Abstract
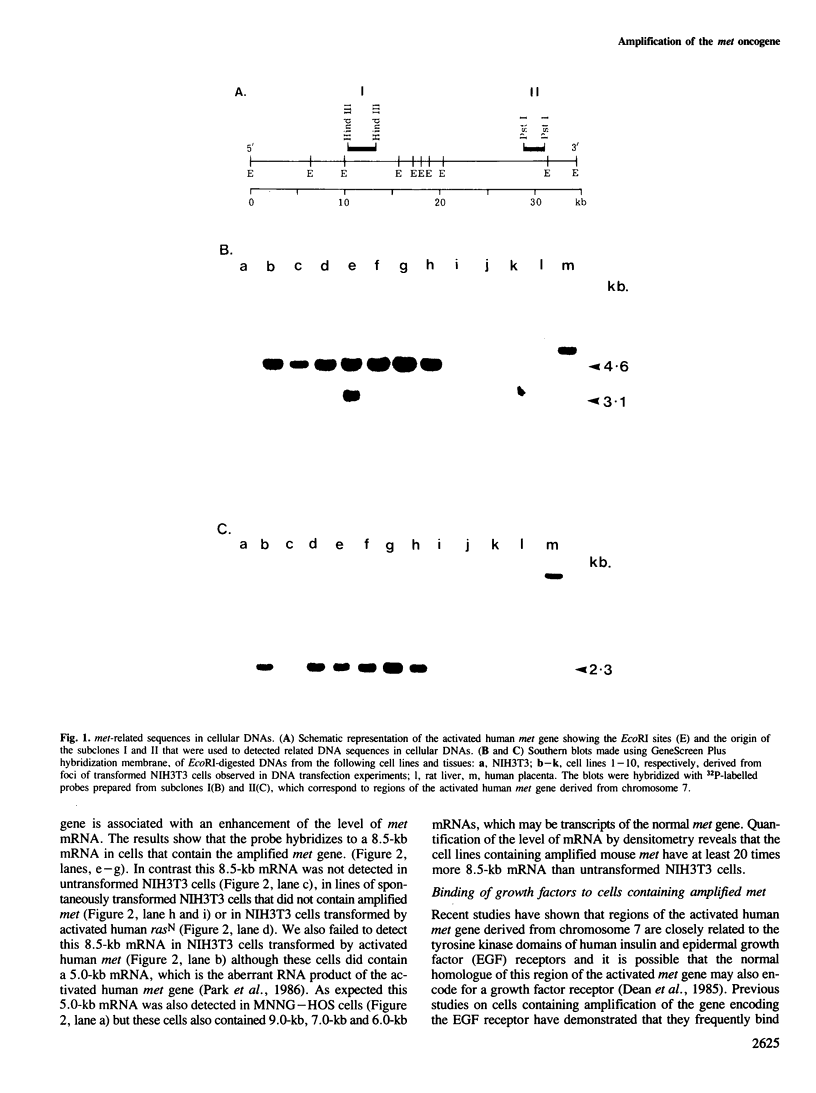
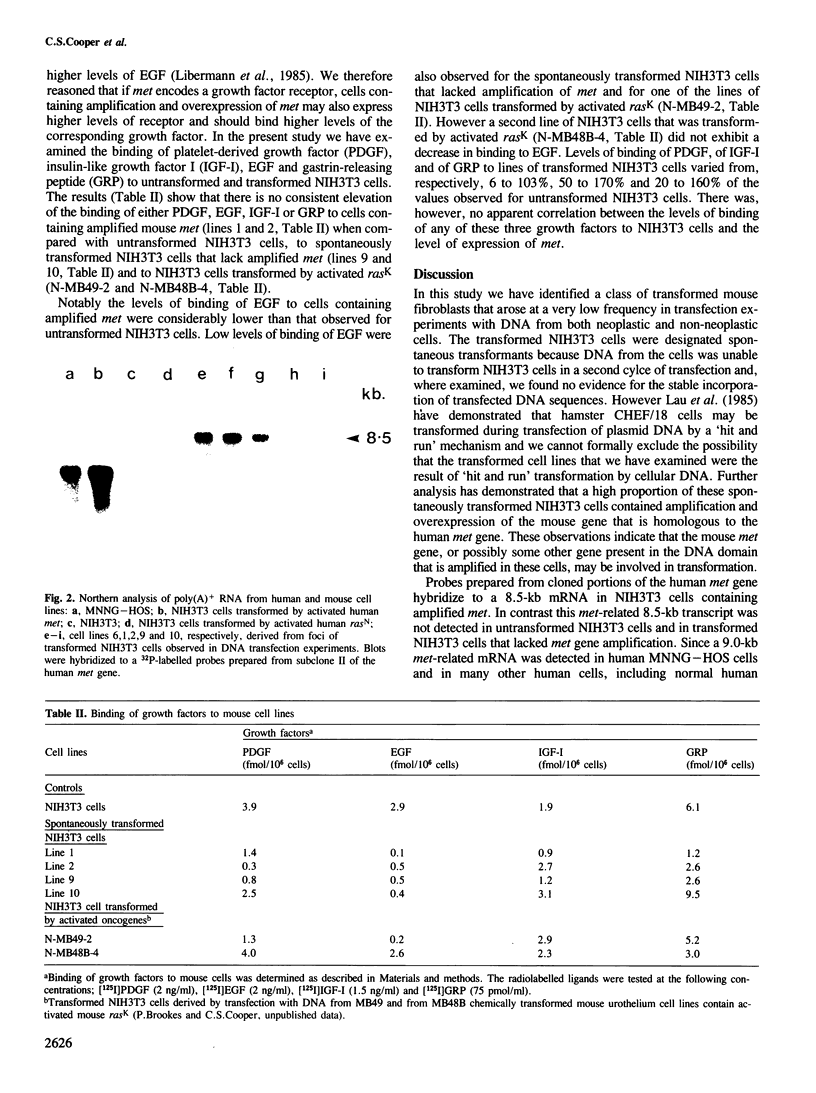
We have identified a class of transformed NIH3T3 mouse fibroblasts that arise at low frequencies in transfection experiments with DNA from both neoplastic and non-neoplastic cells and that may result from a low level of spontaneous transformation of NIH3T3 cells. DNA from the transformed cells was unable to transform NIH3T3 cells in a second cycle of transfection and, where examined, the cells showed no evidence for the uptake of the transfected DNA sequences. The results of Southern analyses demonstrate that a mouse homologue of the human met oncogene is amplified 4- to 8-fold in 7 of 10 lines of these transformed NIH3T3 mouse fibroblasts. The cells containing the amplified gene also exhibit at least a 20-fold overexpression of an 8.5-kb mRNA that is homologous to met. To test the hypothesis that met encodes a growth factor receptor, we examined the binding of platelet-derived growth factor, epidermal growth factor, insulin-like growth factor I and gastrin-releasing peptide to transformed and non-transformed NIH3T3 cells. The results show that there is no significant elevation of the binding of these growth factors to cells containing amplification and overexpression of met.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain A., Pragnell I. B. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983 May 5;303(5912):72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Cooper C. S., Oskarsson M. K., Eader L. A., Vande Woude G. F. New method for detecting cellular transforming genes. Science. 1982 Dec 10;218(4577):1122–1125. doi: 10.1126/science.6293052. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Collins S., Groudine M. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature. 1982 Aug 12;298(5875):679–681. doi: 10.1038/298679a0. [DOI] [PubMed] [Google Scholar]
- Cooper C. S., Blair D. G., Oskarsson M. K., Tainsky M. A., Eader L. A., Vande Woude G. F. Characterization of human transforming genes from chemically transformed, teratocarcinoma, and pancreatic carcinoma cell lines. Cancer Res. 1984 Jan;44(1):1–10. [PubMed] [Google Scholar]
- Cooper C. S., Park M., Blair D. G., Tainsky M. A., Huebner K., Croce C. M., Vande Woude G. F. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984 Sep 6;311(5981):29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Neiman P. E. Transforming genes of neoplasms induced by avian lymphoid leukosis viruses. Nature. 1980 Oct 16;287(5783):656–659. doi: 10.1038/287656a0. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Okenquist S., Silverman L. Transforming activity of DNA of chemically transformed and normal cells. Nature. 1980 Apr 3;284(5755):418–421. doi: 10.1038/284418a0. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- Dean M., Park M., Le Beau M. M., Robins T. S., Diaz M. O., Rowley J. D., Blair D. G., Vande Woude G. F. The human met oncogene is related to the tyrosine kinase oncogenes. 1985 Nov 28-Dec 4Nature. 318(6044):385–388. doi: 10.1038/318385a0. [DOI] [PubMed] [Google Scholar]
- Diamond A., Cooper G. M., Ritz J., Lane M. A. Identification and molecular cloning of the human Blym transforming gene activated in Burkitt's lymphomas. Nature. 1983 Sep 8;305(5930):112–116. doi: 10.1038/305112a0. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Gullino P. M., Pettigrew H. M., Grantham F. H. N-nitrosomethylurea as mammary gland carcinogen in rats. J Natl Cancer Inst. 1975 Feb;54(2):401–414. [PubMed] [Google Scholar]
- HUGGINS C., GRAND L. C., BRILLANTES F. P. Mammary cancer induced by a single feeding of polymucular hydrocarbons, and its suppression. Nature. 1961 Jan 21;189:204–207. doi: 10.1038/189204a0. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Specific receptors for platelet-derived growth factor on cells derived from connective tissue and glia. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3664–3668. doi: 10.1073/pnas.78.6.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl N. E., Kanda N., Schreck R. R., Bruns G., Latt S. A., Gilbert F., Alt F. W. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell. 1983 Dec;35(2 Pt 1):359–367. doi: 10.1016/0092-8674(83)90169-1. [DOI] [PubMed] [Google Scholar]
- Lau C. C., Gadi I. K., Kalvonjian S., Anisowicz A., Sager R. Plasmid-induced "hit-and-run" tumorigenesis in Chinese hamster embryo fibroblast (CHEF) cells. Proc Natl Acad Sci U S A. 1985 May;82(9):2839–2843. doi: 10.1073/pnas.82.9.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leof E. B., Wharton W., van Wyk J. J., Pledger W. J. Epidermal growth factor (EGF) and somatomedin C regulate G1 progression in competent BALB/c-3T3 cells. Exp Cell Res. 1982 Sep;141(1):107–115. doi: 10.1016/0014-4827(82)90073-8. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Nusbaum H. R., Razon N., Kris R., Lax I., Soreq H., Whittle N., Waterfield M. D., Ullrich A., Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985 Jan 10;313(5998):144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- Lin C. R., Chen W. S., Kruiger W., Stolarsky L. S., Weber W., Evans R. M., Verma I. M., Gill G. N., Rosenfeld M. G. Expression cloning of human EGF receptor complementary DNA: gene amplification and three related messenger RNA products in A431 cells. Science. 1984 May 25;224(4651):843–848. doi: 10.1126/science.6326261. [DOI] [PubMed] [Google Scholar]
- Marshall C. J., Hall A., Weiss R. A. A transforming gene present in human sarcoma cell lines. Nature. 1982 Sep 9;299(5879):171–173. doi: 10.1038/299171a0. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Xu Y. H., Ishii S., Clark A. J., Semba K., Toyoshima K., Yamamoto T., Pastan I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984 Apr 27;224(4647):417–419. doi: 10.1126/science.6200934. [DOI] [PubMed] [Google Scholar]
- Nepveu A., Fahrlander P. D., Yang J. Q., Marcu K. B. Amplification and altered expression of the c-myc oncogene in A-MuLV-transformed fibroblasts. Nature. 1985 Oct 3;317(6036):440–443. doi: 10.1038/317440a0. [DOI] [PubMed] [Google Scholar]
- Padua R. A., Barrass N., Currie G. A. A novel transforming gene in a human malignant melanoma cell line. Nature. 1984 Oct 18;311(5987):671–673. doi: 10.1038/311671a0. [DOI] [PubMed] [Google Scholar]
- Park M., Dean M., Cooper C. S., Schmidt M., O'Brien S. J., Blair D. G., Vande Woude G. F. Mechanism of met oncogene activation. Cell. 1986 Jun 20;45(6):895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Lauver A. V., Long L. K., Aaronson S. A., Barbacid M. Oncogenes in solid human tumours. Nature. 1982 Dec 9;300(5892):539–542. doi: 10.1038/300539a0. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Park D. K., Arnstein P., Huebner R. J., Weisburger E. K., Nelson-Rees W. A. Transformation of human cells in culture by N-methyl-N'-nitro-N-nitrosoguanidine. Nature. 1975 Aug 28;256(5520):751–753. doi: 10.1038/256751a0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. M., Houck C. M., Deininger P. L., Friedmann T., Schmid C. W. Partial nucleotide sequence of the 300-nucleotide interspersed repeated human DNA sequences. Nature. 1980 Mar 27;284(5754):372–374. doi: 10.1038/284372a0. [DOI] [PubMed] [Google Scholar]
- Schechter A. L., Stern D. F., Vaidyanathan L., Decker S. J., Drebin J. A., Greene M. I., Weinberg R. A. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984 Dec 6;312(5994):513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Shih C., Shilo B. Z., Goldfarb M. P., Dannenberg A., Weinberg R. A. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Xu Y. H., Ishii S., Clark A. J., Sullivan M., Wilson R. K., Ma D. P., Roe B. A., Merlino G. T., Pastan I. Human epidermal growth factor receptor cDNA is homologous to a variety of RNAs overproduced in A431 carcinoma cells. 1984 Jun 28-Jul 4Nature. 309(5971):806–810. doi: 10.1038/309806a0. [DOI] [PubMed] [Google Scholar]




