Abstract
Objective
Concerns exist about the possible detrimental effects of exercise training on aortic size and valve function in individuals with bicuspid aortic valve (BAV). This multicentre international study aimed to determine the characteristics of aortic size and valve function in athletes versus non-athletes with BAV and athletes with tricuspid aortic valve (TAV).
Methods
We enrolled competitive athletes with BAV and age- and sex-matched athletes with TAV and non-athletes with BAV. We assessed valve function, aortic size and biventricular measures using echocardiography. Individuals with established moderate-severe AV stenosis, regurgitation or significant aortic dilation were excluded from the study.
Results
The study population comprised 504 participants: 186 competitive athletes with BAV (84% males; age 30±11 years), 193 competitive athletes with TAV and 125 non-athletes with BAV. The aortic annulus was greater in athletes with BAV than athletes with TAV and non-athletes with BAV (p<0.001). Both athletic and non-athletic individuals with BAV had greater sinuses of Valsalva, sino-tubular junction and ascending aorta diameters than athletes with TAV (p<0.001). However, no significant differences were found between athletes and non-athletes with BAV. Left ventricular index volumes and mass were greater in athletes with BAV than in the other two groups (p<0.001). Individuals with BAV (athletes and non-athletes) had greater mean gradients than TAV athletes.
Conclusion
This multicentre international study demonstrates no differences between athletes with BAV and non-athletes with BAV regarding aortic valve function or aortic dimensions. However, athletes with BAV have larger aortic diameters and a relatively worse valvular function than athletes with TAV.
Keywords: Athletes, Valve, Aorta, Sports medicine
WHAT IS ALREADY KNOWN ON THIS TOPIC
Concerns exist about the potential negative effects of exercise on valve function and aortic size in athletes with a bicuspid aortic valve (BAV). However, few studies with small sample sizes are available. Therefore, we conducted a multicentre study to enrol a population of athletes and non-athletes with BAV.
WHAT THIS STUDY ADDS
The study demonstrates that competitive athletes with BAV have larger aortic diameters and worse valvular function than athletes with tricuspid aortic valve (TAV). However, no differences were found between athletes and non-athletes with BAV without significant aortic valve stenosis/regurgitation or aortic root dilatation at study entry. Furthermore, athletes with BAV demonstrated a remodelling of the left ventricle that goes beyond usual training-induced adaptation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The present findings suggest that competitive athletes with BAV do not differ from non-athletes with BAV. Close, longitudinal follow-up is needed, considering we observed a greater remodelling of the left ventricle, larger aortic diameters and worse aortic valve function than athletes with TAV.
Introduction
Bicuspid aortic valve (BAV) is the most common congenital heart valve disease in the general population, including young athletes.1 2 Although initially considered a benign entity, it is now recognised as a valvulo-aortopathy of clinical interest. Patients with BAV may develop progressive valve dysfunction in the form of stenosis or regurgitation, dilation of the aortic root and ascending aorta, and reduced aortic elasticity, placing them at risk for dissection.3,5
Age, height, weight and body surface area (BSA) are key determinants of aortic root dimensions in healthy individuals, but also sex and blood pressure may affect aortic root size.4 6 However, these parameters are only partially useful in BAV patients as predictors of disease progression and future occurrence of valve dysfunction and/or aortic dilatation. The progression of valvular disease and/or aortopathy in patients with BAV is caused by multifactorial determinants, and the impact of sports practice remains uncertain.5 Intense exercise may potentially have a detrimental effect on athletes with BAV, placing them at a higher risk for valvular disease progression and aortic dissection or rupture.7 8
Exercise training may affect aortic size due to the haemodynamic stress on the aorta, and elite athletes with tricuspid aortic valve (TAV) exhibit mild aortic enlargement,9 10 although the aortic remodelling is usually consistent with left ventricular (LV) remodelling.11 In athletes with BAV, however, the evidence is conflicting: while some studies demonstrated that athletic training has no impact on the progression of aortic dilatation,1 12 others reported aortic dimensions increasing significantly in athletes with BAV versus TAV in a 5-year follow-up period.13 Unfortunately, data currently available are mostly derived from single-centre studies and are usually restricted to small cohorts of individuals with BAV.12 12,15
This multicentre, international registry enrolled a large cohort of individuals with BAV and aimed to characterise competitive athletes with BAV compared with non-athletes with BAV and competitive athletes with TAV to determine whether these populations differ regarding aortic size and valve function.
Methods
Study design
The SPREAD (Sport PRactice and its Effects on bicuspid aortic valve Disease) is a multicentre study promoted by the University of Siena, the European Association of Preventive Cardiology and the European Association of Cardiovascular Imaging.16 13 international centres with recognised sports cardiology and cardiac imaging expertise participated in the study. The first phase of the project, presented in this manuscript, consisted of a cross-sectional study aiming to evaluate valve function ascending aortic dimensions and establish the prevalence of aortic dilatation and aortic regurgitation or stenosis in athletes with BAV, compared with TAV athletes and BAV non-athletes.
In the second phase of the project, all the participants will be followed up for 3–5 years to evaluate the impact of continued sports practice on the progression of BAV disease.
Study group
Three distinct groups of participants were enrolled: (1) competitive athletes with BAV, (2) competitive athletes with TAV and (3) sedentary patients with BAV (non-athletes). All individuals were ≥18 years old. Competitive athletes (with either BAV or TAV) were defined as individuals training regularly for >5 hours/week and for ≥3 years and participating in competitions in a wide variety of sports disciplines. Non-athletes were defined as individuals who performed less than 3 hours of organised exercise per week, have not practised sport at a professional level in the past, have not practised competitive sports in the previous 5 years, and did not have a history of sports competitions in the previous 15 years. Athletes with TAV and non-athletes with BAV were age- and sex-matched to athletes with BAV. A case–control matching was performed with respect to important confounding factors represented by age and sex.
Our evaluation included the clinical and family history for coronary artery disease and/or sudden cardiac death, exercise history, physical examination and echocardiographic indices. Individuals with a definitive diagnosis of a high suspicion of cardiomyopathy were excluded from the study. Demographic parameters included age, sex, height and weight.
The exclusion criteria were:
Moderate or severe aortic valve stenosis or regurgitation at first evaluation, taking into account that BAV with mild valve dysfunction are the most common forms in the athletic population and that usually athletes with moderate or severe valve dysfunction are considered not eligible for sports competitions in most of the countries.
Significant aortic root or ascending aorta dilatation, defined as >45 mm in men (M) and >41 mm in women (F) at first evaluation. These cut-off values were arbitrarily established, considering the current recommendations, the average values of aortic dimensions collected in male and female competitive athletes, and the clinical practice.7 10 17 18
Moderate or severe mitral, tricuspid or pulmonary valve disease.
Aortic coarctation.
LV or right ventricular (RV) systolic dysfunction.
History of rheumatic fever.
The presence of other cardiovascular comorbidities, including arterial and/or pulmonary hypertension or regular therapy with an effect on the cardiovascular system.
Technically unsatisfactory examinations due to a poor acoustic window.
Individuals with a definitive or borderline diagnosis of Marfan syndrome, a positive family history of aortic aneurysm dissection or first-degree family members with a definitive diagnosis of aortopathy (except for BAV).
According to the exclusion criteria established for enrolling individuals with BAV in the absence of significant aortic dilatation and moderate or severe aortic valve regurgitation or stenosis, based on the different populations evaluated in the different centres, the range of individuals (athletes or non-athletes with BAV) excluded from the initial population varies from 24% to 55%.
In competitive athletes, sports disciplines were classified according to the ESC Sports Cardiology Guideline recommendations7 into four types: (1) skill (ie, golf, table tennis, etc), (2) power (ie, wrestling, boxing, etc), (3) mixed (ie, soccer, tennis, volleyball, etc) and (4) endurance (ie, cycling, triathlon, etc). Information about the lifetime history of exercise, training and sports and the hours of training per week was systematically collected via an interview during the clinical evaluation.
Echocardiography
Echocardiographic examinations were performed by experienced cardiologists or trained sonographers, according to the policy of each centre, using standardised echocardiograms. To harmonise data collection and to reduce heterogeneity, a video tutorial and online materials on how to measure aortic size and function and cardiac dimensions were prepared by the coordinating centre (ie, the Division of Cardiology of the Department of Medical Biotechnologies of the University of Siena, hereafter the ‘echo lab’) and made available for all the participating centres. Given the excellent reproducibility of aortic diameters measured by echocardiography,19 20 we conducted the intraclass correlation coefficient (ICC) analysis in the coordinating centre, demonstrating an excellent inter-observed variability (ICC 0.89–0.92). The inter-centre variability also demonstrated a good agreement (ICC 0.86–0.90).
The aortic annulus, sinuses of Valsalva, sino-tubular junction, ascending aorta and aortic arch diameters were measured as recommended.6 Aortic size and other valve regurgitation and/or stenosis were assessed according to the current guidelines.21 22
The type of BAV was assessed, and the following code was used for the nomenclature: 1 for type 1 (right and left cusps fusion) without raphe; 2 for type 1 with raphe; 3 for type 2 (right and non-coronary cusps fusion) without raphe; 4 for type 2 with raphe; 5 for type 3 (left and non-coronary cusps fusion with raphe).23
LV diameter and LV volumes were obtained as recommended.6 These measures were indexed to BSA. LV ejection fraction (EF) and RV fractional area change were calculated as recommended.6 Doppler interrogation of the tricuspid regurgitant jet was used to estimate pulmonary artery systolic pressure. Pulsed-wave Doppler and tissue Doppler imaging evaluation were recorded to obtain E peak and A peak velocities, E/A and E/e′ ratio and RV s′ velocity.6
Data analysis
Demographic and echocardiographic data were collected using the online platform REDCap software (Research Electronic Data Capture), a browser-based, metadata-driven electronic data capture software and workflow methodology for building and managing online surveys and databases that support online or offline data capture for research studies and specifically for multicentre studies. The Department of Medical Biotechnologies of the University of Siena, Italy, provided access to the online system. Data management (quality control, extraction, etc) was centralised and conducted by the Department of Medical Biotechnologies of the University of Siena. The data collected from different centres were anonymised.
Statistical analysis
The Department of Medical Biotechnologies of the University of Siena performed data analysis. The normality of quantitative variables was verified with the Shapiro-Wilk test. Continuous variables were reported as mean±SD or median and SE, and categorical variables were reported as absolute numbers or percentages. Categorical data were tested with the χ2 test. According to data distribution, the analysis of variance or Friedman test was used to assess the statistical significance of the difference among the different groups. The post hoc analysis of multiple variables was conducted using the Bonferroni or Dunn test as appropriate for data distribution. Correlation analyses were performed to explore the association between aortic size and demographic variables (height, weight, BSA, age and sex) using the Pearson correlation coefficient, according to data distribution. A stepwise linear regression analysis was also used to determine the independent predictors of aortic size. We employed a backward-stepping model. The input variables included in the models were parameters identified as independent predictors in the univariate analysis (p for inclusion <0.10). A p value <0.05 was considered statistically significant for all the analyses. The analysis was conducted using the SPSS V.21.0 (Chicago, Illinois).
The statistical analysis and presentation are consistent with the CHAMP statement.24
Equity, diversity and inclusion statement
The study was designed to include both males and females and is representative of the population of athletes practising competitive sports. Study participants were enrolled regardless of sexual, gender identity or ethnicity. The author team included 15 men and 14 women, including young researchers who actively participated in the research project.
Results
The study population was composed of 504 participants: 186 athletes with BAV (84% males; mean age 30±11 years), 193 athletes with TAV (82% males; mean age 30±11 years), 125 non-athletes with BAV (82% males; mean age 34±10 years). In athletes and non-athletes with BAV, right/left fusion with raphe was the most frequent anomaly (55% and 54%, respectively), followed by right/left fusion without raphe (19% and 20%, respectively).
The demographic characteristics of the study population are reported in table 1. Most athletes were engaged in mixed sports (61% and 63% in the BAV and TAV athletes, respectively), followed by endurance (17% and 22%, respectively), power (16% and 12%, respectively) and skill disciplines (5% and 3%, respectively), with no significant differences between athletes with BAV or TAV.
Table 1. Demographic characteristics of the study population.
| Variables | BAV athletes (n=186) | TAV athletes (n=193) | BAV non-athletes (n=125) | P value overall |
| Age, years | 30±11 | 30±11 | 34±10* | 0.01 |
| Males, n (%) | 157 (84) | 158 (82) | 102 (82) | 0.72 |
| Height, cm | 177±9 | 179±10† | 176±10 | 0.01 |
| Weight, kg | 73±12 | 75±13 | 75±15 | 0.11 |
| BSA, m2 | 1.89±0.19 | 1.94±0.21 | 1.88±0.22 | 0.06 |
| BMI | 23.1±2.5 | 23.3±2.7 | 23.4±3.9 | 0.58 |
| Smoker, n (%) | 17 (9) | 14 (7) | 15 (12) | 0.31 |
| Dyslipidaemia, n (%) | 8 (4) | 5 (3) | 6 (5) | 0.53 |
| Family history CAD, n (%) | 28 (15) | 20 (10) | 10 (8) | 0.14 |
| Sport discipline | NA | |||
| Skill | 10 (5.4) | 6 (3.1) | ||
| Power | 30 (16.1) | 23 (11.9) | ||
| Mixed | 114 (61.3) | 121 (62.7) | ||
| Endurance | 32 (17.2) | 43 (22.3) | ||
| Hours of training per week | 8±5 | 11±6† | 2±1* | <0.001 |
| Years of training | 12±7 | 14±7 | NA | 0.16 |
P<0.005 between other groups.
P<0.005 versus BAV non-athletes.
P<0.005 versus TAV athletes.
P<0.005 versus BAV athletes.
BAVbicuspid aortic valveBMIbody mass indexBSAbody surface areaCADcoronary artery diseaseNAnot applicable
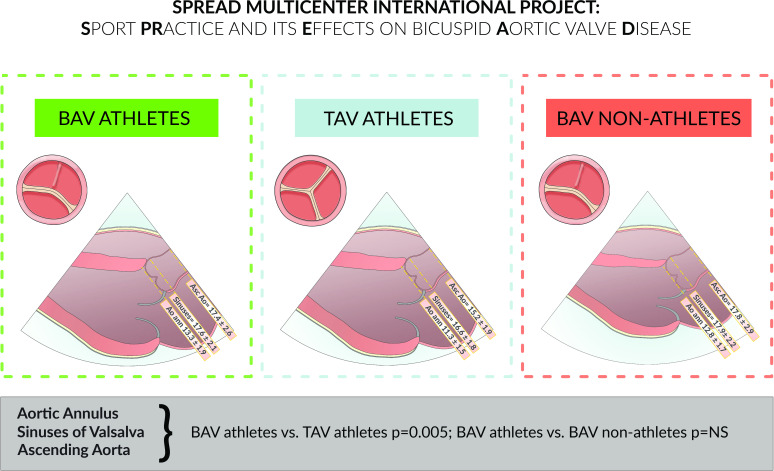
The echocardiographic aortic characteristics are reported in table 2. The aortic annulus, the aortic sinuses of Valsalva, the sino-tubular junction and the proximal ascending aorta indexed diameters were larger in athletes with BAV than in athletes with TAV (<0.001), but there were no significant differences between BAV athletes versus non-athletes.
Table 2. Echocardiographic aortic characteristics.
| Echocardiographic variables | BAV Athletes | TAV athletes | BAV non-athletes | P value |
| Aortic annulus, mm | 25.0±3.6* | 21.7±2.9† | 23.9±3.6 | <0.001 |
| Aortic annulus index, mm/m2 | 13.3±1.9 | 11.3±1.5* | 12.8±1.7 | <0.001 |
| Aortic annulus/height, mm/m | 14.1±1.9 | 12.1±1.6 | 13.5±1.9 | <0.001 |
| Sinuses of valsalva, mm | 33.1±4.2 | 32.0±3.2* | 33.3±4.8 | 0.006 |
| Sinuses of valsalva index, mm/m2 | 17.6±2.1 | 16.6±1.8* | 17.9±2.2 | <0.001 |
| Sinuses of valsalva/height, mm/m | 18.7±2.1 | 17.8±1.6 | 18.9±2.5 | <0.001 |
| Sinotubular junction, mm | 29.4±4.4 | 27.6±3.3* | 28.5±4.6 | <0.001 |
| Sinotubular junction index, mm/m2 | 15.6±2.2 | 14.3±1.8* | 15.2±2.1 | <0.001 |
| Proximal ascending aorta, mm | 32.7±5.1 | 29.4±3.4* | 33.1±5.4 | <0.001 |
| Proximal ascending aorta index, mm/m2 | 17.4±2.6 | 15.2±1.9* | 17.8±2.9 | <0.001 |
| Proximal ascending aorta/height, mm/m | 18.5±2.6 | 16.1±2.2* | 19.0±3.1 | <0.001 |
| Aortic arch, mm | 25.1±4.0 | 24.8±3.0 | 24.2±4.7 | 0.15 |
| BAV typeright and left cusps fusion without raphe, n (%) | 36 (19.3) | NA | 26 (20.8) | 0.46 |
| Right and left cusps fusion with raphe, n (%) | 103 (55.4) | NA | 68 (54.4) | |
| Right and non-coronary cusps fusion without raphe, n (%) | 16 (8.6) | NA | 11 (8.8) | |
| Right and non-coronary cusps fusion with raphe, n (%) | 29 (15.6) | NA | 17 (13.6) | |
| Left and non-coronary cusps fusion with raphe, n (%) | 2 (1.1) | NA | 3 (2.4) | |
| Mean gradient, mm Hg | 8.0±5.3‡ | 3.9±1.5 | 6.3±5.4§ | <0.001 |
| Mild aortic stenosis, n (%) | 31 (16.6) | NA | 13 (10.6) | 0.12 |
| Aortic regurgitation:mild, n (%) | 136 (73.1)‡ | 3 (1.6) | 82 (65.6) | p<0.001 |
| Mild mitral regurgitation, n (%) | 57 (31.0)† | 39 (20.5) | 18 (14.6) | 0.02 |
P<0.005 between other groups.
P<0.005 versus BAV non-athletes.
P<0.005 versus TAV athletes.
P<0.05 versus TAV athletes.
P<0.005 versus BAV athletes.
BAVbicuspid aortic valveNAnot applicableTAVtricuspid aortic valve
Individuals with BAV, athletes and non-athletes, had a greater mean gradient and higher prevalence of mild stenosis or regurgitation compared with TAV athletes, but there were no significant differences between the BAV groups.
The echocardiographic data of the LV, RV and left atrial (LA) are described in table 3. LV end-diastolic and end-systolic volume index and LV mass were significantly greater in competitive athletes with BAV than in the other two groups. LV EF did not differ among the study groups. E and A wave velocities and E/A ratio did not differ among the groups, but E/e′ was lower in TAV athletes than in BAV individuals (p<0.001). RV outflow tract diameters did not differ among the groups; however, athletes with TAV had greater inferior vena cava diameters than individuals with BAV (p<0.001).
Table 3. Echocardiographic characteristics of the study population.
| Echocardiographic variables | BAV athletes | TAV athletes | BAV non-athletes | P value |
| LV end-diastolic diameter, mm | 50.9±4.8 | 50.6±5.3 | 48.6±5.2* | <0.001 |
| LV end-systolic diameter, mm | 32.3±4.4 | 32.5±5.3 | 32.0±4.3 | 0.75 |
| Interventricular septum, mm | 9.2±1.6 | 8.9±1.5 | 8.8±1.8 | 0.12 |
| Posterior wall, mm | 8.9±1.6 | 8.8±1.3 | 8.2±1.5* | <0.01 |
| RWT | 0.35±0.06 | 0.35±0.05 | 0.34±0.06 | 0.34 |
| LV end-diastolic volume, mL | 128.2±33.1* | 116.8±31.9 | 115.3±28.7 | <0.001 |
| LV end-diastolic volume index, mL/m2 | 66.8±15.2* | 60.1±14.2 | 60.8±13.5 | <0.001 |
| LV end-systolic volume, mL | 49.9±15.6 | 47.5±15.1 | 46.3±14.6 | 0.13 |
| LV end-systolic volume index, mL/m2 | 26.3±7.5* | 24.4±7.1 | 24.3±6.7 | 0.02 |
| LV ejection fraction, % | 61±4 | 60±4 | 60±5 | 0.06 |
| LV mass | 171.1±54.3* | 154.7±47.0† | 139±41.5 | 0.001 |
| LV mass index | 90.0±25.0* | 79.1±20.7 | 73.2±18.5 | <0.001 |
| E wave velocity, m/s | 0.83±0.19 | 0.82±0.18 | 0.81±0.18 | 0.71 |
| A wave velocity, m/s | 0.56±0.17 | 0.50±0.11 | 0.53±0.14 | 0.24 |
| E/A ratio | 1.64±0.58 | 1.65±0.54 | 1.61±0.63 | 0.84 |
| e′ lateral wall velocity, m/s | 0.16±0.4 | 0.17±0.4 | 0.15±0.7 | 0.08 |
| e′ septal velocity, m/s | 0.12±0.3 | 0.14±0.3* | 0.11±0.3 | <0.001 |
| E/e′ ratio | 6.1±1.8 | 5.6±1.4* | 6.5±1.7 | <0.001 |
| RV fractional area change, % | 45.0±6.3‡ | 48.1±8.0 | 46.0±6.0 | 0.01 |
| TAPSE, mm | 24.9±3.6† | 24.8±3.6 | 23.8±4.4 | 0.04 |
| s′, m/s | 0.14±0.03 | 0.14±0.03 | 0.13±0.03 | 0.09 |
| PAPs, mm Hg | 21.4±7.4 | 21.8±4.5 | 23.9±3.9 | 0.62 |
| IVC, mm | 16.8±3.8 | 18.9±3.8* | 15.9±4.4 | <0.001 |
P<0.005 between other groups.
P<0.005 versus BAV non-athletes.
P<0.005 versus TAV athletes.
P<0.005 versus BAV athletes.
IVCinferior vena cavaLAleft atrialLVleft ventricularPLAXparasternal long-axis viewRVright ventricularRVOTRV outflow tractRWTrelative wall thickness
Figure 1 summarises the main findings of the study.
Figure 1. Central illustration. Aortic dimensions are adjusted for BSA. Values shown represent indexed diameters (mm)/BSA (m2). BAV, bicuspid aortic valve; TAV, tricuspid aortic valve.
Correlation and regression analysis
In the entire cohort, the univariate correlation analysis demonstrated that age, sex, weight, height and BSA were significantly associated with aortic annulus size (R=0.38, R=−0.39, R=0.45, R=0.39, R=0.46, respectively; p<0.001 for all the parameters), aortic root size (R=0.37, R=−0.27, R=0.31, R=0.22, R=0.30, respectively; p<0.001 for all the parameters) and ascending aorta diameters (R=0.38, R=−0.39, R=0.45, R=0.39, R=0.46, respectively; p<0.001 for all the parameters). The practice of competitive sports or specific disciplines did not correlate with aortic size.
In the population of competitive athletes with BAV, BSA, weight and height were significantly associated with aortic annulus size (R=0.36, R=0.34 and R=0.33, respectively; p<0.001 for all the parameters), aortic root diameters (R=0.47, R=0.42 and R=0.46, respectively; p<0.001 for all the parameters), ascending aorta size (R=0.38, R=0.35 and R=0.36, respectively; p<0.001 for all the parameters) and aortic arch diameters (R=0.37, R=0.35 and R=0.32, respectively; p<0.001 for all the parameters). In this population, a multivariate regression analysis was performed to determine the independent predictors of aortic size. In the multivariate regression analysis, the model with height+age+sex accounted for 59% of the variability of the aortic root and 45% of the ascending aorta explained by the model.
Discussion
This is the first international, multicentre study on a large cohort of 504 individuals, comparing competitive athletes with BAV with athletes with normal aorta and with non-athletes with BAV. Considering the haemodynamic load associated with intense physical training, there are concerns that competitive sports may worsen the anatomic and clinical presentation of valvular disease and aortopathy in athletes with BAV.5 8 The present study is largely reassuring in this context by showing that: (1) competitive athletes with BAV had similar aortic dimensions to non-athletes with BAV, but larger than athletes with TAV, demonstrating no differences in aortic dimensions between athletes and non-athletes with BAV; (2) individuals with BAV, athletes and non-athletes, had a greater mean gradient and higher prevalence of mild stenosis or regurgitation compared with TAV athletes but there were no significative differences within the BAV groups; (3) athletes with BAV demonstrated greater LV volumes and mass compared with both athletes with TAV and non-athletes with BAV, suggesting a peculiar LV remodelling in athletes with BAV.
Aortic dimensions
Our study did not find significant differences in the aortic size between BAV athletes and BAV non-athletes without significant aortic valve stenosis/regurgitation or aortic root dilatation, suggesting the absence of a significant impact of regular training and competitive sport on aortic diameters in this specific population. In a similar study, Boraita et al compared 41 BAV athletes with 41 BAV non-athletes and 41 TAV athletes. In agreement with our results, the authors reported that the aortic diameters were similar in both elite athletes and non-athletes with BAV but significantly larger compared with elite athletes with TAV, concluding that athletic activity, per se, had a limited impact on the aortic diameters.1 Similarly, Stefani et al observed that although aortic root dimensions were significantly greater in athletes with BAV compared with TAV athletes, no association existed between aortic size and years of training other than age, BSA or aortic regurgitation.2
As described in previous studies,17 25 TAV athletes show only a mild increase in aortic dimension compared with healthy sedentary controls, suggesting that sports, including the most intense endurance disciplines, per se, have only limited influence on aortic dimensions. Significant enlargement of the aortic root in highly conditioned athletes is uncommon and unlikely to represent the physiological sequelae of exercise training.17 25 26 Instead, a high prevalence of aortic dilatation is observed in sedentary BAV, suggesting that aortic enlargement is closely related to the pathophysiology of valve disease.3
In our study, where we enrolled individuals with BAV without significant aortic valve stenosis/regurgitation or aortic root dilatation, BAV athletes and BAV non-athletes had more frequently mild aortic stenosis or aortic regurgitation and higher mean gradients than competitive athletes with a normal aorta, without significative differences between the two BAV groups. In the study by Boraita et al, aortic valve regurgitation was the only functional abnormality detected through Doppler echocardiography in BAV athletes and was less frequent (65%) compared with the non-athletic BAV population (84%).1
LV remodelling
In our study, competitive athletes with BAV demonstrated significantly greater LV end-diastolic (and end-systolic) volumes and LV mass than the other two groups. Although the difference with BAV non-athletes is expected, the greater LV dimensions observed in athletes with BAV compared with competitive athletes with a trileaflet aortic valve requires further consideration. The difference in LV dimensions and mass cannot be considered uniquely as the expression of training-induced cardiac remodelling, because both groups participated in similar sports, and athletes with TAV reported an even higher training volume per week. The difference in LV mass may be, at least in part, explained by the specific haemodynamic pressure and volume overload imposed by BAV in addition to the increased cardiac output dictated by exercise conditioning. Furthermore, previous evidence demonstrated that, in patients with BAV, the increase in LV mass and the type of LV remodelling (ie, concentric remodelling, concentric or eccentric hypertrophy) were independently associated with clinical outcomes as AV repair/replacement and all-cause mortality, further supporting the importance of a comprehensive echocardiographic analysis of all cardiac chambers to obtain relevant prognostic information.27 However, our data are insufficient to support a hypothesis of adverse remodelling of the LV in competitive athletes with BAV when exposed to the haemodynamic load induced by intensive training. Similarly, in a large population of athletes with BAV, Stefani et al observed a progressive enlargement of the end-diastolic (and end-systolic) LV dimensions as well as an increase of the LV mass over a 5-year observation period.14 Further, longitudinal studies with prolonged follow-up are therefore needed to confirm our results and such a hypothesis.
Limitations
The first limitation of the study is that this is a cross-sectional evaluation of athletes with BAV compared with non-athletes with BAV and athletes with a normal aorta. Although definitive conclusions regarding a cause–effect relationship cannot be made, the comparison across the three distinct groups, that were sufficiently powered, strengthens the hypothesis that exercise may be less harmful than previously thought. Future prospective studies are warranted to investigate this possibility and provide directions for future guidelines. The second phase of the study, including the follow-up, will answer the questions raised by this manuscript and the current literature, further understanding whether the sport may have an impact on valve function or aortic dilatation, as recently demonstrated in a longitudinal study enrolling collegiate athletes with TAV.28
Another limitation is that, in this study, we excluded individuals (athletes and non-athletes) with BAV and significant aortic valve stenosis/regurgitation or aortic root dilatation. Therefore, this selected cohort does not reflect the entire spectrum of patients with BAV, potentially underestimating the impact of exercise in the more severe forms of the disease. However, a recent paper by Schreurs et al15 demonstrated, in a large population of BAV patients with no exclusion criteria for disease severity, that lifelong exercise does not appear to induce adverse cardiovascular effects in patients with a BAV.
In this study, although the population of competitive athletes was selected according to specific criteria, and we inquired about the training volume per week, the training intensity was not objectively measured. Therefore, we cannot conclusively exclude that the differences between the groups of athletes are due to different training intensities or volumes. Moreover, the study population of competitive athletes was primarily represented by athletes engaged in mixed sports, as they are the most popular sports disciplines practised in Europe. Although other sports disciplines were represented, the small number of athletes engaged in different disciplines represents a limitation in analysing the impact of different sports on aortic size and valvular dysfunction. Similarly, we cannot obtain information regarding the impact of endurance and resistance training protocols on aortic remodelling in athletes with BAV.
Echocardiographic data were not reviewed by a core lab. However, a video tutorial and online materials on how to measure aortic size and function and cardiac dimensions were prepared by the coordinating centre and available for all the collaborating centres. These resources, together with the recognised expertise of the international centres, help us harmonise data collection and reduce heterogeneity.
Conclusions
This multicentre cross-sectional study demonstrates no differences between athletes with BAV and non-athletes with BAV regarding aortic valve function or dimensions. However, athletes with BAV demonstrated greater LV volumes and LV mass than athletes with TAV and non-athletes with BAV, suggesting that competitive sports may cause a greater LV remodelling in individuals with BAV. The exact significance of this observation remains to be determined by the longitudinal follow-up of the SPREAD registry.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Patient consent for publication: Consent obtained directly from patient(s).
Ethics approval: This study involves human participants and was approved by local ethics committee of the University of Siena and all the local ethics committees of all collaborating centres. After the rationale and protocol of the study were explained, the participants gave their written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Flavio D'Ascenzi, Email: flavio.dascenzi@unisi.it.
Luna Cavigli, Email: luna.cavigli2@unisi.it.
Matteo Cameli, Email: matteo.cameli@unisi.it.
Guido Claessen, Email: guido.claessen@uzleuven.be.
Emeline M van Craenenbroeck, Email: emeline.vancraenenbroeck@uantwerpen.be.
Elena Cavarretta, Email: elena.cavarretta@uniroma1.it.
Antonello D'Andrea, Email: antonellodandrea@libero.it.
Maria Sanz De la Garza, Email: msanzdelagarza@gmail.com.
Thijs M H Eijsvogels, Email: thijs.eijsvogels@radboudumc.nl.
Roland R J van Kimmenade, Email: Roland.vanKimmenade@radboudumc.nl.
Laura Galian-Gay, Email: galiangay@hospitalclinic.es.
Martin Halle, Email: martin.Halle@mri.tum.de.
Valentina Mantegazza, Email: Valentina.Mantegazza@cardiologicomonzino.it.
Antonella Moreo, Email: antonella.moreo@ospedaleniguarda.it.
Bibi Schreurs, Email: Bibi.Schreurs@radboudumc.nl.
Laura Stefani, Email: laura.stefani@unifi.it.
Jose L Zamorano, Email: zamorano@secardiologia.es.
Antonio Pelliccia, Email: ant.pelliccia@gmail.com.
Michael Papadakis, Email: mipapada@sgul.ac.uk.
Data availability statement
Data are available upon reasonable request.
References
- 1.Boraita A, Morales-Acuna F, Marina-Breysse M, et al. Bicuspid aortic valve behaviour in elite athletes. Eur Heart J Cardiovasc Imaging. 2019;20:772–80. doi: 10.1093/ehjci/jez001. [DOI] [PubMed] [Google Scholar]
- 2.Stefani L, Galanti G, Toncelli L, et al. Bicuspid aortic valve in competitive athletes. Br J Sports Med. 2008;42:31–5. doi: 10.1136/bjsm.2006.033530. [DOI] [PubMed] [Google Scholar]
- 3.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–12. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 4.Iskandar A, Thompson PD. Diseases of the aorta in elite athletes. Clin Sports Med. 2015;34:461–72. doi: 10.1016/j.csm.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 5.D’Ascenzi F, Valentini F, Anselmi F, et al. Bicuspid aortic valve and sports: from the echocardiographic evaluation to the eligibility for sports competition. Scand J Med Sci Sports. 2021;31:510–20. doi: 10.1111/sms.13895. [DOI] [PubMed] [Google Scholar]
- 6.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Pelliccia A, Sharma S, Gati S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa605. [DOI] [PubMed] [Google Scholar]
- 8.Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370:1920–9. doi: 10.1056/NEJMra1207059. [DOI] [PubMed] [Google Scholar]
- 9.Iskandar A, Thompson PD. A meta-analysis of aortic root size in elite athletes. Circulation. 2013;127:791–8. doi: 10.1161/CIRCULATIONAHA.112.000974. [DOI] [PubMed] [Google Scholar]
- 10.Pelliccia A, Di Paolo FM, Quattrini FM. Aortic root dilatation in athletic population. Prog Cardiovasc Dis. 2012;54:432–7. doi: 10.1016/j.pcad.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Cavigli L, Ragazzoni GL, Quer L, et al. Aortic root/left ventricular diameters golden ratio in competitive athletes. Int J Cardiol. 2023;390:131202. doi: 10.1016/j.ijcard.2023.131202. [DOI] [PubMed] [Google Scholar]
- 12.Spataro A, Pelliccia A, Rizzo M, et al. The natural course of bicuspid aortic valve in athletes. Int J Sports Med. 2008;29:81–5. doi: 10.1055/s-2007-965110. [DOI] [PubMed] [Google Scholar]
- 13.Galanti G, Stefani L, Toncelli L, et al. Effects of sports activity in athletes with bicuspid aortic valve and mild aortic regurgitation. Br J Sports Med. 2010;44:275–9. doi: 10.1136/bjsm.2008.047407. [DOI] [PubMed] [Google Scholar]
- 14.Stefani L, Galanti G, Innocenti G, et al. Exercise training in athletes with bicuspid aortic valve does not result in increased dimensions and impaired performance of the left ventricle. Cardiol Res Pract. 2014;2014:238694. doi: 10.1155/2014/238694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreurs BA, Hopman MTE, Bakker CM, et al. Associations of lifelong exercise characteristics with valvular function and aortic diameters in patients with a bicuspid aortic valve. J Am Heart Assoc. 2024;13:e031850. doi: 10.1161/JAHA.123.031850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavigli L, Ragazzoni GL, Boncompagni A, et al. Rationale and design of the SPREAD study: sport practice and its effects on aortic size and valve function in bicuspid aortic valve disease. J Sports Med Phys Fitness. 2024 doi: 10.23736/S0022-4707.24.16051-3. [DOI] [PubMed] [Google Scholar]
- 17.Limongelli G, Monda E, Lioncino M, et al. Aortic root diameter in highly-trained competitive athletes: reference values according to sport and prevalence of aortic enlargement. Can J Cardiol. 2023;39:889–97. doi: 10.1016/j.cjca.2023.02.010. [DOI] [PubMed] [Google Scholar]
- 18.van Buuren F, Gati S, Sharma S, et al. Athletes with valvular heart disease and competitive sports: a position statement of the sport cardiology section of the European association of preventive cardiology. Eur J Prev Cardiol. 2021;28:1569–78. doi: 10.1093/eurjpc/zwab058. [DOI] [PubMed] [Google Scholar]
- 19.Asch FM, Yuriditsky E, Prakash SK, et al. The need for standardized methods for measuring the aorta: multimodality core lab experience from the GenTAC registry. JACC Cardiovasc Imaging. 2016;9:219–26. doi: 10.1016/j.jcmg.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Servato ML, Teixidó-Turá G, Sabate-Rotes A, et al. Are aortic root and ascending aorta diameters measured by the pediatric versus the adult American society of echocardiography guidelines interchangeable? J Clin Med. 2021;10:5290. doi: 10.3390/jcm10225290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgartner H, Hung J, Bermejo J, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European association of cardiovascular imaging and the American society of echocardiography. Eur Heart J Cardiovasc Imaging. 2017;18:254–75. doi: 10.1093/ehjci/jew335. [DOI] [PubMed] [Google Scholar]
- 22.Lancellotti P, Pibarot P, Chambers J, et al. Multi-modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging. 2022;23:e171–232. doi: 10.1093/ehjci/jeab253. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer BM, Lewin MB, Stout KK, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart. 2008;94:1634–8. doi: 10.1136/hrt.2007.132092. [DOI] [PubMed] [Google Scholar]
- 24.Mansournia MA, Collins GS, Nielsen RO, et al. A checklist for statistical assessment of medical papers (the CHAMP statement): explanation and elaboration. Br J Sports Med. 2021;55:1009–17. doi: 10.1136/bjsports-2020-103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelliccia A, Di Paolo FM, De Blasiis E, et al. Prevalence and clinical significance of aortic root dilation in highly trained competitive athletes. Circulation. 2010;122:698–706. doi: 10.1161/CIRCULATIONAHA.109.901074. [DOI] [PubMed] [Google Scholar]
- 26.Gati S, Malhotra A, Sedgwick C, et al. Prevalence and progression of aortic root dilatation in highly trained young athletes. Heart. 2019;105:920–5. doi: 10.1136/heartjnl-2018-314288. [DOI] [PubMed] [Google Scholar]
- 27.Butcher SC, Pio SM, Kong WKF, et al. Left ventricular remodelling in bicuspid aortic valve disease. Eur Heart J Cardiovasc Imaging. 2022;23:1669–79. doi: 10.1093/ehjci/jeab284. [DOI] [PubMed] [Google Scholar]
- 28.Tso JV, Turner CG, Liu C, et al. Longitudinal aortic root dilatation in collegiate American-style football athletes. J Am Heart Assoc. 2023;12:e030314. doi: 10.1161/JAHA.122.030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.