Abstract
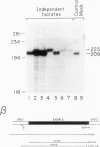
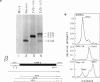
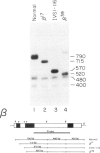
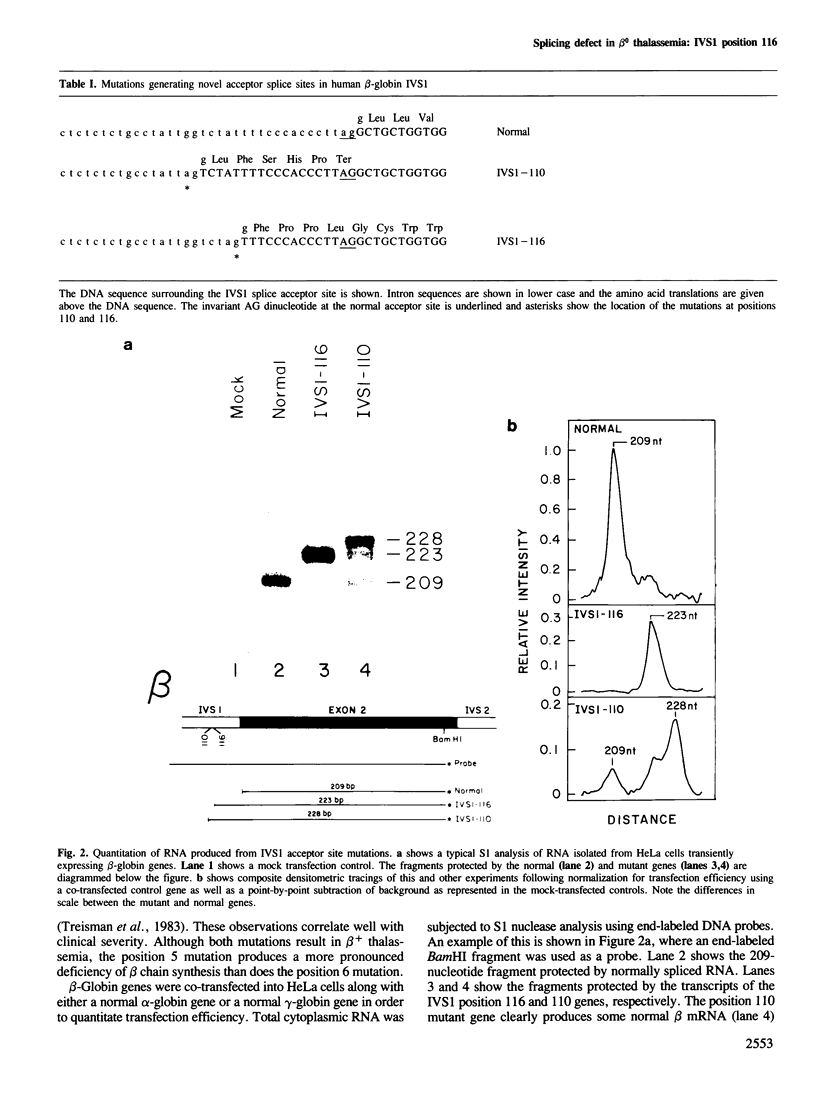
A thalassemic beta-globin gene cloned from a haplotype I chromosome contains a T to G transversion at position 116 of IVS1 which results in the generation of an abnormal alternative acceptor splice site. Transient expression studies revealed a 4-fold decrease in the amount of RNA produced with greater than 99% of it being abnormally spliced despite preservation of the normal acceptor splice site at position 130. These results suggest that the mutation at IVS1 position 116 results in beta zero thalassemia. A closely related mutation at position 110 of IVS1 also generates a novel acceptor site and results in a similar decrease in total mRNA produced, but approximately 20% of the mRNA produced is normally spliced and thus the phenotype is that of beta + thalassemia. These observations suggest that short range position effects may play a dramatic role in the choice of potential splice acceptor sites. We demonstrate the presence of abnormally spliced mRNA in reticulocytes of affected individuals and show the mutation at IVS1 position 116 segregating from the mutation at IVS1 position 110 in a three generation pedigree. The mutation results in the creation of a MaeI restriction site, as do a number of other thalassemic mutations, and we demonstrate some difficulties that may arise in the differential diagnosis of these mutations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atweh G. F., Forget B. G. Identification of a beta-thalassemia mutation associated with a novel haplotype of RFLPs. Am J Hum Genet. 1986 Jun;38(6):855–859. [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Place A. R., Wang N., Pentz E., Sofer W. Deletions at intervening sequence splice sites in the alcohol dehydrogenase gene of Drosophila. Nucleic Acids Res. 1982 Nov 25;10(22):7261–7272. doi: 10.1093/nar/10.22.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz E. J., Jr, Forget B. G. Defect in messenger RNA for human hemoglobin synthesis in beta thalassemia. J Clin Invest. 1971 Dec;50(12):2755–2760. doi: 10.1172/JCI106778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz E. J., Jr, Scarpa A. L., Forget B. G. Defective processing of beta mRNA in different forms of beta +-thalassemia. Trans Assoc Am Physicians. 1982;95:325–333. [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Busslinger M., Moschonas N., Flavell R. A. Beta + thalassemia: aberrant splicing results from a single point mutation in an intron. Cell. 1981 Dec;27(2 Pt 1):289–298. doi: 10.1016/0092-8674(81)90412-8. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Metherall J. E., Yamakawa M., Pan J., Weissman S. M., Forget B. G. A point mutation in the A gamma-globin gene promoter in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):325–326. doi: 10.1038/313325a0. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Stoeckert C. J., Jr, Serjeant G. R., Forget B. G., Weissman S. M. G gamma beta+ hereditary persistence of fetal hemoglobin: cosmid cloning and identification of a specific mutation 5' to the G gamma gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4894–4898. doi: 10.1073/pnas.81.15.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. The molecular genetics of human hemoglobin. Prog Nucleic Acid Res Mol Biol. 1984;31:315–462. doi: 10.1016/s0079-6603(08)60382-7. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Orkin S. H., Hamer D. H. Abnormal RNA splicing causes one form of alpha thalassemia. Cell. 1982 Jul;29(3):895–902. doi: 10.1016/0092-8674(82)90451-2. [DOI] [PubMed] [Google Scholar]
- Frendewey D., Keller W. Stepwise assembly of a pre-mRNA splicing complex requires U-snRNPs and specific intron sequences. Cell. 1985 Aug;42(1):355–367. doi: 10.1016/s0092-8674(85)80131-8. [DOI] [PubMed] [Google Scholar]
- Fukumaki Y., Ghosh P. K., Benz E. J., Jr, Reddy V. B., Lebowitz P., Forget B. G., Weissman S. M. Abnormally spliced messenger RNA in erythroid cells from patients with beta+ thalassemia and monkey cells expressing a cloned beta+-thalassemic gene. Cell. 1982 Mar;28(3):585–593. doi: 10.1016/0092-8674(82)90213-6. [DOI] [PubMed] [Google Scholar]
- Grabowski P. J., Padgett R. A., Sharp P. A. Messenger RNA splicing in vitro: an excised intervening sequence and a potential intermediate. Cell. 1984 Jun;37(2):415–427. doi: 10.1016/0092-8674(84)90372-6. [DOI] [PubMed] [Google Scholar]
- Grabowski P. J., Seiler S. R., Sharp P. A. A multicomponent complex is involved in the splicing of messenger RNA precursors. Cell. 1985 Aug;42(1):345–353. doi: 10.1016/s0092-8674(85)80130-6. [DOI] [PubMed] [Google Scholar]
- Humphries R. K., Ley T., Turner P., Moulton A. D., Nienhuis A. W. Differences in human alpha-, beta- and delta-globin gene expression in monkey kidney cells. Cell. 1982 Aug;30(1):173–183. doi: 10.1016/0092-8674(82)90023-x. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Nathan D. G. Mild thalassemia: the result of interactions of alpha and beta thalassemia genes. J Clin Invest. 1970 Apr;49(4):635–642. doi: 10.1172/JCI106274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Orkin S. H., Markham A. F., Chapman C. R., Youssoufian H., Waber P. G. Quantification of the close association between DNA haplotypes and specific beta-thalassaemia mutations in Mediterraneans. Nature. 1984 Jul 12;310(5973):152–154. doi: 10.1038/310152a0. [DOI] [PubMed] [Google Scholar]
- Kühne T., Wieringa B., Reiser J., Weissmann C. Evidence against a scanning model of RNA splicing. EMBO J. 1983;2(5):727–733. doi: 10.1002/j.1460-2075.1983.tb01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang K. M., Spritz R. A. RNA splice site selection: evidence for a 5' leads to 3' scanning model. Science. 1983 Jun 24;220(4604):1351–1355. doi: 10.1126/science.6304877. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Anagnou N. P., Ley T. J. Advances in thalassemia research. Blood. 1984 Apr;63(4):738–758. [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr The mutation and polymorphism of the human beta-globin gene and its surrounding DNA. Annu Rev Genet. 1984;18:131–171. doi: 10.1146/annurev.ge.18.120184.001023. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Konarska M. M., Grabowski P. J., Hardy S. F., Sharp P. A. Lariat RNA's as intermediates and products in the splicing of messenger RNA precursors. Science. 1984 Aug 31;225(4665):898–903. doi: 10.1126/science.6206566. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Thomm M., Laminet A., Laue F. G., Kessler C., Stetter K. O., Schmitt R. Three new restriction endonucleases MaeI, MaeII and MaeIII from Methanococcus aeolicus. Nucleic Acids Res. 1984 Mar 26;12(6):2619–2628. doi: 10.1093/nar/12.6.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. BKV splice sequences based on analysis of preferred donor and acceptor sites. Nucleic Acids Res. 1979 Jul 25;6(10):3387–3398. doi: 10.1093/nar/6.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Solnick D. An adenovirus mutant defective in splicing RNA from early region 1A. Nature. 1981 Jun 11;291(5815):508–510. doi: 10.1038/291508a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Jagadeeswaran P., Choudary P. V., Biro P. A., Elder J. T., deRiel J. K., Manley J. L., Gefter M. L., Forget B. G., Weissman S. M. Base substitution in an intervening sequence of a beta+-thalassemic human globin gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2455–2459. doi: 10.1073/pnas.78.4.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritz R. A., Van Santen V. L. Deletion analysis of the human gamma-globin IVS2: sequence requirements for RNA splicing. Ann N Y Acad Sci. 1985;445:10–19. doi: 10.1111/j.1749-6632.1985.tb17170.x. [DOI] [PubMed] [Google Scholar]
- Takanami M. RNA polymerase nascent product analysis. Methods Enzymol. 1980;65(1):497–499. doi: 10.1016/s0076-6879(80)65058-7. [DOI] [PubMed] [Google Scholar]
- Takeshita K., Forget B. G., Scarpa A., Benz E. J., Jr Intranuclear defect in beta-globin mRNA accumulation due to a premature translation termination codon. Blood. 1984 Jul;64(1):13–22. [PubMed] [Google Scholar]
- Thein S. L., Wainscoat J. S., Lynch J. R., Weatherall D. J., Sampietro M., Fiorelli G. Direct detection of beta zero 39 thalassaemic mutation with Mae 1. Lancet. 1985 May 11;1(8437):1095–1095. doi: 10.1016/s0140-6736(85)92390-6. [DOI] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Westaway D., Williamson R. An intron nucleotide sequence variant in a cloned beta +-thalassaemia globin gene. Nucleic Acids Res. 1981 Apr 24;9(8):1777–1788. doi: 10.1093/nar/9.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa B., Hofer E., Weissmann C. A minimal intron length but no specific internal sequence is required for splicing the large rabbit beta-globin intron. Cell. 1984 Jul;37(3):915–925. doi: 10.1016/0092-8674(84)90426-4. [DOI] [PubMed] [Google Scholar]
- Wieringa B., Meyer F., Reiser J., Weissmann C. Unusual splice sites revealed by mutagenic inactivation of an authentic splice site of the rabbit beta-globin gene. Nature. 1983 Jan 6;301(5895):38–43. doi: 10.1038/301038a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]