] [Google Scholar]
- 8.Talib WH, Awajan D, Alqudah A, Alsawwaf R, Althunibat R, Abu AlRoos M, et al. Targeting cancer hallmarks with Epigallocatechin Gallate (EGCG): mechanistic basis and therapeutic targets. Molecules. 2024;29(6):1373. 10.3390/molecules29061373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siriphap A, Kiddee A, Duangjai A, Yosboonruang A, Pook-In G, Saokaew S, et al. Antimicrobial activity of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against clinical isolates of multidrug-resistant Vibrio cholerae. Antibiotics. 2022;11(4):518. 10.3390/antibiotics11040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh NA, Mandal AKA, Khan ZA. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr J. 2015;15:1–17. 10.1186/s12937-016-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cascella M, Bimonte S, Muzio MR, Schiavone V, Cuomo A. The efficacy of Epigallocatechin-3-gallate (green tea) in the treatment of Alzheimer’s disease: An overview of pre-clinical studies and translational perspectives in clinical practice. Infect Agents Cancer. 2017;12(1):1–7. 10.1186/s13027-017-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury A, Sarkar J, Chakraborti T, Pramanik PK, Chakraborti S. Protective role of epigallocatechin-3-gallate in health and disease: a perspective. Biomed Pharmacother. 2016;78:50–9. 10.1016/j.biopha.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Zhu Q, Chen J-Y, OuYang D, Lu J-H. The pharmacological activity of epigallocatechin-3-gallate (EGCG) on Alzheimer’s disease animal model: a systematic review. Phytomedicine. 2020;79:153316. 10.1016/j.phymed.2020.153316. [DOI] [PubMed] [Google Scholar]
- 14.Singh R, Akhtar N, Haqqi TM. Green tea polyphenol epigallocatechi3-gallate: Inflammation and arthritis. Life Sci. 2010;86(25–26):907–18. 10.1016/j.lfs.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalatbary AR, Khademi E. The green tea polyphenolic catechin epigallocatechin gallate and neuroprotection. Nutr Neurosci. 2020;23(4):281–94. 10.1080/1028415X.2018.1500124. [DOI] [PubMed] [Google Scholar]
- 16.Youn K, Ho C-T, Jun M. Multifaceted neuroprotective effects of (-)-epigallocatechin-3-gallate (EGCG) in Alzheimer’s disease: An overview of pre-clinical studies focused on β-amyloid peptide. Food Sci Human Wellness. 2022;11(3):483–93. 10.1016/j.fshw.2021.12.006. [Google Scholar]
- 17.Nasb M, Li F, Dayoub L, Wu T, Wei M, Chen N. Bridging the gap: Integrating exercise mimicry into chronic disease management through suppressing chronic inflammation. J Adv Res. 2024;3(24):00176. 10.1016/j.jare.2024.04.034. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Ge S, Ahammed GJ, Gao H, Shen K, Wang Q, et al. Epigallocatechin-3-gallate-induced tolerance to cadmium stress involves increased flavonoid synthesis and nutrient homeostasis in tomato roots. Plant Physiol Biochem. 2024;208:108468. 10.1016/j.plaphy.2024.108468. S0981-9428(24)00136-0. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Xu S, Luo J, Zhang S, Wu D, Yang Q, et al. Epigallocatechin-3-gallate alleviates ethanol-induced endothelia cells injury partly through alteration of NF-kappaB translocation and activation of the Nrf2 signaling pathway. Biol Pharm Bull. 2024;47(7):1248–54. 10.1248/bpb.b23-00773. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Fei X, Li Y, Zhang H, Chen L, Ruan J, et al. Epigallocatechin-3-gallate attenuates fluoride induced apoptosis via PI3K/FoxO1 pathway in ameloblast-like cells. Toxicon. 2024;247:107857. 10.1016/j.toxicon.2024.107857. S0041-0101(24)00429-X. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Wang Q, Zhu F. Epigallocatechin-3-gallate attenuates the sulfamethoxazole-induced immunotoxicity and reduces SMZ residues in Procambarus clarkia. J Hazard Mater 2024 472;134602.10.1016/j.jhazmat.2024.134602 S0304-3894(24)01181-6 [DOI] [PubMed]
- 22.Kim E, Hwang K, Lee J, Han SY, Kim E-M, Park J, et al. Skin protective effect of epigallocatechin gallate. Int J Mol Sci. 2018;19(1):173. 10.3390/ijms19010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem. 2007;55(22):8896–907. 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- 24.Vishnoi H, Bodla RB, Kant R, Bodla R. Green Tea (Camellia sinensis) and its antioxidant property: A review. International Journal of Pharmaceutical Sciences and Research. 2018 9(5);1723–36. 10.13040/IJPSR.0975-8232
- 25.Nobari H, Saedmocheshi S, Chung LH, Suzuki K, Maynar-Mariño M, Pérez-Gómez J. An overview on how exercise with green tea consumption can prevent the production of reactive oxygen species and improve sports performance. Int J Environ Res Public Health. 2021;19(1):218. 10.3390/ijerph19010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alam M, Shamsi A, Hassan MI. 11 Biological roles and mechanism of phytochemicals in disease prevention and treatment. 2023. 10.1201/b22842-11
- 27.Dube A, Ng K, Nicolazzo JA, Larson I. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem. 2010;122(3):662–7. 10.1016/j.foodchem.2010.03.027. [Google Scholar]
- 28.Nagle DG, Ferreira D, Zhou Y-D. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006;67(17):1849–55. 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochman J, Jakubczyk K, Antoniewicz J, Mruk H, Janda K. Health benefits and chemical composition of matcha green tea: a review. Molecules. 2020;26(1):85. 10.3390/molecules26010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuskavage JK. Epigallocatechin gallate in the regulation of insulin secretion: Virginia Tech; 2008. http://hdl.handle.net/10919/32761
- 31.Mokra D, Joskova M, Mokry J. Therapeutic effects of green tea polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) in relation to molecular pathways controlling inflammation, oxidative stress, and apoptosis. Int J Mol Sci. 2022;24(1):340. 10.1039/C3RA45933K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makarewicz M, Drożdż I, Tarko T, Duda-Chodak A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants. 2021;10(2):188. 10.3390/antiox10020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mokra D, Adamcakova J, Mokry J. Green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG): a time for a new player in the treatment of respiratory diseases? Antioxidants. 2022;11(8):1566. 10.3390/antiox11081566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitichalermkiat A, Katsuki M, Sato J, Sonoda T, Masuda Y, Honjoh K-i, et al. Effect of epigallocatechin gallate on gene expression of Staphylococcus aureus. J Glob Antimicrob Resist. 2020;22:854–9. 10.1016/j.jgar.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol. 2013;168(5):1059–73. 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali A, Parisi A, Normanno G. Polyphenols as emerging antimicrobial agents. Emerging Modalities in Mitigation of Antimicrobial Resistance. Springer; 2022. p. 219–59. 10.1007/978-3-030-84126-3_10.
- 37.Gemma S, Brogi S, Patil PR, Giovani S, Lamponi S, Cappelli A, et al. From (+)-epigallocatechin gallate to a simplified synthetic analogue as a cytoadherence inhibitor for P. falciparum. Rsc Adv. 2014;4(9):4769–81. 10.1039/C3RA45933K. [Google Scholar]
- 38.Serra DO, Mika F, Richter AM, Hengge R. The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and downregulating the biofilm regulator CsgD via the σE-dependent sRNA RybB. Mol Microbiol. 2016;101(1):136–51. 10.1111/mmi.13379. [DOI] [PubMed] [Google Scholar]
- 39.Gao T, Ye F, Tan Y, Peng M, Yuan F, Liu Z, et al. Metabolomics and proteomics analyses revealed mechanistic insights on the antimicrobial activity of epigallocatechin gallate against Streptococcus suis. Front Cell Infect Microbiol. 2022;12:973282. 10.3389/fcimb.2022.973282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S-B, Choi E-H, Jeong K-H, Kim K-S, Shim S-M, Kim G-H. Effect of catechins and high-temperature-processed green tea extract on scavenging reactive oxygen species and preventing Aβ1–42 fibrils’ formation in brain microvascular endothelium. Nutr Neurosci. 2020;23(5):363–73. 10.1080/1028415X.2018.1507618. [DOI] [PubMed] [Google Scholar]
- 41.Cano A, Ettcheto M, Chang J-H, Barroso E, Espina M, Kühne BA, et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J Contr Rel. 2019;301:62–75. 10.1016/j.jconrel.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh AP, Biswas A, Shukla A, Maiti P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct Target Ther. 2019;4(1):33. 10.1038/s41392-019-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cano A, Ettcheto M, Chang JH, Barroso E, Espina M, Kuhne BA et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer's disease mice model. J Control Release. 2019;301:62–75 10.1016/j.jconrel.2019.03.010 S0168-3659(19)30157-9 [DOI] [PMC free article] [PubMed]
- 44.Zheng M, Pan M, Zhang W, Lin H, Wu S, Lu C, et al. Poly (α-l-lysine)-based nanomaterials for versatile biomedical applications: Current advances and perspectives. Bioact Mat. 2021;6(7):1878–909. 10.1016/j.bioactmat.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alam M, Kashyap T, Mishra P, Panda AK, Nagini S, Mishra R. Role and regulation of proapoptotic Bax in oral squamous cell carcinoma and drug resistance. Head Neck. 2019;41(1):185–97. 10.1002/hed.25471. [DOI] [PubMed] [Google Scholar]
- 46.Alam M, Ali S, Mohammad T, Hasan GM, Yadav DK, Hassan MI. B cell lymphoma 2: a potential therapeutic target for cancer therapy. Int J Mol Sci. 2021;22(19):10442. 10.3390/ijms221910442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almatroodi SA, Almatroudi A, Khan AA, Alhumaydhi FA, Alsahli MA, Rahmani AH. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules. 2020;25(14):3146. 10.3390/molecules25143146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Wu S, Li Q, Lang W, Li W, Jiang X, et al. Epigallocatechin-3-gallate: a phytochemical as a promising drug candidate for the treatment of Parkinson’s disease. Front Pharmacol. 2022;13:977521. 10.3389/fphar.2022.977521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonçalves PB, Sodero ACR, Cordeiro Y. Green tea epigallocatechin-3-gallate (EGCG) targeting protein misfolding in drug discovery for neurodegenerative diseases. Biomolecules. 2021;11(5):767. 10.3390/biom11050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bakun P, Mlynarczyk DT, Koczorowski T, Cerbin-Koczorowska M, Piwowarczyk L, Kolasiński E, et al. Tea-break with epigallocatechin gallate derivatives - powerful polyphenols of great potential for medicine. Eur J Med Chem. 2023;261(115820):14. 10.1016/j.ejmech.2023.115820. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Liu W, You H, Chen Z, Xu L, He H. Assessing the analgesic efficacy of oral epigallocatechin-3-gallate on epidural catheter analgesia in patients after surgical stabilisation of multiple rib fractures: a prospective double-blind, placebo-controlled clinical trial. Pharm Biol. 2020;58(1):741–4. 10.1080/13880209.2020.1797123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chu C, Deng J, Man Y, Qu Y. Green tea extracts epigallocatechin-3-gallate for different treatments. BioMed Res Int. 2017;2017:5615647. 10.1155/2017/5615647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alam M, Naqvi AAT, Hassan MI. Emerging Role of Structural and Systems Biology in Anticancer Therapeutics. Systems Biomedicine Approaches in Cancer Research. Springer; 2022. p. 97–114. 10.1007/978-981-19-1953-4_5
- 54.Lecumberri E, Dupertuis YM, Miralbell R, Pichard C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin Nutr. 2013;32(6):894–903. 10.1016/j.clnu.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Alam M, Ali S, Ahmed S, Elasbali AM, Adnan M, Islam A, et al. Therapeutic potential of ursolic acid in cancer and diabetic neuropathy diseases. Int J Mol Sci. 2021;22(22):12162. 10.3390/ijms222212162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali S, Alam M, Hassan MI. Kinase inhibitors: An overview. Protein Kinase Inhibitors. 2022:1–22. 10.1016/B978-0-323-91287-7.00026-0
- 57.Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J Neurochem. 2003;87(1):172–81. 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- 58.Huang Y-J, Wang K-L, Chen H-Y, Chiang Y-F, Hsia S-M. Protective effects of epigallocatechin gallate (EGCG) on endometrial, breast, and ovarian cancers. Biomolecules. 2020;10(11):1481. 10.3390/biom10111481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delabar JM, Gomes MAG, Fructuoso M, Sarrazin N, George N, Fleary-Roberts N, et al. EGCG-like non-competitive inhibitor of DYRK1A rescues cognitive defect in a down syndrome model. Eur J Med Chem. 2024;265:116098. 10.1016/j.ejmech.2023.116098. [DOI] [PubMed] [Google Scholar]
- 60.Sharma SK, Parasuraman P, Kumar G, Surolia N, Surolia A. Green tea catechins potentiate triclosan binding to enoyl-ACP reductase from Plasmodium falciparum (PfENR). J Med Chem. 2007;50(4):765–75. 10.1021/jm061154d. [DOI] [PubMed] [Google Scholar]
- 61.Meng J, Chen Y, Wang J, Qiu J, Chang C, Bi F, et al. EGCG protects vascular endothelial cells from oxidative stress-induced damage by targeting the autophagy-dependent PI3K-AKT-mTOR pathway. Ann Transl Med. 2020;8(5):200. 10.21037/atm.2020.01.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu H, Wang L, Li F, Jiang Y, Guan H, Wang D, et al. The synergistic protection of EGCG and quercetin against streptozotocin (STZ)-induced NIT-1 pancreatic β cell damage via upregulation of BCL-2 expression by miR-16-5p. J Nutr Biochem. 2021;96:108748. 10.1016/j.jnutbio.2021.108748. [DOI] [PubMed] [Google Scholar]
- 63.Liu T, Shi W, Ding Y, Wu Q, Zhang B, Zhang N, et al. (−)-Epigallocatechin gallate is a noncompetitive inhibitor of NAD kinase. ACS Med Chem Lett. 2022;13(11):1699–706. 10.1021/acsmedchemlett.2c00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo C, Huang Q, Wang Y, Yao Y, Li J, Chen J, et al. Therapeutic application of natural products: NAD+ metabolism as potential target. Phytomedicine. 2023;114:154768. 10.1016/j.phymed.2023.154768. [DOI] [PubMed] [Google Scholar]
- 65.Sharma C, Sadek B, Goyal SN, Sinha S, Kamal MA, Ojha S. Small molecules from nature targeting G-protein coupled cannabinoid receptors: potential leads for drug discovery and development. Evid-Based Complement Altern Med. 2015;2015(1):238482. 10.1155/2015/238482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cunningham CW. Plant-based modulators of endocannabinoid signaling. J Nat Prod. 2019;82(3):636–46. 10.1021/acs.jnatprod.8b00874. [DOI] [PubMed] [Google Scholar]
- 67.Abdel-Hamid NM, Abass SA. Matrix metalloproteinase contribution in management of cancer proliferation, metastasis and drug targeting. Mol Biol Rep. 2021;48(9):6525–38. 10.1007/s11033-021-06635-z. [DOI] [PubMed] [Google Scholar]
- 68.Wang W, Zhang Q, Xiong X, Zheng Y, Yang W, Du L. Investigation on the influence of galloyl moiety to the peptidyl prolyl cis/trans isomerase Pin1: a spectral and computational analysis. J Mol Liq. 2020;316:113870. 10.1016/j.molliq.2020.113870. [Google Scholar]
- 69.Zueva IV, Vasilieva EA, Gaynanova GA, Moiseenko AV, Burtseva AD, Boyko KM, et al. Can activation of acetylcholinesterase by β-Amyloid peptide decrease the effectiveness of cholinesterase inhibitors? Int J Mol Sci. 2023;24(22):16395. 10.3390/ijms242216395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu S, Li Y, Li Z, Ma C, Lou Z, Yokoyama W, et al. Lipase-catalyzed synthesis of acetylated EGCG and antioxidant properties of the acetylated derivatives. Food Res Int. 2014;56:279–86. 10.1016/j.foodres.2013.10.026. [Google Scholar]
- 71.El-Mowafy A, Salem H, Al-Gayyar M, El-Mesery M, El-Azab M. Evaluation of renal protective effects of the green-tea (EGCG) and red grape resveratrol: role of oxidative stress and inflammatory cytokines. Nat Prod Res. 2011;25(8):850–6. 10.1080/14786419.2010.533669. [DOI] [PubMed] [Google Scholar]
- 72.Chen W-C, Hsieh S-R, Chiu C-H, Hsu B-D, Liou Y-M. Molecular identification for epigallocatechin-3-gallate-mediated antioxidant intervention on the H 2 O 2-induced oxidative stress in H9c2 rat cardiomyoblasts. J Biomed Sci. 2014;21(1):1–12. 10.1186/1423-0127-21-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao Z, Han Y, Hu Y, Wu X, Wang Y, Zhang X, et al. Targeting HO-1 by epigallocatechin-3-gallate reduces contrast-induced renal injury via anti-oxidative stress and anti-inflammation pathways. PLoS ONE. 2016;11(2):e0149032. 10.1371/journal.pone.0149032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simos YV, Verginadis II, Toliopoulos IK, Velalopoulou AP, Karagounis IV, Karkabounas SC, et al. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox Rep. 2012;17(5):181–6. 10.1179/1351000212Y.0000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang W, Zhang M, Qiu Q, Zhang J, Hua C, Chen G, et al. Metabolomics of human umbilical vein endothelial cell-based analysis of the relationship between hyperuricemia and dyslipidemia. Nutr Metab Cardiovasc Dis. 2024;34(6):1528–37. 10.1016/j.numecd.2024.02.001. [DOI] [PubMed] [Google Scholar]
- 76.Xie H, Sun J, Chen Y, Zong M, Li S, Wang Y. EGCG attenuates uric acid-induced inflammatory and oxidative stress responses by medicating the NOTCH pathway. Oxid Med Cell Longev. 2015;2015(1):214836. 10.1155/2015/214836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cai J, Qiao Y, Chen L, Lu Y, Zheng D. Regulation of the Notch signaling pathway by natural products for cancer therapy. J Nutr Biochem. 2023;123:109483. 10.1016/j.jnutbio.2023.109483. [DOI] [PubMed] [Google Scholar]
- 78.Farooqi AA, Pinheiro M, Granja A, Farabegoli F, Reis S, Attar R, et al. EGCG mediated targeting of deregulated signaling pathways and non-coding RNAs in different cancers: focus on JAK/STAT, Wnt/β-Catenin, TGF/SMAD, NOTCH, SHH/GLI, and TRAIL mediated signaling pathways. Cancers. 2020;12(4):951. 10.3390/cancers12040951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kiesel VA, Stan SD. Modulation of notch signaling pathway by bioactive dietary agents. Int J Mol Sci. 2022;23(7):3532. 10.3390/ijms23073532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gadapa S, Battula SN, Mor S, Pushpadass HA, Naik LN, Emerald ME. Green tea catechin loaded niosomes: Formulation and their characterization for food fortification. J Food Sci Technol. 2022;59(9):3669–82. 10.1007/s13197-022-05384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Liu E, Gao H, He Q, Chen A, Pang Y, et al. Natural products for the treatment of hypertrophic scars: preclinical and clinical studies. Heliyon. 2024;10(17):e37059. 10.1016/j.heliyon.2024.e37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garcia Garcia JM, Vannuzzi V, Donati C, Bernacchioni C, Bruni P, Petraglia F. Endometriosis: cellular and molecular mechanisms leading to fibrosis. Reprod Sci. 2023;30(5):1453–61. 10.1007/s43032-022-01083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang B, Wang B, Cao S, Wang Y. Epigallocatechin-3-gallate (EGCG) attenuates traumatic brain injury by inhibition of edema formation and oxidative stress. Kor J Physiol Pharmacol. 2015;19(6):491–7. 10.1201/9781003402374-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abo-Salem OM, Ali TM, Harisa GI, Mehanna OM, Younos IH, Almalki WH. Beneficial effects of (−)-epigallocatechin-3-O-gallate on diabetic peripheral neuropathy in the rat model. J Biochem Mol Toxicol. 2020;34(8):e22508. 10.1002/jbt.22508. [DOI] [PubMed] [Google Scholar]
- 85.Arafa MH, Atteia HH. Protective role of epigallocatechin gallate in a rat model of cisplatin-induced cerebral inflammation and oxidative damage: impact of modulating NF-κB and Nrf2. Neurotox Res. 2020;37(2):380–96. 10.1007/s12640-019-00095-x. [DOI] [PubMed] [Google Scholar]
- 86.Payne A, Nahashon S, Taka E, Adinew GM, Soliman KF. Epigallocatechin-3-Gallate (EGCG): New therapeutic perspectives for neuroprotection, aging, and neuroinflammation for the modern age. Biomolecules. 2022;12(3):371. 10.3390/biom12030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sang S, Lambert JD, Ho C-T, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64(2):87–99. 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 88.Jude S, Gopi S. Multitarget approach for natural products in inflammation. Inflammation and natural products. Elsevier; 2021. p. 39–67. 10.1016/j.drudis.2014.08.006
- 89.Sah DK, Khoi PN, Li S, Arjunan A, Jeong J-U, Jung YD. (-)-Epigallocatechin-3-gallate prevents IL-1β-Induced uPAR expression and invasiveness via the suppression of NF-κB and AP-1 in human bladder cancer cells. Int J Mol Sci. 2022;23(22):14008. 10.3390/ijms232214008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pesapane A, Di Giovanni C, Rossi FW, Alfano D, Formisano L, Ragno P, et al. Discovery of new small molecules inhibiting 67 kDa laminin receptor interaction with laminin and cancer cell invasion. Oncotarget. 2015;6(20):18116. 10.18632/oncotarget.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y, Li D, Chen J, Wang S. A polysaccharide from Pinellia ternata inhibits cell proliferation and metastasis in human cholangiocarcinoma cells by targeting of Cdc42 and 67 kDa Laminin Receptor (LR). Int J Biol Macromol. 2016;93:520–5. 10.1016/j.ijbiomac.2016.08.069. [DOI] [PubMed] [Google Scholar]
- 92.Farkhondeh T, Pourbagher-Shahri AM, Ashrafizadeh M, Folgado SL, Rajabpour-Sanati A, Khazdair MR, et al. Green tea catechins inhibit microglial activation which prevents the development of neurological disorders. Neural Regen Res. 2020;15(10):1792–8. 10.4103/1673-5374.280300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fujimura Y, Sumida M, Sugihara K, Tsukamoto S, Yamada K, Tachibana H. Green tea polyphenol EGCG sensing motif on the 67-kDa laminin receptor. PLoS ONE. 2012;7(5):e37942. 10.1371/journal.pone.0037942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu H-N, Zhang L-C, Yang J-G, Das UN, Shen S-R. Effect of laminin tyrosine–isoleucine–glycine–serine–arginine peptide on the growth of human prostate cancer (PC-3) cells in vitro. Eur J Pharmacol. 2009;616(1):251–5. 10.1371/journal.pone.0037942. [DOI] [PubMed] [Google Scholar]
- 95.Fujimura Y, Yamada K, Tachibana H. A lipid raft-associated 67 kDa laminin receptor mediates suppressive effect of epigallocatechin-3-O-gallate on FcεRI expression. Biochem Biophys Res Commun. 2005;336(2):674–81. 10.1016/j.bbrc.2005.08.146. [DOI] [PubMed] [Google Scholar]
- 96.Jha A, Alam M, Kashyap T, Nath N, Kumari A, Pramanik KK, et al. Crosstalk between PD-L1 and Jak2-Stat3/MAPK-AP1 signaling promotes oral cancer progression, invasion and therapy resistance. Int Immunopharmacol. 2023;124:110894. 10.1016/j.intimp.2023.110894. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Z-X, Li Y-B, Zhao R-P. Epigallocatechin gallate attenuates β-amyloid generation and oxidative stress involvement of PPARγ in N2a/APP695 cells. Neurochem Res. 2017;42:468–80. 10.1007/s11064-016-2093-8. [DOI] [PubMed] [Google Scholar]
- 98.Zhou H, Fu L-X, Li L, Chen Y-Y, Zhu H-Q, Zhou J-L, et al. The epigallocatechin gallate derivative Y6 reduces the cardiotoxicity and enhances the efficacy of daunorubicin against human hepatocellular carcinoma by inhibiting carbonyl reductase 1 expression. J Ethnopharmacol. 2020;261:113118. 10.1016/j.jep.2020.113118. [DOI] [PubMed] [Google Scholar]
- 99.Zan L, Chen Q, Zhang L, Li X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered. 2019;10(1):374–82. 10.1080/21655979.2019.1657327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang L, Li P, Feng K. EGCG adjuvant chemotherapy: Current status and future perspectives. Eur J Med Chem. 2023;250:115197. 10.1016/j.ejmech.2023.115197. [DOI] [PubMed] [Google Scholar]
- 101.Yen C, Zhao F, Yu Z, Zhu X, Li CG. Interactions between natural products and tamoxifen in breast cancer: a comprehensive literature review. Front Pharmacol. 2022;13:847113. 10.3389/fphar.2022.847113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xie L-W, Cai S, Zhao T-S, Li M, Tian Y. Green tea derivative (−)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free Radical Biol Med. 2020;161:175–86. 10.1016/j.freeradbiomed.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 103.Zhao Y, Hu X, Zuo X, Wang M. Chemopreventive effects of some popular phytochemicals on human colon cancer: a review. Food Funct. 2018;9(9):4548–68. 10.1039/C8FO00850G. [DOI] [PubMed] [Google Scholar]
- 104.Singla RK, Behzad S, Khan J, Tsagkaris C, Gautam RK, Goyal R, et al. Natural kinase inhibitors for the treatment and management of endometrial/uterine cancer: preclinical to clinical studies. Front Pharmacol. 2022;13:801733. 10.3389/fphar.2022.801733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Phosrithong N, Ungwitayatorn J. Molecular docking study on anticancer activity of plant-derived natural products. Med Chem Res. 2010;19:817–35. 10.1007/s00044-009-9233-5. [Google Scholar]
- 106.Errachid A, Nohawica M, Wyganowska-Swiatkowska M. A comprehensive review of the influence of Epigallocatechin gallate on Sjögren’s syndrome associated molecular regulators of exocytosis. Biomed Rep. 2021;15(5):95. 10.3892/br.2021.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Remely M, Ferk F, Sterneder S, Setayesh T, Roth S, Kepcija T, et al. EGCG prevents high fat diet-induced changes in gut microbiota, decreases of DNA strand breaks, and changes in expression and DNA methylation of Dnmt1 and MLH1 in C57BL/6J male mice. Oxid Med Cell Longev. 2017;2017(1):3079148. 10.1155/2017/3079148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dharshini LCP, Mandal AKA. Regulation of gene expression by modulating microRNAs through Epigallocatechin-3-gallate in cancer. Mol Biol Rep. 2024;51(1):023–09145. 10.1007/s11033-023-09145-2. [DOI] [PubMed] [Google Scholar]
- 109.Li Y, Zhao Y, Han J, Wang Y, Lei S. Effects of epigallocatechin gallate (EGCG) on the biological properties of human dental pulp stem cells and inflammatory pulp tissue. Arch Oral Biol. 2021;123:105034. 10.1016/j.archoralbio.2020.105034. [DOI] [PubMed] [Google Scholar]
- 110.Demeule M, Brossard M, Pagé M, Gingras D, Béliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochim et Biophys Acta (BBA)-Protein Struct Mol Enzymol. 2000;1478(1):51–60. 10.1016/S0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 111.Huang Q, Zhu Y, Lv L, Sang S. Translating in vitro acrolein-trapping capacities of tea polyphenol and soy genistein to in vivo situation is mediated by the bioavailability and biotransformation of individual polyphenols. Mol Nutr Food Res. 2020;64(1):1900274. 10.1002/mnfr.201900274. [DOI] [PubMed] [Google Scholar]
- 112.Kong C, Zhang H, Li L, Liu Z. Effects of green tea extract epigallocatechin-3-gallate (EGCG) on oral disease-associated microbes: a review. J Oral Microbiol. 2022;14(1):2131117. 10.1080/20002297.2022.2131117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mosaddad SA, Tahmasebi E, Yazdanian A, Rezvani MB, Seifalian A, Yazdanian M, et al. Oral microbial biofilms: an update. Eur J Clin Microbiol Infect Dis. 2019;38:2005–19. 10.1007/s10096-019-03641-9. [DOI] [PubMed] [Google Scholar]
- 114.Vyas T, Nagi R, Bhatia A, Bains SK. Therapeutic effects of green tea as an antioxidant on oral health-a review. J Fam Med Prim Care. 2021;10(11):3998. 10.4103/jfmpc.jfmpc_943_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taylor PW, Hamilton-Miller J, Stapleton PD. Antimicrobial properties of green tea catechins. Food Sci Technol Bull. 2006;2:71–8. 10.1616/1476-2137.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ni D, Ai Z, Munoz-Sandoval D, Suresh R, Ellis PR, Yuqiong C, et al. Inhibition of the facilitative sugar transporters (GLUTs) by tea extracts and catechins. FASEB J. 2020;34(8):9995–10010. 10.1096/fj.202000057RR. [DOI] [PubMed] [Google Scholar]
- 117.Han JH, Kim M, Kim HJ, Jang SB, Bae S-J, Lee I-K, et al. Targeting lactate dehydrogenase a with catechin resensitizes SNU620/5FU gastric cancer cells to 5-Fluorouracil. Int J Mol Sci. 2021;22(10):5406. 10.3390/ijms22105406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manikandan S, Behera S, Karthikeyan R, Niranjana A, Bharathan R, Mohammed OFB. Effect of green tea extract mouthrinse and probiotic mouthrinse on salivary pH in a group of schoolchildren: an in vivo study. J Pharm Bioallied Sci. 2020;12(Suppl 1):S404. 10.4103/jpbs.jpbs_119_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dakshinamoorthy M, Subramanian M, Padmavathi K, Mahalakshmi K, Arumugam K, Paramasivam V. Effect of probiotic chocolate in the reduction of Streptococcus Mutans count. Biomed Pharmacol J. 2016;9(3). 10.13005/bpj/1051.
- 120.Morin M-P, Bedran TBL, Fournier-Larente J, Haas B, Azelmat J, Grenier D. Green tea extract and its major constituent epigallocatechin-3-gallate inhibit growth and halitosis-related properties of Solobacterium moorei. BMC Complement Altern Med. 2015;15:1–11. 10.1186/s12906-015-0557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kwon OS, Jung JH, Shin EA, Park JE, Park WY, Kim S-H. Epigallocatechin-3-gallate induces apoptosis as a TRAIL sensitizer via activation of caspase 8 and death receptor 5 in human colon cancer cells. Biomedicines. 2020;8(4):84. 10.3390/biomedicines8040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pramanik KK, Nagini S, Singh AK, Mishra P, Kashyap T, Nath N, et al. Glycogen synthase kinase-3β mediated regulation of matrix metalloproteinase-9 and its involvement in oral squamous cell carcinoma progression and invasion. Cell Oncol. 2018;41(1):47–60. 10.1007/s13402-017-0358-0. [DOI] [PubMed] [Google Scholar]
- 123.Pramanik KK, Singh AK, Alam M, Kashyap T, Mishra P, Panda AK, et al. Reversion-inducing cysteine-rich protein with Kazal motifs and its regulation by glycogen synthase kinase 3 signaling in oral cancer. Tumor Biol. 2016;37(11):15253–64. 10.1007/s13277-016-5362-x. [DOI] [PubMed] [Google Scholar]
- 124.Ho YC, Yang SF, Peng CY, Chou MY, Chang YC. Epigallocatechin-3-gallate inhibits the invasion of human oral cancer cells and decreases the productions of matrix metalloproteinases and urokinase-plasminogen activator. J Oral Pathol Med. 2007;36(10):588–93. 10.1111/j.1600-0714.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 125.Koh YW, Choi EC, Kang SU, Hwang HS, Lee MH, Pyun J, et al. Green tea (−)-epigallocatechin-3-gallate inhibits HGF-induced progression in oral cavity cancer through suppression of HGF/c-Met. J Nutr Biochem. 2011;22(11):1074–83. 10.1016/j.jnutbio.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 126.Xie L, Yi J, Song Y, Zhao M, Fan L, Zhao L. Suppression of GOLM1 by EGCG through HGF/HGFR/AKT/GSK-3β/β-catenin/c-Myc signaling pathway inhibits cell migration of MDA-MB-231. Food Chem Toxicol. 2021;157:112574. 10.1016/j.fct.2021.112574. [DOI] [PubMed] [Google Scholar]
- 127.Chen P-N, Chu S-C, Kuo W-H, Chou M-Y, Lin J-K, Hsieh Y-S. Epigallocatechin-3 gallate inhibits invasion, epithelial− mesenchymal transition, and tumor growth in oral cancer cells. J Agri Food Chem. 2011;59(8):3836–44. 10.1021/jf1049408. [DOI] [PubMed] [Google Scholar]
- 128.Yuan J-M. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6):1676S-S1681. 10.3945/ajcn.113.058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu X, Dai Z, Zhang Z, Kou X, You X, Sun H, et al. Fabrication of oral nanovesicle in-situ gel based on Epigallocatechin gallate phospholipid complex: Application in dental anti-caries. Eur J Pharmacol. 2021;897:173951. 10.1016/j.ejphar.2021.173951. [DOI] [PubMed] [Google Scholar]
- 130.Shinde S, Lee LH, Chu T. Inhibition of biofilm formation by the synergistic action of EGCG-S and antibiotics. Antibiotics. 2021;10(2):102. 10.3390/antibiotics10020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schneider-Rayman M, Steinberg D, Sionov RV, Friedman M, Shalish M. Effect of epigallocatechin gallate on dental biofilm of Streptococcus mutans: an in vitro study. BMC Oral Health. 2021;21:1–11. 10.1186/s12903-021-01798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aragão MGB, He X, Aires CP, Corona SAM. Epigallocatechin gallate reduces the virulence of cariogenic Streptococcus mutans biofilm by affecting the synthesis of biofilm matrix components. Arch Oral Biol. 2024;164:105990. 10.1016/j.archoralbio.2024.105990. [DOI] [PubMed] [Google Scholar]
- 133.Lagha AB, Grenier D. Tea polyphenols protect gingival keratinocytes against TNF-α-induced tight junction barrier dysfunction and attenuate the inflammatory response of monocytes/macrophages. Cytokine. 2019;115:64–75. 10.1016/j.cyto.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 134.Messire G, Serreau R, Berteina-Raboin S. Antioxidant effects of catechins (EGCG), andrographolide, and curcuminoids compounds for skin protection, cosmetics, and dermatological uses: an update. Antioxidants. 2023;12(7):1317. 10.3390/antiox12071317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ben Lagha A, Haas B, Grenier D. Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum. Sci Rep. 2017;7(1):44815. 10.1038/srep44815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang X, Liu Y, Wu Z, Zhang P, Zhang X. Tea polyphenols: a natural antioxidant regulates gut flora to protect the intestinal mucosa and prevent chronic diseases. Antioxidants. 2022;11(2):253. 10.3390/antiox11020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moghadam ET, Yazdanian M, Tahmasebi E, Tebyanian H, Ranjbar R, Yazdanian A, et al. Current herbal medicine as an alternative treatment in dentistry: In vitro, in vivo and clinical studies. Eur J Pharmacol. 2020;889:173665. 10.1016/j.ejphar.2020.173665. [DOI] [PubMed] [Google Scholar]
- 138.Guo Y, Li Z, Chen F, Chai Y. Polyphenols in oral health: homeostasis maintenance, disease prevention, and therapeutic applications. Nutrients. 2023;15(20):4384. 10.1016/j.ejphar.2020.173665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aggarwal V, Tuli HS, Tania M, Srivastava S, Ritzer EE, Pandey A et al., editors. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Seminars in cancer biology; 2022: Elsevier. 10.1016/j.semcancer.2020.05.011. [DOI] [PubMed]
- 140.da Silva-Júnior EF, Silva LR. Multi-target approaches of Epigallocatechin-3-O-gallate (EGCG) and its derivatives against Influenza Viruses. Curr Top Med Chem. 2022;22(18):1485–500. 10.2174/1568026622666220127112056. [DOI] [PubMed] [Google Scholar]
- 141.Tang G, Xu Y, Zhang C, Wang N, Li H, Feng Y. Green Tea and Epigallocatechin Gallate (EGCG) for the management of Nonalcoholic Fatty Liver Diseases (NAFLD): insights into the role of oxidative stress and antioxidant mechanism. Antioxidants. 2021;10(7):1076. 10.3390/antiox10071076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhu W, Tang H, Cao L, Zhang J, Li J, Ma D, et al. Epigallocatechin-3-O-gallate ameliorates oxidative stress-induced chondrocyte dysfunction and exerts chondroprotective effects via the Keap1/Nrf2/ARE signaling pathway. Chem Biol Drug Des. 2022;100(1):108–20. 10.1111/cbdd.14056. [DOI] [PubMed] [Google Scholar]
- 143.Wang Y, Huang M, Zhou X, Li H, Ma X, Sun C. Potential of natural flavonoids to target breast cancer angiogenesis. Br J Pharmacol. 2023. 10.1111/bph.16275. [DOI] [PubMed] [Google Scholar]
- 144.Marín V, Burgos V, Pérez R, Maria DA, Pardi P, Paz C. The potential role of Epigallocatechin-3-Gallate (EGCG) in breast cancer treatment. Int J Mol Sci. 2023;24(13):10737. 10.3390/ijms241310737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chu M, Zheng C, Chen C, Song G, Hu X, Wang Z-w, editors. Targeting cancer stem cells by nutraceuticals for cancer therapy. Seminars in cancer biology; 2022: Elsevier. 10.1016/j.semcancer.2021.07.008. [DOI] [PubMed]
- 146.Park IS, Cho JH, Han Y, Lee KW, Song YS. Targeting cancer stem cells with phytoceuticals for cancer therapy. Functional Foods in Cancer Prevention and Therapy. Elsevier; 2020. p. 329–57. 10.1016/j.jnutbio.2021.108843.
- 147.Wei H, Ge Q, Zhang L-Y, Xie J, Gan R-H, Lu Y-G, et al. EGCG inhibits growth of tumoral lesions on lip and tongue of K-Ras transgenic mice through the Notch pathway. J Nutr Biochem. 2022;99:108843. 10.1016/j.jnutbio.2021.108843. [DOI] [PubMed] [Google Scholar]
- 148.Lu X, Friedrich LJ, Efferth T. Natural products targeting tumour angiogenesis. Br J Pharmacol. 2023. 10.1111/bph.16232. [DOI] [PubMed] [Google Scholar]
- 149.Tanabe H, Suzuki T, Ohishi T, Isemura M, Nakamura Y, Unno K. Effects of epigallocatechin-3-gallate on matrix metalloproteinases in terms of its anticancer activity. Molecules. 2023;28(2):525. 10.3390/molecules28020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Alam M, Alam S, Shamsi A, Adnan M, Elasbali AM, Al-Soud WA, et al. Bax/Bcl-2 cascade is regulated by the EGFR pathway: therapeutic targeting of non-small cell lung cancer. Front Oncol. 2022;12:869672. 10.3389/fonc.2022.869672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pan Y, Lv H, Feng X, Zhou S, Hu H, Chen S, et al. Epigallocatechin gallate (EGCG) alleviates the inflammatory response and recovers oral microbiota in acetic acid-induced oral inflammation mice. Food Funct. 2023;14(22):10069–82. 10.1039/D3FO03107A. [DOI] [PubMed] [Google Scholar]
- 152.Bag N, Bag A. Antimetastatic properties of tea polyphenols. Nutr Cancer. 2020;72(3):365–76. 10.1080/01635581.2019.1638426. [DOI] [PubMed] [Google Scholar]
- 153.Yang L, Zhang W, Chopra S, Kaur D, Wang H, Li M, et al. The epigenetic modification of epigallocatechin gallate (EGCG) on cancer. Curr Drug Targets. 2020;21(11):1099–104. 10.2174/1389450121666200504080112. [DOI] [PubMed] [Google Scholar]
- 154.Bakhshandeh N, Mohammadi M, Mohammadi P, Nazari E, Damchi M, Khodabandelu S, et al. Increased expression of androgen receptor and PSA genes in LNCaP (prostate cancer) cell line due to high concentrations of EGCG, an active ingredient in green tea. Horm Mol Biol Clin Invest. 2023;44(2):181–6. 10.1515/hmbci-2022-0054. [DOI] [PubMed] [Google Scholar]
- 155.Thomas P, Dong J. (-)-Epicatechin acts as a potent agonist of the membrane androgen receptor, ZIP9 (SLC39A9), to promote apoptosis of breast and prostate cancer cells. J Steroid Biochem Mol Biol. 2021;211:105906. 10.1016/j.jsbmb.2021.105906. [DOI] [PubMed] [Google Scholar]
- 156.Ferrari E, Bettuzzi S, Naponelli V. The potential of epigallocatechin gallate (EGCG) in targeting autophagy for cancer treatment: a narrative review. Int J Mol Sci. 2022;23(11):6075. 10.3390/ijms23116075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Man GCW, Wang J, Song Y, Wong JH, Zhao Y, Lau TS, et al. Therapeutic potential of a novel prodrug of green tea extract in induction of apoptosis via ERK/JNK and Akt signaling pathway in human endometrial cancer. BMC Cancer. 2020;20:1–14. 10.1186/s12885-020-07455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dev SS, Farghadani R, Abidin SAZ, Othman I, Naidu R. Flavonoids as receptor tyrosine kinase inhibitors in lung cancer. J Funct Foods. 2023;110:105845. 10.1016/j.jff.2023.105845. [Google Scholar]
- 159.Li F, Hao S, Gao J, Jiang P. EGCG alleviates obesity-exacerbated lung cancer progression by STAT1/SLC7A11 pathway and gut microbiota. J Nutr Biochem. 2023;120:109416. 10.1016/j.jnutbio.2023.109416. [DOI] [PubMed] [Google Scholar]
- 160.Bernini R, Velotti F. Natural polyphenols as immunomodulators to rescue immune response homeostasis: Quercetin as a research model against severe COVID-19. Molecules. 2021;26(19):5803. 10.3390/molecules26195803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dey P, Olmstead BD, Sasaki GY, Vodovotz Y, Yu Z, Bruno RS. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. J Nutr Biochem. 2020;84:108455. 10.1016/j.jnutbio.2020.108455. [DOI] [PubMed] [Google Scholar]
- 162.Suhail M, Rehan M, Tarique M, Tabrez S, Husain A, Zughaibi TA. Targeting a transcription factor NF-κB by green tea catechins using in silico and in vitro studies in pancreatic cancer. Front Nutr. 2023;9:1078642. 10.3389/fnut.2022.1078642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khan H, Ullah H, Castilho PCMF, Gomila AS, D’Onofrio G, Filosa R, et al. Targeting NF-κB signaling pathway in cancer by dietary polyphenols. Crit Rev Food Sci Nutr. 2020;60(16):2790–800. 10.1080/10408398.2019.1661827. [DOI] [PubMed] [Google Scholar]
- 164.Niloy MS, Shakil MS, Alif MM, Rosengren RJ. Using natural compounds to target KRAS mutated non-small cell lung cancer. Curr Med Chem. 2021;28(39):8098–115. 10.2174/0929867328666210301105856. [DOI] [PubMed] [Google Scholar]
- 165.Eng QY, Thanikachalam PV, Ramamurthy S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J Ethnopharmacol. 2018;210:296–310. 10.1016/j.jep.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 166.Momose Y, Maeda-Yamamoto M, Nabetani H. Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans. Int J Food Sci Nutr. 2016;67(6):606–13. 10.1080/09637486.2016.1196655. [DOI] [PubMed] [Google Scholar]
- 167.Chen I-J, Liu C-Y, Chiu J-P, Hsu C-H. Therapeutic effect of high-dose green tea extract on weight reduction: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr. 2016;35(3):592–9. 10.1016/j.clnu.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 168.Li G, Pan B, Liu L, Xu X, Zhao W, Mou Q, et al. Epigallocatechin-3-gallate restores mitochondrial homeostasis impairment by inhibiting HDAC1-mediated NRF1 histone deacetylation in cardiac hypertrophy. Mol Cell Biochem. 2024;479(4):963–73. 10.1007/s11010-023-04768-2. [DOI] [PubMed] [Google Scholar]
- 169.Wang M-H, Chang W-J, Soung H-S, Chang K-C. (−)-Epigallocatechin-3-gallate decreases the impairment in learning and memory in spontaneous hypertension rats. Behav Pharmacol. 2012;23(8):771–80. 10.1097/fbp.0b013e32835a3bc8. [DOI] [PubMed] [Google Scholar]
- 170.Muhammed I, Sankar S, Govindaraj S. Ameliorative effect of epigallocatechin gallate on cardiac hypertrophy and fibrosis in aged rats. J Cardiovasc Pharmacol. 2018;71(2):65–75. 10.1097/fjc.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 171.Chen K, Chen W, Liu SL, Wu TS, Yu KF, Qi J, et al. Epigallocatechingallate attenuates myocardial injury in a mouse model of heart failure through TGF-β1/Smad3 signaling pathway. Mol Med Rep. 2018;17(6):7652–60. 10.3892/mmr.2018.8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cai Y, Yu S-S, Chen T-T, Gao S, Geng B, Yu Y, et al. EGCG inhibits CTGF expression via blocking NF-κB activation in cardiac fibroblast. Phytomedicine. 2013;20(2):106–13. 10.1016/j.phymed.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 173.Pan B, Quan J, Liu L, Xu Z, Zhu J, Huang X, et al. Epigallocatechin gallate reverses cTnI-low expression-induced age-related heart diastolic dysfunction through histone acetylation modification. J Cell Mol Med. 2017;21(10):2481–90. 10.1111/jcmm.13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chan D, Woo J, Ho S, Pang C, Law L, Ng P, et al. Genetic and environmental risk factors for Parkinson’s disease in a Chinese population. J Neurol Neurosurg Psychiatry. 1998;65(5):781–4. 10.1136/jnnp.65.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ono K, Tsuji M, Yamasaki TR, Pasinetti GM. Anti-aggregation effects of phenolic compounds on α-synuclein. Molecules. 2020;25(10):2444. 10.3390/molecules25102444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xu Y, Zhang Y, Quan Z, Wong W, Guo J, Zhang R, et al. Epigallocatechin gallate (EGCG) inhibits alpha-synuclein aggregation: a potential agent for Parkinson’s disease. Neurochem Res. 2016;41:2788–96. 10.1007/s11064-016-1995-9. [DOI] [PubMed] [Google Scholar]
- 177.Kumar S, Kumar R, Kumari M, Kumari R, Saha S, Bhavesh NS, et al. Ellagic acid inhibits α-synuclein aggregation at multiple stages and reduces its cytotoxicity. ACS Chem Neurosci. 2021;12(11):1919–30. 10.1021/acschemneuro.1c00001. [DOI] [PubMed] [Google Scholar]
- 178.Xu Q, Langley M, Kanthasamy A, Reddy M. Neurorescue effect of EGCG in an animal model of Parkinson's disease. The FASEB Journal. 2016 30;1174.11-11. 10.1007/s11064-016-1995-9.
- 179.Kujawska M, Jodynis-Liebert J. Polyphenols in Parkinson’s disease: a systematic review of in vivo studies. Nutrients. 2018;10(5):642. 10.3390/nu10050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen P, Totten M, Zhang Z, Bucinca H, Erikson K, Santamaría A, et al. Iron and manganese-related CNS toxicity: mechanisms, diagnosis and treatment. Expert Rev Neurother. 2019;19(3):243–60. 10.3390/nu10050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chu AJ. Quarter-century explorations of bioactive polyphenols: Diverse health benefits. Front Biosci-Landmark. 2022;27(4):134. 10.31083/j.fbl2704134. [DOI] [PubMed] [Google Scholar]
- 182.Mandel S, Youdim MB. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radical Biol Med. 2004;37(3):304–17. 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 183.Mandel SA, Amit T, Weinreb O, Youdim MB. Understanding the broad-spectrum neuroprotective action profile of green tea polyphenols in aging and neurodegenerative diseases. J Alzheimers Dis. 2011;25(2):187–208. 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 184.Alam M, Ahmed S, Abid M, Hasan GM, Islam A, Hassan MI. Therapeutic targeting of microtubule affinity-regulating kinase 4 in cancer and neurodegenerative diseases. J Cell Biochem. 2023;124(9):1223–40. 10.1002/jcb.30468. [DOI] [PubMed] [Google Scholar]
- 185.Iqbal K, Grundke-Iqbal I. Alzheimer neurofibrillary degeneration: significance, etiopathogenesis, therapeutics and prevention. J Cell Mol Med. 2008;12(1):38–55. 10.1111/j.1582-4934.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xue B, DasGupta D, Alam M, Khan MS, Wang S, Shamsi A, et al. Investigating binding mechanism of thymoquinone to human transferrin, targeting Alzheimer’s disease therapy. J Cell Biochem. 2022;123(8):1381–93. 10.1002/jcb.30299. [DOI] [PubMed] [Google Scholar]
- 187.Hasan GM, Shamsi A, Sohal SS, Alam M, Hassan MI. Structure-Based Identification of Natural Compounds as Potential RET-Kinase Inhibitors for Therapeutic Targeting of Neurodegenerative Diseases. Journal of Alzheimer's disease. 2023(Preprint):1–15. 10.3233/jad-230698. [DOI] [PubMed]
- 188.Cascella M, Muzio MR. Potential application of the Kampo medicine goshajinkigan for prevention of chemotherapy-induced peripheral neuropathy. J Integr Med. 2017;15(2):77–87. 10.1016/S2095-4964(17)60313-3. [DOI] [PubMed] [Google Scholar]
- 189.Soeda S, Aritake K, Urade Y, Sato H, Shoyama Y. Neuroprotective activities of saffron and crocin. The benefits of natural products for neurodegenerative diseases. 2016:275–92. 10.1007/978-3-319-28383-8_14 [DOI] [PubMed]
- 190.Grabska-Kobyłecka I, Szpakowski P, Król A, Książek-Winiarek D, Kobyłecki A, Głąbiński A, et al. Polyphenols and their impact on the prevention of neurodegenerative diseases and development. Nutrients. 2023;15(15):3454. 10.3390/nu15153454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Manzine PR, Ettcheto M, Cano A, Busquets O, Marcello E, Pelucchi S, et al. ADAM10 in Alzheimer’s disease: Pharmacological modulation by natural compounds and its role as a peripheral marker. Biomed Pharmacother. 2019;113:108661. 10.1016/j.biopha.2019.108661. [DOI] [PubMed] [Google Scholar]
- 192.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):1–8. 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 194.Martin MA, Goya L, Ramos S. Protective effects of tea, red wine and cocoa in diabetes. Evidences from human studies. Food Chem Toxicol. 2017;109:302–14. 10.1016/j.fct.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 195.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–88. 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 196.Bulboaca AE, Boarescu PM, Porfire AS, Dogaru G, Barbalata C, Valeanu M, et al. The effect of nano-epigallocatechin-gallate on oxidative stress and matrix metalloproteinases in experimental diabetes mellitus. Antioxidants (Basel). 2020;9(2):172. 10.3390/antiox9020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu C-Y, Huang C-J, Huang L-H, Chen I-J, Chiu J-P, Hsu C-H. Effects of green tea extract on insulin resistance and glucagon-like peptide 1 in patients with type 2 diabetes and lipid abnormalities: a randomized, double-blinded, and placebo-controlled trial. PLoS ONE. 2014;9(3):e91163. 10.1371/journal.pone.0091163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ali F, Ismail A, Kersten S. Molecular mechanisms underlying the potential antiobesity-related diseases effect of cocoa polyphenols. Mol Nutr Food Res. 2014;58(1):33–48. 10.1002/mnfr.201300277. [DOI] [PubMed] [Google Scholar]
- 199.Chen L, Pu Y, Xu Y, He X, Cao J, Ma Y, et al. Anti-diabetic and anti-obesity: Efficacy evaluation and exploitation of polyphenols in fruits and vegetables. Food Res Int. 2022;157:111202. 10.1016/j.foodres.2022.111202. [DOI] [PubMed] [Google Scholar]
- 200.Rodríguez-Daza MC, de Vos WM. Polyphenols as drivers of a homeostatic gut microecology and immuno-metabolic traits of Akkermansia muciniphila: from mouse to man. Int J Mol Sci. 2022;24(1):45. 10.3390/ijms24010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wu Z, Huang S, Li T, Li N, Han D, Zhang B, et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome. 2021;9:1–22. 10.1186/s40168-021-01115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Legeay S, Rodier M, Fillon L, Faure S, Clere N. Epigallocatechin gallate: a review of its beneficial properties to prevent metabolic syndrome. Nutrients. 2015;7(7):5443–68. 10.3390/nu7075230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript and are attached to this article.