Abstract
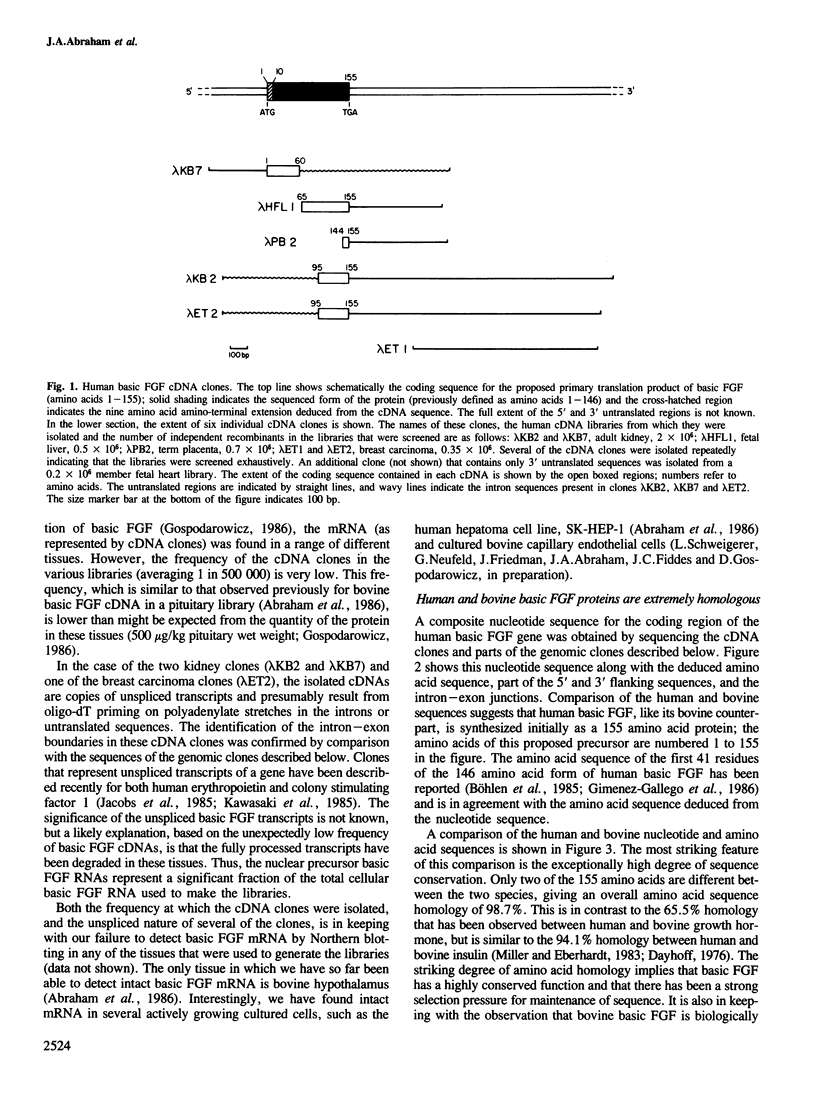
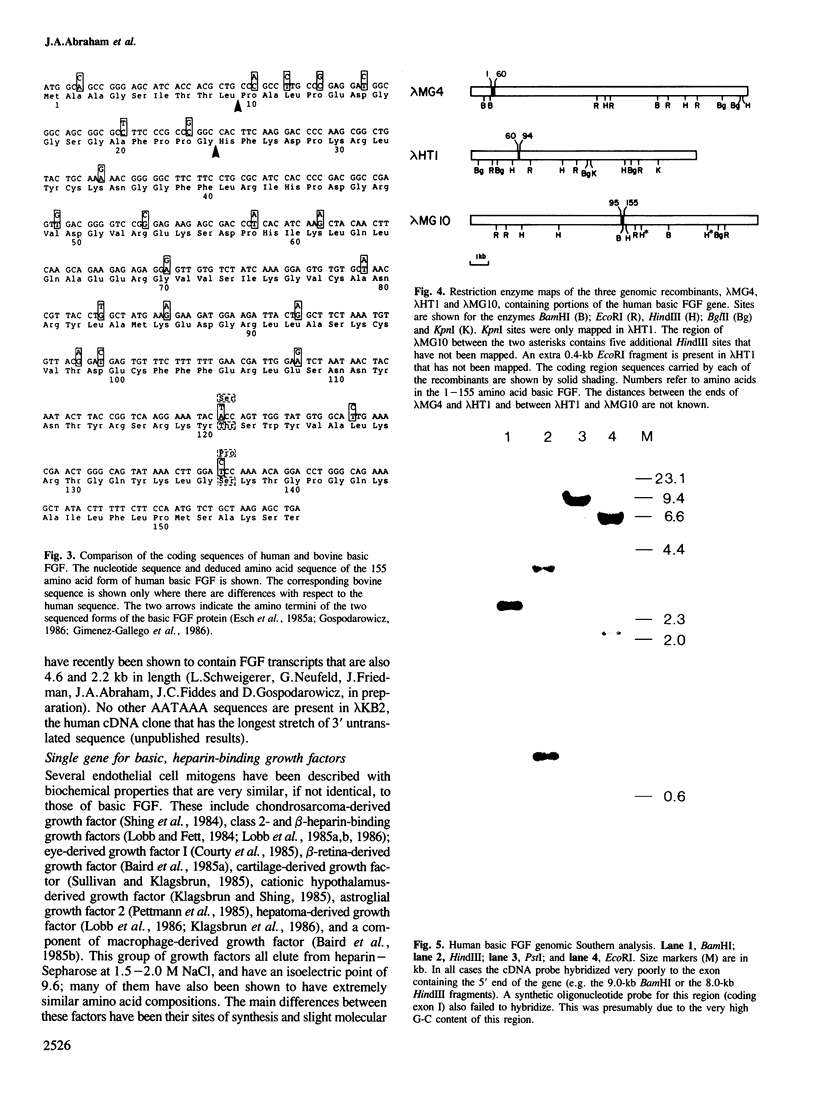
Clones encoding the angiogenic endothelial cell mitogen, basic fibroblast growth factor (FGF), have been isolated from human cDNA libraries made from kidney, fetal heart, fetal liver, term placenta, and a breast carcinoma. Basic FGF cDNA clones are present in these libraries at very low levels when compared to the quantity of the growth factor in the tissues. This observation, combined with the fact that several of the clones represent unspliced transcripts, suggests that cytoplasmic basic FGF mRNA is unstable and that the protein is stored in tissues. The amino acid sequence of human basic FGF, deduced from the sequence of these cDNAs and from genomic clones, is 99% homologous to that of bovine basic FGF, implying a strong selection pressure for maintenance of function and structure. As with the bovine factor, human basic FGF does not appear to have a signal peptide sequence. Southern blot analysis of human genomic DNA and mapping of the cloned gene shows that there is only one basic FGF gene. All of the basic, heparin-binding endothelial cell mitogens of similar amino acid composition that have been described must therefore be products of this single gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Baird A., Esch F., Gospodarowicz D., Guillemin R. Retina- and eye-derived endothelial cell growth factors: partial molecular characterization and identity with acidic and basic fibroblast growth factors. Biochemistry. 1985 Dec 31;24(27):7855–7860. doi: 10.1021/bi00348a001. [DOI] [PubMed] [Google Scholar]
- Baird A., Mormède P., Böhlen P. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun. 1985 Jan 16;126(1):358–364. doi: 10.1016/0006-291x(85)90614-x. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Esch F., Baird A., Jones K. L., Gospodarowicz D. Human brain fibroblast growth factor. Isolation and partial chemical characterization. FEBS Lett. 1985 Jun 3;185(1):177–181. doi: 10.1016/0014-5793(85)80765-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Courty J., Loret C., Moenner M., Chevallier B., Lagente O., Courtois Y., Barritault D. Bovine retina contains three growth factor activities with different affinity to heparin: eye derived growth factor I, II, III. Biochimie. 1985 Feb;67(2):265–269. doi: 10.1016/s0300-9084(85)80056-0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F., Ueno N., Baird A., Hill F., Denoroy L., Ling N., Gospodarowicz D., Guillemin R. Primary structure of bovine brain acidic fibroblast growth factor (FGF). Biochem Biophys Res Commun. 1985 Dec 17;133(2):554–562. doi: 10.1016/0006-291x(85)90942-8. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis and its inhibitors. Important Adv Oncol. 1985:42–62. [PubMed] [Google Scholar]
- Gimenez-Gallego G., Conn G., Hatcher V. B., Thomas K. A. Human brain-derived acidic and basic fibroblast growth factors: amino terminal sequences and specific mitogenic activities. Biochem Biophys Res Commun. 1986 Mar 13;135(2):541–548. doi: 10.1016/0006-291x(86)90028-8. [DOI] [PubMed] [Google Scholar]
- Gimenez-Gallego G., Rodkey J., Bennett C., Rios-Candelore M., DiSalvo J., Thomas K. Brain-derived acidic fibroblast growth factor: complete amino acid sequence and homologies. Science. 1985 Dec 20;230(4732):1385–1388. doi: 10.1126/science.4071057. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Thakral T. K. The angiogenic activity of the fibroblast and epidermal growth factor. Exp Eye Res. 1979 May;28(5):501–514. doi: 10.1016/0014-4835(79)90038-1. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs K., Shoemaker C., Rudersdorf R., Neill S. D., Kaufman R. J., Mufson A., Seehra J., Jones S. S., Hewick R., Fritsch E. F. Isolation and characterization of genomic and cDNA clones of human erythropoietin. 1985 Feb 28-Mar 6Nature. 313(6005):806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Sasse J., Sullivan R., Smith J. A. Human tumor cells synthesize an endothelial cell growth factor that is structurally related to basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2448–2452. doi: 10.1073/pnas.83.8.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Shing Y. Heparin affinity of anionic and cationic capillary endothelial cell growth factors: analysis of hypothalamus-derived growth factors and fibroblast growth factors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):805–809. doi: 10.1073/pnas.82.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lobb R. R., Alderman E. M., Fett J. W. Induction of angiogenesis by bovine brain derived class 1 heparin-binding growth factor. Biochemistry. 1985 Sep 10;24(19):4969–4973. doi: 10.1021/bi00340a001. [DOI] [PubMed] [Google Scholar]
- Lobb R. R., Fett J. W. Purification of two distinct growth factors from bovine neural tissue by heparin affinity chromatography. Biochemistry. 1984 Dec 18;23(26):6295–6299. doi: 10.1021/bi00321a001. [DOI] [PubMed] [Google Scholar]
- Lobb R. R., Strydom D. J., Fett J. W. Comparison of human and bovine brain derived heparin-binding growth factors. Biochem Biophys Res Commun. 1985 Sep 16;131(2):586–592. doi: 10.1016/0006-291x(85)91277-x. [DOI] [PubMed] [Google Scholar]
- Lobb R., Sasse J., Sullivan R., Shing Y., D'Amore P., Jacobs J., Klagsbrun M. Purification and characterization of heparin-binding endothelial cell growth factors. J Biol Chem. 1986 Feb 5;261(4):1924–1928. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Eberhardt N. L. Structure and evolution of the growth hormone gene family. Endocr Rev. 1983 Spring;4(2):97–130. doi: 10.1210/edrv-4-2-97. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. Basic and acidic fibroblast growth factors interact with the same cell surface receptors. J Biol Chem. 1986 Apr 25;261(12):5631–5637. [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. The identification and partial characterization of the fibroblast growth factor receptor of baby hamster kidney cells. J Biol Chem. 1985 Nov 5;260(25):13860–13868. [PubMed] [Google Scholar]
- Pettmann B., Weibel M., Sensenbrenner M., Labourdette G. Purification of two astroglial growth factors from bovine brain. FEBS Lett. 1985 Sep 9;189(1):102–108. doi: 10.1016/0014-5793(85)80851-6. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
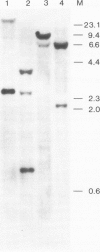
- Sullivan R., Klagsbrun M. Purification of cartilage-derived growth factor by heparin affinity chromatography. J Biol Chem. 1985 Feb 25;260(4):2399–2403. [PubMed] [Google Scholar]