Abstract
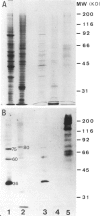
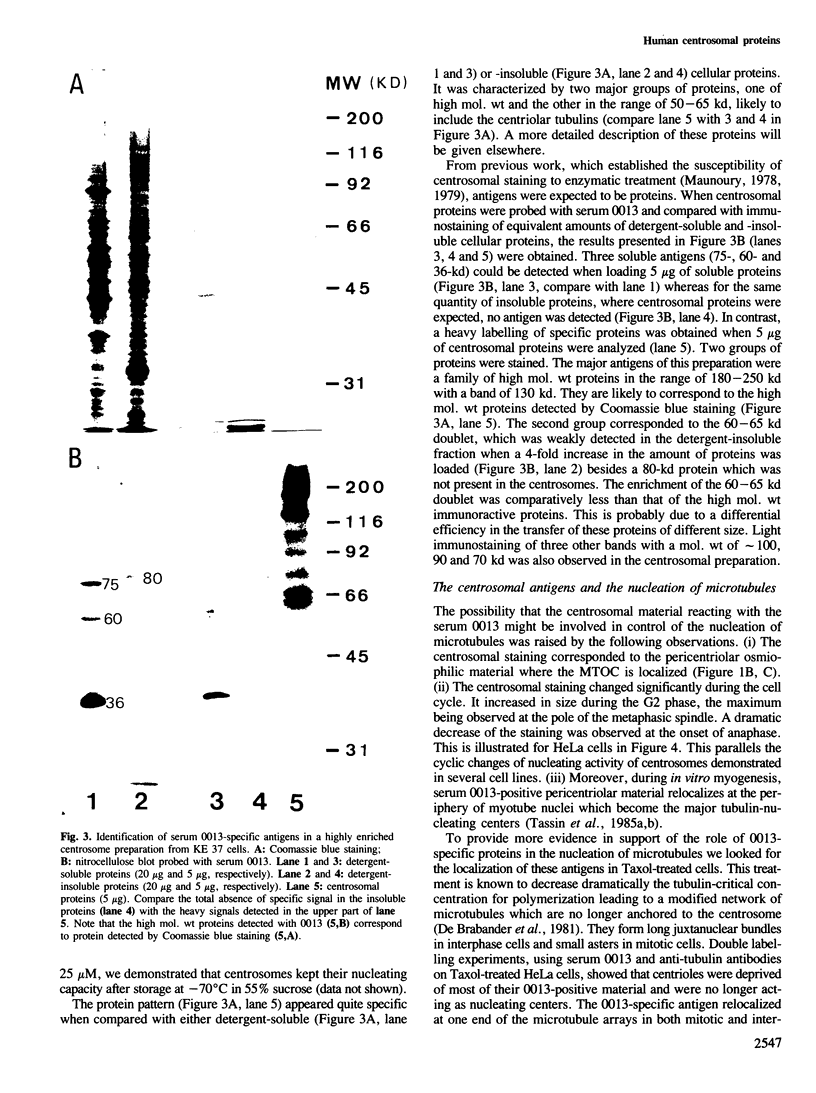
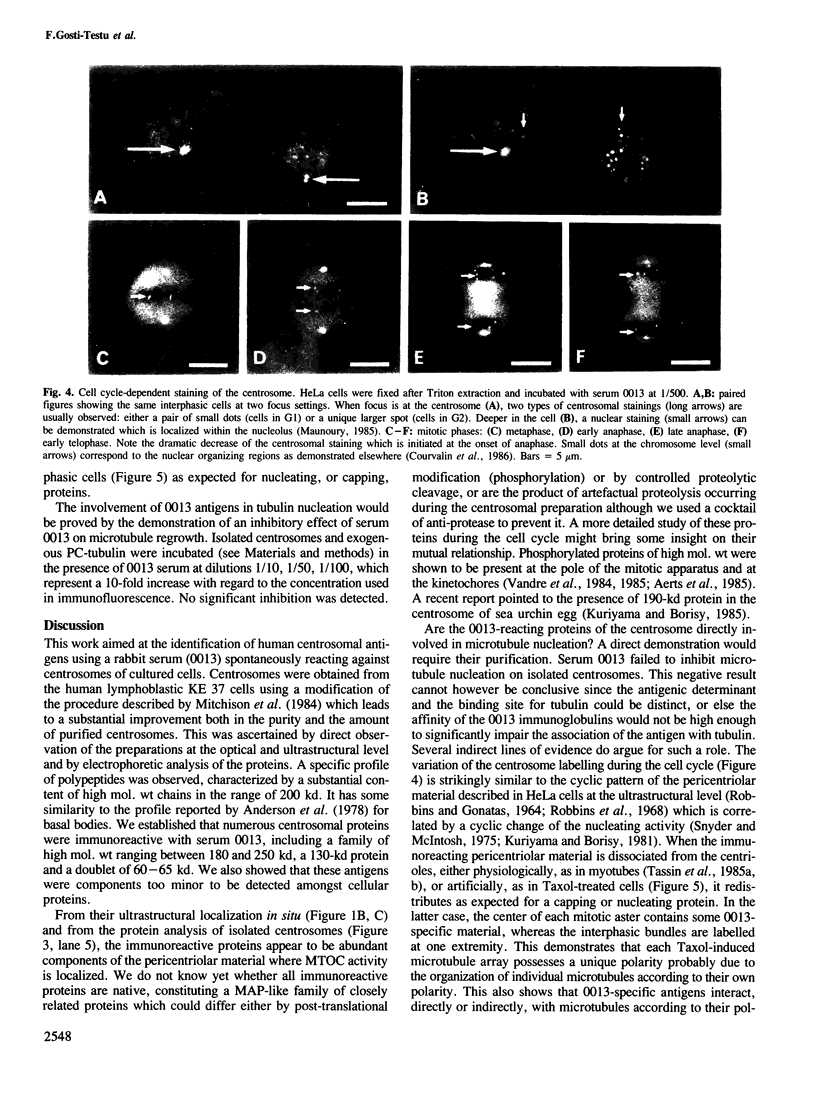
Highly enriched preparations of centrosomes from human T-lymphoblasts KE 37 were analyzed for their protein content. The specific pattern of polypeptides was characterized by an abundant subset of high mol. wt proteins and a major group of proteins with mol. wt ranging from 50 to 65 kd. Several immunoreactive proteins were identified, using a rabbit serum spontaneously reacting with human centrosomes. They include a family of high mol. wt ranging from 180 to 250 kd, a 130-kd protein and a 60-65 kd doublet. These antigens have the following properties: they are localized within the pericentriolar material; their abundance, as judged by centrosome labelling, changes significantly during the cell cycle, the maximum being observed at the pole of the metaphasic spindle; in Taxol-treated cells where the centrosome is no longer acting as a nucleating center, they redistribute at one end of the microtubule arrays in both mitotic and interphasic cells, as expected for nucleating, or capping, proteins. All these properties are compatible with their involvement in microtubule nucleation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B., Osborn M., Weber K. Specific visualization of the distribution of the calcium dependent regulatory protein of cyclic nucleotide phosphodiesterase (modulator protein) in tissue culture cells by immunofluorescence microscopy: mitosis and intercellular bridge. Cytobiologie. 1978 Aug;17(2):354–364. [PubMed] [Google Scholar]
- Anderson R. G., Floyd A. K. Electrophoretic analysis of basal body (centriole) proteins. Biochemistry. 1980 Nov 25;19(24):5625–5631. doi: 10.1021/bi00565a026. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Klausner R. D., Sandoval I. V. A widely distributed nuclear protein immunologically related to the microtubule-associated protein MAP1 is associated with the mitotic spindle. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1146–1150. doi: 10.1073/pnas.82.4.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C., Borisy G. G. Microtubule-associated proteins from cultured HeLa cells. Analysis of molecular properties and effects on microtubule polymerization. J Biol Chem. 1980 Dec 10;255(23):11570–11576. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Calarco-Gillam P. D., Siebert M. C., Hubble R., Mitchison T., Kirschner M. Centrosome development in early mouse embryos as defined by an autoantibody against pericentriolar material. Cell. 1983 Dec;35(3 Pt 2):621–629. doi: 10.1016/0092-8674(83)90094-6. [DOI] [PubMed] [Google Scholar]
- Clayton L., Black C. M., Lloyd C. W. Microtubule nucleating sites in higher plant cells identified by an auto-antibody against pericentriolar material. J Cell Biol. 1985 Jul;101(1):319–324. doi: 10.1083/jcb.101.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977 Oct 25;116(2):207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I. Visualization of centrioles and basal bodies by fluorescent staining with nonimmune rabbit sera. J Cell Biol. 1978 Nov;79(2 Pt 1):526–532. doi: 10.1083/jcb.79.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brabander M., Geuens G., Nuydens R., Willebrords R., De Mey J. Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5608–5612. doi: 10.1073/pnas.78.9.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellagi K., Brouet J. C., Perreau J., Paulin D. Human monoclonal IgM with autoantibody activity against intermediate filaments. Proc Natl Acad Sci U S A. 1982 Jan;79(2):446–450. doi: 10.1073/pnas.79.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U., McIntosh J. R. Structural polarity of kinetochore microtubules in PtK1 cells. J Cell Biol. 1981 May;89(2):338–345. doi: 10.1083/jcb.89.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. Organization and energy-dependent growth of microtubules in cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2798–2802. doi: 10.1073/pnas.73.8.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. R., Borisy G. G. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J Cell Biol. 1977 Jun;73(3):601–615. doi: 10.1083/jcb.73.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti E., Guilbert B., Bornens M., Avrameas S. Antibodies to tubulin in normal nonimmunized animals. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3997–4001. doi: 10.1073/pnas.74.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti E., Kobayashi S., Mitchison T., Kirschner M. Role of the centrosome in organizing the interphase microtubule array: properties of cytoplasts containing or lacking centrosomes. J Cell Biol. 1984 May;98(5):1763–1776. doi: 10.1083/jcb.98.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R., Borisy G. G. Identification of molecular components of the centrosphere in the mitotic spindle of sea urchin eggs. J Cell Biol. 1985 Aug;101(2):524–530. doi: 10.1083/jcb.101.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R., Borisy G. G. Microtubule-nucleating activity of centrosomes in Chinese hamster ovary cells is independent of the centriole cycle but coupled to the mitotic cycle. J Cell Biol. 1981 Dec;91(3 Pt 1):822–826. doi: 10.1083/jcb.91.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro B., Howlett S. K., Webb M. Non-spindle microtubule organizing centers in metaphase II-arrested mouse oocytes. J Cell Biol. 1985 Nov;101(5 Pt 1):1665–1672. doi: 10.1083/jcb.101.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro B., Paintrand M., Sauron M. E., Paulin D., Bornens M. Vimentin filaments and centrosomes. Are they associated? Exp Cell Res. 1984 Feb;150(2):452–458. doi: 10.1016/0014-4827(84)90589-5. [DOI] [PubMed] [Google Scholar]
- Maunoury M. R. Localisation immunocytochimique de la centrosphère de cellules tumorales humaines par utilisation d'anticorps naturels de lapin. C R Acad Sci Hebd Seances Acad Sci D. 1978 Feb 13;286(6):503–506. [PubMed] [Google Scholar]
- Maunoury R. Mise en évidence immunocytochimique simultanée de la centrosphére et de l'organisateur nucléolaire (NOR) dans des cellules humaines en culture. C R Acad Sci III. 1985;300(18):679–684. [PubMed] [Google Scholar]
- Mayer L., Fu S. M., Kunkel H. G. Human T cell hybridomas secreting factors for IgA-specific help, polyclonal B cell activation, and B cell proliferation. J Exp Med. 1982 Dec 1;156(6):1860–1865. doi: 10.1084/jem.156.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984 Nov 15;312(5991):232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- Moroi Y., Murata I., Takeuchi A., Kamatani N., Tanimoto K., Yokohari R. Human anticentriole autoantibody in patients with scleroderma and Raynaud's phenomenon. Clin Immunol Immunopathol. 1983 Dec;29(3):381–390. doi: 10.1016/0090-1229(83)90041-7. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Schäfer G., Hilz H., Eppenberger H. M. Cyclic-AMP-dependent protein kinase type II is associated with the Golgi complex and with centrosomes. Cell. 1985 Jul;41(3):1039–1051. doi: 10.1016/s0092-8674(85)80084-2. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Osborne W. R., Pfeiffer J. R., Child F. M., Berlin R. D. Purine nucleoside phosphorylase is associated with centrioles and basal bodies. J Cell Biol. 1981 Dec;91(3 Pt 1):837–847. doi: 10.1083/jcb.91.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Cytoplasmic microtubules in tissue culture cells appear to grow from an organizing structure towards the plasma membrane. Proc Natl Acad Sci U S A. 1976 Mar;73(3):867–871. doi: 10.1073/pnas.73.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. THE ULTRASTRUCTURE OF A MAMMALIAN CELL DURING THE MITOTIC CYCLE. J Cell Biol. 1964 Jun;21:429–463. doi: 10.1083/jcb.21.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins E., Jentzsch G., Micali A. The centriole cycle in synchronized HeLa cells. J Cell Biol. 1968 Feb;36(2):329–339. doi: 10.1083/jcb.36.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski A. A., Singer S. J. Associations of elements of the Golgi apparatus with microtubules. J Cell Biol. 1984 Sep;99(3):1092–1100. doi: 10.1083/jcb.99.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherline P., Mascardo R. N. Epidermal growth factor induces rapid centrosomal separation in HeLa and 3T3 cells. J Cell Biol. 1982 May;93(2):507–512. doi: 10.1083/jcb.93.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J. A., McIntosh J. R. Initiation and growth of microtubules from mitotic centers in lysed mammalian cells. J Cell Biol. 1975 Dec;67(3):744–760. doi: 10.1083/jcb.67.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin A. M., Maro B., Bornens M. Fate of microtubule-organizing centers during myogenesis in vitro. J Cell Biol. 1985 Jan;100(1):35–46. doi: 10.1083/jcb.100.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin A. M., Paintrand M., Berger E. G., Bornens M. The Golgi apparatus remains associated with microtubule organizing centers during myogenesis. J Cell Biol. 1985 Aug;101(2):630–638. doi: 10.1083/jcb.101.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer B. R., Haimo L. T. Decoration of spindle microtubules with Dynein: evidence for uniform polarity. J Cell Biol. 1981 May;89(2):373–378. doi: 10.1083/jcb.89.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandre D. D., Davis F. M., Rao P. N., Borisy G. G. Phosphoproteins are components of mitotic microtubule organizing centers. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4439–4443. doi: 10.1073/pnas.81.14.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev I. A., Chentsov YuS Centrioles in the cell cycle. I. Epithelial cells. J Cell Biol. 1982 Jun;93(3):938–949. doi: 10.1083/jcb.93.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherbee J. A., Sherline P., Mascardo R. N., Izant J. G., Luftig R. B., Weihing R. R. Microtubule-associated proteins of HeLa cells: heat stability of the 200,000 mol wt HeLa MAPs and detection of the presence of MAP-2 in HeLa cell extracts and cycled microtubules. J Cell Biol. 1982 Jan;92(1):155–163. doi: 10.1083/jcb.92.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]