Abstract
Purpose
The blood concentrations of some tyrosine kinase inhibitors are known to decrease with long-term administration. We evaluated the variability in the serum concentrations of sunitinib and its metabolites in patients receiving long-term sunitinib treatment.
Methods
This study prospectively recruited patients who received sunitinib for metastatic renal cell carcinoma at the Showa University Hospital between March 2020 and January 2022. Bivariate correlations between the serum concentration/dose (C/D) ratios of sunitinib and its metabolites (i.e., N-desethyl sunitinib and sunitinib N-oxide) and treatment duration were evaluated using Pearson’s correlation coefficient.
Results
Seven patients were enrolled, and 79 blood samples were collected. Among six patients who received sunitinib for > 1 year, three showed a decreasing trend in the C/D ratio of sunitinib (Pt1: r = -0.608, p = 0.047; Pt2: r = -0.555, p = 0.077; Pt6: r = -0.590, p = 0.073). In these patients, the median annual decrease in the C/D ratio of sunitinib was 55.8% (26.5–63.2%). Additionally, two of the three patients also showed a decrease in the C/D ratio of N-desethyl sunitinib. The ratio of N-desethyl sunitinib/sunitinib concentration at baseline and the end of follow-up was similar between the C/D-decreased and C/D-non-decreased groups.
Conclusion
This study showed that the C/D ratio of sunitinib decreased by half over time in half of the patients who received long-term sunitinib treatment despite continuing the same dose. Therefore, serum concentrations of sunitinib and its metabolites should be monitored periodically in patients receiving long-term treatment to prevent decrease in serum sunitinib concentrations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00280-024-04741-w.
Keywords: Long-term treatment, Sunitinib, Metabolites, Therapeutic drug monitoring, Metastatic renal cell carcinoma
Introduction
Sunitinib, a multi-targeted tyrosine kinase inhibitor (multi-TKI), has been used as first-line treatment for all risk groups of metastatic renal cell carcinoma (mRCC) and has shown high efficacy [1–3]. In contrast, owing to the high incidence of serious adverse events, such as hand-foot skin reaction and thrombocytopenia [4], dose adjustments are required for each patient.
Sunitinib is primarily metabolized by CYP3A4 to the active metabolite N-desethyl sunitinib, and some is metabolized to sunitinib N-oxide [5, 6]. Recent studies have suggested that controlling the serum concentrations of sunitinib and its active metabolite (N-desethyl sunitinib) is important [7–9]. Additionally, there is large interpatient variability in the pharmacokinetics of sunitinib [10–12]. Therefore, therapeutic drug monitoring (TDM) is considered a useful approach for sunitinib treatment, and sunitinib administration design based on TDM is widely recommended in clinical practice [13, 14].
A previous study showed that the area under the plasma concentration-time curve (AUC) of sunitinib tended to decrease with disease progression compared with the early stages of treatment, although the difference was not statistically significant [15]. Additionally, long-term exposure (> 12 months) to imatinib, another multi-TKI, has been reported to increase clearance and decrease the AUC [16]. Similarly, in patients receiving long-term sorafenib, another multi-TKI, AUC and drug concentrations have been reported to decrease over time, even when sorafenib was continued at the same dose [17, 18]. Thus, long-term administration of TKIs may result in substantial variations in blood drug concentrations compared with that at initial drug administration.
In this study, we evaluated the variability in the serum concentrations of sunitinib and its metabolites in patients receiving long-term sunitinib treatment to investigate the need for periodic TDM and dose adjustments based on serum concentration. Furthermore, we discussed the factors that influence the variability in serum concentrations of sunitinib and its metabolites.
Materials and methods
Patients and treatment
This study prospectively recruited from patients receiving sunitinib for mRCC at the Showa University Hospital between March 2020 and January 2022. The eligibility criteria were patients aged ≥ 20 years with an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of ≤ 2.
All patients were diagnosed with mRCC based on pathology, ultrasonography, computed tomography, and magnetic resonance imaging. The initial dose of sunitinib was individually determined by each attending physician based on the patient’s age, body mass index, and ECOG PS. Patients were treated for mRCC according to the regimen approved at our institution, with 2 weeks on and 1 week off. Additionally, the decision to reduce the dose or discontinue treatment was made by a physician based on adverse events or disease progression.
This study was conducted with the approval of the Ethics Committee of Showa University (approval number: 299) and the Ethics Committee of Gunma University Hospital (approval number: HS2021-131). All participants provided written informed consent before enrolment.
Blood sample collection
Blood samples (5 mL) were collected at each outpatient visit until treatment was discontinued. Outpatient visits were conducted between 7 and 14 days after the start of each sunitinib treatment cycle. Blood samples were collected 24 h after sunitinib administration or immediately before the next administration. All samples were centrifuged at 1,500 ×g and 4 °C for 10 min and stored at -25 °C until analysis.
Measurement of serum concentrations of sunitinib and its metabolites
By analyzing fluctuations in the concentrations of as many metabolites as possible within the body, it becomes possible to gain a detailed understanding of variations in the metabolic pathways of a drug in vivo. Therefore, in this study, in addition to sunitinib and N-desethyl sunitinib, we analyzed sunitinib N-oxide, which has been identified as a metabolite present in serum. The serum concentrations of sunitinib, N-desethyl sunitinib, and sunitinib N-oxide were measured using liquid chromatography–tandem mass spectrometry, as previously reported [19]. The lower limits of quantitation for sunitinib, N-desethyl sunitinib, and sunitinib N-oxide were 2.5, 2.5, and 0.1 ng/mL, respectively.
Statistical analysis
Bivariate correlations between the serum concentration/dose (C/D) ratios of sunitinib and its metabolites and treatment duration were evaluated using Pearson’s correlation coefficient. Statistical analyses were performed using the SPSS software version 27 (IBM, Tokyo, Japan). P < 0.05 was considered statistically significant.
Results
Patients
Seven patients were enrolled, and 79 blood samples were collected. Two patients were enrolled within 1 year of starting sunitinib treatment, and five patients were enrolled more than 1 year after starting sunitinib treatment. The overall characteristics of the study population and individual patient characteristics are shown in Table 1 and Supplementary Table 1, respectively. None of the patients received cytokine therapy or targeted therapy before sunitinib treatment, and all patients had clear cell histology. The median duration of sunitinib treatment was 44.6 months (8.8–67.1 months), and the median follow-up period of serum concentration measurements was 18.6 months (1.7–25.3 months).
Table 1.
Patient characteristics
| Characteristics (n = 7) | N | % | Characteristics | N | % | |
|---|---|---|---|---|---|---|
| Age | MSKCC risk group | |||||
| Median (range), years | 69 (53–78) | Favorable | 4 | 57.1 | ||
| Sex | intermediate | 3 | 42.9 | |||
| Male | 6 | 85.7 | poor | 0 | 0 | |
| ECOG-PS | Previous Treatment | |||||
| 0 | 4 | 57.1 | No | 7 | 100 | |
| 1 | 2 | 28.6 | Initial dose | |||
| 2 | 1 | 14.3 | 50 mg | 3 | 42.9 | |
| Body weight | 37.5 mg | 3 | 42.9 | |||
| Median (range), kg | 59.7 (46.0–103) | 25 mg | 1 | 14.2 | ||
| Body surface area | Maintenance dose | |||||
| Median (range), m2 | 1.7 (1.4–2.2) | 50 mg | 1 | 14.3 | ||
| AST | 37.5 mg | 1 | 14.3 | |||
| Median (range), U/L | 21.0 (15.0–41.0) | 25 mg | 5 | 71.4 | ||
| ALT | Treatment schedule | |||||
| Median (range), U/L | 16.0 (7.0–41.0) | 2-week on / 1-week off | 7 | 100 | ||
| sCr | Concomitant medications | |||||
| Median (range), mg/dL | 0.9 (0.8–1.9) | PPI | 3 | 42.9 | ||
| eGFR | H2RA | 0 | 0 | |||
| Median (range), mL/min/1.73m2 | 49.1 (28.2–71.5) | Antacid | 2 | 28.6 | ||
| Histology type | CYP3A4 inhibitor | 0 | 0 | |||
| Clear cell | 7 | 100 | CYP3A4 inducer | 0 | 0 | |
| Prior nephrectomy | ||||||
| Yes | 6 | 85.7 | ||||
ECOG PS Eastern Cooperative Oncology Group Performance Status, AST aspartate aminotransferase, ALT alanine aminotransferase, sCr serum creatinine, eGFR estimated glomerular filtration rate, MSKCC Memorial Sloan Kettering Cancer Center, PPI proton pump inhibitor, H2RA histamine-2 receptor antagonist, CYP3A4 cytochrome P450 3A4
Variations in serum concentrations of sunitinib and its metabolites
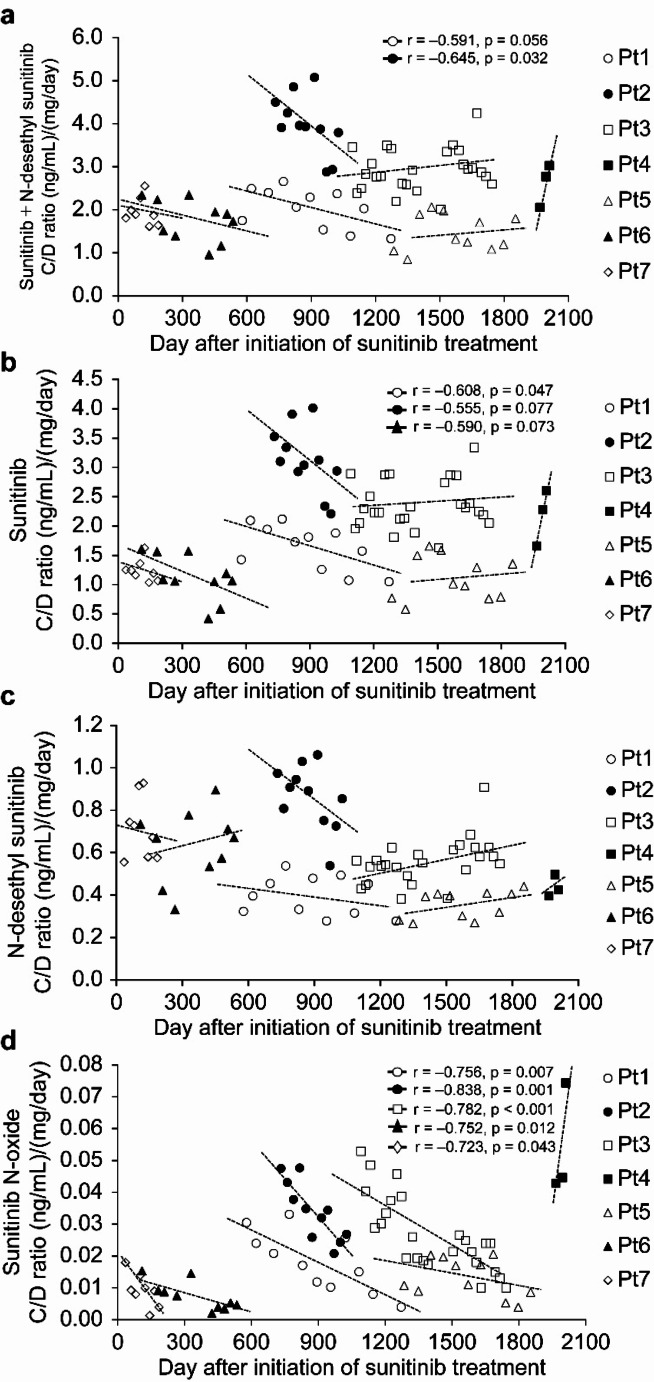
The changes in the C/D ratios of sunitinib and its metabolites are shown in Fig. 1. The serum concentrations of sunitinib and its metabolites by time after initiation of sunitinib treatment in individual patients are shown in Table 2. The serum concentrations and C/D ratios of sunitinib and its metabolites in individual patients are shown in Supplementary Table 2. Among six patients who received sunitinib for > 1 year, two showed a decreasing trend in the C/D ratio of sunitinib plus N-desethyl sunitinib (Fig. 1-a). One of the two patients showed a significant decrease in the C/D ratio of sunitinib plus N-desethyl sunitinib with increasing treatment duration (Pt1: r = -0.591, p = 0.056; Pt2: r = -0.645, p = 0.032). Among six patients who received sunitinib for > 1 year, three showed a decreasing trend in the C/D ratio of sunitinib (Fig. 1-b). One of the three patients showed a significant decrease in the C/D ratio of sunitinib with increasing treatment duration (Pt1: r = -0.608, p = 0.047; Pt2: r = -0.555, p = 0.077; Pt6: r = -0.590, p = 0.073). In patients who showed a decreasing trend in the C/D ratio of sunitinib, the median annual decrease ratio was 55.8% (26.5–63.2%). Two of the three patients also showed a decrease in the C/D ratio of N-desethyl sunitinib (Pt1: r = -0.282, p = 0.401; Pt2: r = -0.500, p = 0.117, Fig. 1-c). All three patients also showed a significant decrease in the C/D ratio of sunitinib N-oxide with increasing treatment duration (Pt1: r = -0.756, p = 0.007; Pt2: r = -0.838, p = 0.001; Pt6: r = -0.752, p = 0.012, Fig. 1-d).
Fig. 1.
Changes in serum concentration/dose ratios of (a) sunitinib plus N-desethyl sunitinib, (b) sunitinib, (c) N-desethyl sunitinib, and (d) sunitinib N-oxide in patients receiving long-term sunitinib treatment (n = 7 patients and 79 samples). C/D serum concentration/dose. *Correlation coefficients are listed with P-values < 0.1
Table 2.
Serum concentration of sunitinib plus N-desethyl sunitinib, sunitinib, N-desethyl sunitinib, and sunitinib N-oxide by time after initiation of sunitinib treatment in individual patients receiving long-term sunitinib treatment
| Serum concentration (ng/mL) after initiation of sunitinib treatment | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–210 days | 211–420 days | 421–630 days | 631–840 days | 841–1050 days | 1051–1260 days | 1261–1470 days | 1471–1680 days | 1681–1890 days | 1891–2100 days | ||||||||||
| Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | ||||||||||
| Sunitinib + N-desethyl sunitinib | |||||||||||||||||||
| Pt1 | 53.0 | 59.3 | 51.7 | 42.7 | 33.2 | ||||||||||||||
| Pt2 | 109.5 | 94.4 | |||||||||||||||||
| Pt3 | 72.7 | 67.3 | 79.5 | 68.7 | |||||||||||||||
| Pt4 | 65.4 | ||||||||||||||||||
| Pt5 | 54.8 | 56.8 | 54.1 | ||||||||||||||||
| Pt6 | 101.4 | 93.5 | 67.9 | ||||||||||||||||
| Pt7 | 48.9 | ||||||||||||||||||
| Sunitinib | |||||||||||||||||||
| Pt1 | 44.0 | 48.3 | 41.2 | 33.1 | 26.2 | ||||||||||||||
| Pt2 | 86.7 | 73.5 | |||||||||||||||||
| Pt3 | 59.5 | 54.9 | 64.1 | 54.1 | |||||||||||||||
| Pt4 | 54.5 | ||||||||||||||||||
| Pt5 | 42.2 | 44.7 | 39.4 | ||||||||||||||||
| Pt6 | 71.0 | 65.8 | 37.5 | ||||||||||||||||
| Pt7 | 31.1 | ||||||||||||||||||
| N-desethyl sunitinib | |||||||||||||||||||
| Pt1 | 9.0 | 11.0 | 10.4 | 9.6 | 6.9 | ||||||||||||||
| Pt2 | 22.7 | 20.9 | |||||||||||||||||
| Pt3 | 13.2 | 12.5 | 15.5 | 14.6 | |||||||||||||||
| Pt4 | 11.0 | ||||||||||||||||||
| Pt5 | 12.6 | 12.1 | 14.7 | ||||||||||||||||
| Pt6 | 30.4 | 27.7 | 30.4 | ||||||||||||||||
| Pt7 | 17.8 | ||||||||||||||||||
| Sunitinib N-oxide | |||||||||||||||||||
| Pt1 | 0.68 | 0.59 | 0.40 | 0.29 | 0.10 | ||||||||||||||
| Pt2 | 1.10 | 0.71 | |||||||||||||||||
| Pt3 | 0.99 | 0.58 | 0.53 | 0.32 | |||||||||||||||
| Pt4 | 1.35 | ||||||||||||||||||
| Pt5 | 0.56 | 0.57 | 0.36 | ||||||||||||||||
| Pt6 | 0.56 | 0.55 | 0.17 | ||||||||||||||||
| Pt7 | 0.24 | ||||||||||||||||||
Relationship between the patient characteristics and C/D ratio of sunitinib
The relationship between the patient characteristics and C/D ratio of sunitinib is shown in Table 3. The CV (%) of albumin (Alb) levels in individual patients are shown in Supplementary Table 3. The rate of weight loss and decrease in Alb levels were similar between the C/D-decreased and C/D-non-decreased groups. The ratio of N-desethyl sunitinib/sunitinib concentration or sunitinib N-oxide/sunitinib concentration at baseline and the end of follow-up was similar between the C/D-decreased and C/D-non-decreased groups. Five patients (71.4%) had grade 2 or higher anorexia, a common adverse event associated with sunitinib. However, no difference was observed between the C/D-decreased and C/D-non-decreased groups.
Table 3.
Relationship between sunitinib serum concentration/dose ratio and patients’ characteristics
| Sunitinib C/D ratio | ||
|---|---|---|
| Decreased group | Non-decreased group | |
| Total patient, n | 3 | 4 |
| Age (years) | 55 (53–69) | 73 (69–78) |
| Male/female, n | 2/1 | 4/0 |
| ECOG PS (0/1/2/3), n | 2/0/1/0 | 2/2/0/0 |
| Baseline, median (min–max) | ||
| Body weight (kg) | 86.6 (59.7–103) | 52.6 (46.0–73.0) |
| Body surface area (m2) | 2.04 (1.59–2.16) | 1.58 (1.44–1.81) |
| Body mass index | 27.5 (24.5–34.6) | 19.3 (17.6–26.5) |
| Weight loss (%), median (min–max) | 0 (0–3.3) | -0.7 (-4.5–12.9) |
| Baseline, median (min–max) | ||
| AST (U/L) | 22.0 (15.0–28.0) | 19.5 (17.0–41.0) |
| ALT (U/L) | 18.0 (13.0–40.0) | 13.5 (7.0–41) |
| sCr (mg/dL) | 0.94 (0.91–1.61) | 0.98 (0.82–1.88) |
| eGFR (mL/min/1.73m2) | 49.1 (34.2–67.7) | 59.9 (28.2–71.5) |
| Hb (g/dL) | 11.5 (11.5–13.6) | 11.4 (10.9–14.3) |
| Alb (g/dL) | 4.4 (3.8–4.5) | 4.0 (3.8–4.4) |
| Alb loss (%), median (min–max) | 11.1 (2.3–18.4) | 2.1 (-15.4–7.5) |
| CV (%) of Alb, median (min–max) | 4.3 (2.9–7.5) | 4.4 (3.4–8.0) |
| eGFR loss (%), median (min–max) | -0.6 (-1.8–47.4) | 5.3 (-3.4–21.2) |
| CV (%) of eGFR, median (min–max) | 6.0 (5.9–23.6) | 6.2 (4.8–13.4) |
| Maintenance dose, n | ||
| 50 mg/day | 1 | 0 |
| 37.5 mg/day | 0 | 1 |
| 25 mg/day | 2 | 3 |
| Baseline, median (min–max) | ||
| N-desethyl sunitinib/sunitinib | 0.28 (0.23–0.46) | 0.30 (0.19–0.44) |
| Sunitinib N-oxide/sunitinib | 0.013 (0.010–0.021) | 0.016 (0.014–0.026) |
| After follow-up, median (min–max) | ||
| N-desethyl sunitinib/sunitinib | 0.29 (0.26–0.63) | 0.30 (0.16–0.54) |
| Sunitinib N-oxide/sunitinib | 0.004 (0.004–0.009) | 0.006 (0.004–0.029) |
| Concomitant medications, n | ||
| PPI | 2 | 1 |
| Antacid | 1 | 1 |
| Adverse events, n | ||
| Anorexia | ||
| Grade 0 | 1 | 0 |
| Grade 1 | 0 | 1 |
| Grade 2 | 1 | 2 |
| Grade 3 | 1 | 1 |
| Diarrhea | ||
| Grade 0 | 2 | 2 |
| Grade 1 | 1 | 2 |
C/D serum concentration/dose, ECOG PS Eastern Cooperative Oncology Group Performance Status, AST aspartate aminotransferase, ALT alanine aminotransferase, sCr serum creatinine, eGFR estimated glomerular filtration rate, Hb Hemoglobin, Alb Albumin, CV Coefficient of Variation, PPI proton pump inhibitor
Discussion
We showed that the C/D ratio of sunitinib decreased by half over time in half of the patients who received sunitinib for > 1 year despite continuing the same dose. To the best of our knowledge, this is the first study to reveal the relationship between the maintenance dose and variations in the serum concentrations of sunitinib and its metabolites in patients who received sunitinib treatment for > 1 year. A decrease in the serum concentration of sunitinib may weaken its antitumor effects; thus, serum concentrations of sunitinib and its metabolites should be periodically monitored when administering sunitinib for a long period.
We discussed the factors that led to a decrease in the C/D ratio of sunitinib in patients who received long-term sunitinib treatment. Judson et al. noted that the decreased plasma concentrations of imatinib with long-term treatment were due to increased clearance and a decreased AUC [16]. Sunitinib is metabolized by cytochrome P450 (CYP) 3A4 to N-desethyl sunitinib [20]; thus, the ratio of N-desethyl sunitinib/sunitinib concentration varies depending on CYP3A4 activity [21]. However, in this study, the ratio of N-desethyl sunitinib/sunitinib concentration did not vary significantly, and the effect of metabolic clearance on the decrease in the C/D ratio of sunitinib was considered small. Additionally, since no patients were found to be using concomitant CYP3A4 inducers or inhibitors during the sunitinib treatment period of this study, it is considered unlikely that sunitinib metabolic activity changed during treatment. Judson et al. also clarified that high Alb and hemoglobin (Hb) levels were correlated with increased clearance of imatinib [16]. However, in this study, Alb and Hb levels were similar between the C/D-decreased and C/D-non-decreased groups. Therefore, it was considered that factors other than the increased clearance and decreased AUC, observed with long-term administration of imatinib, were involved in the decrease in the C/D ratio of sunitinib associated with long-term treatment.
Moreover, reduced absorption was considered as another factor. Burger et al. noted that the decreased absorption of imatinib was due to increased expression of the drug excretion transporters, ATP-binding cassette transporter, sub-family G, member 2 (ABCG2/BCRP) and ATP-binding cassette transporter, sub-family B, member 1 (ABCB1/MDR1), in the gastrointestinal tract with long-term administration of imatinib [22]. As sunitinib is a substrate of ABCG2/BCRP and ABCB1/MDR1, long-term sunitinib administration may have increased the expression of these transporters in the intestinal tract, resulting in decreased absorption of sunitinib.
It is known that the absorption rate of itraconazole, the same basic drug as sunitinib, is greatly affected by intragastric pH [23]. Additionally, because TKIs are weakly basic, concomitant use with gastric acid suppressants increases gastric pH, resulting in decreases bioavailability [24, 25]. Many TKIs have pH-dependent solubility, thus affecting absorption in the gastrointestinal tract [25, 26]. However, in this study, although some patients were using proton pump inhibitors (PPIs) or antacids before the start of sunitinib treatment, no patients received these drugs during the sunitinib treatment period examined in this study. Therefore, the concomitant use of sunitinib and PPIs or antacids was considered unlikely to be a factor in reducing absorption in the gastrointestinal tract.
Anorexia is an adverse event of sunitinib, which has been reported to reduce gastric acid secretion and increase intragastric pH [27]. Anorexia was observed in six patients (85.7%) who received long-term sunitinib treatment, five of whom (71.4%) had grade 2 or higher anorexia. However, the number of patients with grade 2 or higher anorexia was similar between the C/D-decreased and C/D-non-decreased groups. Therefore, the effect of anorexia on decreased absorption of sunitinib remains unclear.
Boudou-Rouquette et al. reported that long-term administration of sorafenib decreases the absorption of sorafenib, a vascular endothelial growth factor receptor-TKI (VEGFR-TKI), from the gastrointestinal tract, resulting in a decreased AUC [28]. Therefore, it is possible that sunitinib, a VEGFR-TKI, may also decrease absorption from the gastrointestinal tract with long-term administration. Additionally, all patients had good medication adherence to medication, it was considered unlikely that medication status had any influence on the decrease in blood concentrations.
Finally, the effect of increased excretory clearance was considered as another factor. However, Khosravan et al. reported that the pharmacokinetics of sunitinib and N-desethyl sunitinib are largely unaffected by renal function [29]. Additionally, in this study, the variability of the estimated glomerular filtration rate was similar between the C/D-decreased and C/D-non-decreased groups. Although these findings could not rule out the influence of increased excretory clearance associated with changes in ABCG2/BCRP or ABCB1/MDR1 expression, the influence of variability in renal clearance on the decreased C/D ratio of sunitinib observed in this study was considered small. Therefore, the cause of the decrease in the sunitinib C/D ratio could not be clarified; however, it was thought that the influences of metabolic and renal clearance were low, whereas the influence of decreased absorption or variability of ABCG2/BCRP and ABCB1/MDR1 expression was high.
The baseline body weight of the C/D-decreased group was higher than that of the C/D-non-decreased group. Additionally, obesity is known to affect the variability of drug absorption [30]. Therefore, body weight and obesity may have influenced the decrease in serum concentrations. On the other hand, in their population pharmacokinetic (PPK) analysis of sunitinib, Houk et al. reported a maximum variation in Vd/F of 14% for a patient weighing 100 kg [31]. Since this is comparable to general inter-individual variation, weight variations are likely to have a minimal effect on the decrease in serum concentration. However, due to a lack of clinical data, the impact of weight variations on the serum concentration and absorption of sunitinib should be examined in detail in the future.
A rapid increase in the serum concentration of sunitinib and its metabolites was observed in one patient (Patient 4). This patient did not receive any additional concomitant medications during the treatment period but experienced a weight loss of approximately 13%. When we calculated the decrease in the volume of distribution (Vd/F) based on the PPK analysis of sunitinib reported by Houk et al. [31]., the decrease rate of Vd/F was 6.4%. Therefore, although weight loss may have affected Vd/F, it is unlikely to have been the main cause of the rapid increase in serum concentrations in this patient. On the other hand, transient increases in serum concentrations were also observed in other patients, and it is possible that the increase in this patient may have been a transient fluctuation. However, because the observation period for this patient was short, it was not possible to evaluate subsequent variations in serum concentrations. Therefore, it is necessary to increase the number of patients and investigate the cause of the rapid increase in serum concentration.
This study has two limitations. First, we could not clarify the cause of the decreased C/D ratio of sunitinib. Therefore, future studies should analyze variations in the intragastric pH and ABCG2 expression. Second, the number of patients treated with sunitinib was small. Therefore, future studies should increase the sample size and conduct similar analyses to clarify the cause of the decrease in the serum sunitinib concentration, which will lead to the maintenance of appropriate serum concentration during sunitinib treatment.
Conclusion
This study showed that the C/D ratio of sunitinib decreased by half over time in half of the patients who received long-term sunitinib treatment despite continuing the same dose. Therefore, serum concentrations of sunitinib and its metabolites should be periodically monitored when administering sunitinib for a long period to prevent a reduction in its antitumor effect associated with decreased serum sunitinib concentrations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients who participated in this study.
Author contributions
All authors contributed to the conception and design of the study. MTS, JM, and YM performed data collection. MTS, TA, HY, and KY performed data analysis. MTS, TA, HY, YI, JM, YM, MO, NK, YO, TF, KY, and MK performed interpretation of the data. MTS wrote the first draft of the manuscript, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by a Grant-in-Aid for Scientific Research (20K16454) from the Japan Society for the Promotion of Science.
Data availability
The datasets generated during and analyzed during the current study are not publicly available owing to ethical reasons but are available from the corresponding author on reasonable request.
Declarations
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of Showa University (approval number: 299) and the Ethics Committee of Gunma University Hospital (approval number: HS2021-131).
Informed consent
All patients participating in the study provided written informed consent before enrolment.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27(22):3584–3590. 10.1200/JCO.2008.20.1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Jonasch E, Agarwal N, Alva A, Bagshaw H, Baine M, Beckermann K, Carlo MI, Choueiri TK, Costello BA, Derweesh IH, Desai A, Ged Y, George S, Gore JL, Gunn A, Haas N, Johnson M, Kapur P, King J, Kyriakopoulos C, Lam ET, Lara PN, Lau C, Lewis B, Madoff DC, Manley B, Michaelson MD, Mortazavi A, Ponsky L, Ramalingam S, Shuch B, Smith ZL, Sosman J, Sweis R, Zibelman M, Schonfeld R, Stein M, Gurski LA (2024) NCCN guidelines® Insights: Kidney cancer, Version 2.2024. J Natl Compr Canc Netw 22(1):4–16. 10.6004/jnccn.2024.0008 [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356(2):115–124. 10.1056/NEJMoa065044 [DOI] [PubMed] [Google Scholar]
- 4.Akaza H, Naito S, Ueno N, Aoki K, Houzawa H, Pitman Lowenthal S, Lee SY (2015) Real-world use of sunitinib in Japanese patients with advanced renal cell carcinoma: efficacy, safety and biomarker analyses in 1689 consecutive patients. Jpn J Clin Oncol 45(6):576–583. 10.1093/jjco/hyv045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speed B, Bu HZ, Pool WF, Peng GW, Wu EY, Patyna S, Bello C, Kang P (2012) Pharmacokinetics, distribution, and metabolism of [14 C]sunitinib in rats, monkeys, and humans. Drug Metab Dispos 40(3):539–555. 10.1124/dmd.111.042853 [DOI] [PubMed] [Google Scholar]
- 6.Takenaka M, Takahashi Y, Yashima H, Araki T, Yamamoto K (2019) The Impact of Sunitinib N-oxide as a Photodegradation Product of Sunitinib. Indonesian J Pharm 1(1):19–25. 10.24198/idjp.v1i1.19908 [Google Scholar]
- 7.Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ (2010) Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 66(2):357–371. 10.1007/s00280-009-1170-y [DOI] [PubMed] [Google Scholar]
- 8.Noda S, Otsuji T, Baba M, Yoshida T, Kageyama S, Okamoto K, Okada Y, Kawauchi A, Onishi H, Hira D, Morita SY, Terada T (2015) Assessment of sunitinib-induced toxicities and clinical outcomes based on therapeutic drug monitoring of sunitinib for patients with renal cell carcinoma. Clin Genitourin Cancer 13(4):350–358. 10.1016/j.clgc.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 9.Numakura K, Fujiyama N, Takahashi M, Igarashi R, Tsuruta H, Maeno A, Huang M, Saito M, Narita S, Inoue T, Satoh S, Tsuchiya N, Niioka T, Miura M, Habuchi T (2018) Clinical implications of pharmacokinetics of sunitinib malate and N-desethyl-sunitinib plasma concentrations for treatment outcome in metastatic renal cell carcinoma patients. Oncotarget 9(38):25277–25284. 10.18632/oncotarget.25423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabanathan D, Zhang A, Fox P, Coulter S, Gebski V, Balakrishnar B, Chan M, Liddle C, Gurney H (2017) Dose individualization of sunitinib in metastatic renal cell cancer: toxicity-adjusted dose or therapeutic drug monitoring. Cancer Chemother Pharmacol 80(2):385–393. 10.1007/s00280-017-3362-1 [DOI] [PubMed] [Google Scholar]
- 11.Lankheet NAG, Knapen LM, Schellens JHM, Beijnen JH, Steeghs N, Huitema ADR (2014) Plasma concentrations of tyrosine kinase inhibitors imatinib, erlotinib, and sunitinib in routine clinical outpatient cancer care. Ther Drug Monit 36(3):326–334. 10.1097/FTD.0000000000000004 [DOI] [PubMed] [Google Scholar]
- 12.Takasaki S, Kawasaki Y, Kikuchi M, Tanaka M, Suzuka M, Noda A, Sato Y, Yamashita S, Mitsuzuka K, Saito H, Ito A, Yamaguchi H, Arai Y, Mano N (2018) Relationships between sunitinib plasma concentration and clinical outcomes in Japanese patients with metastatic renal cell carcinoma. Int J Clin Oncol 23(5):936–943. 10.1007/s10147-018-1302-7 [DOI] [PubMed] [Google Scholar]
- 13.Mueller-Schoell A, Groenland SL, Scherf-Clavel O, van Dyk M, Huisinga W, Michelet R, Jaehde U, Steeghs N, Huitema ADR, Kloft C (2021) Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur J Clin Pharmacol 77(4):441–464. 10.1007/s00228-020-03014-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verheijen RB, Yu H, Schellens JHM, Beijnen JH, Steeghs N, Huitema ADR (2017) Practical recommendations for therapeutic drug monitoring of kinase inhibitors in oncology. Clin Pharmacol Ther 102(5):765–776. 10.1002/cpt.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabel L, Blanchet B, Thomas-Schoemann A, Huillard O, Bellesoeur A, Cessot A, Giroux J, Boudou-Rouquette P, Coriat R, Vidal M, Saidu NEB, Golmard L, Alexandre J, Goldwasser F (2018) Drug monitoring of sunitinib in patients with advanced solid tumors: a monocentric observational French study. Fundam Clin Pharmacol 32(1):98–107. 10.1111/fcp.12327 [DOI] [PubMed] [Google Scholar]
- 16.Judson I, Ma P, Peng B, Verweij J, Racine A, di Paola ED, van Glabbeke M, Dimitrijevic S, Scurr M, Dumez H, van Oosterom A (2005) Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: a retrospective population pharmacokinetic study over time. EORTC Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol 55(4):379–386. 10.1007/s00280-004-0876-0 [DOI] [PubMed] [Google Scholar]
- 17.Arrondeau J, Mir O, Boudou-Rouquette P, Coriat R, Ropert S, Dumas G, Rodrigues MJ, Rousseau B, Blanchet B, Goldwasser F (2012) Sorafenib exposure decreases over time in patients with hepatocellular carcinoma. Invest New Drugs 30(5):2046–2049. 10.1007/s10637-011-9764-8 [DOI] [PubMed] [Google Scholar]
- 18.Fukudo M, Ito T, Mizuno T, Shinsako K, Hatano E, Uemoto S, Kamba T, Yamasaki T, Ogawa O, Seno H, Chiba T, Matsubara K (2014) Exposure-toxicity relationship of sorafenib in Japanese patients with renal cell carcinoma and hepatocellular carcinoma. Clin Pharmacokinet 53(2):185–196. 10.1007/s40262-013-0108-z [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa Y, Araki T, Sato MT, Yashima H, Nagano D, Yamamoto K (2021) Development of a quantitative method for sunitinib N-oxide. Indo J Pharm 3(2). 10.24198/idjp.v3i2.37368
- 20.Kim A, Balis FM, Widemann BC (2009) Sorafenib and sunitinib. Oncologist 14(8):800–805. 10.1634/theoncologist.2009-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Cui X, Cheng C, Wang Y, Sun W, Huang CK, Chen RJ, Wang Z (2021) Effects of CYP3A inhibitors ketoconazole, voriconazole, and itraconazole on the pharmacokinetics of sunitinib and its main metabolite in rats. Chem Biol Interact 338:109426. 10.1016/j.cbi.2021.109426 [DOI] [PubMed] [Google Scholar]
- 22.Burger H, van Tol H, Brok M, Wiemer EAC, de Bruijn EA, Guetens G, de Boeck G, Sparreboom A, Verweij J, Nooter K (2005) Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther 4(7):747–752. 10.4161/cbt.4.7.1826 [DOI] [PubMed] [Google Scholar]
- 23.Poirier JM, Cheymol G (1998) Optimisation of itraconazole therapy using target drug concentrations. Clin Pharmacokinet 35:461–473. 10.2165/00003088-199835060-00004 [DOI] [PubMed] [Google Scholar]
- 24.van Leeuwen RWF, Jansman FGA, Hunfeld NG, Peric R, Reyners AKL, Imholz ALT, Brouwers J, Aerts JG, van Gelder T, Mathijssen RHJ (2017) Tyrosine Kinase Inhibitors and Proton Pump Inhibitors: An Evaluation of Treatment Options. Clin Pharmacokinet 56(7):683–688. 10.1007/s40262-016-0503-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ, Holden SN, Benet LZ, Ware JA (2012) Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther 92(2):203–213. 10.1038/clpt.2012.73 [DOI] [PubMed] [Google Scholar]
- 26.Raoul JL, Edeline J, Simmet V, Moreau-Bachelard C, Gilabert M, Frénel JS (2022) Long-Term Use of Proton Pump Inhibitors in Cancer Patients: An Opinion Paper. Cancers (Basel) 14(5):1156. 10.3390/cancers14051156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubois A, Gross HA, Ebert MH, Castell DO (1979) Altered gastric emptying and secretion in primary anorexia nervosa. Gastroenterology 77(2):319–323. 10.1016/0016-5085(79)90285-3 [PubMed] [Google Scholar]
- 28.Boudou-Rouquette P, Ropert S, Mir O, Coriat R, Billemont B, Tod M, Cabanes L, Franck N, Blanchet B, Goldwasser F (2012) Variability of sorafenib toxicity and exposure over time: a pharmacokinetic/pharmacodynamic analysis. Oncologist 17(9):1204–1212. 10.1634/theoncologist.2011-0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khosravan R, Toh M, Garrett M, La Fargue J, Ni G, Marbury TC, Swan SK, Lunde NM, Bello CL (2010) Pharmacokinetics and safety of sunitinib malate in subjects with impaired renal function. J Clin Pharmacol 50(4):472–481. 10.1177/0091270009347868 [DOI] [PubMed] [Google Scholar]
- 30.Stillhart C, Vučićević K, Augustijns P, Basit AW, Batchelor H, Flanagan TR, Gesquiere I, Greupink R, Keszthelyi D, Koskinen M, Madla CM, Matthys C, Miljuš G, Mooij MG, Parrott N, Ungell AL, de Wildt SN, Orlu M, Klein S, Müllertz A (2020) Impact of gastrointestinal physiology on drug absorption in special populations–An UNGAP review. Eur J Pharm Sci 147:105280. 10.1016/j.ejps.2020.105280 [DOI] [PubMed] [Google Scholar]
- 31.Houk BE, Bello CL, Kang D, Amantea M (2009) A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res 15(7):2497–2506. 10.1158/1078-0432.Ccr-08-1893 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the current study are not publicly available owing to ethical reasons but are available from the corresponding author on reasonable request.