Abstract
Background
10% of postmenopausal breast cancer cases are attributed to a high body mass index (BMI). BMI underestimates body fat, particularly in older women, and therefore the cancer burden attributable to obesity may be even higher. However, this is not clear. CUN-BAE (Clínica Universidad de Navarra–Body Adiposity Estimator) is an accurate validated estimator of body fat, taking into account sex and age. The objective of this study was to compare the burden of postmenopausal breast cancer attributable to excess body fat calculated using BMI and CUN-BAE.
Methods
This case–control study included 1033 cases of breast cancer and 1143 postmenopausal population controls from the multicase–control MCC-Spain study. Logistic regression models were used to calculate odds ratios (ORs). The population attributable fraction (PAF) of excess weight related to breast cancer was estimated with both anthropometric measures. Stratified analyses were carried out for hormone receptor type.
Results
Excess body weight attributable to the risk of breast cancer was 23.0% when assessed using a BMI value ≥30 kg/m2 and 38.0% when assessed using a CUN-BAE value of ≥40% body fat. Hormone receptor stratification showed that these differences in PAFs were only observed in hormone receptor positive cases, with an estimated burden of 19.9% for BMI and 41.9% for CUN-BAE.
Conclusion
These findings suggest that the significance of excess body fat in postmenopausal hormone receptor positive breast cancer could be underestimated when assessed using only BMI. Accurate estimation of the cancer burden attributable to obesity is crucial for planning effective prevention initiatives.
Keywords: BREAST NEOPLASMS, OBESITY, HEALTH IMPACT ASSESSMENT
WHAT IS ALREADY KNOWN ON THIS TOPIC
Obesity is a well known risk factor for postmenopausal breast cancer, and body mass index (BMI) is the most commonly used measure.
However, BMI underestimates body fat in older women, leading to underestimation of the cancer burden attributable to obesity.
It is important to compare the cancer burden attributable to obesity calculated using BMI with more accurate measures of body fat.
WHAT THIS STUDY ADDS
Our study estimated that 38% of incident postmenopausal breast cancer cases in Spain might be attributable to high body weight.
The burden of postmenopausal hormone receptor positive breast cancer attributable to excess body weight was higher when assessed with the Clínica Universidad de Navarra–Body Adiposity Estimator (CUN-BAE) than with BMI.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The findings of this study highlight the importance of considering more accurate measures of body fat than BMI to estimate the cancer burden attributable to obesity in postmenopausal breast cancer.
This information could potentially influence the planning of effective cancer prevention initiatives.
Introduction
Breast cancer is the most common cancer among women, with an estimated >2.2 million new cases worldwide (46.8 per 100 000 persons incident) in 2022.1 Body mass index (BMI) is a well established risk factor for postmenopausal breast cancer.2 It is estimated that about 10% of cases can be due to a BMI >24.9 kg/m2.3 Most studies that have assessed the relationship between body fat and breast cancer were based on calculations of BMI.3,6
However, it is widely known that the correlation between body fat and BMI is not linear because it is influenced by several factors, such as sex, age and race. Therefore, BMI tends to underestimate the percentage of body fat, particularly in older women.7 8 Several alternative anthropometric measures have been proposed, either independently or in addition to BMI.9,11 One of these more accurate measures of body fat is CUN-BAE (Clínica Universidad de Navarra–Body Adiposity Estimator), a body fat estimator developed in a white population by Gómez-Ambrosi et al.12 This estimator uses BMI values, sex and age, and correlates better with body fat and metabolic disorders than BMI.13,16
Hormonal factors have an important role in the relationship between body weight and breast cancer. Obesity is a well established risk factor for oestrogen receptor positive breast cancer, because the production of oestrogen in fatty breast tissue is a key factor in tumour development.4 6 17 Nevertheless, previous studies have not evaluated the impact of body fat on the burden of breast cancer according to different types of tumour receptors. It is essential to determine the impact of excess fat on the risk of breast cancer to justify interventions aiming to prevent and control obesity, particularly in postmenopausal women.18,20
The aim of this study was to compare the burden of breast cancer cases that can be attributed to excess body fat in postmenopausal women, calculated with BMI and CUN-BAE, and to examine this relation in terms of different tumour receptors.
Materials and methods
Study design and participants
MCC-Spain is a population-based multicenter case-control study, carried out in September 2008 to December 2013 in 13 Spanish provinces (Asturias, Barcelona, Cantabria, Girona, Granada, Guipúzcoa, Huelva, León, Madrid, Murcia, Navarra and Valencia). The overall objective of the study was to evaluate the environmental and genetic factors associated with colorectal cancer, breast cancer, gastric cancer, prostate cancer and chronic lymphocytic leukaemia.21
Inclusion criteria were participants aged 20–85 years, residenccy in the recruitment area for at least 6 months before recruitment and physically/mentally capable of answering the epidemiological survey. Recruitment of cases and controls was carried out simultaneously. Only incident breast cancer cases confirmed histologically in the 23 collaborating hospitals were included. The population controls were selected randomly and matched by age, sex and region to the cases. Controls were invited to participate, after being selected from the administrative registers of the primary care centres located within each hospital’s catchment area.21 MCC-Spain includes 1738 breast cancer cases and 1910 matched population controls. More details on the project can be found at https://www.mccspain.org.
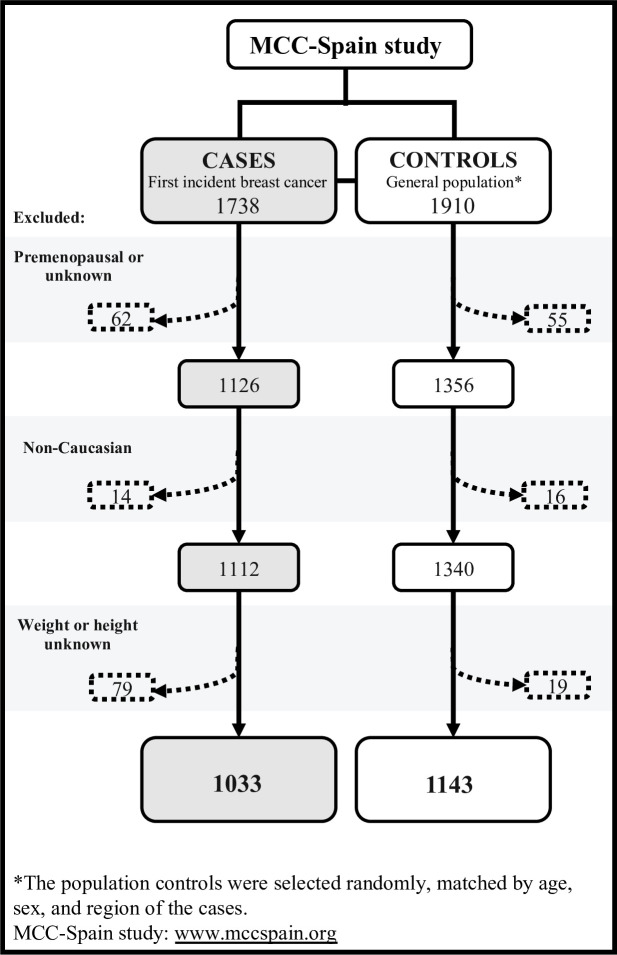
This study included postmenopausal white women with available anthropometric information. The final sample included 1033 breast cancer cases and 1143 controls. Figure 1 shows the flowchart of the selection process. Non-white participants were excluded because BMI cut-off points differ in other races, and CUN-BAE has been validated only in white populations.
Figure 1. Flowchart of the selection process of breast cancer cases in postmenopausal women, and in population controls, in the MCC-Spain study.
Data collection
Data on sociodemographic factors, lifestyle, personal/family medical history and reproductive history were collected by a computerised structured epidemiological questionnaire, administered by trained personnel in a face-to-face interview.21 Dietary information was obtained with a validated semi-structured 140 item food frequency questionnaire,22 which assessed dietary intake in the previous year. This questionnaire was provided to and self-administered by participants after the first interview (response rate 88%). Also, information about past alcohol consumption (for ages 30–40 years) was collected mainly through a self-administered questionnaire. Clinicopathological information on the cancer cases was obtained and validated from medical records, including the hormone receptor status of the tumour, degree of differentiation and histological type.
Definition of breast cancer cases
The main outcome variable was incident breast cancer confirmed histologically. The International Classification of Diseases, 10th revision codes used were C50 (invasive breast cancer), D05.1 and D05.7 (breast cancer in situ). The subtype of breast cancer was classified according to the pressence of positive hormone receptors (HR+=oestrogen positive or progesterone positive) or positive human epidermal growth factor (Erb2+), irrespective of oestrogen or progesterone status, or triple negative tumours (HR−=none of the three receptors expressed).12
Anthropometric measures
In face-to-face interviews carried out by trained personnel, participants provided data on weight and height. BMI of the cancer cases was obtained 1 year before diagnosis and BMI for controls was measured at the time of the interview. BMI was calculated as the ratio of weight (kg) to height squared (kg/m2) and was categorised using the WHO standards23: <25; 25–29.9; 30–34.9; and ≥35 kg/m2.
CUN-BAE was calculated with the equation developed by Gómez-Ambrosi et al12: % BF=−44.988+(0.503×age)+(10 689×sex)+(3.172×BMI)–(0.026×BMI2+(0.181×BMI×sex)–(0.02×BMI×age)–(0.005×BMI2×sex)+(0.00021×BMI2×age), where age is expressed in years and sex is encoded as men=0 and women=1. CUN-BAE was categorised according to the estimated percentage of body fat: <35%, 35–39.9%, 40–44.9% and ≥45%.
Statistical analysis
A descriptive analysis of the characteristics of participant was carried out using arithmetic mean (SD) for quantitative variables, and absolute and relative frequencies (%) for categorical variables. To test differences in general characteristics between cases and controls, the χ2 test was used.
Odds ratios (ORs) were estimated with 95% CIs for both measures (BMI and CUN-BAE) for breast cancer cases using unconditional logistic regression. The multivariate model included the following covariates: age at recruitment (years) ≤50, 51–60, 61–70 or >70 years; age of menarche <12, 12–13, ≥14 years or unknown; nulliparity (number of children) none, 1, 2, ≥3 or unknown; breastfeeding time (months) none, <6, ≥6 or unknown; energy intake (calories/day) <1500, 1500–2000, ≥2000 or unknown; family history of breast cancer no or yes; socioeconomic status low, medium or high; alcohol consumption (past or present g/day) 0, <12, ≥12 or unknown; smoking status never, yes or former; physical activity (METS×hours/week for mean year) 0, <8, 8–16, >16 or unknown; anti-inflammatory drug use never, some or unknown; oral contraceptive treatment never, some or unknown; and oral supplementary hormone treatment never, some, unknown.
A priori modifying effect by hormone receptor type in breast cancer was conducted, stratifying by tumour receptor status: hormone receptor positive (oestrogen or progesterone or both receptors), Erb2+ and triple negative.
Finally, the population attributable fraction (PAF) with 95% CIs was estimated as an epidemiological measure to assess the impact of both exposures (BMI vs CUN-BAE) on the burden of breast cancer in our Spanish population. PAFs were calculated in the MCC-Spain study datasets using the formula for exposures with k+1 levels:
, where pr is the proportion of cases in each body fat category and OR is the adjusted OR for each category.24 All analyses were performed with the statistical software package Stata MP V.15 (StataCorp). Statistical significance was set at a two sided p value of <0.05.
Results
We included 1033 incident postmenopausal breast cancer cases and 1143 population controls from the MCC-Spain study. Table 1 shows the characteristics of participants. Compared with controls, breast cancer cases presented at a lower mean age, had a smaller number of children and higher energy intake. Cases more often had a first degree family history of breast cancer and low socioeconomic status. Mean BMI was 26.3 (SD 4.8) kg/m2 in controls and 27.2 (4.9) kg/m2 in cases. Mean CUN-BAE was 39.7 (5.5)% in controls and 40.4 (5.5)% in cases.
Table 1. Descriptive characteristics of study participants.
| Characteristics | Breast cancer cases(n=1033) | Controls(n=1143) | P value* | |||
| n | % | n | % | |||
| Age (years) | <50 | 92 | 8.9 | 71 | 6.2 | <0.001 |
| 51–60 | 373 | 36.1 | 345 | 30.2 | ||
| 61–70 | 355 | 34.4 | 400 | 35.0 | ||
| >70 | 213 | 20.6 | 327 | 28.6 | ||
| Menarche age (years) | <12 | 211 | 20.4 | 221 | 19.3 | 0.068 |
| 12–13 | 472 | 45.7 | 512 | 44.8 | ||
| ≥14 | 333 | 32.2 | 403 | 35.3 | ||
| Unknown | 17 | 1.7 | 7 | 0.6 | ||
| No of children | Never | 182 | 17.6 | 174 | 15.2 | 0.020 |
| 1 | 149 | 14.4 | 141 | 12.3 | ||
| 2 | 414 | 40.1 | 464 | 40.6 | ||
| ≥3 | 282 | 27.3 | 363 | 31.8 | ||
| Unknown | 6 | 0.6 | 1 | 0.1 | ||
| Breast feeding | Never | 335 | 32.4 | 355 | 31.1 | 0.510 |
| <6 months | 354 | 34.3 | 423 | 37.0 | ||
| ≥6 months | 224 | 21.7 | 228 | 19.9 | ||
| Unknown | 120 | 11.6 | 137 | 12.0 | ||
| Total energy intake (calories/day) | <1500 | 273 | 26.4 | 348 | 30.4 | 0.134 |
| 1500–2000 | 332 | 32.1 | 370 | 32.4 | ||
| ≥2000 | 268 | 25.9 | 267 | 23.4 | ||
| Unknown | 160 | 15.5 | 158 | 13.8 | ||
| Family history of breast cancer | No | 877 | 85.7 | 1018 | 89.3 | 0.010 |
| Yes | 146 | 14.3 | 122 | 10.7 | ||
| Socioeconomic status | Low | 403 | 39.0 | 411 | 36.0 | 0.258 |
| Medium | 506 | 49.0 | 576 | 50.4 | ||
| High | 124 | 12.0 | 156 | 13.6 | ||
| Alcohol intake (g/day) | 0 | 240 | 23.2 | 277 | 24.2 | 0.190 |
| <12 | 487 | 47.1 | 574 | 50.2 | ||
| ≥12 | 146 | 14.1 | 134 | 11.7 | ||
| Unknown | 160 | 15.5 | 158 | 13.8 | ||
| Smoking status | Never | 653 | 63.2 | 730 | 63.9 | 0.750 |
| Yes | 380 | 36.8 | 413 | 36.1 | ||
| Physical activity (METS×hour/week) | 0 | 407 | 39.4 | 424 | 37.1 | 0.015 |
| <8 | 165 | 16.0 | 192 | 16.8 | ||
| 8–16 | 127 | 12.3 | 152 | 13.3 | ||
| >16 | 334 | 32.23 | 363 | 31.8 | ||
| Unknown | 0 | 0.0 | 12 | 1.0 | ||
| Anti-inflammatory drugs | Never | 413 | 40.0 | 527 | 46.1 | 0.013 |
| Sometimes | 581 | 56.2 | 572 | 50.0 | ||
| Unknown | 39 | 3.8 | 44 | 3.9 | ||
| Oral contraceptives | Never | 631 | 61.1 | 657 | 57.5 | 0.010 |
| Sometime | 397 | 38.4 | 486 | 42.5 | ||
| Unknown | 5 | 0.5 | 0 | 0.0 | ||
| Hormone replacement therapy | Never | 883 | 45.5 | 962 | 84.2 | 0.350 |
| Sometime | 110 | 10.6 | 122 | 10.7 | ||
| Unknown | 40 | 3.9 | 59 | 5.1 | ||
p=0.05 was considered statistically significant.
Differences between cases and controls were tested using the χ2 test.
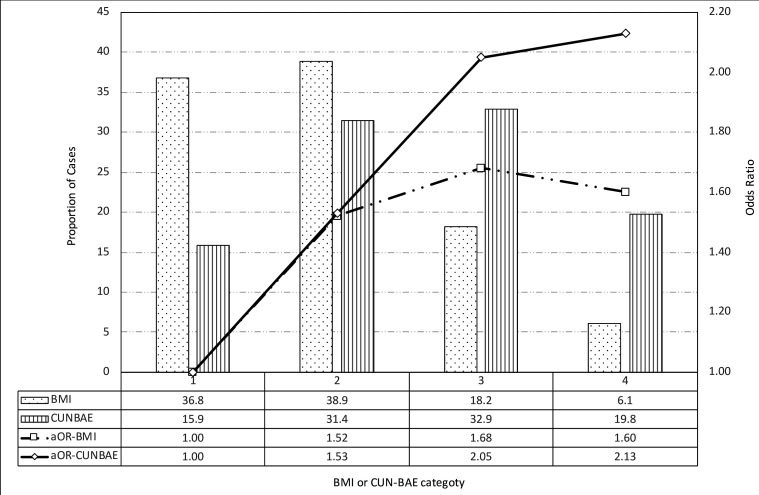
Figure 2 shows the distribution of obesity index exposure by BMI and CUN-BAE category. BMI <25 kg/m2 (reference) was observed in 45.0% of controls and in 36.8% of breast cancer cases, whereas a BMI ≥30 kg/m2 was observed in 19.9% of controls and 24.3% of breast cancer cases. CUN-BAE <35% (reference) was observed in 20.6% of controls and in 15.9% of cases, and CUN-BAE ≥40% was observed in 46.3% of controls and in 52.7% of cases.
Figure 2. Prevalence of body fat, according to body mass index (BMI) and Clínica Universidad de Navarra–Body Adiposity Estimator (CUN-BAE) category, and ORs for postmenopausal breast cancer. BMI was classified as <25 (reference), 25–29.9, 30–34.9 and ≥35 kg/m2. CUN-BAE was classified as <35 (reference), 35–39.9, 40–44.9 and ≥45 percentage body fat. ORs were adjusted (aOR) for age at recruitment (years) ≤50, 51–60, 61–70 or >70 years; age of menarche <12, 12–13, ≥14 or unknown; nulliparity (number of children) none, 1, 2, ≥3 or unknown; breastfeeding time (months) no, <6, ≥ 6 or unknown; energy intake (calories/day) <1500, 1500 to 2000, ≥2000 or unknown; family history of breast cancer no or yes; socioeconomic status low, medium or high; alcohol consumption (past or present g/day) 0, <12, ≥12 or unknown; smoking status never, yes or former; physical activity (METS×hours/week for mean year) 0, <8, 8–16, >16 or unknown; anti-inflammatory drug use never, some or unknown; oral contraceptive treatment never, some or unknown; and oral supplementary hormonal treatment never, some or unknown.
The highest categories of CUN-BAE showed an increase in the risk of postmenopausal breast cancer (OR 2.13 for body fat ≥45% compared with the reference category <35%). However, no similar trend was observed for BMI, because the gradient declined after a BMI of ≥35 kg/m2. Because of the different distributions of exposure together with the small increase in the estimated OR for BMI, we showed that the estimated PAFs were 23.0% (95% CI 12.2% to 31.4%) for BMI and 38.0% (95% CI 23.1% to 49.6%) for CUN-BAE, as presented in table 2.
Table 2. Burden of postmenopausal breast cancer due to body fat using body mass index (BMI) and Clínica Universidad de Navarra–Body Adiposity Estimator (CUN-BAE).
| Controls | Postmenopausal breast cancer cases | |||||||
| No | % | No | % | OR | 95% CI for OR | PAF | 95% CI for PAF | |
| BMI | ||||||||
| <25 | 521 | 45.6 | 380 | 36.8 | 1 | 0.23 | 0.122 to 0.314 | |
| 25–29.9 | 395 | 34.5 | 402 | 38.9 | 1.52 | 1.24 to 1.86 | ||
| 30.0–34.9 | 168 | 14.7 | 188 | 18.2 | 1.68 | 1.30 to 2.19 | ||
| ≥35 | 59 | 5.2 | 63 | 6.1 | 1.6 | 1.08 to 2.40 | ||
| CUN-BAE | ||||||||
| <35 | 235 | 20.6 | 164 | 15.9 | 1 | 0.38 | 0.231 to 0.496 | |
| 35.0–39.9 | 379 | 33.2 | 324 | 31.4 | 1.53 | 1.17 to 2.01 | ||
| 40.0–44.9 | 331 | 29.0 | 340 | 32.9 | 2.05 | 1.54 to 2.72 | ||
| ≥45 | 198 | 17.3 | 205 | 19.8 | 2.13 | 1.54 to 2.93 | ||
ORs were adjusted (aOR) for age at recruitment (years) ≤50, 51–60, 61–70 or >70 years; age of menarche <12, 12–13, ≥14 or unknown; nulliparity (number of children) none, 1, 2, ≥3 or unknown; breastfeeding time (months) no, <6, ≥ 6 or unknown; energy intake (calories/day) <1500, 1500 to 2000, ≥2000 or unknown; family history of breast cancer no or yes; socioeconomic status low, medium or high; alcohol consumption (past or present g/day) 0, <12, ≥12 or unknown; smoking status never, yes or former; physical activity (METS×hours/week for mean year) 0, <8, 8–16, >16 or unknown; anti-inflammatory drug use never, some or unknown; oral contraceptive treatment never, some or unknown; and oral supplementary hormonal treatment never, some or unknown.
Bold typeface indicates significance at p=0.05.
PAFspopulation attributable fractions
Estimated PAFs for BMI and CUN-BAE by breast cancer hormone receptor type are shown in table 3. For breast cancer cases with positive hormone receptors (n=680 cases), a higher BMI contributed to a PAF of 19.9% (95% CI 9.1% to 27.8%) while with CUN-BAE, PAF was 41.9% (95% CI 26.3% to 61.2%). PAFs for breast cancer cases with positive hormone receptors were similar to those for overall breast cancer cases. These similar trends in PAFs reflected differences in the distribution of BMI versus CUN-BAE categories, as well as differences in OR values. For Erb2+ (178 cases) or triple negative (79 cases) cases, the PAF burden was the same when estimating PAF using BMI or CUN-BAE (%PAF for Erb2+ 23.4% vs 24.6%; %PAF for triple negative breast cancer 23.0% vs 26.3%, with BMI and CUN-BAE, respectively).
Table 3. Fraction of postmenopausal breast cancer cases attributable to body fat using body mass index (BMI) and Clínica Universidad de Navarra–Body Adiposity Estimator (CUN-BAE), by hormone receptor type.
| BMI | CUN-BAE | |||||||
| <25 | 25–29.9 | 30–34.9 | ≥35 | <35 | 35–39.9 | 40–44.9 | ≥45 | |
| Controls | ||||||||
| No | 521 | 395 | 168 | 59 | 235 | 379 | 331 | 198 |
| % | 45.6 | 34.5 | 14.7 | 5.2 | 20.5 | 33.2 | 29 | 17.3 |
| Positive hormone receptors | ||||||||
| No | 248 | 264 | 126 | 42 | 100 | 216 | 228 | 136 |
| % | 36.5 | 38.8 | 18.5 | 6.2 | 14.7 | 31.8 | 33.5 | 20.0 |
| OR | 1 | 1.53 | 1.68 | 1.61 | 1 | 1.66 | 2.20 | 2.23 |
| OR (95% CI) | 1 | 1.21 to 1.92 | 1.25 to 2.25 | 1.13 to 3.56 | 1 | 1.22 to 2.27 | 1.59 to 3.05 | 1.54 to 3.22 |
| PAFs (95% CI) | 0.199 (0.091 to 0.278) | 0.419 (0.263 to 0.612) | ||||||
| Erb2+ | ||||||||
| No | 68 | 75 | 27 | 8 | 35 | 56 | 59 | 28 |
| % | 38.2 | 42.1 | 15.2 | 4.5 | 19.7 | 31.5 | 33.1 | 15.7 |
| OR | 1 | 1.75 | 1.43 | 1.20 | 1 | 1.26 | 1.73 | 1.39 |
| OR (95% CI) | 1 | 1.20 to 2.56 | 0.86 to 2.39 | 0.52 to 2.74 | 1 | 0.77 to 2.05 | 1.04 to 2.88 | 0.76 to 2.55 |
| PAFs (95% CI) | 0.234 (0.004 to 0.374) | 0.249 (0.000 to 0.473) | ||||||
| Triple negative hormone receptors | ||||||||
| No | 27 | 27 | 19 | 6 | 12 | 25 | 19 | 23 |
| % | 34.2 | 34.2 | 24 | 7.6 | 15.2 | 31.6 | 24.1 | 29.1 |
| OR | 1 | 1.25 | 2.13 | 1.84 | 1 | 1.33 | 1.09 | 2.30 |
| OR (95% CI) | 1 | 0.70 to 2.22 | 1.11 to 4.09 | 0.68 to 4.96 | 1 | 0.62 to 2.85 | 0.48 to 2.48 | 0.99 to 5.39 |
| PAFs (95% CI) | 0.230 (0.000 to 0.430) | 0.263 (0.000 to 0.586) | ||||||
ORs were adjusted (aOR) for age at recruitment (years) ≤50, 51–60, 61–70 or >70 years; age of menarche <12, 12–13, ≥14 or unknown; nulliparity (number of children) none, 1, 2, ≥3 or unknown; breastfeeding time (months) no, <6, ≥ 6 or unknown; energy intake (calories/day) <1500, 1500 to 2000, ≥2000 or unknown; family history of breast cancer no or yes; socioeconomic status low, medium or high; alcohol consumption (past or present g/day) 0, <12, ≥12 or unknown; smoking status never, yes or former; physical activity (METS×hours/week for mean year) 0, <8, 8–16, >16 or unknown; anti-inflammatory drug use never, some or unknown; oral contraceptive treatment never, some or unknown; and oral supplementary hormonal treatment never, some or unknown.
Bold typeface indicates significance at p=0.05.
Erb2+positive human epidermal growth factorPAFspopulation attributable fractions
Discussion
The burden of breast cancer attributable to excess body fat is likely to be underestimated if assessed with BMI in postmenopausal women, especially in hormone receptor positive tumours. Excess body fat is a well established risk factor for postmenopausal breast cancer,6 although why excess body weight is suggested as a protective factor in premenopausal cancer and a risk factor in postmenopausal cancer are not clear.4 25 There are several possible explanations, including low oestrogen levels in postmenopausal women.26 27 The 2018 Third Expert Report from the World Cancer Research Fund (WCRF) and the Global Cancer Update Programme (CUP) showed that the risk increased by 1.12 for every 5 kg/m2 increase in BMI, but with higher risks in Asian populations, hormone receptor positive cancers, and in those receiving hormone replacement therapy.6 This positive association in postmenopausal women was consistent when using different indicators of excess body fat (eg, waist circumference or waist-to-hip ratio) or changes in weight throughout life.9 28 Our study showed that body fat was associated with an increased risk when measured with a different anthropometric index (ie, CUN-BAE). Although further research is necessary to determine the underlying mechanisms, there are plausible biological explanations linking obesity to carcinogenesis, including associations between obesity and circulating hormone concentrations (eg, insulin, growth factors, oestrogens and adipokines), as well as low grade chronic inflammation.26 27
Quantifying the burden of cancer attributable to lifestyle factors is important for preventive programmes and public health decisions. To accurately estimate the burden caused by diseases that can be attributed to a risk factor, a thorough understanding of that risk factor is required. Although we acknowledge that the results of our case–control study cannot establish causal association (because PAF is typically assessed as relative risk), we have tried to provide insight into the impact of differences in the exposure level according to both anthropometric measures. Arnold et al3 previously estimated that 10% of postmenopausal breast cancers were attributable to a high BMI based on prevalence data from 2002 and GLOBOCAN 2012 to calculate PAFs. In our study, we showed higher estimates of postmenopausal breast cancer cases in Spain attributable to BMI. This difference may be due to the increased prevalence of obesity and the different methods used to calculate risks.3 29
Most previous studies that assessed the contribution of excess body fat to breast cancer were carried out using BMI.4 It is well known that BMI and its correlation with body fat is affected by race, sex and age, and therefore it tends to underestimate percentage body fat, especially in women and in older persons.7 In contrast, after adjusting BMI for age and sex, CUN-BAE showed a better correlation with body fat and also showed better relationship with cardiovascular risk factors and diabetes.12 15 Similarly, it has been observed that body fat assessed with BMI might also underestimate the risk of severe cases of influenza compared with body fat assessed with CUN-BAE.20 In terms of clinical implementation, CUN-BAE has the simplicity of BMI with improved assessment of body fat, and can be used in primary care with a simple colour scale.19
In this study, we found differences in the proportion of postmenopausal breast cancers attributable to body fat when using CUN-BAE (38.0%) compared with BMI (23.0%). These differences were caused by discrepancies in the distribution of the prevalence of overweight and obesity with each method. Moreover, higher ORs estimates were found in the category with a higher percentage of body fat, which suggests that the association between body fat and cancer risk was better stratified using CUN-BAE.
Breast cancer is a heterogeneous disease. As well as differences between premenopausal and postmenopausal diagnoses, various hormone receptors have also been seen to have different aetiologies, and their prognosis and response to treatment may vary. Most breast tumours have hormone receptors, which usually have a better prognosis and response to treatment. Other studies have found an association between BMI and risk of breast cancer exclusively in tumours that expressed a hormone receptor but not in triple negative or Erb2+ tumours.2 This is in line with our analysis based on hormone receptors, which showed that differences in attributable fractions according to the method used were observed exclusively in tumours with hormone receptors.
Our results should be interpreted with caution because of the case–control design of the study, although in the MCC-Spain project, population controls were selected and data collection was carried out by trained personnel. BMI was self-reported at the time of the interview for controls and 1 year before diagnosis for cancer cases. Regarding CUN-BAE, one of its limitations is that the formula was calculated from a sedentary convenience sample. The small sample size of cases that did not express hormone receptors is another limitation.
The strengths of the study include its originality; to the best of our knowledge, no previous study has carried out this comparison. Furthermore, it was a multicentre study with population controls and a relatively large sample size, which allowed us to examine specific subtypes of breast tumours, as well as different subgroups (eg, postmenopausal women, different physical activity levels and oral anti-inflammatory drug use). The results obtained with CUN-BAE were independent of its components (sex, age and BMI).15
Conclusions
The results of our study indicate that excess body fat is a significant risk factor for hormone receptor positive breast cancer in postmenopausal women. Our findings suggest that the population impact could be underestimated when using traditional BMI estimates, and that more accurate measures of body fat, such as CUN-BAE, should be considered when estimating the cancer burden attributable to obesity in postmenopausal breast cancer. This information could influence cancer prevention initiatives by highlighting the role of excess body fat in the development of breast cancer and by raising awareness among healthcare professionals and the public.
Acknowledgements
The authors thank all patients and families for their participation in the MCC-Spain study, as well as all study centres and collaborators.
Footnotes
Funding: This research was supported by Acción Transversal del Cáncer (approved by the Spanish Council of Ministers on 11 October 2007), Carlos III Health Institute-FEDER (PI08/1770, PI08/0533, PI08/1359, PS09/00773, PS09/01286, PS09/01903, PS09/02078, PS09/01662, PI11/01403, PI11/01889-FEDER, PI11/00226, PI11/01810, PI11/02213, PI12/00488, PI12/00265, PI12/01270, PI12/00715, PI12/00150, PI14/01219, PI14/0613, PI15/00069, PI15/00914, PI15/01032, PI11/01810, PI14/01219, PI11/02213, PIE16/00049, PI17/01179, PI17/00092), Fundación Marqués de Valdecilla (API 10/09), ICGC International Cancer Genome Consortium CLL (the ICGC CLL-Genome Project is funded by the Spanish Ministry of Economy and Competitiveness through the Carlos III Health Institute (ISCIII)), ISCIII Red Temática de Investigación del Cáncer (RTICC) (RD12/0036/0036), Regional Government of Castilla y León (LE22A10-2), Regional Health Ministry of Andalucía (PI-0571-2009, PI-0306-2011, salud201200057018tra), Regional Health Ministry of Valencia (AP_061/10), Recercaixa (2010ACUP00310), Regional Government of the Basque Country, Regional Health Ministry of Murcia, European Commission (grants FOOD-CT-2006–036224-HIWATE), Spanish Association Against Cancer (AECC) Scientific Foundation (GCTRA18022MORE), Agency for Management of University and Research Grants (AGAUR) of the Catalan Regional Government (2014SGR647, 2014SGR850 and 2017SGR723), Fundación Caja de Ahorros de Asturias and University of Oviedo. ISGlobal is a member of the CERCA Program, Regional Government of Catalonia. VD-B is contracted with the competitive national postdoctoral 'Sara Borrell' fellowship programme (CD21/00025) funded by Instituto de Salud Carlos III (ISCIII) and the European Regional Development Funds/European Social Fund.
Patient consent for publication: Consent obtained directly from patient(s).
Ethics approval: This study involves human participants and the database was registered with the Spanish data protection agency (No 210267217118). Ethics committee was Institut Municipal d’Investigació Mèdica (IMIM-Hospital del Mar)-Centre for Research in Environmental Epidemiology (CREAL). MK (PI). Participants gave informed consent to participate in the study before taking part.
Provenance and peer review: Not commissioned; externally peer reviewed.
Collaborators: MCC-Spain group.
Contributor Information
Naiara Cubelos-Fernández, Email: ncubelos@saludcastillayleon.es.
Verónica Dávila-Batista, Email: veronica.davila@ulpgc.es.
Tania Fernández-Villa, Email: tferv@unileon.es.
Gemma Castaño-Vinyals, Email: gemma.castano@isglobal.org.
Beatriz Perez-Gomez, Email: bperez@isciii.es.
Pilar Amiano, Email: epicss-san@euskadi.eus.
Eva Ardanaz, Email: me.ardanaz.aicua@cfnavarra.es.
Irene Delgado Sillero, Email: delgadosillero@gmail.com.
Javier Llorca, Email: javier.llorca@unican.es.
Guillermo Fernández Tardón, Email: fernandeztguillermo@uniovi.es.
Juan Alguacil, Email: alguacil@dbasp.uhu.es.
Mercedes Vanaclocha Espí, Email: vanaclocha_mer@gva.es.
Rafael Marcos-Gragera, Email: rmarcos@iconcologia.net.
Víctor Moreno, Email: v.moreno@iconcologia.net.
Nuria Aragones, Email: nuria.aragones@salud.madrid.org.
Ane Dorronsoro, Email: a-dorronsoroerauskin@euskadi.eus.
Marcela Guevara, Email: mp.guevara.eslava@navarra.es.
Sofía Reguero Celada, Email: sreguero@saludcastillayleon.es.
Marina Pollan, Email: mpollan@isciii.es.
Manolis Kogevinas, Email: manolis.kogevinas@isglobal.org.
Vicente Martín, Email: vmars@unileon.es.
Linda Gough, Email: lindamgough@gmail.com.
Data availability statement
Data are available on reasonable request.
References
- 1.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund / American Institute of Cancer Research . London: 2018. [5-Jun-2018]. Third expert report. Diet, nutrition, physical activity and cancer: a global perspective. continuous update project report.https://www.wcrf.org/dietandcancer Available. accessed. [Google Scholar]
- 3.Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Liu L, Zhou Q, et al. Body mass index had different effects on premenopausal and postmenopausal breast cancer risks: a dose-response meta-analysis with 3,318,796 subjects from 31 cohort studies. BMC Public Health. 2017;17:936. doi: 10.1186/s12889-017-4953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold M, Leitzmann M, Freisling H, et al. Obesity and cancer: an update of the global impact. Cancer Epidemiol. 2016;41:8–15. doi: 10.1016/j.canep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 6.World Cancer Research Fund/American Institute for Cancer Research Diet, nutrition, physical activity and cancer: a global perspective. Continuous Update Project Expert Report 2018. 2018 www.wcrf.org/diet-activity-and-cancer/
- 7.Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes. 2010;34:791–9. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 8.Lennon H, Sperrin M, Badrick E, et al. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18:56. doi: 10.1007/s11912-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christakoudi S, Tsilidis KK, Muller DC, et al. A body shape index (ABSI) achieves better mortality risk stratification than alternative indices of abdominal obesity: results from a large European cohort. Sci Rep. 2020;10:1–15. doi: 10.1038/s41598-020-71302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayawardena R, Ranasinghe P, Ranathunga T, et al. Novel anthropometric parameters to define obesity and obesity-related disease in adults: a systematic review. Nutr Rev. 2020;78:498–513. doi: 10.1093/nutrit/nuz078. [DOI] [PubMed] [Google Scholar]
- 11.Cui Z, Truesdale KP, Cai J, et al. Evaluation of anthropometric equations to assess body fat in adults: NHANES 1999-2004. Med Sci Sports Exerc. 2014;46:1147–58. doi: 10.1249/MSS.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Ambrosi J, Silva C, Catalán V, et al. Clinical usefulness of a new equation for estimating body fat. Diabetes Care. 2012;35:383–8. doi: 10.2337/dc11-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Głuszek S, Ciesla E, Głuszek-Osuch M, et al. Anthropometric indices and cut-off points in the diagnosis of metabolic disorders. PLoS ONE. 2020;15:e0235121. doi: 10.1371/journal.pone.0235121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davila-Batista V, Molina AJ, Fernández-Villa T, et al. The relation of CUN-BAE Index with body mass index and waist circumference in adults aged 50 to 85 years: the MCC-Spain study. Nutrients. 2020;12:996. doi: 10.3390/nu12040996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davila-Batista V, Molina AJ, Vilorio-Marqués L, et al. Net contribution and predictive ability of the CUN-BAE body fatness index in relation to cardiometabolic conditions. Eur J Nutr. 2019;58:1853–61. doi: 10.1007/s00394-018-1743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinknes KJ, Nurk E, Tell GS, et al. The relation of CUN-BAE index and BMI with body fat, cardiovascular events and diabetes during a 6-year follow-up: the Hordaland health study. Clin Epidemiol. 2017;9:555–66. doi: 10.2147/CLEP.S145130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhardwaj P, Au CC, Benito-Martin A, et al. Estrogens and breast cancer: mechanisms involved in obesity-related development, growth and progression. J Steroid Biochem Mol Biol. 2019;189:161–70. doi: 10.1016/j.jsbmb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macek P, Biskup M, Terek-Derszniak M, et al. Optimal cut-off values for anthropometric measures of obesity in screening for cardiometabolic disorders in adults. Sci Rep. 2020;10:11253. doi: 10.1038/s41598-020-68265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dávila-Batista V, Gómez-Ambrosi J, Fernández-Villa T, et al. Escala colorimétrica del porcentaje de grasa corporal según el estimador de adiposidad CUN-BAE. Aten Prim. 2016;48:422–3. doi: 10.1016/j.aprim.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dávila-Batista V, Carriedo D, Díez F, et al. [Estimation of the population attributable fraction due to obesity in hospital admissions for flu valued according to Body Mass Index (BMI) and CUN-BAE] Semergen. 2018;44:100–6. doi: 10.1016/j.semerg.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Castaño-Vinyals G, Aragonés N, Pérez-Gómez B, et al. Population-based multicase-control study in common tumors in Spain (MCC-Spain): rationale and study design. Gac Sanit. 2015;29:308–15. doi: 10.1016/j.gaceta.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Solans M, Fernández-Barrés S, Romaguera D, et al. Consumption of ultra-processed food and drinks and chronic lymphocytic leukemia in the MCC-Spain study. Int J Environ Res Public Health. 2021;18:5457. doi: 10.3390/ijerph18105457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sociedad Española para el Estudio de la Obesidad. (SEEDO) Consenso SEEDO’2000 para la evaluación del sobrepeso y la obesidad y el establecimiento de criterios de intervención terapéutica. Med Clín. 2000;115:587–97. doi: 10.1016/S0025-7753(00)71632-0. [DOI] [PubMed] [Google Scholar]
- 24.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–9. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Recalde M, Davila-Batista V, Díaz Y, et al. Body mass index and waist circumference in relation to the risk of 26 types of cancer: a prospective cohort study of 3.5 million adults in Spain. BMC Med. 2021;19:1–14. doi: 10.1186/s12916-020-01877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wild CP, Weiderpass E, Stewart BW, editors. World cancer report: cancer research for cancer prevention. France: Lyon: International Agency for Research on Cancer; 2020. [30-Jun-2021]. https://publications.iarc.fr/586 Available. accessed. [PubMed] [Google Scholar]
- 27.Amadou A, Hainaut P, Romieu I. Role of obesity in the risk of breast cancer: lessons from anthropometry. J Oncol. 2013;2013:906495. doi: 10.1155/2013/906495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Z, Willett WC, Colditz GA, et al. Waist circumference, waist:hip ratio, and risk of breast cancer in the nurses’ health study. Am J Epidemiol. 1999;150:1316–24. doi: 10.1093/oxfordjournals.aje.a009963. [DOI] [PubMed] [Google Scholar]
- 29.Bedogni G, Pietrobelli A, Heymsfield SB, et al. Is body mass index a measure of adiposity in elderly women? Obes Res. 2001;9:17–20. doi: 10.1038/oby.2001.3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.