ABSTRACT
Despite the widespread use of currently available serum phosphate management options, elevated serum phosphate is common in patients with end‐stage kidney disease on dialysis. Characteristics of currently available phosphate binders that lead to poor patient experiences such as large drug volume size of required daily medication (e.g., many large tablets) and adverse gastrointestinal effects may decrease compliance to labeled dosing instructions, thus decreasing their efficacy. Oxylanthanum carbonate is a new molecule yielding the same phosphate‐binding capacity as lanthanum carbonate, but in a much smaller drug volume and tablet size. It is formulated as small tablets that can be easily swallowed. In a double‐blind dose‐escalation phase 1 study, healthy volunteers (n = 32) were randomly divided into four treatment arms and randomly assigned to receive oxylanthanum carbonate tablets or a placebo over a period of 4 days to evaluate safety, urinary and fecal excretion of phosphorus, and pharmacokinetics. Each treatment arm evaluated a different dose of oxylanthanum carbonate: 500, 1000, 1500, or 2000 mg three times a day (TID). The study drug was well‐tolerated. Oxylanthanum carbonate effectively decreased dietary phosphorus absorption, demonstrated by decreased urinary phosphorus excretion and increased fecal phosphorus excretion. Systemic absorption of oxylanthanum carbonate was minimal, with lanthanum serum concentration values below the level of quantification (0.500 ng/mL) in all subjects receiving 500 mg TID and did not exceed 0.7 ng/mL at other doses. Future studies should evaluate and confirm the ability of oxylanthanum carbonate to reduce pill burden and improve dose administration, patient tolerability, adherence, and treatment outcomes.
Trial Registration: ClinicalTrials.gov identifier: NCT01560884
Keywords: chronic kidney disease, end‐stage kidney disease, hyperphosphatemia, oxylanthanum carbonate, phosphate binder
Summary.
- What is the current knowledge on the topic?
-
○Currently available phosphate binders reduce phosphate absorption by binding to phosphate in the GI tract, forming insoluble complexes that are then excreted in the stool. Despite the widespread use of these available serum phosphate management options, uncontrolled hyperphosphatemia is highly prevalent in patients with CKD who are on dialysis. Characteristics of currently available phosphate binders can lead to poor patient experiences and decreased treatment adherence, resulting in inadequate efficacy. Thus, a phosphate binder option that maintains efficacy while reducing tablet size and adverse effects may increase adherence, thereby improving clinical outcomes. Oxylanthanum carbonate is a new molecule that has the same phosphate binding capacity as lanthanum carbonate but has a much smaller drug volume size. It is formulated as small tablets that can be easily swallowed.
-
○
- What question did this study address?
-
○This is a phase 1, dose‐escalation study investigating the safety/tolerability, pharmacodynamic, and pharmacokinetic of oxylanthanum carbonate in healthy volunteers.
-
○
- What does this study add to our knowledge?
-
○Oxylanthanum carbonate effectively decreased urinary phosphorus excretion. Systemic absorption of oxylanthanum carbonate was minimal, and the study drug was well‐tolerated.
-
○
- How might this change clinical pharmacology or translational science?
-
○Since there is a need for a new phosphate binder that is easy to ingest, exhibits minimal adverse effects, and maintains serum phosphate control, oxylanthanum carbonate may be a welcome choice for patients as it is effective at binding intestinal phosphorus and has been formulated as a small tablet that is swallowed.
-
○
1. Introduction
More than 43% of patients undergoing dialysis in the US have hyperphosphatemia [1], defined as serum phosphate values > 5.5 mg/dL and commonly associated with an increased risk of death. Serum phosphate management options include restricting dietary phosphorus intake, enhancing phosphate elimination with dialysis, and use of phosphate binders [2]. Currently, available phosphate binders reduce phosphate absorption by binding to phosphate in the gastrointestinal (GI) tract, forming insoluble complexes that are then excreted in the stool [2]. Despite the widespread use of these available serum phosphate management options, uncontrolled hyperphosphatemia (> 5.5 mg/dL) is highly prevalent in patients with end‐stage kidney disease (ESKD) who are on dialysis [3, 4]. Based on average serum phosphate values over 3 months, 44.3% of patients on dialysis exhibit values that exceed the recommended target upper threshold of 5.5 mg/dL, and 74.9% have values above the normal level of 4.5 mg/dL [3]. Furthermore, National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines suggest a serum phosphate target of between 3.5 and 5.5 mg/dL [5]. This lack of adequate real‐world serum phosphate management is concerning because elevated levels have been associated with negative clinical consequences. Kestenbaum et al. found that each 1 mg/dL increase in serum phosphate > 3.5 mg/dL was associated with an estimated 23% increased mortality risk in patients with chronic kidney disease (CKD) [6]. Therefore, achieving and maintaining adequate phosphate management is a crucial part of ESKD and CKD treatment.
Characteristics of currently available phosphate binders can lead to poor patient experiences and decreased treatment adherence resulting in inadequate efficacy. Patients are required to ingest several large tablets multiple times each day. Chiu et al. reported that patients on dialysis ingested a median of nine phosphate binder tablets daily and that only 38% of them were adherent to therapy [7]. A separate study of phosphate binder adherence reported that 20% of patients experienced intake inconvenience and low preference, mainly related to tablet size [8]. Additionally, phosphate binders often cause adverse GI effects. In clinical trials, GI adverse events were observed in 45%, 34%, and 56% of patients taking sucroferric oxyhydroxide [9], sevelamer [9], and lanthanum carbonate [10], respectively. Nausea was reported in 7%, 11%, and 26% of patients treated with sucroferric oxyhydroxide [9], sevelamer [9], and lanthanum carbonate [11], respectively. An analysis of reasons for phosphate binder discontinuation found that, of the 11% of patients who discontinued treatment due to an inability to tolerate the medication, 48% specifically cited GI upset [8]. Thus, a phosphate binder option that maintains efficacy while reducing tablet size and adverse effects may increase adherence, thereby improving clinical outcomes.
Oxylanthanum carbonate is a new treatment in development that contains lanthanum, the same active moiety as the approved phosphate binder lanthanum carbonate, and forms the same insoluble phosphate complex, lanthanum phosphate, in the GI tract. However, oxylanthanum carbonate is produced via a proprietary nanoparticle technology that yields the same binding capacity as lanthanum carbonate, but in a much lower drug volume and consequently tablet size. This formulation innovation translates to small tablets that can be easily swallowed compared to lanthanum carbonate and other phosphate binders that are formulated as large tablets that must be chewed. In preclinical studies, oxylanthanum carbonate demonstrated a good efficacy and safety profile. A study in a rat model comparing equivalent doses of oxylanthanum carbonate and lanthanum carbonate found that both compounds lowered serum phosphate concentrations on day 1 and were equally effective in maintaining low serum phosphate levels throughout the treatment cycle, with oxylanthanum carbonate delivering the same amount of active elemental lanthanum in a lower drug volume and tablet size vs. lanthanum carbonate [12]. All doses of oxylanthanum carbonate (200, 600, and 2000 mg/kg/day) were well‐tolerated in rat [13] and dog [14] acute and chronic toxicology studies. A previously published abstract of this phase 1 study reported that subjects receiving oxylanthanum carbonate demonstrated statistically significant reductions in daily urinary phosphorus in a dose‐dependent fashion concomitant with increased fecal phosphorus excretion [15]. The pharmacodynamics profile suggested that the binding capacity of oxylanthanum carbonate exceeds that of currently available phosphate binders [15].
We present the safety, tolerability, pharmacodynamic, and pharmacokinetic results from a phase 1, dose‐escalation study of oxylanthanum carbonate in healthy volunteers, with the aim of investigating oxylanthanum carbonate's potential for further clinical development in ESKD and CKD.
2. Methods
2.1. Ethics
The trial protocol, informed consent document, subject recruitment procedures, and any amendments were approved by a properly constituted Institutional Review Board in compliance with current regulations of the U.S. FDA, International Council for Harmonization (ICH) guidelines, and any country‐specific regulations. Prior to the performance of any protocol‐specific procedures, informed consent was obtained and documented by the use of a written Informed Consent Form that fulfilled requirements as contained in the U.S. Code of Federal Regulations [16], the ICH guidelines [17], and the Declaration of Helsinki [18]. The study was conducted in accordance with Good Clinical Practices as defined in the U.S. FDA Code of Federal Regulations [16] and ICH guidelines [17]. The trial is registered with ClinicalTrials.gov (Registration Number: NCT01560884).
2.2. Subjects
The study took place over a period of 5 months (May 7 to September 18, 2012) in Baltimore Early Phase Clinical Unit Harbor Hospital (Baltimore, Maryland). Eligible subjects were male or female, ≥ 18 years of age with an approximate body weight between 70 and 80 kg, and in good general health prior to study participation (no clinically relevant abnormalities as assessed by the investigator and determined by medical history, physical examination, blood chemistry, hematology, urinalysis, and electrocardiogram). Female subjects were required to be either postmenopausal for at least 1 year, documented surgically sterile, or using two forms of medically acceptable contraception, including at least one barrier method. All subjects had to be able to swallow the study medication tablets and follow a standardized phosphate‐controlled diet and/or meal schedule per protocol.
Any of the following was regarded as criterion for exclusion: evidence of a current or recurrent condition that could affect the action, absorption, or disposition of the investigational product, or could in any way affect the clinical or laboratory assessments; current or past medical history of physical or psychiatric illness, or any medical disorder that may require treatment or make the subject unlikely to fully complete the study; current and chronic use of any antacids, H2 blockers and proton pump inhibitors within 14 days of the first dose of the investigational product (Day 6); known or suspected intolerance or hypersensitivity to the investigational product or any related compound (e.g., lanthanum carbonate); history of alcohol or drug abuse within the past year; a positive HIV, hepatitis B surface antigen (HBsAg), or hepatitis C virus (HCV) antibody screen; use of another investigational product within 30 days prior to the screening visit; active peptic ulcer disease, gastroesophageal reflux disease (GERD), irritable bowel syndrome (IBS), lactose intolerance and malabsorption syndrome; elevated liver enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT) and serum bilirubin ≥ 2 times the upper normal limit (UNL); and history of chronic constipation.
2.3. Study Design
Thirty‐two healthy volunteers were planned for enrollment in this double‐blind, within‐arm randomized, dose escalation, single‐center study (n = 8 for each treatment arm). Within each treatment arm, subjects were randomly assigned to receive oxylanthanum carbonate tablets (n = 6) or a placebo (n = 2) (Figure 1). Data from each of the treatment dose groups (n = 6 each) and the pooled data from the subjects receiving the placebo (n = 8) are presented. Each treatment arm evaluated a different dose of oxylanthanum carbonate: 500, 1000, 1500, and 2000 mg three times a day (TID) with meals (i.e., 1500, 3000, 4500, and 6000 mg/day, respectively) (Figure 1).
FIGURE 1.
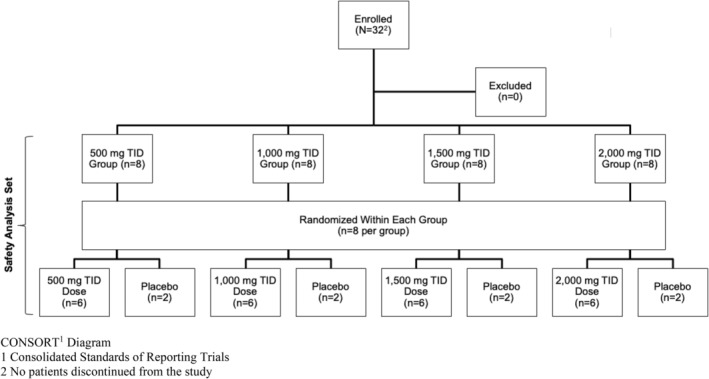
CONSORT1 diagram—This study enrolled 32 healthy volunteers (n = 8 for each treatment arm). Within each treatment arm, subjects were randomly assigned to receive oxylanthanum carbonate tablets (n = 6) or a placebo (n = 2). Each treatment arm evaluated a different dose of oxylanthanum carbonate: 500, 1000, 1500, and 2000 mg TID/day, respectively.
2.4. Study Treatment
On Days 6 through 10, oxylanthanum carbonate or placebo was administered orally within 15 min after each meal (three meals per day). Tablets were to be swallowed whole without being chewed. Each tablet contained 500 mg of lanthanum as oxylanthanum carbonate, the active ingredient, and other inactive ingredients. Placebo tablets were visually identical to oxylanthanum carbonate and contained only inactive ingredients; nanoparticle technology was not incorporated into the placebo tablets. The placebo and treatment arms both received the same number of tablets. Concomitant medications were avoided during the study, with the exception of hormonal replacement therapy or hormonal contraceptives.
2.5. Study Schedule
Subjects were admitted to the clinical research unit 1 day before the study started and remained at the clinical research unit until Day 13. From Day 1 to Day 10, subjects were placed on a controlled phosphate diet approved by a qualified dietician. The phosphate‐controlled diet (three meals and one snack) was designed to provide 37.5 mmol/day (1200 mg/day) of elemental phosphorus. The mean phosphorus content of each meal was 12.1 mmol (387 mg), 8.6 mmol (275 mg), 12.0 mmol (416 mg), and 2.6 mmol (83 mg) for breakfast, lunch, dinner, and snack, respectively. With breakfast, the majority of the phosphorus was administered in milk. With the other meals, phosphorus was primarily administered in solid food. During this 10‐day phosphate‐controlled diet period, each meal was consumed at the same time each day and subjects were required to ingest all meals in their entirety.
All excreted urine and feces for each 24‐h period were collected from Day 1 (morning) to Day 6 (morning) to establish baseline daily phosphorus excretion and from Day 8 (morning) to Day 13 (morning) to evaluate on‐treatment and post‐treatment phosphorus daily excretion. During the treatment phase (Day 6 to 10), subjects received either oxylanthanum carbonate or the placebo orally in three divided doses within 15 min after meals. Subjects were discharged on Day 13 and returned for an End‐of‐Study‐Visit on Day 20 for measurement of serum phosphate and electrolytes.
Initiation of each dosing treatment arm was sequential, and all safety data were reviewed prior to escalation to the next treatment arm. If no Grade 3 adverse events were observed in the first dosing treatment arm (500 mg TID vs. placebo) by Day 20, treatment of the next treatment arm (1000, 1500, or 2000 mg TID, respectively, vs. placebo) began. Grade 3 adverse events (vomiting and nausea) were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 [19].
2.6. Study Endpoints
The primary endpoint was the safety of oxylanthanum carbonate in healthy subjects, as assessed by the incidence and severity (as graded by the CTCAE version 4.03) of adverse events (AEs) and lab abnormalities. The secondary endpoints were the phosphate‐binding capacity, using the surrogate markers of phosphorus excreted in urine and feces, and pharmacokinetics of oxylanthanum carbonate.
2.7. Study Assessments and Analysis
The components of primary (safety) endpoints were the incidence and severity of AEs and serious AEs (SAEs), time to onset and duration of selected AEs, concomitant medication use, laboratory parameters, physical examination, and vital signs.
The secondary endpoint of phosphate‐binding capacity was evaluated by comparing the two‐component vector of differences concerning phosphorus excretion (in mg/day), as measured during defined periods before and during treatment. Urinary and fecal phosphorus excretion are considered surrogate markers of phosphate absorption. Urine and fecal phosphorus concentrations were measured by validated ICP‐MS assays with lower limits of quantification (LLOQ) of < 100 mg/day for urinary phosphorus excretion and < 5 mg/day for fecal phosphorus excretion. Values below the LLOQ were imputed as 0. (Table 1). Baseline phosphate content was determined using 24‐h urine and feces collected at each voiding and pooled in separate containers from the morning of Day 1 to the morning of Day 6. Phosphate content during treatment and posttreatment was assessed using 24‐h urine and feces collected from Day 8 to 10 and Day 11 to 13, respectively.
TABLE 1.
Comparison of factors for urinary and fecal phosphorus—on‐treatment.
| On‐treatment | ||
|---|---|---|
| Urinary phosphorus | Fecal phosphorus | |
| Analysis methodology | ANOVA and visit‐wise ANOVA | |
| Imputed as 0 (below level of quantification) | < 100 mg/day | < 5 mg/day |
The secondary endpoint of pharmacokinetic parameters was determined based on serum concentrations of lanthanum in blood samples drawn from Day 6 to 11 at the following time points: 0 (within 1 h pre‐dose), 1, 2, 4, 7, 11, 24, 48, 72, 96, and 120 h after the first dose. Blood samples were collected prior to subsequent doses of oxylanthanum carbonate (trough values). An additional pharmacokinetic blood sample was collected at the End‐of‐Study Visit (Day 20). Lanthanum serum concentrations were measured by a validated ICP‐MS assay with an LLOQ of < 0.500 ng/mL. The mean highest observed trough concentrations (Ctrough) and time to the highest observed Ctrough are presented. The mean highest observed Ctrough were calculated using the imputed concentrations of 0 for individuals with concentrations below LLOQ. Time to highest observed Ctrough was calculated using only data from individuals with quantifiable samples.
2.8. Statistical Methods
The mean on‐treatment urinary and fecal phosphorus excretion was calculated for each treatment arm. The change in urinary and fecal phosphorus excretion from baseline to the on‐treatment period was analyzed by repeated measures analysis of variance model (ANOVA) and visit wise ANOVA. The repeated variables for the time points were Day 8, Day 9, Day 10, Day 11, and Day 12 (n = 5), and doses of the drug were 500, 1000, 1500, and 2000 mg TID (n = 4). The mean on‐treatment phosphorus excretion was first calculated for each individual patient. These individual mean on‐treatment phosphorus excretion values were averaged across all patients for each treatment arm. The data for eight subjects taking placebo across treatment arms were pooled for analyses.
Mean, median, standard deviation (SD), standard error (SE), and min‐max were calculated to assess urine and fecal phosphorus excretion. P‐values were calculated using paired t‐test. All statistical analyses were performed using the SAS Version 9.3 or higher software package (SAS Institute Inc. Cary, North Carolina, USA) [20].
3. Results
3.1. Demographics
Demographics were well‐balanced between treatment arms. Overall, the mean (SD) age was 40 (12.2) years and 81% of subjects were male. The majority of subjects were Black or African American (66%), followed by White or Caucasian (31%) and Asian (3%) (Table 2). 19% of subjects were of Hispanic or Latino ethnicity. All subjects were healthy and none had a history of urinary or renal disorders. Baseline biochemistry data were similar across treatment arms (Table S1).
TABLE 2.
Baseline characteristics of study subjects.
| Placebo (n = 8) | Oxylanthanum carbonate (mg TID) | Total (n = 32) | |||||
|---|---|---|---|---|---|---|---|
| 500 (n = 6) | 1000 (n = 6) | 1500 (n = 6) | 2000 (n = 6) | ||||
| Age (years) | Mean (SD) | 31 (6.9) | 42 (9.7) | 48 (14.2) | 39 (14.0) | 42 (11.6) | 40 (12.2) |
| Median | 29 | 40 | 52 | 41 | 40 | 39 | |
| Min, Max | 23, 43 | 32, 60 | 23, 60 | 22, 57 | 24, 57 | 22, 60 | |
| Race | Black or African American, n (%) | 7 (88) | 3 (50) | 4 (67) | 4 (67) | 3 (50) | 21 (66) |
| White or Caucasian, n (%) | 1 (13) | 2 (33) | 2 (33) | 2 (33) | 3 (50) | 10 (31) | |
| Asian, n (%) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | |
| Ethnicity | Hispanic or Latino, n (%) | 2 (25) | 1 (17) | 0 (0) | 2 (33) | 1 (17) | 6 (19) |
| Gender | Male, n (%) | 7 (88) | 6 (100) | 4 (67) | 3 (50) | 6 (100) | 26 (81) |
| Weight (kg) | Mean (SD) | 76.0 (1.9) | 76.7 (2.5) | 75.3 (2.0) | 75.2 (2.9) | 77.7 (2.4) | — |
| Median | 76.4 | 76.7 | 75.5 | 74.8 | 78.3 | — | |
| Min, Max | 71.8, 78.0 | 72.6, 80.0 | 72.0, 78.2 | 72.4, 80.0 | 73.2, 80.0 | — | |
3.2. Safety
Oxylanthanum carbonate was well tolerated during the treatment period. Overall, there were no SAEs, severe or life‐threatening AEs (Grade 3–4), deaths, or AEs leading to discontinuation (Table S2). Most treatment‐emergent adverse events (TEAE) were mild in severity with only one report of moderately severe headache. Most treatment‐related TEAEs were in the GI Disorders System Organ Class (31%). Nausea was the most common treatment‐related TEAE across all patients (18.8%), followed by abdominal pain (9.4%), and flatulence (9.4%) (Table 3). A complete listing of AEs by de‐identified subject is shown in Table S3.
TABLE 3.
Summary of treatment related TEAEs in ≥ 1 subject by preferred term.
| Adverse event (AE) preferred term | Placebo (n = 8) | Oxylanthanum carbonate daily dose (mg TID) | |||
|---|---|---|---|---|---|
| 500 (n = 6) | 1000 (n = 6) | 1500 (n = 6) | 2000 (n = 6) | ||
| Any related AE | 2 (25.0) | 1 (16.7) | 4 (66.7) | 2 (33.3) | 4 (66.7) |
| Nausea | 0 (0.0) | 0 (0.0) | 2 (33.3) | 1 (16.7) | 3 (50.0) |
| Abdominal pain | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (16.7) | 1 (16.7) |
| Flatulence | 0 (0.0) | 0 (0.0) | 1 (16.7) | 2 (33.3) | 0 (0.0) |
| Headache | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (33.3) |
| Abdominal pain upper | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (16.7) |
| Fatigue | 1 (12.5) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Note: TEAE is an AE which occurred on or after the first administration of study drug.
3.3. Phosphate Binding Capacity
Across all treatment arms, based on the mechanism of action of oxylanthanum carbonate and reduced absorption of phosphate, urinary phosphorus excretion decreased from baseline in a dose‐dependent manner during treatment with oxylanthanum carbonate and fecal phosphorus excretion increased from baseline. The change in mean urinary phosphorus excretion from baseline to the evaluation period (Day 8 to 10) in subjects administered placebo was −12.0 mg/day (Table 4). In subjects administered oxylanthanum carbonate, reductions in urinary phosphorus excretion from baseline to the evaluation period were 244.9, 421.1, 633.6, and 713.4 mg/day for the 500, 1000, 1500, and 2000 mg TID treatment arms, respectively. The mean change in urinary phosphorus excretion from baseline showed a dose‐dependent trend (Figure 2). After the end of treatment on Day 12, urinary phosphorus excretion was similar to baseline values across treatment arms.
TABLE 4.
Summary of change in mean phosphorus excretion (mg/day) [urine] (A) urine.
| Placebo (n = 8) | Oxylanthanum carbonate (mg TID) | |||||
|---|---|---|---|---|---|---|
| 500 (n = 6) | 1000 (n = 6) | 1500 (n = 6) | 2000 (n = 6) | |||
| Baseline a | Mean (SD) | 630.2 (254.7) | 689.1 (379.6) | 640.4 (319.7) | 670.2 (319.0) | 741.5 (192.4) |
| Evaluation a | Mean (SD) | 618.2 (216.7) | 444.2 (255.1) | 219.4 (282.9) | 36.6 (91.0) | 28.1 (85.8) |
| Change from baseline | −12.0 | −244.9 | −421.1 | −633.6 | −713.4 | |
| p‐value b | 0.789 | 0.0008 | < 0.0001 | < 0.0001 | < 0.0001 | |
Note: Urine phosphorus concentrations for each day are recorded on the morning of the following day at a 24‐h interval; phosphorus values <LLOQ are imputed as 0; methodology: For every treatment‐dose arm, the change from baseline was determined.
Baseline is the mean of phosphorus from study day 1 to day 5; evaluation period is the mean of phosphorus from study day 8 to day 10.
p‐Values were calculated using paired t‐test.
FIGURE 2.
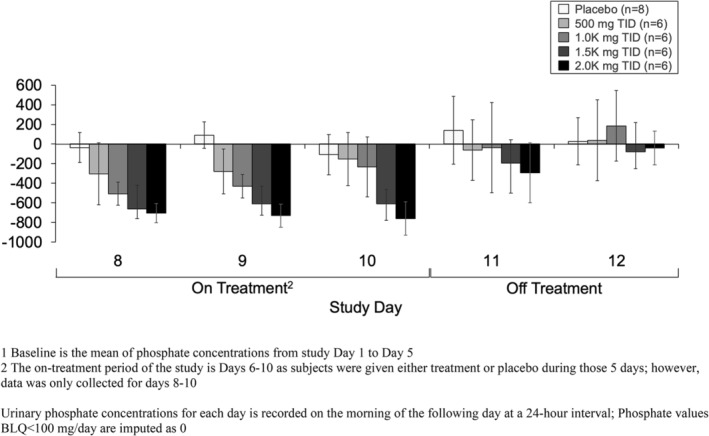
Mean (±SE) urinary phosphorus excretion change from baseline1 by oxylanthanum carbonate dose—oxylanthanum carbonate therapy demonstrated decreased urinary phosphorus excretion from baseline in a dose‐dependent manner.
Overall, fecal phosphorus excretion increased with higher doses of oxylanthanum carbonate (Table 5, Figure 3). The mean increase in fecal phosphorus excretion from baseline was significant in subjects treated with 500, 1500, and 2000 mg oxylanthanum carbonate TID.
TABLE 5.
Summary of change in mean phosphorus excretion (mg/day) [fecal].
| Placebo (n = 8) | Oxylanthanum carbonate (mg TID) | |||||
|---|---|---|---|---|---|---|
| 500 (n = 6) | 1000 (n = 6) | 1500 (n = 6) | 2000 (n = 6) | |||
| Baseline a | Mean (SD) | 337.9 (559.8) | 277.6 (369.0) | 326.1 (566.1) | 428.9 (470.0) | 177.5 (366.4) |
| Treatment period a | Mean (SD) | 370.0 (487.1) | 608.6 (540.5) | 847.5 (852.6) | 930.1 (622.0) | 1137.1 (412.6) |
| Change from baseline a | 32.1 | 331.0 | 521.4 | 501.2 | 959.5 | |
| p‐value b | 0.784 | 0.027 | 0.058 | 0.004 | < 0.0001 | |
Note: Urine phosphorus concentrations for each day are recorded on the morning of the following day at a 24‐h interval; phosphorus values <LLOQ are imputed as 0; methodology: For every treatment‐dose arm, the change from baseline was determined by subtracting the mean baseline value from the corresponding mean evaluation value for all patients.
Baseline is the mean of phosphorus from study day 1 to day 5; evaluation period is the mean of phosphorus from study day 8 to day 10.
p‐Values were calculated using paired t‐test.
FIGURE 3.
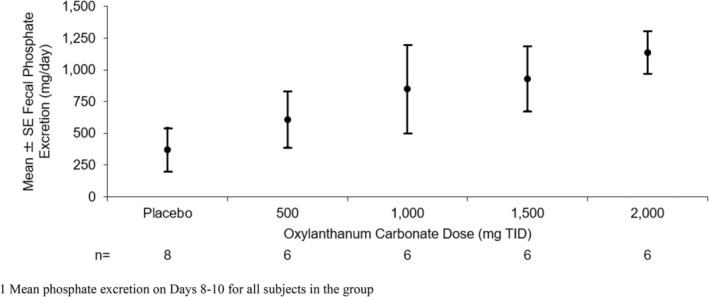
Mean (±SE) fecal phosphorus excretion on‐treatment1 by oxylanthanum carbonate dose—oxylanthanum carbonate therapy achieved increased fecal phosphorus excretion in a general dose‐dependent manner.
3.4. Serum Phosphate and Electrolyte Concentrations
Serum concentrations of phosphate and other electrolytes at Day 8 and 10 were not significantly different from baseline in any treatment arms (Table S3). Serum phosphate levels were unchanged from baseline compared to the end of treatment and ranged from +0.09 ± 0.20 mg/dL in the placebo group to −0.62 ± 0.36 mg/dL in the 2000 mg TID group. Serum calcium, magnesium, sodium, and potassium concentrations did not change throughout treatment in any treatment arm.
3.5. Lanthanum Exposure
Systemic absorption of lanthanum was minimal in most oxylanthanum carbonate‐treated subjects, prohibiting the quantification of systemic exposure in the 500 and 1500 mg TID groups. The highest serum lanthanum concentrations were observed in those receiving the highest dose (2000 mg TID). Lanthanum serum concentrations values were below the level of quantification in all subjects receiving 500 mg TID (Table 6). Of 384 serum samples, 53 (13.8%) had measurable lanthanum concentrations (above the assay lower limit of quantification: 0.5 ng/mL), with 86% of these measurable lanthanum serum samples being collected from the 2000 mg TID treatment arm. Additionally, 1 subject in the 1000 mg TID group, 2 subjects in the 1500 mg TID group, and 4 subjects in the 2000 mg TID group had measurable lanthanum concentrations. The time of the highest observed Ctrough was 48 to 120 h after the first dose. The mean highest observed Ctrough were 0.1, 0.2, and 0.7 ng/mL in subjects receiving 1000, 1500, and 2000 mg TID, respectively. The mean plasma concentration–time profiles for each treatment group are shown in Figure S1.
TABLE 6.
Mean (SD) highest observed Ctrough a and time of highest observed Ctrough by dose group following administration of oxylanthanum carbonate.
| Oxylanthanum carbonate (mg TID) | |||||
|---|---|---|---|---|---|
| 500 | 1000 | 1500 | 2000 | ||
| Highest observed Ctrough a (ng/mL) | Mean (SD) | BLQ | 0.131 (0.321) | 0.196 (0.304) | 0.665 (0.542) |
| n | — | 6 | 6 | 6 | |
| Time of highest observed Ctrough (Hour) | Median (Min, Max) | BLQ | 72 (72–72) | 108 (96–120) | 72 (48–120) |
| n | — | 1 | 2 | 4 | |
Abbreviations: BLQ, below the limit of quantification; NC, not calculable.
Concentrations less than the lower limit of quantitation (LLOQ < 0.500 ng/mL) were reported as and set to zero in the calculations.
4. Discussion
The ultimate goal of serum phosphate management in patients with ESKD is to achieve and maintain recommended serum phosphate concentrations of < 5.5 mg/dL, and ideally normal levels of < 4.5 mg/dL, to avoid the negative clinical outcomes associated with long‐term hyperphosphatemia [6, 21, 22]. However, a large proportion of patients receiving chronic hemodialysis fail to achieve serum phosphate level goals despite widespread treatment with oral binders [3, 4]. Some characteristics of currently available phosphate binders (e.g., high pill burden, large tablet size, tablets must be chewed before ingestion, GI adverse effects) [23] negatively impact the patient experience, which likely contributes to decreased patient adherence and treatment efficacy. Therapies that can minimize these burdens have the highest likelihood of positively impacting serum phosphate concentrations. However, all oral phosphate binders have the same limitation of only binding to ingested phosphates and do little to affect the overall positive body burden.
Oxylanthanum carbonate is a new compound that forms an insoluble phosphate complex, lanthanum phosphate, in the GI tract. Unlike lanthanum carbonate, oxylanthanum carbonate is formulated as tablets that are swallowed whole rather than chewed. The use of nanotechnology allows the tablet size containing 500 mg of lanthanum to be much smaller for oxylanthanum carbonate (0.35 cm3) than lanthanum carbonate (1.33 cm3) [24]. Consistent with this observation, a preclinical study showed that the drug volume required to bind 1 g of phosphate is much lower for oxylanthanum carbonate (5.6 mL) than lanthanum carbonate (19.8 mL) [24].
The current study was the first in‐human phase 1 clinical trial of oxylanthanum carbonate. Overall, oxylanthanum carbonate was effective at binding dietary phosphorus in the GI tract, as demonstrated by the increase in fecal phosphorus. Dose‐dependent changes in urinary and fecal phosphorus excretion were observed and the tablets were well‐tolerated. Consistent with lanthanum carbonate [25], systemic absorption of oxylanthanum carbonate was extremely low with all values below the level of quantification in subjects receiving 500 mg TID. When measurable, the time to highest observed trough concentrations of oxylanthanum carbonate following multiple‐dose administration was 48–120 h after the first dose.
Overall, due to reduced absorption of phosphate, oxylanthanum carbonate achieved significant decreases in urinary phosphorus excretion and significant increases in fecal phosphorus excretion compared to placebo. Both reductions in urinary phosphorus excretion and increases in fecal phosphorus excretion followed a dose‐dependent trend. The ability of oxylanthanum carbonate to reduce serum phosphate concentrations could not be evaluated in this study because the study population consisted of healthy volunteers. Given that these patients have a normal serum phosphate level to begin with, a non‐absorbed drug that is effective at binding phosphate in the GI tract would be expected to increase the amount of phosphate excreted in feces, which is consistent with our study results, and decrease the amount of phosphate absorbed into the serum. Healthy volunteers with functional kidneys would then be expected to excrete less phosphate in the urine thus maintaining homeostasis, which is again consistent with our study results. The consistent and significant decreases and increases in urinary and fecal phosphorus excretion, respectively, suggest that oxylanthanum carbonate will effectively reduce serum phosphate concentrations in the target population. Additionally, a recent randomized crossover study found that both oxylanthanum carbonate and lanthanum carbonate decreased urinary phosphorus excretion similarly and were found to be bioequivalent [26].
Although the study design was consistent with FDA clinical trial guidelines for non‐oncology phase 1 first‐in‐human studies, a limitation was the small sample size (which did not endow the study with enough formal statistical power to yield definitive safety or activity results) and lack of inclusion of patients with CKD and hyperphosphatemia. The healthy volunteer population with normal phosphate concentrations did not allow for evaluation of the serum phosphate‐lowering effects of oxylanthanum carbonate. An additional limitation was the low number of female subjects in the study, reducing the ability of this study to evaluate sex as a biological variable in tolerability and urinary and fecal phosphorus excretion. These limitations will be addressed in future studies with larger sample sizes of patients with CKD and hyperphosphatemia. Another limitation is that this study structure was not conducive to assessing liver accumulation of lanthanum and chronic liver damage with oxylanthanum carbonate usage: the study design did not include long‐term administration of oxylanthanum carbonate, liver imaging was not performed, and the lack of measurable serum lanthanum concentrations does not preclude accumulation on first pass. However, these potential risks are counter‐balanced by a 10‐year safety study of lanthanum carbonate that reported no clinically relevant changes in liver enzyme or bilirubin levels and no association of lanthanum carbonate with adverse safety outcomes in patients with ESKD [27]. A final potential limitation was selection bias stemming from the eligibility criteria that patients had to be able to swallow the study medication and follow a standardized phosphate‐controlled diet and/or meal schedule. In practice, swallowing tablets may be difficult for some patients (e.g., those with dysphagia) [28], and patients enrolled in a clinical study may not be representative of the broader population. However, oxylanthanum carbonate tablets are small in drug volume and tablet size (0.35 cm3 per 500 mg tablet [24]) and may be easier to swallow compared to currently available phosphate binders with larger drug volume and tablet sizes (1.33 cm3 per 500 mg and 2.67cm3 per 1000 mg tablet of lanthanum carbonate, and 0.75 cm3 per 667 mg calcium acetate tablet [24]). Additionally, the imperfect representation of real‐world circumstances (e.g., scheduled meals) and broader populations is an inherent limitation of all clinical trials.
Because there is a need for a new phosphate binder that is easy to ingest, exhibits minimal adverse effects, and maintains target serum phosphate levels, oxylanthanum carbonate may be a welcome choice for patients as it is effective at binding intestinal phosphorus and has been formulated as a small tablet that is swallowed. Compared to currently available formulations of chewable lanthanum carbonate, the novel oxylanthanum carbonate tablet has the potential to improve treatment outcomes. Future studies will assess the ability of oxylanthanum carbonate to reduce pill burden and enhance patient quality of life via easier dose administration, as well as the potential positive impact of a lower pill burden and easier administration on treatment adherence.
5. Conclusions
Oxylanthanum carbonate was formulated using nanoparticle technology that enables the tablet to be swallowed whole. In this phase 1 first‐in‐human study, the drug was well‐tolerated, demonstrated a favorable safety profile, and limited systemic exposure. Oxylanthanum carbonate effectively decreased urinary phosphorus excretion and increased fecal phosphorus excretion in a dose‐dependent manner. No changes in serum phosphate values were observed, which was expected given that healthy subjects were enrolled in this phase 1 clinical study.
Author Contributions
P.P., M.S.J., A.G., S.J.H., A.K., G.R., P.G., and W.F. wrote the manuscript, designed the research, performed the research, and analyzed the data.
Conflicts of Interest
Pablo Pergola is an employee at Renal Associates, PA, consults for Ardelyx, AstraZeneca, Bayer, Furoscix, GSK, Lilac, Novo Nordisk, Renibus, and Unicycive Therapeutics Inc. has ownership interest at Unicycive Therapeutics Inc. and has advisory or leadership roles at Ardelyx and Unicycive Therapeutics Inc. Through contracts with his practice, not individual contracts, Pablo E. Pergola receives research funding as principal or sub investigator on multiple clinical trials. Armando Garsd is a consultant for Unicycive Therapeutics Inc.; William F. Finn is a consultant for Unicycive Therapeutics Inc.; Atul Khare, Guru Reddy, and Pramod Gupta are employees at Unicycive Therapeutics Inc. All other authors declared no competing interests for this work.
Supporting information
Data S1.
Acknowledgments
Editorial support was provided by Xelay Acumen Group Inc. (funded by Unicycive Therapeutics Inc.). All authors have authorized the submission of their manuscript via Xelay Acumen Group Inc. and have approved all statements and declarations, including conflicting interests and funding.
Funding: The study was funded by Unicycive Therapeutics Inc.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. United States Renal Data System , USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States (Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020). [Google Scholar]
- 2. Barreto F. C., Barreto D. V., Massy Z. A., and Drüeke T. B., “Strategies for Phosphate Control in Patients With CKD,” Kidney International Reports 4 (2019): 1043–1056, 10.1016/j.ekir.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Serum phosphorus (3 month average), categories , “DOPPS Practice Monitor,” (2022), https://www.dopps.org/DPM‐HD/Files/meanphosphmgdl_c_overallTAB.htm.
- 4. Phosphate binder use, last 3 months , “DOPPS Practice Monitor,” (2022), https://www.dopps.org/DPM‐HD/Files/maxPBINDER_use_c_overallTAB.htm.
- 5. National Kidney Foundation , “K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease,” American Journal of Kidney Diseases 42 (2003): S1–S201. [PubMed] [Google Scholar]
- 6. Kestenbaum B., Sampson J. N., Rudser K. D., et al., “Serum Phosphate Levels and Mortality Risk Among People With Chronic Kidney Disease,” Journal of the American Society of Nephrology 16 (2005): 520–528, 10.1681/asn.2004070602. [DOI] [PubMed] [Google Scholar]
- 7. Chiu Y. W., Teitelbaum I., Misra M., de Leon E. M., Adzize T., and Mehrotra R., “Pill Burden, Adherence, Hyperphosphatemia, and Quality of Life in Maintenance Dialysis Patients,” Clinical Journal of the American Society of Nephrology 4 (2009): 1089–1096, 10.2215/cjn.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Camp Y. P., Vrijens B., Abraham I., Van Rompaey B., and Elseviers M. M., “Adherence to Phosphate Binders in Hemodialysis Patients: Prevalence and Determinants,” Journal of Nephrology 27 (2014): 673–679, 10.1007/s40620-014-0062-3. [DOI] [PubMed] [Google Scholar]
- 9. Floege J., Covic A. C., Ketteler M., et al., “A Phase III Study of the Efficacy and Safety of a Novel Iron‐Based Phosphate Binder in Dialysis Patients,” Kidney International 86 (2014): 638–647, 10.1038/ki.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joy M. S. and Finn W. F., “Randomized, Double‐Blind, Placebo‐Controlled, Dose‐Titration, Phase III Study Assessing the Efficacy and Tolerability of Lanthanum Carbonate: A New Phosphate Binder for the Treatment of Hyperphosphatemia,” American Journal of Kidney Diseases 42 (2003): 96–107, 10.1016/s0272-6386(03)00554-7. [DOI] [PubMed] [Google Scholar]
- 11. Finn W. F. and Joy M. S., “A Long‐Term, Open‐Label Extension Study on the Safety of Treatment With Lanthanum Carbonate, a New Phosphate Binder, in Patients Receiving Hemodialysis,” Current Medical Research and Opinion 21 (2005): 657–664, 10.1185/030079905x41453. [DOI] [PubMed] [Google Scholar]
- 12. Khare A., Gupta P., Reddy G., and Gupta S., “WCN23‐0106 In‐Vivo Phosphate Reduction: Lanthanum Dioxycarbonate vs Lanthanum Carbonate Tetrahydrate,” Kidney International Reports 8 (2023): S132, 10.1016/j.ekir.2023.02.301. [DOI] [Google Scholar]
- 13. Khare A. and Pramod G., “MO557: Lanthanum Dioxycarbonate Is Safe in Rats,” Nephrology, Dialysis, Transplantation 37 (2022): i409–i410, 10.1093/ndt/gfac074.002. [DOI] [Google Scholar]
- 14. Khare A. and Gupta P., “129 Lanthanum Dioxycarbonate Is Safe in Dogs,” American Journal of Kidney Diseases 79 (2022): S39–S40, 10.1053/j.ajkd.2022.01.134. [DOI] [Google Scholar]
- 15. Finn W. F., Denu‐Ciocca C. J., Joy M. S., et al., “TH‐PO756: Double‐Blind, Dose‐Ranging, Study of Lanthanum Dioxycarbonate (SPI‐014, RenaZorb) in Healthy Volunteers Shows High Phosphorus Binding Capacity,” Journal of the American Society of Nephrology 24 (2013): 271A. [Google Scholar]
- 16. Government, U.F , “Code of Federal Regulations.” (2022).
- 17. Guideline, I.H.T , “Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E2A.” (1994).
- 18. Association, W.M. World Medical Association Declaration of Helsinki , “Ethical Principles for Medical Research Involving Human Subjects,” Bulletin of the World Health Organization 79 (2001): 373–374. [PMC free article] [PubMed] [Google Scholar]
- 19. Services, U.D.o.H.a.H , “Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0.” (v.4.03: 14 June 2010), (2009).
- 20. SAS® 9.3 Software , http://support.sas.com/software/93/. (2024).
- 21. Lopes M. B., Karaboyas A., Bieber B., et al., “Impact of Longer Term Phosphorus Control on Cardiovascular Mortality in Hemodialysis Patients Using an Area Under the Curve Approach: Results From the DOPPS,” Nephrology, Dialysis, Transplantation 35 (2020): 1794–1801, 10.1093/ndt/gfaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Block G. A., Hulbert‐Shearon T. E., Levin N. W., and Port F. K., “Association of Serum Phosphorus and Calcium × Phosphate Product With Mortality Risk in Chronic Hemodialysis Patients: A National Study,” American Journal of Kidney Diseases 31 (1998): 607–617, 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 23. Wang S., Anum E. A., Ramakrishnan K., Alfieri T., Braunhofer P., and Newsome B., “Reasons for Phosphate Binder Discontinuation Vary by Binder Type,” Journal of Renal Nutrition 24 (2014): 105–109, 10.1053/j.jrn.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 24. Sprague S. M., Reddy G., Jermasek D., and Gupta P., “High Phosphate‐Binding Capacity of Oxylanthanum Carbonate With a Low Medication Volume: Comparison With Commercially Available Phosphate Binders,” American Journal of Nephrology 54 (2023): 219–223, 10.1159/000530989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pierce D., Hossack S., Robinson A., Zhang P., and Martin P., “Assessment of Pharmacodynamic Equivalence and Tolerability of Lanthanum Carbonate Oral Powder and Tablet Formulations: A Single‐Center, Randomized, Open‐Label, 2‐Period Crossover Study in Healthy Subjects,” Clinical Therapeutics 34 (2012): 1290–1300.e1292, 10.1016/j.clinthera.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 26. Mathur V., Walker M., Hasal S., Reddy G., and Gupta S., “Two‐Way Randomized Crossover Study to Establish Pharmacodynamic Bioequivalence Between Oxylanthanum Carbonate and Lanthanum Carbonate,” Clinical Therapeutics (2024): S0149–2918(24)00351‐5, 10.1016/j.clinthera.2024.11.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27. Hutchison A. J., Wilson R. J., Garafola S., and Copley J. B., “Lanthanum Carbonate: Safety Data After 10 Years,” Nephrology (Carlton, Vic.) 21 (2016): 987–994, 10.1111/nep.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carnaby‐Mann G. and Crary M., “Pill Swallowing by Adults With Dysphagia,” Archives of Otolaryngology – Head & Neck Surgery 131 (2005): 970–975, 10.1001/archotol.131.11.970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


