Abstract
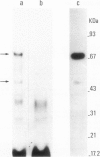
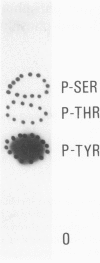
Estradiol receptor from rat uteri incubated with [32P] orthophosphate has been purified by diethylstilbestrol--Sepharose followed by heparin--Sepharose chromatography. The purified receptor, analyzed by centrifugation through sucrose gradients after incubation with monoclonal antibodies against purified estradiol receptor, appears to be labeled with 32P. The receptor preparation has been further purified by immunoaffinity chromatography and submitted to SDS--poly-acrylamide gel electrophoresis. A heavily 32P-labeled 68 kd protein and a very lightly 32P-labeled 48 kd protein, probably a proteolytic product of the 68 kd protein, were detected. Phosphoamino acid analysis of the receptor eluted from the immunoaffinity column shows that its 32P-labeling occurs exclusively on tyrosine. This is the first report on phosphorylation on tyrosine of a steroid receptor in tissue. It is consistent with our previous finding that a uterus estradiol receptor-kinase, which confers hormone binding ability to the estradiol receptor, in vitro phosphorylates this receptor exclusively on tyrosine. Calf uterus receptor binds with high specificity and affinity to monoclonal anti-phosphotyrosine antibodies covalently bound to Sepharose (Kd = 0.28 nM). Dephosphorylation of the receptor by nuclei containing the calf uterus nuclear phosphatase abolishes the interaction with antibodies. These results suggest that also in calf uterus, estradiol receptor is phosphorylated on tyrosine. Anti-phosphotyrosine antibodies bound to Sepharose have been used to partially purify the estradiol receptor from calf uterus.
Full text
PDF
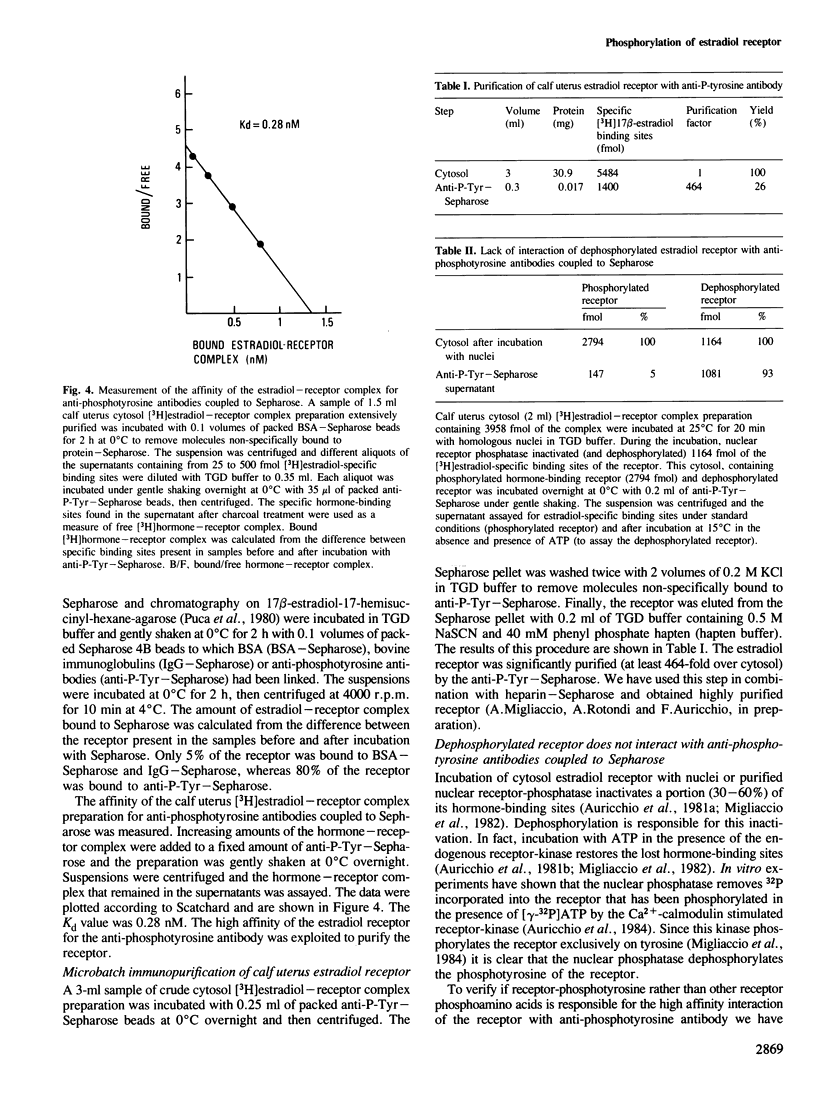



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auricchio F., Migliaccio A., Castoria G., Lastoria S., Rotondi A. Evidence that in vivo estradiol receptor translocated into nuclei is dephosphorylated and released into cytoplasm. Biochem Biophys Res Commun. 1982 May 14;106(1):149–157. doi: 10.1016/0006-291x(82)92070-8. [DOI] [PubMed] [Google Scholar]
- Auricchio F., Migliaccio A., Castoria G., Lastoria S., Schiavone E. ATP-dependent enzyme activating hormone binding of estradiol receptor. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1171–1178. doi: 10.1016/0006-291x(81)91571-0. [DOI] [PubMed] [Google Scholar]
- Auricchio F., Migliaccio A., Castoria G., Rotondi A., Lastoria S. Direct evidence of in vitro phosphorylation-dephosphorylation of the estradiol-17 beta receptor. Role of Ca2+-calmodulin in the activation of hormone binding sites. J Steroid Biochem. 1984 Jan;20(1):31–35. doi: 10.1016/0022-4731(84)90185-7. [DOI] [PubMed] [Google Scholar]
- Auricchio F., Migliaccio A. In vitro inactivation of oestrogen receptor by nuclei: prevention by phosphatase inhibitors. FEBS Lett. 1980 Aug 11;117(1):224–226. doi: 10.1016/0014-5793(80)80950-1. [DOI] [PubMed] [Google Scholar]
- Auricchio F., Migliaccio A., Rotondi A. Inactivation of oestrogen receptor in vitro by nuclear dephosphorylation. Biochem J. 1981 Feb 15;194(2):569–574. doi: 10.1042/bj1940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Czech M. P. The nature and regulation of the insulin receptor: structure and function. Annu Rev Physiol. 1985;47:357–381. doi: 10.1146/annurev.ph.47.030185.002041. [DOI] [PubMed] [Google Scholar]
- Daniel T. O., Tremble P. M., Frackelton A. R., Jr, Williams L. T. Purification of the platelet-derived growth factor receptor by using an anti-phosphotyrosine antibody. Proc Natl Acad Sci U S A. 1985 May;82(9):2684–2687. doi: 10.1073/pnas.82.9.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Foulkes J. G., Chow M., Gorka C., Frackelton A. R., Jr, Baltimore D. Purification and characterization of a protein-tyrosine kinase encoded by the Abelson murine leukemia virus. J Biol Chem. 1985 Jul 5;260(13):8070–8077. [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Ross A. H., Eisen H. N. Characterization and use of monoclonal antibodies for isolation of phosphotyrosyl proteins from retrovirus-transformed cells and growth factor-stimulated cells. Mol Cell Biol. 1983 Aug;3(8):1343–1352. doi: 10.1128/mcb.3.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen J. A., Carlson K. E., Heiman D. F., Robertson D. W., Wei L. L., Katzenellenbogen B. S. Efficient and highly selective covalent labeling of the estrogen receptor with [3H]tamoxifen aziridine. J Biol Chem. 1983 Mar 25;258(6):3487–3495. [PubMed] [Google Scholar]
- Krust A., Green S., Argos P., Kumar V., Walter P., Bornert J. M., Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986 May;5(5):891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A., Lastoria S., Moncharmont B., Rotondi A., Auricchio F. Phosphorylation of calf uterus 17 beta-estradiol receptor by endogenous Ca2+-stimulated kinase activating the hormone binding of the receptor. Biochem Biophys Res Commun. 1982 Dec 15;109(3):1002–1010. doi: 10.1016/0006-291x(82)92039-3. [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Rotondi A., Auricchio F. Calmodulin-stimulated phosphorylation of 17 beta-estradiol receptor on tyrosine. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5921–5925. doi: 10.1073/pnas.81.19.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncharmont B., Su J. L., Parikh I. Monoclonal antibodies against estrogen receptor: interaction with different molecular forms and functions of the receptor. Biochemistry. 1982 Dec 21;21(26):6916–6921. doi: 10.1021/bi00269a046. [DOI] [PubMed] [Google Scholar]
- Puca G. A., Medici N., Molinari A. M., Moncharmont B., Nola E., Sica V. Estrogen receptor of calf uterus: an easy and fast purification procedure. J Steroid Biochem. 1980 Jan;12:105–113. doi: 10.1016/0022-4731(80)90259-9. [DOI] [PubMed] [Google Scholar]
- Van Oosbree T. R., Kim U. H., Mueller G. C. Affinity chromatography of estrogen receptors on diethylstilbestrol-agarose. Anal Biochem. 1984 Feb;136(2):321–327. doi: 10.1016/0003-2697(84)90224-0. [DOI] [PubMed] [Google Scholar]
- Walter P., Green S., Greene G., Krust A., Bornert J. M., Jeltsch J. M., Staub A., Jensen E., Scrace G., Waterfield M. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]