Abstract
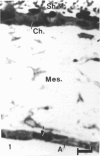
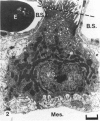
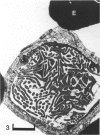
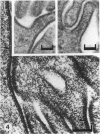
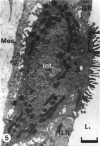
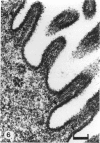
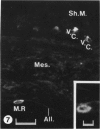
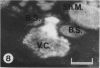
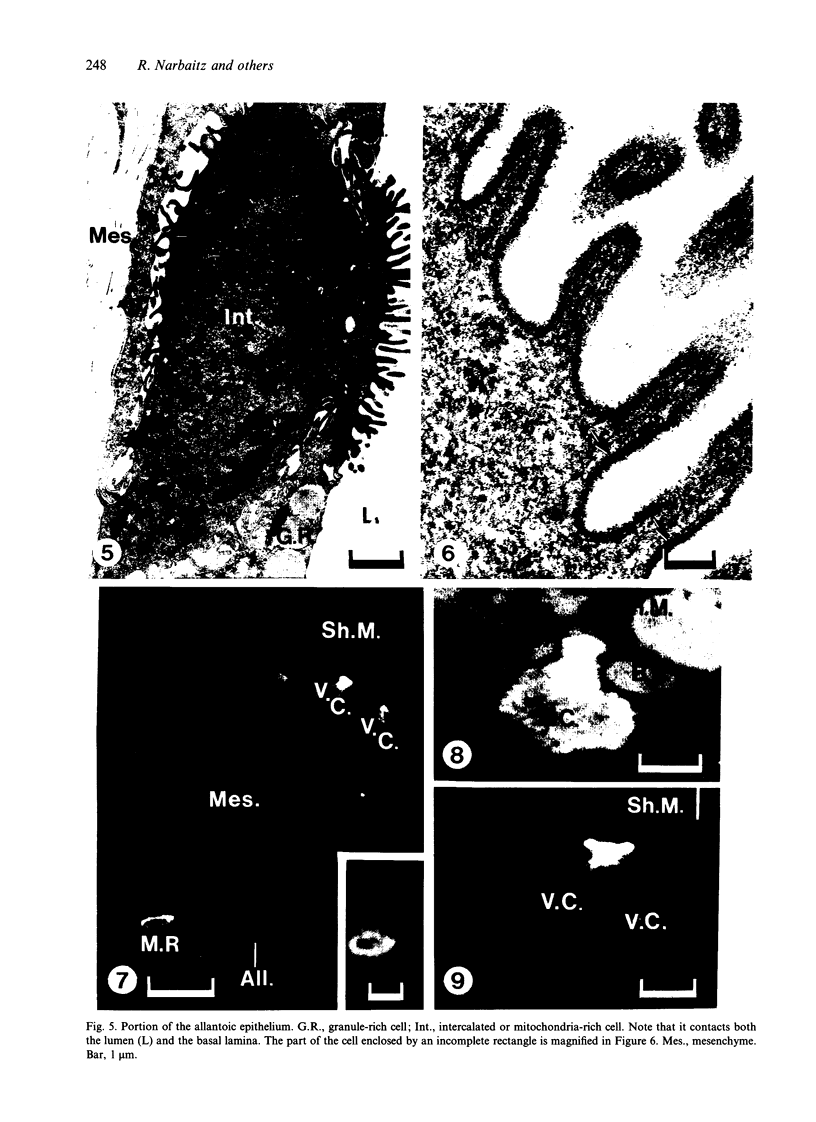
The chick embryo, confined in the eggshell, has to dispose/buffer the acid generated by its metabolism, as well as to release calcium from the shell which is used for growth. To localise H(+)-ATPase, electron microscope and immunocytochemical studies were conducted on chorioallantoic membranes of 15-17 d chick embryos. Ultrastructural studies of the villus cavity (VC) cells in the chorionic epithelium demonstrated that their apical plasma membrane, juxtaposed with the shell membranes, contains microvilli as well as microplicae which possess 9-10 nm studs at a density of 16,700 particles/micron2, a characteristic feature of the polarised H(+)-ATPase pump. Immunocytochemical staining, using a monoclonal antibody to the 31 kDa subunit of H(+)-ATPase, confirmed the presence of large amounts of the vacuolar H(+)-ATPase in the VC shells with a distribution highly polarised towards the eggshell membranes. Immunoelectron-microscopic localisation studies using a rabbit antiserum to whole bovine H(+)-ATPase and immunogold technique, confirmed the localisation of H(+)-ATPase at the apical microvilli/microplicae as well as in the subapical vesicles. In the allantoic epithelium, the presence of mitochondria-rich (MR) cells was confirmed; it was shown that these cells extend through the full thickness of this epithelium. The MR cells also contained large numbers of 9-10 nm studs, typical of proton secreting cells, in their apical plasma membrane. This was confirmed by immunocytochemical staining which showed abundant localisation of H(+)-ATPase in these cells; this localisation was, however, diffuse rather than apical.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovici A. L'evolution du pH du plasma et des liquides extraembryonnaires de l'embryon de poulet au cours du développement normal. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jul 24;265(4):336–339. [PubMed] [Google Scholar]
- Anderson R. E., Gay C. V., Schraer H. Ultrastructural localization of carbonic anhydrase in the chorioallantoic membrane by immunocytochemistry. J Histochem Cytochem. 1981 Oct;29(10):1121–1127. doi: 10.1177/29.10.6170666. [DOI] [PubMed] [Google Scholar]
- Brown D., Gluck S., Hartwig J. Structure of the novel membrane-coating material in proton-secreting epithelial cells and identification as an H+ATPase. J Cell Biol. 1987 Oct;105(4):1637–1648. doi: 10.1083/jcb.105.4.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Hirsch S., Gluck S. An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature. 1988 Feb 18;331(6157):622–624. doi: 10.1038/331622a0. [DOI] [PubMed] [Google Scholar]
- Brown D., Hirsch S., Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest. 1988 Dec;82(6):2114–2126. doi: 10.1172/JCI113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. Membrane recycling and epithelial cell function. Am J Physiol. 1989 Jan;256(1 Pt 2):F1–12. doi: 10.1152/ajprenal.1989.256.1.F1. [DOI] [PubMed] [Google Scholar]
- Crooks R. J., Simkiss K. Respiratory acidosis and eggshell resorption by the chick embryo. J Exp Biol. 1974 Aug;61(1):197–202. doi: 10.1242/jeb.61.1.197. [DOI] [PubMed] [Google Scholar]
- Dawes C. M. Acid-base relationships within the avian egg. Biol Rev Camb Philos Soc. 1975 Aug;50(3):351–371. doi: 10.1111/j.1469-185x.1975.tb00834.x. [DOI] [PubMed] [Google Scholar]
- Dawes C. M., Simkiss K. The effects of respiratory acidosis in the chick embryo. J Exp Biol. 1971 Aug;55(1):77–84. doi: 10.1242/jeb.55.1.77. [DOI] [PubMed] [Google Scholar]
- Dunn B. E., Fitzharris T. P. Endocytosis in the embryonic chick chorionic epithelium. J Exp Zool Suppl. 1987;1:75–79. [PubMed] [Google Scholar]
- Gluck S., Al-Awqati Q. An electrogenic proton-translocating adenosine triphosphatase from bovine kidney medulla. J Clin Invest. 1984 Jun;73(6):1704–1710. doi: 10.1172/JCI111378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON P. M., COMAR C. L. Distribution and contribution of calcium from the albumen, yolk and shell to the developing chick embryo. Am J Physiol. 1955 Dec;183(3):365–370. doi: 10.1152/ajplegacy.1955.183.3.365. [DOI] [PubMed] [Google Scholar]
- LEESON T. S., LEESON C. R. THE CHORIO-ALLANTOIS OF THE CHICK. LIGHT AND ELECTRON MICROSCOPIC OBSERVATIONS AT VARIOUS TIMES OF INCUBATION. J Anat. 1963 Oct;97:585–595. [PMC free article] [PubMed] [Google Scholar]
- Madsen K. M., Tisher C. C. Structure-function relationships in H+-secreting epithelia. Fed Proc. 1985 Aug;44(11):2704–2709. [PubMed] [Google Scholar]
- Madsen K. M., Verlander J. W., Tisher C. C. Relationship between structure and function in distal tubule and collecting duct. J Electron Microsc Tech. 1988 Jun;9(2):187–208. doi: 10.1002/jemt.1060090206. [DOI] [PubMed] [Google Scholar]
- Narbaitz R. Cytological and cytochemical study of the chick chorionic epithelium. Rev Can Biol. 1972 Dec;31(4):259–267. [PubMed] [Google Scholar]
- Narbaitz R., Kacew S., Sitwell L. Carbonic anhydrase activity in the chick embryo chorioallantois: regional distribution and vitamin D regulation. J Embryol Exp Morphol. 1981 Oct;65:127–137. [PubMed] [Google Scholar]
- Narbaitz R. Role of vitamin D in the development of the chick embryo. J Exp Zool Suppl. 1987;1:15–23. [PubMed] [Google Scholar]
- Narbaitz R., Stumpf W., Sar M., DeLuca H. F., Tanaka Y. Autoradiographic demonstration of target cells for 1,25-dihydroxycholecalciferol in the chick embryo chorioallantoic membrane, duodenum, and parathyroid glands. Gen Comp Endocrinol. 1980 Nov;42(3):283–289. doi: 10.1016/0016-6480(80)90156-2. [DOI] [PubMed] [Google Scholar]
- Narbaitz R., Vandorpe D., Levine D. Z. Differentiation of renal intercalated cells in fetal and postnatal rats. Anat Embryol (Berl) 1991;183(4):353–361. doi: 10.1007/BF00196836. [DOI] [PubMed] [Google Scholar]
- Owczarzak A. Calcium-absorbing cell of the chick chorioallantoic membrane. I. Morphology, distribution and cellular interactions. Exp Cell Res. 1971 Sep;68(1):113–129. doi: 10.1016/0014-4827(71)90593-3. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder E., Gay C. V., Schraer H. Autoradiographic localization of carbonic anhydrase in the developing chorioallantoic membrane. Anat Embryol (Berl) 1980;159(1):17–31. doi: 10.1007/BF00299252. [DOI] [PubMed] [Google Scholar]
- SKALINSKY E. I., KONDALENKO V. F. ELECTRON MICROSCOPIC STUDIES OF THE CHICK CHORIO-ALLANTOIS DURING EMBRYOGENESIS. Acta Morphol Acad Sci Hung. 1964;12:247–259. [PubMed] [Google Scholar]
- Steinmetz P. R. Cellular organization of urinary acidification. Am J Physiol. 1986 Aug;251(2 Pt 2):F173–F187. doi: 10.1152/ajprenal.1986.251.2.F173. [DOI] [PubMed] [Google Scholar]
- Tiedemann K. The allantoic and amniotic epithelia of the pig: SEM and TEM studies. Anat Embryol (Berl) 1979 May 3;156(1):53–72. doi: 10.1007/BF00315715. [DOI] [PubMed] [Google Scholar]
- Verlander J. W., Madsen K. M., Galla J. H., Luke R. G., Tisher C. C. Response of intercalated cells to chloride depletion metabolic alkalosis. Am J Physiol. 1992 Feb;262(2 Pt 2):F309–F319. doi: 10.1152/ajprenal.1992.262.2.F309. [DOI] [PubMed] [Google Scholar]
- Wangensteen O. D., Rahn H. Respiratory gas exchange by the avian embryo. Respir Physiol. 1970;11(1):31–45. doi: 10.1016/0034-5687(70)90100-3. [DOI] [PubMed] [Google Scholar]